Abstract
Background
Optimal treatment strategies for patients with heart failure with preserved ejection fraction (HFpEF) remain uncertain. The goal of this study was to compare the treatment effects of different therapeutic agents for patients with HFpEF.
Methods
Randomized controlled trials (RCTs) published before June 2022 were searched from PubMed, Clinical Trials gov, and the Cochrane Central Register databases. Combined odds ratios (ORs) with 95% confidence intervals (CI) were calculated for the primary and secondary outcomes. All-cause death was the primary endpoint and cardiac death, hospitalization for HF, and worsening HF (WHF) events were secondary endpoints in this meta-analysis.
Results
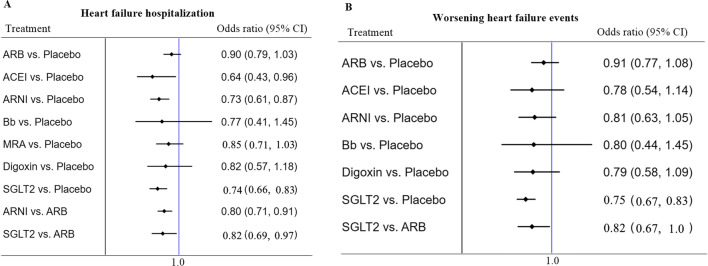
Fifteen RCTs including 31,608 patients were included in this meta-analysis. All-cause and cardiac death were not significantly correlated between drug treatments and placebo. Compared with placebo, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor neprilysin inhibitors (ARNIs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors significantly reduced HF hospitalizations [odds ratio (OR) = 0.64, (95% confidence interval (95%CI 0.43 − 0.96), OR = 0.73, (95%CI 0.61 − 0.86), and OR = 0.74, (95%CI 0.66 − 0.83), respectively] without heterogeneity among studies. Only SGLT2 inhibitors significantly reduced WHF events [OR = 0.75, (95%CI 0.67 − 0.83)].
Conclusions
No treatments were effective in reducing mortality, but ARNIs, ACEIs or SGLT2 inhibitors reduced HF hospitalizations and only SGLT2 inhibitors reduced WHF events for patients with HFpEF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01679-2.
Keywords: Heart failure with preserved ejection fraction, All-cause death, Cardiac death, HF hospitalization, Worsening HF events, Randomized control trials, Registered in PROSPERO (CRD42021247034)
Background
Heart failure (HF) is associated with substantially high morbidity and mortality as well as high rates of rehospitalization [1, 2]. HF with preserved ejection (HFpEF) has evolved into a major type of HF [3, 4]. HFpEF was previously defined as HF accompanied by left ventricular ejection fraction (LVEF) > 40%, but is currently defined as LVEF > 50% with no history of improved LVEF from < 40% [5]. Mortality (defined as 1-year mortality of approximately 10 − 30% and 5-year mortality of > 50%) and readmission rates of patients with HFpEF are higher compared to those patients with HF with reduced ejection fraction (HFrEF) [6]. Due to the pathophysiological heterogeneity of HFpEF, there is a lack of therapeutic agents that effectively treat these outcomes, which presents a major clinical challenge for patients with HFpEF.
The 2021 European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic HF recommend the use of diuretics, angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor neprilysin inhibitors (ARNI), and mineralocorticoid receptor antagonists (MRA) for HFpEF. However, there is a lack of convincing evidence for the ability of these treatments to reduce mortality7. Recently, the EMPEROR-Preserved and the DELIVER study showed that sodium-glucose cotransporter-2 (SGLT2) inhibitors (empagliflozin and dapagliflozin) have a positive effect on composite outcomes in HFpEF [8, 9]. Accordingly, we conducted a network meta-analysis to compare the effects of these drugs with placebo for the treatment of HFpEF.
Materials
Search strategy
PubMed, Clinical Trial gov, and the Cochrane Central Register were searched to identify articles published before May 30, 2022 (Additional file 1: Tables S1, S2, S3). The MeSH terms included “heart failure with preserved ejection fraction”, “diastolic heart failure”, “angiotensin receptor neprilysin inhibitor or sacubitril-valsartan”, “angiotensin converting enzyme inhibitors”, “angiotensin receptor blockers”, “beta blockers”, “mineralocorticoid receptor antagonists”, “digoxin”, “phosphodiesterase-5 inhibition or sidenafi”, “vericiguat”, “sodium-glucose cotransporter-2”, and “diuretic”.
