Abstract
Background
As a cardiometabolic disease, hypertension has shown an obvious upward trend, becoming a global epidemic chronic disease. Lifestyle intervention is a fundamental method for lowering blood pressure. This systematic review and meta-analysis aimed to evaluate the effects of time-restricted eating (TRE) on blood pressure.
Methods
Studies were retrieved from the PubMed, Embase, Cochrane Library, and Web of Science databases to evaluate the effects of TRE on blood pressure. The time frame of search was from the start of database construction until July 14, 2022.There were no language restrictions. Meta-analysis and meta-regression were performed using Stata version 16. The weighted mean difference with 95% CI was used to assess the effect of TRE on blood pressure, heart rate, weight, blood glucose, total cholesterol, HDL-C, LDL-C, and triglycerides. The main ending of this article were blood pressure and heart rate, while the secondary ending were weight, blood glucose, total cholesterol, HDL-C, LDL-C, and triglycerides.
Results
Ten randomized controlled trials involving 694 patients were identified. TRE significantly reduced systolic blood pressure (SBP) (mean difference = −4.15; 95% CI: −6.73, −2.30; P < 0.0001), but had no significant effect on diastolic blood pressure (DBP) (mean difference = −2.06; 95% CI: −4.16, 0.02; P = 0.053) and no beneficial effect on heart rate (mean difference = 0.36; 95% CI: −2.83, 3.54; P = 0.0825). TRE promoted weight loss (mean difference = −1.63; 95% CI: −2.61, −0.64; P = 0.001) and decreased blood glucose levels (mean difference = −2.80; 95% CI: −4.64, −0.96; P = 0.003), but had no significant effect on total cholesterol (mean difference = 0.03, 95% CI: −10.01, 10.08; P = 0.995), HDL-C (mean difference = 0.85, 95% CI: −1.80, 3.49; P = 0.531), LDL-C (mean difference = −0.86, 95% CI: −6.47, 4.76; P = 0.764), or triglycerides (mean difference = −3.524, 95% CI: −9.49, 2.45; P = 0.248). In a separate meta-regression analysis, the degree of SBP change was related to weight loss (P = 0.044) but not to glucose improvement (P = 0.867).
Conclusions
The present meta-analysis suggests that TRE significantly reduced SBP, while no effect of reducing DBP was seen. The observed lower blood pressure may be attributed to significant weight loss. The effects of TRE on heart rate and blood lipid levels were not apparent.
Keywords: Time-restricted eating, Blood pressure, Meta-analysis
Background
On a global scale, modern humans face numerous complex chronic health challenges, such as obesity, diabetes, metabolic disease, and cardiometabolic disease. The presence of artificial light enables humans to remain active for 24 h a day. The chaotic activity-rest cycle indirectly disrupts the natural daily cycle of eating and fasting while unconsciously encouraging excess caloric intake. Chronically disrupted temporal regulation not only leads to metabolic disease, but also accelerates the aging process [1–3].
Recently, it has been suggested that intermittent and periodic fasting may be promising methods for optimizing longevity [4]. Fasting enables the body to enter alternative metabolic phases which are less dependent on glucose and more dependent on ketone body carbon sources. Nearly ten years ago, large-scale clinical trials [5–8] confirmed that intermittent fasting (IF) can reduce body weight and body fat, improve insulin sensitivity, reduce glucose and insulin levels, lower blood pressure (BP), improve lipid profiles, and reduce markers of inflammation and oxidative stress. The forms of IF investigated include alternate-day fasting, the 5:2 diet, and time-restricted eating (TRE). TRE, in which feeding times are restricted to specific times of the day, produced a similar effect to IF and conveyed benefits ranging from prevention to treatment of metabolic diseases. As a metabolic disease of the heart,, hypertension has shown an obvious upward trend, becoming a global epidemic chronic disease [9]. Lifestyle intervention is an essential method for lowering BP. However, thus far no systematic review or meta-analysis has directly linked TRE to BP . Therefore, the aim of this systematic review and meta-analysis was to evaluate the effects of TRE on BP.
Methods
This meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.
Search strategy
Based on the Population, Intervention, Comparator, Outcomes, and Study (PICOS) design framework, we searched the PubMed, Embase, Cochrane Library, and Web of Science databases to determine the effects of TRE on BP. Keywords, truncation symbols, medical subject heading (MeSH) terms, and Boolean operators (AND/OR) were used in the search strategy. The MeSH table retrieval formula was as follows: ‘time-restricted eating’ [MeSH] OR ‘blood pressure’ [MeSH]. The keyword search included the following terms: Time Restricted Feeding [Title/Abstract] OR Time Restricted Fasting [Title/Abstract] OR Intermittent Fasting [Title/Abstract] OR Fasting, Intermittent [Title/Abstract] OR Intermittent Fasting [Title/Abstract] OR Feeding, Time Restricted [Title/Abstract] OR Time Restricted Feedings [Title/Abstract] OR Ramadan [Title/Abstract] combined with Arterial Pressure [Title/Abstract] OR Hemodynamics [Title/Abstract] OR Systolic Blood Pressure (SBP) [Title/Abstract] OR Diastolic Blood Pressure (DBP) [Title/Abstract]. The most recent search was performed on July 14, 2022.
