Abstract
Background
Species in genus Amomum always have important medicinal and economic values. Classification of Amomum using morphological characters has long been a challenge because they exhibit high similarity. The main goals of this study were to mine genetic markers from cp genomes for Amomum species identification and discover their evolutionary history through comparative analysis.
Results
Three species Amomum villosum, Amomum maximum and Amomum longipetiolatum were sequenced and annotated for the complete chloroplast (cp) genomes, and the cp genomes of A. longipetiolatum and A. maximum were the first reported. Three cp genomes exhibited typical quadripartite structures with 163,269-163,591 bp in length. Each genome encodes 130 functional genes including 79 protein-coding, 26 tRNAs and 3 rRNAs genes. 113-152 SSRs and 99 long repeats were identified in the three cp genomes. By designing specific primers, we amplified the highly variable loci and the mined genetic marker ccsA exhibited a relatively high species identification resolution in Amomum. The nonsynonymous and synonymous substitution ratios (Ka/Ks) in Amomum and Alpinia showed that most genes were subjected to a purifying selection. Phylogenetic analysis revealed the evolutionary relationships of Amomum and Alpinia species and proved that Amomum is paraphyletic. In addition, the sequenced sample of A. villosum was found to be a hybrid, becoming the first report of natural hybridization of this genus. Meanwhile, the high-throughput sequencing-based ITS2 analysis was proved to be an efficient tool for interspecific hybrid identification and with the help of the chloroplast genome, the hybrid parents can be also be determined.
Conclusion
The comparative analysis and mined genetic markers of cp genomes were conducive to species identification and evolutionary relationships of Amomum.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03898-x.
Keywords: Amomum species, Chloroplast genome, Comparative analysis, Species identification markers, Evolutionary history, Hybridization
Background
Amomum Roxb. is the second largest genus after Alpinia Roxb. in Zingiberaecae family, which includes approximately 150-180 species that is widely distributed in Southeast Asia [1, 2]. There are 24 species and two variants in China and most of them have medicinal and culinary values [3]. Some famous species in this genus are used as traditional Chinese medicine and included in the Chinese Pharmacopoeia: Amomum villosum, Amomum villosum var. xanthioides, Amomum longiligulare, Amomum tsao-ko, Amomum kravanh and Amomum compactum [4]; other 16 species of this genus are found to be important folk medicines in southeast Asian countries [5]. The plants of this genus have been usually used in the treatment of antibiotic-associated diarrhea (AAD), steatohepatitis and functional dyspepsia (FD), etc. [5]. Therefore, a great deal of studies about the phytochemistry and pharmacology of the plants from Amomum genus have been carried out [5]. Among these species, A. villosum is one of the authentic species of a famous traditional Chinese medicine Amomi Fructus. Higher active ingredient content of borenol acetate in the fruit of A. villosum than in A. villosum var. xanthioides and A. longiligulare was confirmed by studies, which is the presumable cause of better therapeutic effect of A. villosum [6]. Amomum maximum Roxb. rhizome is a folk medicine mainly used in South and Southeast Asia [7]. Amomum longipetiolatum is distributed in Guangxi and Hainan provinces of China and the neighboring country Vietnam, which is not studied that much.
Classification of Amomum using morphological characters has long been a challenge as they exhibit high similarity among different species in this genus [1]. This problem also leads to the confusion of Amomum species in commercial herbal market, and authentic species are often misidentified; for example, the ripe fruits of A. villosum var. xanthioides, A. longiligulare and other species from Amomum or even Alpinia have been adulterated or substituted for A. villosum during the sales process [8, 9]. And this phenomenon severely hinders the clinical use and scientific research of Amomum species. Previous studies have conducted the molecular classification and identification of Amomum species. Shi et al. [10] evaluated eight candidate DNA barcodes, finding ITS/ITS2 were more suitable for Amomum species identification. And ITS proved to be able to differentiate Amomi Fructus from its adulterants, however, it couldn’t distinguish the three authentic plant species [9]. Based on the analysis of ITS and matK, Xia [11] found that Amomum was polyphyletic with three major groups of species. Further, de Boer [1] recircumscribed the 9 clades of the paraphyletic Amomum as a monophyletic genus by combining molecular data and morphological characters. These results indicated that more informative sites were needed to determine the relationships among species and accurately identifying species within the genus.