Eligibility criteria
The inclusion criteria were as follows: (i) types of studies: randomized controlled trials (RCTs); (ii) types of participants: HF with LVEF ≥ 40%; (iii) types of interventions: treatment groups received oral drugs; (iv) types of comparators: placebo or no drugs; (v) types of outcome measures: data on all-cause and cardiac mortality, HF hospitalizations, or worsening HF (WHF) events; and (vi) articles published in English. Exclusion criteria were as follows: (i) duplicate publications; (ii) subgroup studies; and (iii) lack of data on endpoint.
Study outcomes
All-cause death was the primary endpoint, and cardiac death, HF hospitalization, and WHF events (defined as deterioration in heart failure symptoms and signs requiring an intensification of therapy) were the secondary endpoints.
Data extraction and quality assessment
Data were extracted independently by two authors (Y.W.L. and Z.G.C.) through a detailed review of the full text. Any disagreement between the two researchers was discussed and decided by a third author (S.H.D.). We assessed the risk of bias, publication bias, and overall quality of the literature using the Cochrane Collaboration tool, funnel plots, and confidence in network meta-analysis (CINeMA), respectively.
Statistical analysis
We used STATA software (version 16.0) for statistical analysis. Pooled odds ratios (OR) and their 95% confidence intervals (CI) were adopted to assess endpoint events. The surface under the cumulative ranking (SUCRA) curve was used to assess the best treatment strategy. Heterogeneity between studies was analyzed using the I2 statistic.
Results
Characteristics of included studies
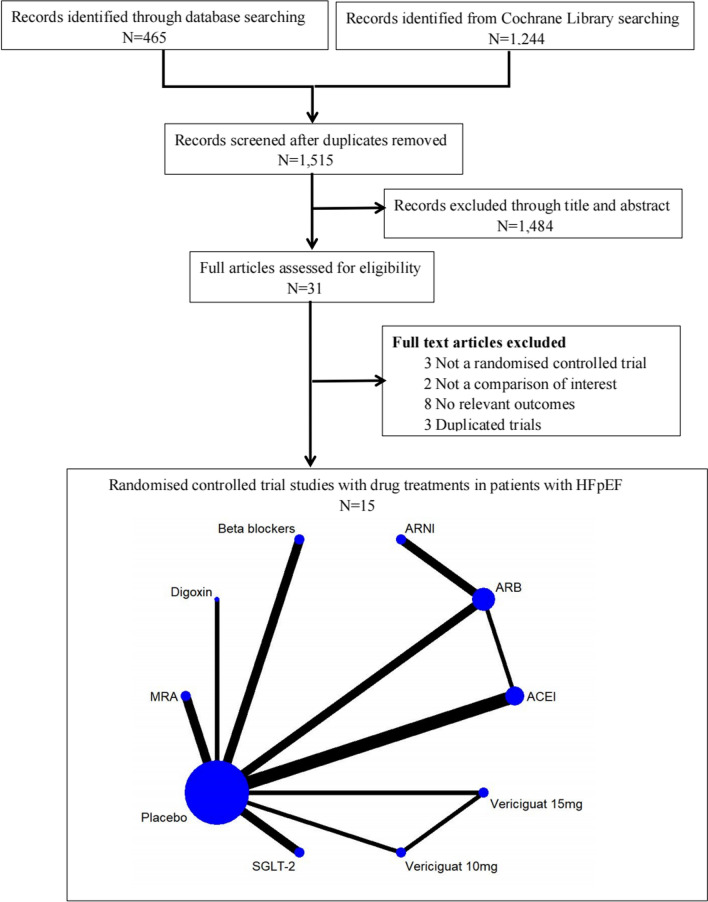
A total of 1709 related articles were identified through the database search, of which 168 articles were duplicates. After reading the titles and abstracts, 1214 articles were removed because they did not meet the inclusion criteria. In addition, 16 articles were removed due to lack of endpoint data. Ultimately, 15 RCTs [9–23] (Fig. 1) were included in this meta-analysis. Eight were double-blind, two were single-blind, and five were open-label studies. Finally, 31,608 patients were included (15,969 in the intervention group; 15,639 in the control group) with a follow-up period of 0.5 to 4 years. All patients enrolled were elderly (mean age 66.7 to 89 years) without differences in baseline profiles (Table 1).