Inclusion criteria and data extraction
Studies were included in our meta-analysis if (1) the participants included mostly adults with metabolic diseases such as obesity, (2) intervention involved limiting daily meal times to 4–12 hours, (3) A normal dietary eating strategy group was set as the control group, (4) the study outcomes/metrics included BP, including baseline and post-intervention values, expressed as the mean and standard deviation (SD), (5) Priority was given to randomized control trials (RCT), but observational studies were also accepted, provided they included a control group without TRE. Articles were excluded based on the following criteria: (1) animal experiments rather than human adult studies; (2) editorials, letters, reviews, commentaries, or interviews; (3) lack of control group, such as only one experimental group; (4) the intervention method was not TRE; (5) BP values were incomplete.
Using the aforementioned inclusion/exclusion criteria, two independent researchers (Weihao Wang and Ran Wei) reviewed the titles and abstracts of each retrieved paper. If there were any uncertainties regarding qualifications, a third researcher studied the full text. A consensus was reached for all studies.
Data collection and registered protocols
Potential eligible articles were collected based on the inclusion and exclusion criteria. The following data were acquired from the selected articles: data source and setting, study design, participants, study duration, TRE regimen (Fasting: Feeding), total number, age, sex, body mass index (BMI), and indicators, including SBP, DBP, weight, glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C).
Because this study collected data from articles published by others for reporting, no ethics approval was required. We registered the systematic review and meta-analysis protocols at inplasy.com under the registration number Inplasy Protocol 202280057 (https://doi.org/10.37766/inplasy2022.8.0057).
Statistical analysis
Meta-analysis and meta-regression were performed using Stata version 16 (StataCorp, College Station, TX, USA). The weighted mean difference with 95% CI was used to assess the effect of TRE on BP, heart rate, weight, blood glucose, cholesterol, HDL-C, LDL-C, and triglycerides. We used the I2 index to quantify statistical heterogeneity. I2>50% indicated apparent heterogeneity, and <50% indicated no apparent heterogeneity. A random-effects model was used if apparent heterogeneity was observed. Otherwise, a fixed effects model was used. Subgroup analyses were performed to identify sources of heterogeneity. A sensitivity analysis was performed by individually excluding documents to assess the stability of the results. Publication bias was evaluated using a filled funnel plot. Egger’s test was employed to assess the probability of publication bias at a significance level of 10%. We used the GRADE software to evaluate the quality of evidence and evaluated the quality of the included studies using the risk of bias tool in Revman 5.4.
Results
Literature search
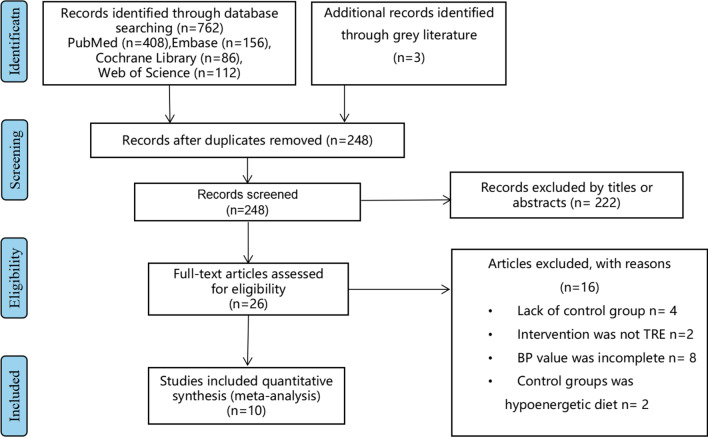
A total of 762 articles were retrieved (408 from PubMed, 86 from the Cochrane Library, 112 from the Web of Science, and 156 from Embase), of which 26 articles met the inclusion criteria. Sixteen articles were further excluded: 4 lacked a normal eating dietary strategy group [10–13]; in 2 articles, the intervention method was not TRE [14, 15]; in 8 articles, the BP value was incomplete [16–23]; and in 2 articles, the control group was a hypoenergetic diet [24, 25]. Therefore, 10 eligible studies [26–35] were included in the final meta-analysis. Of these, reference 24 included three groups, with two different TRE intervention groups. A flowchart of the study selection process is shown in Fig. 1.
Fig. 1.
Flowchart of the study selection
Study characteristics
The study characteristics are listed in Table 1, which includes data source and setting, study design, participants, study duration, TRE regimen (Fasting: Feeding), total number, age, sex, and BMI.
Table 1.