The chloroplast (cp) is an important organelle providing essential energy for plants, which mainly conduct photosynthesis that converts solar energy into chemical energy and releases oxygen. The cp has its own genome comprising a circular DNA that is independent from nuclear one. In angiosperms, cp genomes are highly conserved in gene content and genome structure and usually uniparental inheritance, and have low nucleotide substitution mutation rates [12]. With the improvement of high throughput sequencing, they are more conveniently obtained and widely used for species identification [13] and investigation of phylogenetic evolution [14, 15] in recent years. Although there has been over 2200 complete plant cp-genomes deposited in the public database by 2021 [16], it’s still insufficient and only cp genomes of six species included in the Chinese Pharmacopoeia for Amomum are publicly available at present. And these Amomum species with cp genomes published were all in the villosum and tsao-ko groups according to Xia’s study [11]. Here, we sequnced and anotated cp genomes of A. maximum and A. longipetiolatum in the other maximum group besides of A. villosum in the villosum group, then conducted phylogenetic analysis to evaluate plant relationships among Amomum and even Zingiberaceae.
Most studies of cp genomes explored the variable regions for species identification by bioinformatics analysis [17], whereas, more and more studies selected and tested the specific barcodes through experiments verification in recent years. Scanning cp genomes for more variable markers has improved identification success in several plant groups [18–20]. For species in Zingiberaecae, Cui et al. [8] sequenced and annotated cp genomes of A. villosum, A. villosum var. xanthioides and A. longiligulare. However, they found that highly divergent regions screened from cp genomes could not be used to distinguish Amomum species. Zhang et al. [21] reported the cp genomes of Alpinia galanga and Alpinia kwangsiensis. Finally, five candidate markers were found for species-level identification of Alpinia through interspecific comparisons. In this study, we explored the highly divergent regions of cp genomes for Amomum species identification using different species from those in Cui’s study [8].
Overall, we report the complete cp genomes of A. villosum, A. longipetiolatum and A. maximum in this study. Then, we conducted comparative analysis among these cp genomes with other published cp genomes in Amomum. Our main objectives were: (1) to explore the molecular structures of three chloroplast genomes; (2) to examine the repeat sequences among Amomum chloroplast genomes; (3) to discover sequence variations and highly divergent regions, and select specific DNA markers for Amomum species identification; (4) to assess the evolutionary history and explore the phylogenetic relationships of Amomum.
Results
Chloroplast genome features and organizations
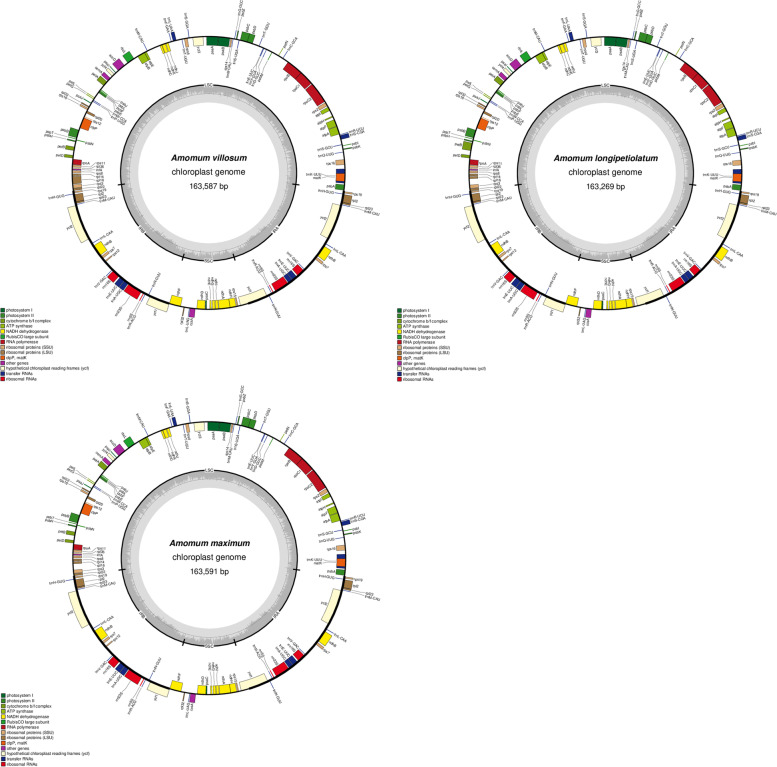