Fig. 1.
Flow diagram of the study selection process HF heart failure, MRA mineralocorticoid receptor antagonist, SLGT2 sodium-glucose cotransporter-2, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, ARNI angiotensin receptor neprilysin inhibitor, MRA mineralocorticoid receptor antagonist
Table 1.
Baseline characteristics of included RCTs
| Study | Year/country | Study | Intervention group | Control group | Design | Age, y | NYHA III-IV, % | All-cause mortality | Cardiovascular mortality | Heart failure hospitalization | Worsening heart failure events | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aronow WS | 1997 USA | Open-label | Propranolol (n = 79) | Placebo (n = 79) | LVEF ≥ 40%, > 62 years age | 81 ± 8 vs. 81 ± 7 | 47 vs. 49 | 44/79 vs. 60/797 | / | / | / | 35 months |
| Yusuf, S | 2003 Canada | Double-blind | Candesartan (n = 1514) | Placebo (n = 1509) | CHARM preserved trial, LVEF ≥ 40% |
67.2 ± 11.1 vs 67.1 ± 11.1 |
38.5 vs. 40 | / | 170/1514 vs. 170/1509 | 241/1514vs.276/1509 | / | 36.6 months |
| Zi M | 2003 UK | Double-blind | Quinapril (n = 36) | Placebo (n = 38) | LVEF ≥ 40%, > 62 years age | 77 ± 7 vs. 78 ± 7 | 16.7 vs. 26.3 | 1/36 vs. 1/38 | 1/36 vs. 1/38 | 2/36 vs. 5/38 | 0/36 vs. 4/38 | 6 months |
| Cleland, JG | 2006 UK | Double-blind | perindopril (n = 424) | Placebo (n = 426) | PEP-CHF, LVEF ≥ 40%, > 70 years age | 75 (72,79) vs. 75 (72,79) | 23 vs. 26 | 17/424 vs. 19 /4267 | 10/424 vs. 17/426 | 34/424 vs. 53 /426 | 59/424 vs. 71/426 | 12 months |
| Ahmed, A | 2006 USA | Open-label | Digoxin (n = 492) | Placebo (n = 496) | LVEF ≥ 45%, > 45 years age | 66.7 ± 10.7 vs. 66.9 ± 9.9 | 21.5 vs. 22.6 | 115/492 vs. 116/496 | 81/492 vs. 81/496 | 61/492 vs. 73/496 | 89/492 vs. 108/496 | 37 months |
| Massie,BM | 2008 USA | Single-blind | Irbesartan (n = 2067) | Placebo (n = 2061) | PRESERVE, LVEF ≥ 45%, > 60 years age | 72 ± 7 vs. 72 ± 7 | 80 vs. 79 | 445/2067 vs. 436 /2061 | 311/2067 vs. 302 /2061 | 325/2067 vs. 336 /2061 | 291/2067 vs. 314 /2061 | 49.5 months |
| Yip, GW | 2008 Hong Kong | Open-label | Irbesartan (n = 53) vs. Ramipril (n = 39) | Placebo (n = 47) | HK-PROBE, LVEF ≥ 45%, > 18 years age | 75 ± 8.5 vs. 74 ± 6.1 vs. 73 ± 8.4 | 30.4 vs. 33.3 vs. 28.0 | 1/53 vs. 0/39 vs. 3/47 | 1/53 vs. 0/39 vs. 1/47 | 6/53 vs. 5/39 vs. 6/47 | / | 12 months |