Characteristics of the studies investigating the effects of Time-Restricted Eating on blood pressure
| Reference | Study design | Participants | Study duration | TRE regimen (fasting: feeding) | Total number | Age (year) |
Sex: Male (%) | BMI (kg/m 2 ) |
|---|---|---|---|---|---|---|---|---|
| Cienfuegos [26] | RCT | Obesity | 8 weeks | 4-h TRF (20:4), 6-h TRF (18:6), or a control group | 58 | 47 [3] | 8.6 | 37 [1] |
| Phillips [27] | RCT | Metabolic syndrome | 6 months |
12 h time-restricted eating (12:12), standard dietary advice |
213 | 40.1 (13.3) | 28.6 | 24.9 (22.6-29.1) |
| Gabel [28] | – | Obese adults | 12 weeks | 8-h time restricted feeding(16:8) , matched historical control group | 46 | 50 [2] | 89.1 | 35 (1) |
| Aliasghari [29] | Observational trial | Nafld patients | – | fast for Ramadan(16:8) ,not to fast for Ramadan | 83 | 37.59 (7.06) | 68.7 | 30.09 (4.49) |
| Dewanti [30] | – | Male outdoor workers | 1 month | fast for Ramadan(16:8) ,not to fast for Ramadan | 100 | – | 100 | 24.2 (3.2) |
| Lowe [31] | RCT | Overweight and obesity | 12 weeks | 8-h time restricted feeding(16:8),consistent meal timing group | 50 | 43.8 (11.2) | 56.0 | 31.4 (4.0) |
| Chow [32] | – | Overweight | 12 weeks |
TRE (8-hour window), non-TRE(unrestricted eating) |
20 | 46.5 (12.4) | 15 | 33.8 (7.6) |
| Kotarsky [33] | RCT | Overweight and obese adults | 8 weeks | TRE consumed all calories between 12:00 p.m. and 8:00 p.m(16:8) ,normal eating (NE) dietary strategy group | 21 | 45 (3) | 14.3 | 29.8 (0.8) |
| Tinsley [34] | RCT | Active females | 8 weeks | TRF(~7.5 h/d),control diet | 40 | 22.0 (2.4) | 0 | – |
| Lin [35] | Randomized,open-label,parallel-group design, | Middle-aged women | 8 weeks | TRF group (limit 8 h of eating time and fasting for 16 h) , a non-TRF group | 63 | 50.1 (7.5) | 0 | 25.9 (3.7) |
Age and BMI values are expressed as mean ± SD.BMI, body mass index; RCT,randomized controlled trial;NAFLD, nonalcoholic fatty liver disease
Main outcomes
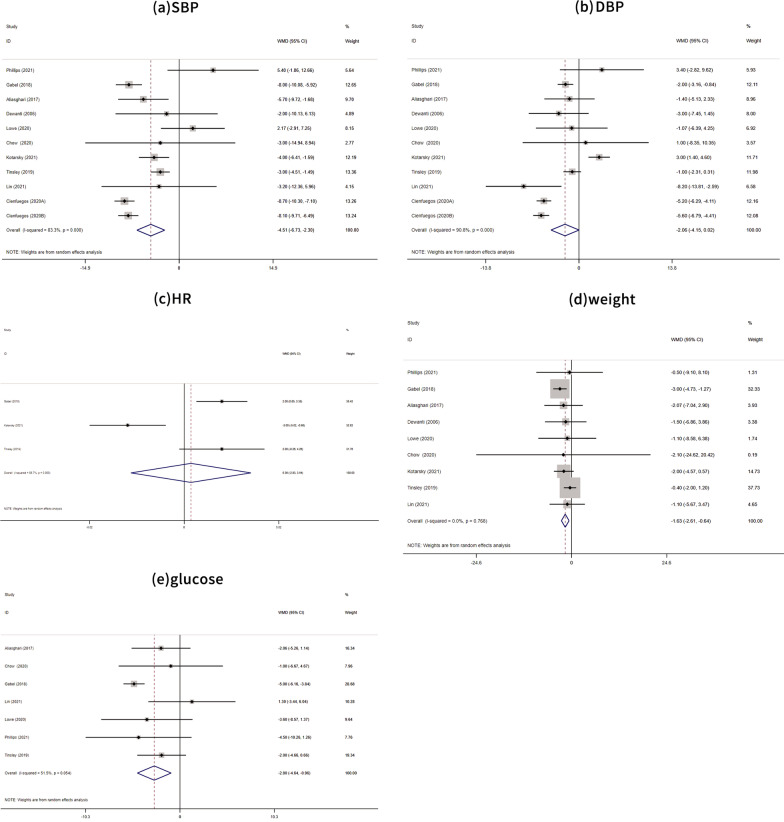
A meta-analysis of ten studies [26–35] showed that TRE significantly reduced SBP (mean difference = −4.15, 95% CI: −6.73, −2.30; P < 0.0001), as shown in Fig. 2a. Meanwhile, TRE had no significant effect on DBP (mean difference = −2.06, 95% CI: −4.16, 0.02; P = 0.053), as shown in Fig. 2b. Although 10 studies were included, the graphs have 11 rows because reference 24 includes three groups, including two different TRE intervention groups.
Fig. 2.
Forest plots of TRE vs. normal dietary eating in overall analyses, and a based on SBP changes b based on SBP changes c based on heart rate changes; d based on weight changes; and e based on blood glucose. TRE, time-restricted eating; SBP, systolic blood pressure
Secondary outcomes
A meta-analysis of three studies [28, 33, 34] showed that TRE was unable to lower the heart rate (mean difference =0.36, 95% CI: −2.83, 3.54; P = 0.825), as shown in Fig. 2c.
The results of a meta-analysis of nine [27–35] studies showed that TRE significantly reduced weight (mean difference = −1. 63, 95% CI: −2.61, −0.64; P = 0.001), as shown in Fig. 2d.
A meta-analysis of seven studies [27–29, 31, 32, 34, 35] showed that TRE significantly reduced blood glucose levels (mean difference = −2.80, 95% CI: −4.64, −0.96; P = 0.003), as shown in Fig. 2e.
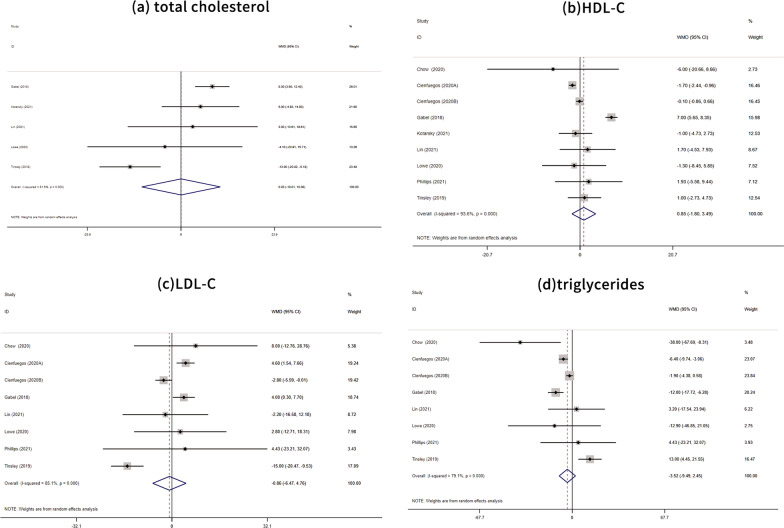
A meta-analysis of five studies [28, 31, 33–35] showed that TRE had no significant effect on total cholesterol (mean difference = 0.03, 95% CI: −10.01, 10.08; P = 0.995), as shown in Fig. 3a. A meta-analysis of eight studies [26–28, 31–35] showed that TRE insignificantly increased HDL-C (mean difference = 0.85, 95% CI: −1.80, 3.49; P = 0.531), as shown in Fig. 3b. A meta-analysis of seven studies [26–28, 31, 32, 34, 35] showed that TRE decreased LDL-C (mean difference = −0.86, 95% CI: −6.47, 4.76; P = 0.764), as shown in Fig. 3c. A meta-analysis of seven studies [26–28, 31, 32, 34, 35] showed that TRE decreased triglyceride levels (mean difference = −3.52, 95% CI: −9.49, 2.45; P = 0.248), as shown in Fig. 3d.
Fig. 3.
Forest plots of TRE vs. normal eating dietary on blood lipids a based on cholesterol levels; b based on HDL-C; c based on LDL-C; and d based on triglycerides. TRE time-restricted eating; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol
Subgroup analysis
As shown in Table 2, upon stratification by the duration of TRE intervention, we divided the studies into two groups 8 weeks (n = 3) and 12 weeks (n = 4). DBP was significantly reduced in patients with an intervention time of 12 weeks (WMD = −1.916 mmHg, 95% CI: −3.037, −0.794, P = 0.001), with low heterogeneity (I2= 0.0%).
Table 2.
Subgroup Analysis. SBP, systolic blood pressure; DBP, diastolic blood pressure; WMD, weighted mean difference; 95% CI, 95% confidence interval
| Index | Subgroup (weeks) | Num. of trials | WMD | 95% CI | P | I2 (P) |
|---|---|---|---|---|---|---|
| SBP | 12 | 4 | −3.197 | −11.140 to 4.745 | 0.430 | 85.2% (0.001) |
| 8 | 3 | −5.794 | −8.610 to −2.979 | 0.000 | 88.7% (0.000) | |
| DBP | 12 | 4 | −1.916 | −3.037 to−0.794 | 0.001 | 0.0% (0.782) |
| 8 | 3 | −3.054 | −6.422 to 0.314 | 0.076 | 96.0% (0.000) |
Sensitivity analysis
As shown in Table 3, sensitivity analysis was performed on nine indicators, including SBP, DBP, heart rate, weight, glucose, total cholesterol, HDL-C, LDL-C, and triglycerides. After excluding studies individually, the combined effect size before and after did not change significantly, indicating that the results of the meta-analysis were relatively stable.