| Solomon,SD | 2012 USA | Double-blind | Sacubitril–valsartan (n = 149) | Valsartan (n = 152) | PARAMOUNT, LVEF ≥ 45%, > 18 years age | 70.9 ± 9.4 vs. 71.2 ± 8.9 | 19 vs. 21 | 1/149 vs. 2/152 | 1/149 vs. 2/152 | / | 9/149 vs. 12/152 | 36 weeks |
| Edelmann, F | 2013 Austria | Double-blind | Spironolactone (n = 213) | Placebo (n = 209) | Aldo-DHF, LVEF ≥ 50%, > 18 years age | 67 ± 8 vs. 67 ± 8 | 15 vs. 12 | 1/213 vs. 0/209 | 1/213 vs. 0/2091 | 21/213 vs. 15/209 | / | 12 months |
| Yamamoto,K | 2013 Japan | Open-label | Carvedilol (n = 120) | Placebo (n = 125) | DHF, LVEF > 40%, > 20 years age | 73 ± 10 vs. 71 ± 11 | 15 vs. 8 | 18/120 vs. 21/1257 | 8/120 vs. 7/1255 | 21/120 vs. 27/125 | 25/120 vs. 31/125 | 24 months |
| Pitt, B | 2014 USA | Double-blind | Spironolactone (n = 1722) | Placebo (n = 1723) | TOPCAT, LVEF ≥ 45%, > 18 years age | 68.7 (61,76.4) vs. 68.7 (60.7,75.5) | 33.4 vs. 32.6 | 252/1722 vs. 274/1723 | 160/1722 vs. 176/1723 | 206/1722 vs. 245/1723 | / | 3.3 years |
| Solomon, SD | 2019 USA | Single-blind | Sacubitril–valsartan (n = 2407) | Valsartan (n = 2389) | PARAGON-HF, LVEF ≥ 45%, > 18 years age | 72.7 ± 8.3 vs. 72.5 ± 8.5 | 19.3 vs. 20.3 | 342/2407 vs. 349/2389 | 204/2407 vs. 212/2389 | 690/2407 vs. 797/2389 | 202/2407 vs. 221/2389 | 4 years |
| Armstrong, PW | 2020 Canada | Open-label | Vericiguat 15 mg (n = 264) vs. Vericiguat 10 mg (n = 262) | Placebo (n = 262) | VITALITY-HFpEF, LVEF ≥ 45%, > 45 years age | 73.1 ± 9.1 vs. 72.2 ± 9.7 vs. 72.8 ± 9.4 | 42.4 vs. 41.4 vs. 40.5 | 10/264 vs. 15/262 vs. 7/262 | 8/264 vs. 12/262 vs. 4/262 | / | / | 24 weeks |
| Anker SD | 2021 Germany | Double-blind | Empagliflozin (n = 2997) | Placebo (n = 2991) | EMPEROR-Presersed LVEF ≥ 50% | 71.8 ± 9.3 vs. 71.9 ± 9.6 | 422 vs. 427 | 219 vs.244 | 259 vs 352 | 362 vs 485 | 36 months | |
| Solomon, SD | 2022 USA | Double-blind | Dapagliflflozin (n = 3131) | Placebo (n = 3132) | DELIVER LVEF > 40% | 71.8 ± 9.6 vs. 71.5 ± 9.5 | 26.1 vs. 23.4 | 497 vs 526 | 231 vs. 261 | 329 vs 418 | 368 vs. 455 | 2.95 years |
HF heart failure, HFpEF heart failure with preserved ejection fraction, LVEF left ventricular ejection fraction; “/” = no data available
Primary and secondary endpoints
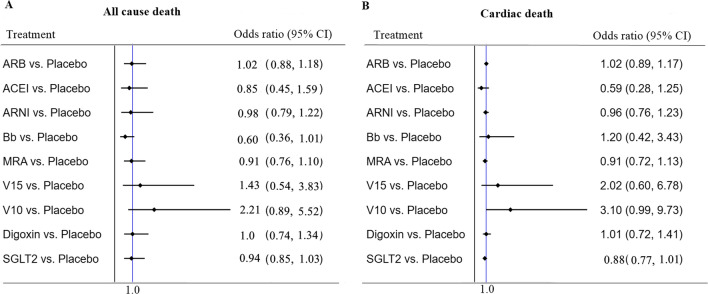
Thirteen of the 15 included RCTs (excluding Yusuf S et al.) reported all-cause death data. No drug treatments were found to significantly reduce all-cause death. Fourteen RCTs (excluding Wilbert S et al.) reported cardiac death data, whereas no difference was found in this network meta-analysis (Fig. 2).
Fig. 2.