Table 3.
Sensitivity Analysis. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR,heart rate;95% CI, 95% confidence interval
| Index | Effect | 95%CI lower | 95% CI upper | Change 95% CI lower | Change 95% CI upper |
|---|---|---|---|---|---|
| SBP | −4.51 | −6.73 | −2.30 | −7.36 | −1.39 |
| DBP | −2.06 | −4.15 | 0.02 | −4.6 | 0.63 |
| HR | 0.36 | −2.8 | 3.54 | −5.43 | 4.46 |
| Weight | −1.63 | −2.61 | −0.64 | −3.62 | 0.23 |
| Glucose | −2.80 | −4.64 | −0.96 | −5.07 | −0.31 |
| Total cholesterol | 0.03 | −10.01 | 10.08 | 14.58 | 11.92 |
| HDL-C | 0.85 | −1.80 | 3.49 | −3.25 | 4.86 |
| LDL-C | −0.86 | −6.47 | 4.76 | −8.64 | 7.15 |
| Triglycerides | −3.52 | −9.49 | 2.45 | −13.29 | 5.63 |
Meta-regression
We included nine studies that included changes in body weight and seven studies that included changes in blood glucose levels using meta-regression. The random-effect meta-regression of the primary meta-analysis on SBP revealed that body weight change (P = 0.044) predicted the size of the estimated treatment effect or explained heterogeneity between studies, while glucose (P = 0.867) did not. We further found that weight loss can predict TRE-induced SBP reduction.
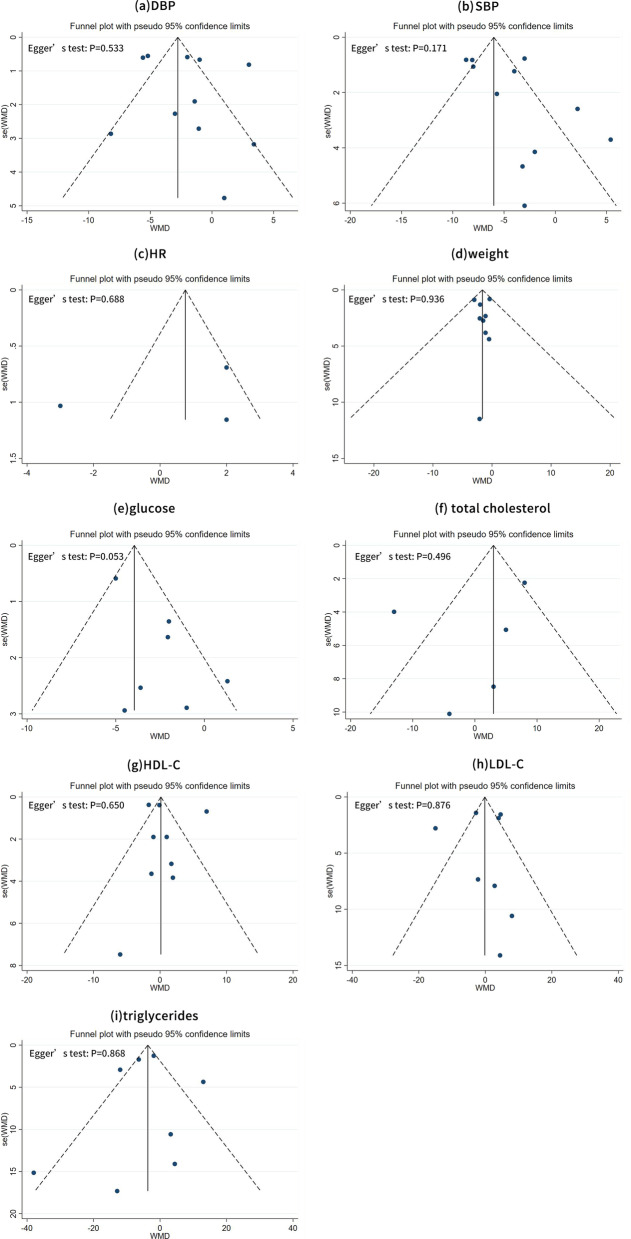
Funnel plots and egger tests
As reflected by the filled funnel plots (Fig. 4), there was obvious heterogeneity in the SBP (Fig. 4b). In addition, as indicated by Egger’s tests, there was a low probability of publication bias for all indexes under study (all P > 0.05).
Fig. 4.
Filled funnel plots of TRE vs. normal dietary eating a based on SBP changes; b based on SBP changes; c based on heart rate changes; d based on weight changes; e based on blood glucose levels; f based on cholesterol; g based on HDL-C; h based on the LDL-C; i based on triglycerides. TRE time-restricted eating; SBP systolic blood pressure; HDL-C high-density lipoprotein cholesterol; LDL-C low-density lipoprotein cholesterol
The quality of evidence assessment
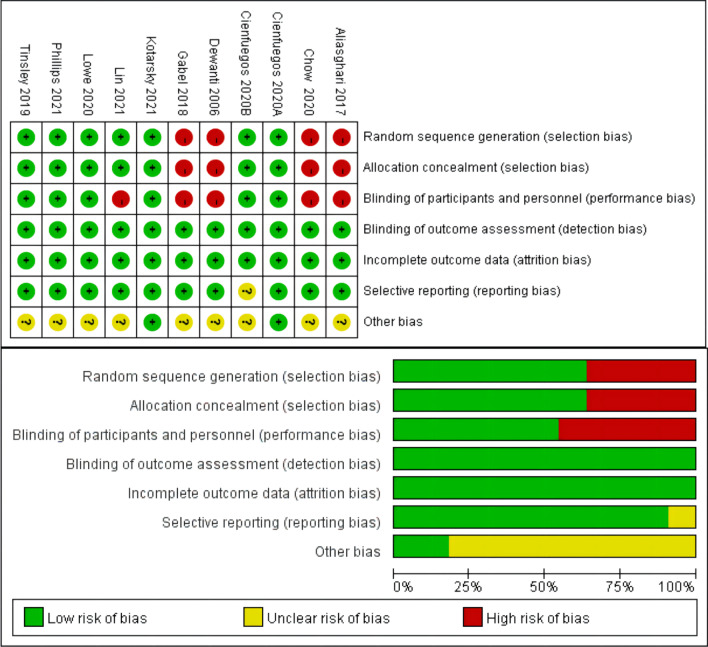
Ten studies included in our analysis were assessed for their quality. As shown in Fig. 5, we evaluated the quality assessment of the included studies using the risk of bias tool in Revman 5.4.
Fig. 5.
The quality of evidence assessment
Discussion
Unlike previous studies, which focused on body weight and blood glucose levels, this meta-analysis primarily focused on the effects of TRE on BP. To the best of our knowledge, this is the first meta-analysis to investigate this relationship. The results of a meta-analysis of ten studies showed that TRE significantly reduced SBP. Furthermore, this relationship remained consistent across the subgroup analyses. Meanwhile, TRE significantly reduced DBP in patients with an intervention duration of 12 weeks. These findings are critical because they provide a new perspective on decreasing BP.
In recent years, TRE, a weight loss method, has shown enormous promise in fighting obesity and metabolic diseases. The concept of TRE stems from studies of the effects of food timing on the circadian system. One of the major adverse consequences of circadian rhythm disturbances is an increased risk of cardiovascular diseases [36]. Cardiovascular diseases remain a leading cause of death globally in the general population; consequently, there is growing interest in the circadian regulation of cardiovascular health.
However, TRE was shown to have little beneficial effect on heart rate. Therefore, lower BP may be attributed to distinct weight loss. As is well known, there is a strong link between body weight and BP in obese patients [37]. Several mechanisms may lead to hypertension, such as insulin and leptin resistance, perivascular adipose tissue dysfunction, renal impairment, renin-angiotensin-aldosterone activation, and sympathetic nervous system activity [37]. Weight loss has beneficial effects on BP. In this study, we further analyzed the effect of TRE on blood lipid levels (total cholesterol, triglycerides, LDL-C, and HDL-C). Unfortunately, we found no obvious effects in our study. Recent studies [38] using metabolomics and lipidomics platforms have shown that the levels of hundreds of lipid species in the plasma are regulated by circadian rhythms, although the timing and magnitude of the lipid rhythms vary widely among individuals. A prior meta-analysis by Chen et al. [39] demonstrated that LDL-C levels were increased in the TRE group, which may partially explain our poor results.
Broadly speaking, the results of previous animal experimentation were consistent with the conclusions of the present study. An animal model conducted by Cote et al. [40] illustrated that limiting feeding in the active phase reduces BP. Hou et al. demonstrated [41] that TRE protects the circadian rhythm of BP in db/db mice by suppressing sympathetic activity during the light phase. Furthermore, Mager et al. [42] demonstrated that intermittent fasting reduces heart rate in rats. The increased variability in heart rate in rats caused by TRE may result from the enhanced activity of brainstem cholinergic cardiovagal neurons [43]. Godar et al. [44, 45] showed that intermittent fasting improved myocardial ischemia-reperfusion injury and reduced circulating cholesterol and triglyceride levels. In general, animal studies have demonstrated significant cardioprotective effects of TRE.