All-cause mortality (primary outcome): Forest plot (estimates as hazard ratio) of all trials. Bb beta blockers, V15 vericiguat 15 mg, V10 vericiguat 10 mg
Compared to placebo, the ACEIs, ARNIs, and SGLT2 inhibitors significantly reduced hospitalization due to HF (HF hospitalization) [OR = 0.64 (95%CI 0.43 − 0.96), OR = 0.73 (95%CI 0.61 − 0.86), and OR = 0.74 (95%CI 0.66 − 0.83), respectively], without heterogeneity among studies. Only the SGLT2 inhibitors significantly reduced WHF events [OR = 0.75 (95%CI 0.67 − 0.83)] (Fig. 3).
Fig. 3.
HF hospitalization and worsening HF events (secondary outcome): Forest plot (estimates as hazard ratio) of all trials
The SUCRA rankogram plots for the primary endpoint showed that beta-blockers were the best treatment strategy for reducing all-cause death, followed by ACEIs, ARNIs, and SGLT2 (Additional file 2: Figure S1). However, no treatment was significantly different compared to placebo.
Risk of bias assessment and publication bias
The Cochrane Collaboration tool was used for quality assessment. There were four studies with high attrition bias and selection bias, one with a large performance bias, and the remaining studies had low risk of bias (Additional file 2: Figure S2). No publication bias was found (Additional file 2: Figure S3). The CINeMA framework showed high quality of the included literature and low bias in the included studies (Additional file 3, 4, 5, 6).
Discussion
The primary findings of this network meta-analysis are as follows: (i) There were no differences in all-cause and cardiac death between the drug treatments in patients with HFpEF; (ii) ARNIs, ACEIs, and SGLT2 inhibitors significantly reduced HF hospitalization, and only SGLT2 inhibitors reduced the risk of WHF events; and (iii) Angiotensin receptor blockers (ARBs) failed to reduce HF hospitalization.
HFpEF results from a complex interaction between risk factors, comorbidities, and cardiac pathology, affecting LV structure, hemodynamics, and systemic organ function. The underlying pathophysiological mechanisms of HFpEF include: (i) increased LV end-diastolic pressure (LVEDP) as evidenced by thickened LV walls and/or enlarged left atria [24, 25]; (ii) pulmonary vascular disease or dysfunction, and right ventricular failure [26]; and (iii) expansion of plasma volume and high plasma levels of natriuretic peptides [27–29]. These complex and diverse pathophysiological mechanisms make it difficult for the current treatment strategies to reduce mortality in HFpEF patients.
Our network meta-analysis showed that none of the current drug treatments have been able to reduce mortality in patients with HFpEF. Large RCTs focused on HFpEF outcomes, including PEP-CHF (perindopril) [13], CHARM-Preserved (candesartan) [11], I-PRESERVE (irbesartan) [15], TOPCAT (spironolactone) [20], DIG-Preserved (digoxin) [14], J-DHF (carvedilol) [19], VITALITY-HFpEF (vericiguat) [22], and PARAGON-HF (sacubitril/valsartan) [21], have failed to achieve their primary endpoints. However, more than 86%, 80%, and 24% of the included patients in the PARAGON-HF study received ACEI/ARB, beta-blockers, and MRA, respectively. The guideline-directed medical therapy (GDMT) may have overlapped with the mechanisms of ARNIs, resulting in no statistical difference in the primary endpoint. Additionally, subgroup analysis suggests a possible reduction (45–57%) in cardiac death in females. The EMPEROR-preserved study showed that empagliflozin significantly reduced the composite risk of cardiac death or HF hospitalization by 21% in patients with HFpEF compared to placebo, regardless of the presence of diabetes (HR 0.79, 95% CI 0.69–0.9, p < 0.0001) [23], while the DELIVER study showed that dapagliflozin significantly reduced the primary composite risk of worsening HF or cardiac death by 18% (HR 0.82, 95% CI 0.73–0.92, p < 0.001) [9]. However, it must be recognized that diabetes is a significant factor for HF hospitalization, and the cardiac mortality factor were not different between groups in the two SGLT2 inhibitor studies [OR = 0.88 (95%CI 0.73 − 1.07) and OR = 0.88 (95%CI 0.74 − 1.05), respectively]. These findings are consistent with a recent meta-analysis that included 12 RCTs (11 studies included subgroup analyses) comparing SGLT2 inhibitors with placebo in 10,883 patients with HFpEF [30].