Our study has several limitations which should be mentioned. Firstly, as shown in Table 1, the vast majority of our subjects were overweight or obese, while patients with hypertension were excluded from the present study. Therefore, it is prudent to conclude that TRE improves BP in obese individuals. Whether this conclusion can be extended to hypertensive populations requires further investigation. Secondly, the heterogeneity of the studies was significant; this was attributed to the different time ranges and durations of the TRE interventions. The longest intervention period in the included studies lasted 12 months, whereas the shortest was 1 month. These factors may have had different degrees of influence on the results. Despite this, our results remained statistically significant. Furthermore, compared with DBP, TRE significantly reduced the SBP. Heart rate and peripheral resistance had a significant effect on DBP. This appears to explain the DBP changes with inconspicuous improvement in heart rate. At the same time, the included participants were generally younger with higher peripheral resistance; therefore, the changes in DBP were not distinct. In addition, intervention components such as calorie restriction or exercise are thought to influence BP, weight, lipids, and glucose. Kotarsky et al. [33] reported that participants in both the TRE and normal eating groups completed eight weeks of aerobic exercise and supervised resistance training. As both the experimental and control groups exercised, we excluded the liability of exercise on BP, weight, lipids, and glucose. Finally, the effect of different TRE restriction times (e.g. 4 h, 6 h, 8 h, 12 h) would have had an effect on the outcome. However, owing to the number of original studies, the TRE restriction time could not be refined further. Our results need to be validated by RCT studies with large samples and long-term follow-up.
There is already a wealth of evidence, both in the basic and clinical fields, to suggest the effects of TRE. However, there is still room for improvement in the following aspects. First, the effects of TRE on the heart rate and blood lipid levels were not apparent in our study, which was not consistent with the results of basic research. More convincing data on the effects of TRE on heart rate and blood lipids are still required. Simultaneously, we generalized our conclusions to patients with prehypertension and hypertension. As a simple and accessible means, TRE promises to improve the lifestyle of prehypertensive and hypertensive patients.
Conclusions
The present meta-analysis suggests that TRE significantly reduced SBP while the effect of reducing DBP was not obvious. The lower BP may be attributed to the significant weight loss. The effects of TRE on heart rate and blood lipid levels were not apparent. There is an urgent need for higher quality RCTs with a longer follow-up time to prove our results.
Acknowledgements
Not applicable.
Abbreviations
- IF
Intermittent fasting
- TRE
Time-restricted eating
- BP
Blood pressure
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analyses
- PICOS
Population, Intervention, Comparator, Outcomes, and Study
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
- SD
Standard deviation
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- BMI
Body mass index
- MeSH
Medical subject heading
- RESET
REStricted Eating Time
Author contributions
RW consulted literature and wrote the manuscript; LG and QP designed the review; WW assisted with writing and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82170848) and Beijing Hospital Project (BJ-2021-200).
Declarations
Ethics approval and consent to participate
This study collected data from published articles by others for reporting, so no ethics statement was required.
Consent for publication
NoT applicable.
Competing interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weihao Wang and Ran Wei have contribute equally
Contributor Information
Qi Pan, Email: panqi621@126.com.
Lixin Guo, Email: glx1218@163.com.
References
- 1.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manoogian ENC, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing ReS Rev. 2017;39:59–67. doi: 10.1016/j.arr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. doi: 10.1016/j.arr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. New Engl J Med. 2019;381(26):2541–51. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- 5.Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract. 2016;122:106–12. doi: 10.1016/j.diabres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity. 2016;24(9):1874–83. doi: 10.1002/oby.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Internal Med. 2017;177(7):930–8. doi: 10.1001/jamainternmed.2017.0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nature Rev Cardiol. 2021;18(11):785–802. doi: 10.1038/s41569-021-00559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92–104.e5. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PloS One. 2021;16(1):e0246186. doi: 10.1371/journal.pone.0246186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnason TG, Bowen MW, Mansell KD. Effects of intermittent fasting on health markers in those with type 2 diabetes: a pilot study. World J Diabetes. 2017;8(4):154–64. doi: 10.4239/wjd.v8.i4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesztyüs D, Cermak P, Gulich M, Kesztyüs T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11(12):2854. doi: 10.3390/nu11122854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh RB, Cornelissen G, Mojto V, Fatima G, Wichansawakun S, Singh M, et al. Effects of circadian restricted feeding on parameters of metabolic syndrome among healthy subjects. Chronobiol Int. 2020;37(3):395–402. doi: 10.1080/07420528.2019.1701817. [DOI] [PubMed] [Google Scholar]
- 16.Prasad M, Fine K, Gee A, Nair N, Popp CJ, Cheng B, et al. A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study. Nutrients. 2021;13(7):2148. doi: 10.3390/nu13072148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–21.e3. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quist JS, Jensen MM, Clemmensen KKB, Pedersen H, Bjerre N, Størling J, et al. Protocol for a single-centre, parallel-group, randomised, controlled, superiority trial on the effects of time-restricted eating on body weight, behaviour and metabolism in individuals at high risk of type 2 diabetes: the REStricted Eating Time (RESET) study. BMJ Open. 2020;10(8):e037166. doi: 10.1136/bmjopen-2020-037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience. 2020;42(2):667–86. doi: 10.1007/s11357-020-00156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moholdt T, Silva CP, Lydersen S, Hawley JA. Isolated and combined effects of high-intensity interval training and time-restricted eating on glycaemic control in reproductive-aged women with overweight or obesity: study protocol for a four-armed randomised controlled trial. BMJ Open. 2021;11(2):e040020. doi: 10.1136/bmjopen-2020-040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. 2020;75:32–43. doi: 10.1016/j.nutres.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Moro T, Tinsley G, Pacelli FQ, Marcolin G, Bianco A, Paoli A. Twelve months of time-restricted eating and resistance training improves inflammatory markers and cardiometabolic risk factors. Med Sci Sports Exercise. 2021;53(12):2577–85. doi: 10.1249/MSS.0000000000002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady AJ, Langton HM, Mulligan M, Egan B. Effects of 8 wk of 16:8 time-restricted eating in male middle- and long-distance runners. Med Sci Sports Exercise. 2021;53(3):633–42. doi: 10.1249/MSS.0000000000002488. [DOI] [PubMed] [Google Scholar]
- 24.Pureza IR, da Silva Junior AE, Praxedes DR, Vasconcelos LG, de Lima MM, de Melo IS, et al. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759–66. doi: 10.1016/j.clnu.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Pureza I, Melo ISV, Macena ML, Praxedes DRS, Vasconcelos LGL, Silva-Júnior AE, et al. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition. 2020;77:110796. doi: 10.1016/j.nut.2020.110796. [DOI] [PubMed] [Google Scholar]
- 26.Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32(3):366–78.e3. doi: 10.1016/j.cmet.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips NE, Mareschal J, Schwab N, Manoogian ENC, Borloz S, Ostinelli G, et al. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients. 2021;13(3):1042. doi: 10.3390/nu13031042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53. doi: 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliasghari F, Izadi A, Gargari BP, Ebrahimi S. The effects of Ramadan fasting on body composition, blood pressure, glucose metabolism, and markers of inflammation in NAFLD patients: an observational trial. J Am College of Nutr. 2017;36(8):640–5. doi: 10.1080/07315724.2017.1339644. [DOI] [PubMed] [Google Scholar]
- 30.Dewanti L, Watanabe C, Sulistiawati OR. Unexpected changes in blood pressure and hematological parameters among fasting and nonfasting workers during Ramadan in Indonesia. Eur J Clin Nutr. 2006;60(7):877–81. doi: 10.1038/sj.ejcn.1602393. [DOI] [PubMed] [Google Scholar]
- 31.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Internal Med. 2020;180(11):1491–9. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow LS, Manoogian ENC, Alvear A, Fleischer JG, Thor H, Dietsche K, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. 2020;28(5):860–9. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotarsky CJ, Johnson NR, Mahoney SJ, Mitchell SL, Schimek RL, Stastny SN, et al. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol Rep. 2021;9(10):e14868. doi: 10.14814/phy2.14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628–40. doi: 10.1093/ajcn/nqz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin YJ, Wang YT, Chan LC, Chu NF. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition. 2022;93:111504. doi: 10.1016/j.nut.2021.111504. [DOI] [PubMed] [Google Scholar]
- 36.Chellappa SL, Vujovic N, Williams JS, Scheer F. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019;30(10):767–79. doi: 10.1016/j.tem.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantin F, Giani A, Zoico E, Rossi AP, Mazzali G, Zamboni M. Weight loss and hypertension in obese subjects. Nutrients. 2019;11(7):1667. doi: 10.3390/nu11071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooley JJ. Circadian regulation of lipid metabolism. Proc Nutr Soc. 2016;75(4):440–50. doi: 10.1017/S0029665116000288. [DOI] [PubMed] [Google Scholar]
- 39.Chen JH, Lu LW, Ge Q, Feng D, Yu J, Liu B, et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2021;2021:1–17. doi: 10.1080/10408398.2021.1974335. [DOI] [PubMed] [Google Scholar]
- 40.Cote I, Toklu HZ, Green SM, Morgan D, Carter CS, Tümer N, et al. Limiting feeding to the active phase reduces blood pressure without the necessity of caloric reduction or fat mass loss. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R751–r8. doi: 10.1152/ajpregu.00076.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou T, Su W, Duncan MJ, Olga VA, Guo Z, Gong MC. Time-restricted feeding protects the blood pressure circadian rhythm in diabetic mice. Proc Natl Acad Sci USA. 2021 doi: 10.1073/pnas.2015873118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20(6):631–7. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 43.Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014;129(4):573–80. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, et al. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy. 2015;11(9):1537–60. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]