In patients with HFpEF, most of whom are older, reducing the risk of HF hospitalization can also significantly improve the quality of life. Accordingly, ACEIs [EF ≥ 40%; OR = 0.64 (95%CI 0.43 − 0.96)], ARNIs [EF ≥ 40%; OR = 0.73 (95%CI 0.61 − 0.87)], and SGLT2 inhibitors [EF ≥ 50%; OR = 0.78 (95%CI 0.67 − 0.91) or EF ≥ 40%; OR = 0.74 (95%CI 0.66 − 0.83)] remain first choice treatments in terms of drug selection for HFpEF. There were no statistical between-group differences in drug interactions for the three drugs for reducing HF hospitalization. However, in the subgroup study of EMPEROR-preserved, SGLT2 inhibitors reduced HF hospitalization regarding of using ACEIs or ARNIs. Accordingly, ACEIs or ARNIs combined with SGLT2 inhibitors may further reduce endpoint events. The PEP-CHF study, which was a randomized double-blind trial, compared placebo with perindopril (4 mg/day) in patients aged 70 years with an EF ≥ 40% and found that perindopril reduced HF hospitalization [OR = 0.628 (95%CI 0.408 − 0.966)] and improved functional classes and the 6-min walk distance (6MWD) [13]. The 2019 PARAGON trial compared ARNI in 4822 patients with symptomatic HFpEF (EF ≥ 45%) and found no difference for reducing the risk of the primary composite outcome of HF hospitalization and cardiac death compared to valsartan [OR = 0.87 (95%CI 0.75–1.01)]. There was a reduction, although not significant, in HF hospitalization [OR = 0.85 (95%CI 0.72 − 1.00)] compared to ARBs in the PARAGON trial, whereas there was a significant reduction compared with placebo for the indirection comparison in our study.
In this meta-analysis, ARBs failed to reduce HF hospitalizations in HFpEF patients compared to placebo. This may be related to the fact that ACEIs are upstream blockers of the angiotensin-converting enzyme, and ARBs are downstream AT1 antagonists. ACEIs also preserve the kinin system, which may lead to the superiority of ACEIs over ARBs in anti-heart failure treatment. Secondly, ARNIs consist of ARBs and enkephalinase inhibitors that enhance the natriuretic peptide system by inhibiting enkephalinase. The PARAMOUNT study showed that ARNIs reduce NT-proBNP by approximately 23% compared to valsartan. Myocardial stiffness and myocardial fibrosis are the main physiological mechanisms in HFpEF [31]. Natriuretic peptides provide relief from breathlessness by rapidly reducing LVEDP and protecting the cardiovascular system by improving myocardial remodeling, including via its anti-hypertrophy anti-fibrotic effects [32]. Therefore, HFpEF patients who cannot tolerate ACEIs (dry cough) should perhaps be treated with ARNIs and not ARBs.
Only SGLT2 inhibitors reduced the risk of WHF events in patients with HFpEF [OR = 0.75 (95%CI 0.67 − 0.83)] following treatment with ACEIs, ARBs, or ARNIs at baseline. SGLT2 inhibitors have been shown to reduce the risk of HF hospitalization by 31% in patients with diabetes [33]. The dapagliflozin reduced the composite outcome of HF hospitalization and cardiac death in HFrEF by 26% in the DAPA-HF trial [34] and reduced the risk of WHF in HFpEF by 21% in the DELIVER study [9]. In the SOLOIST-WHF study, 1222 patients with type 2 diabetes mellitus who presented with WHF symptoms and were treated with intravenous diuretics were included in the study, of which 256 (21%) patients with LVEF ≥ 50% in the subgroup analysis, showing that sotagliflozin significantly reduced the risk of cardiac death and hospitalizations or emergency department visits due to WHF [HR = 0.78 (0.67–0.89)] [35]. Therefore, the 2022 AHA/ACC/HFSA guidelines for the management of heart failure firstly recommends SGLT2 inhibitors as new therapeutic agents in Class IIA, the strongest class into the treatment recommendations for HFpEF patients [36]. The mechanism of SGLT2 inhibitors for lowering the risk of HF hospitalization and adverse cardiovascular events may be explained as follows. (i) Cardiac stress-reducing effect: SGLT2 inhibitors promote the excretion of urinary sodium and glucose and thereby reduce cardiac preload. SGLT2 inhibitors can also block the renin–angiotensin–aldosterone system and reduce hypertension [37, 38]. (ii) Improve myocardial energy metabolism: SGLT2 inhibitors reduce oxidative stress and promote the breakdown of fatty acids into ketone bodies, thereby increasing ketone body levels in the body [39, 40]. This effect is distinct from ACEIs or ARNIs. Thus, the additional use of an SGLT2 inhibitor after ACEI or ARNI treatment may further reduce adverse events. (iii) Improving the pro-inflammatory properties of epicardial lipids (EAT) is an important target for therapeutic intervention in HFpEF. SGLT2i inhibitors can reduce EAT abnormalities, reduce water and sodium retention, and reduce the risk of HFpEF combined with atrial fibrillation. (iv) Anti-myocardial fibrosis and inhibition of ventricular remodeling: SGLT2 inhibitors can reduce the release of inflammatory factors, thereby reducing myocardial fibrosis [41, 42]. SGLT2 inhibitors can also reverse LV remodeling by reducing Na + and NHE-1 receptor activity in cardiomyocytes to slow myocardial fibrosis and cardiac hypertrophy [43].
Several limitations of this meta-analysis should be noted. First, HFpEF was defined as HF symptoms accompanied by an LVEF ≥ 50%. However, previous clinical studies have often included patients with an LVEF of 40–49%. A second limitation is that no study included the use of diuretics. Despite a lack of strong evidence, diuretics have been the first-line drug to relieve and alleviate HF symptoms due to fluid overload. Thus, the use of diuretics to reduce composite endpoints of HF hospitalization and WHF events should be considered in future RCTs. Third, the RELAX trial that included the use of a phosphodiesterase-5 inhibitor (sildenafil) did not provide any data on primary endpoints. Finally, this meta-analysis lacked data on 6MWD and KCCQ outcomes.
Conclusion
No medications have been found to reduce the endpoint of mortality in HFpEF patients. ARNIs, ACEIs, or SGLT2 inhibitors significantly reduced the risk of HF hospitalization, but only SGLT2 inhibitors reduced WHF events.
Supplementary Information
Additional file 1: Table S1. Final search strategy for PubMed. Table 2. Final search strategy for Clinical Trial gov of Controlled Trials. Table 3. Final search strategy for Cochrane Central Register of Controlled Trials
Additional file 2: Figure 1. Treatment strategy for all-cause mortality. Figure 2. Risk of bias in all trials. Figure 3. Assessment of risk of bias of the included studies (I²=0%)
Additional file 3. Heterogeneity of the network meta-analysis.
Additional file 4. Indirectness of the network meta-analysis.
Additional file 5. Incoherence of the network meta-analysis.
Additional file 6. Overal evaluattion of the network meta-analysis.
Acknowledgements
No
Abbreviations
- HFpEF
Heart failure with preserved ejection fraction
- ACEI
Angiotensin-converting enzyme inhibitor
- ARNI
Angiotensin receptor neprilysin inhibitor
- SGLT2
Sodium-glucose cotransporter-2
- MRA
Mineralocorticoid receptor antagonists
- LVEDP
LV end-diastolic pressure
- GDMT
Guideline-directed medical therapy
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- 6MWD
6 Minutes walk distance
- WHF
Worsening heart failure
Author contributions
YL and ZC collected, analyzed and wrote this manuscript. JY, HL, XP and Qiuling Chen assisted in the conduct of study. XT, QG and SD were the principal investigator. All authors read and approved the final manuscript.
Funding
Supported by Shenzhen Foundation (JCYJ20210324113614038), Shenzhen Key Medical Discipline Construction Fund (No. SZXK003) and Sanming Project of Medicine in Shenzhen (No. SZSM201412012).
Declarations
Ethics approval and consent to participate
The protocol was approved by the Ethics Committee of Shenzhen People's Hospital.
Consent for publication
The consent to publish was obtained from all participants in this study.
Availability of data and materials
The data that support the fndings of this study are available from the cor- responding author upon reasonable request.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yaowang Lin and Zhigang Cai are Co-authors
Contributor Information
Xinzheng Tang, Email: xnktangxinzheng@yeah.net.
Qingshan Geng, Email: szgengqingshan@yeah.net.
Shaohong Dong, Email: xnkdsh@yeah.net.
References
- 1.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 4.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–580. doi: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AHA/ACC/HFSA Guideline for the Management of Heart Failure J Card Fail. 2022;28(5):e1–e167. doi: 10.1016/j.cardfail.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11(4):354–365. doi: 10.1007/s11897-014-0223-7. [DOI] [PubMed] [Google Scholar]
- 7.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC). With the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2022;24(1):4–131. doi: 10.1002/ejhf.2333. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. 2021;144(16):1284–1294. doi: 10.1161/CIRCULATIONAHA.121.056824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022 doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 10.Aronow WS, Ahn C, Kronzon I. Effect of propranolol versus no propranolol on total mortality plus nonfatal myocardial infarction in older patients with prior myocardial infarction, congestive heart failure, and left ventricular ejection fraction ≥ 40% treated with diuretics plus angiotensin-converting enzyme inhibitors. Am J Cardiol. 1997;80(2):207–209. doi: 10.1016/s0002-9149(97)00320-2. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362(9386):777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 12.Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther. 2003;17(2):133–139. doi: 10.1023/a:1025387702212. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114(5):397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 16.Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, et al. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94(5):573–580. doi: 10.1136/hrt.2007.117978. [DOI] [PubMed] [Google Scholar]
- 17.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 18.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese diastolic heart failure study (J-DHF) Eur J Heart Fail. 2013;15(1):110–118. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the vitality-HFpEF randomized clinical trial. JAMA. 2020;324(15):1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 24.Morris DA, Boldt LH, Eichstädt H, Ozcelik C, Haverkamp W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ Heart Fail. 2012;5(5):610–620. doi: 10.1161/CIRCHEARTFAILURE.112.966564. [DOI] [PubMed] [Google Scholar]
- 25.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, et al. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation. 2019;140(5):353–365. doi: 10.1161/CIRCULATIONAHA.118.039136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart failure association of the european society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 29.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61(14):1498–1506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Peng W, Li F, Wang Y, Wang B, Ding Y, et al. Effect of sodium-glucose cotransporter 2 inhibitors for heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized clinical trials. Front Cardiovasc Med. 2022;9:875327. doi: 10.3389/fcvm.2022.875327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC, Jr, et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc. 2013;2(6):e000536. doi: 10.1161/JAHA.113.000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73(7):795–806. doi: 10.1016/j.jacc.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 33.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 34.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 35.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 36.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):1757–1780. doi: 10.1016/j.jacc.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press. 2016;25(2):93–103. doi: 10.3109/08037051.2015.1116258. [DOI] [PubMed] [Google Scholar]
- 38.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation. 2016;134(10):752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 39.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 40.Marton A, Kaneko T, Kovalik J-P, Yasui A, Nishiyama A, Kitada K, et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol. 2021;17(1):65–77. doi: 10.1038/s41581-020-00350-x. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Zhang J, Xue M, Li X, Han F, Liu X, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa CM, Campos DHS, Reyes DRA, Damatto FC, Kurosaki LY, Pagan LU, et al. Effects of the SGLT2 inhibition on Cardiac Remodeling in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus. Antioxidants. 2022 doi: 10.3390/antiox11050982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;22(4):618–628. doi: 10.1002/ejhf.1732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Final search strategy for PubMed. Table 2. Final search strategy for Clinical Trial gov of Controlled Trials. Table 3. Final search strategy for Cochrane Central Register of Controlled Trials
Additional file 2: Figure 1. Treatment strategy for all-cause mortality. Figure 2. Risk of bias in all trials. Figure 3. Assessment of risk of bias of the included studies (I²=0%)
Additional file 3. Heterogeneity of the network meta-analysis.
Additional file 4. Indirectness of the network meta-analysis.
Additional file 5. Incoherence of the network meta-analysis.
Additional file 6. Overal evaluattion of the network meta-analysis.
Data Availability Statement
The data that support the fndings of this study are available from the cor- responding author upon reasonable request.