Fresh samples of A. villosum, A. longipetiolatum and A. maximum were used as source for gDNA, and subjected to NGS with Illumina NovaSeq paired-end sequencing. Chloroplast-like sequences were extracted from clean Ilumina reads, by BLAST searches against an in-house constructed chloroplast database, assembled with SOAPdenovo [22] and annotated with CPGAVAS2 [23]. De novo assembled chloroplast genomes were deposited in GenBank with accession numbers (A. villosum MW995976, A. longipetiolatum MW970344, and A. maximum MW995975). The complete cp genomes of three species ranged from 163,269 bp to 163,591 bp in size and exhibited typical quadripartite structures (Fig. 1). The overall GC content for these three species was nearly identical (36.08-36.13%) but was unevenly distributed in the cp genomes (Table 1). In details, the GC content was the highest in IR regions (41.15-41.17%) while the lowest in SSC regions (29.82-30.25%). Furthermore, AT appearance at the third codon position (71.14-72.83%) was higher than that at the first (54.86-55.41%) and second (61.98-62.62%) positions in the protein-coding regions (CDS) of three species. The complete cp genomes of three species encode 130 functional genes. After removing duplicates, 108 unique genes including 79 protein-coding, 26 tRNAs and 3 rRNAs genes were remained for each genome (Table 2). Among these genes, 19 genes were duplicated in IR regions. There were 16 genes containing introns for each cp genomes, and 15 genes were shared among all the species. PetB, rpl16 and ycf1 were distinct intron-containing genes for A. villosum, A. longipetiolatum and A. maximum respectively. For the 16 genes, 14 genes contained one intron, and the other two genes ycf3 and clpP contained two introns. It’s worthy to note that trnK-UUU gene had the longest intron more than 2500 bp.
Fig. 1.
Chloroplast (cp) genome maps of A. villosum, A. longipetiolatum and A. maximum. Genes inside the circle are transcribed clockwise, and those outside are transcribed counter-clockwise. Genes in different functional groups are color-coded. The small (SSC) and large (LSC) single copy regions and inverted repeat (IRa and IRb) regions are noted in the inner circle
Table 1.
Chloroplast genome features of A. villosum, A. longipetiolatum and A. maximum
| Type | A.villosum | A.longipetiolatum | A.maximum |
|---|---|---|---|
| Total Length (bp) | 163,587 | 163,269 | 163,591 |
| LSC length (bp) | 88,659 | 88,461 | 88,615 |
| IR length (bp) | 29,822 | 29,665 | 29,775 |
| SSC length (bp) | 15,284 | 15,478 | 15,426 |
| CDS length (bp) | 81,372 | 82,740 | 69,111 |
| Number of genes | 130 | 130 | 130 |
| Protein-coding genes | 87 | 87 | 87 |
| rRNA genes | 37 | 37 | 37 |
| tRNA genes | 6 | 6 | 6 |
| Total GC content | 36.08% | 36.13% | 36.13% |
| GC content of LSC | 33.71% | 33.77% | 33.84% |
| GC content of IRa | 41.15% | 41.17% | 41.17% |
| GC content of IRb | 41.15% | 41.17% | 41.17% |
| GC content of SSC | 30.06% | 30.25% | 29.82% |
| CDS.AT1 | 55.16% | 55.41% | 54.86% |
| CDS.AT2 | 62.36% | 62.62% | 61.98% |
| CDS.AT3 | 71.14% | 71.17% | 72.83% |
Table 2.
Genes encoded in the cp genomes of A. villosum, A. longipetiolatum and A. maximum
| Category of genes | Group of genes |
|---|---|
| photosystem I | psaA, psaB, psaC, psaI, psaJ |
| photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, |
| psbK, psbL, psbM, psbN, psbT, psbZ, ycf3** | |
| cytochrome b/f complex | petA, petBa, petD, petG, petL, petN |
| ATP synthase | atpA, atpB, atpE, atpF*, atpH, atpI |
| NADH-dehydrogenase | ndhA*, ndhB (×2)*, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| rubisco | rbcL |
|
DNA dependent RNA polymerase |
rpoA, rpoB, rpoC1*, rpoC2 |
| Small subunit of ribosome | rps2, rps3, rps4, rps7 (×2), rps8, rps11, rps12 (× 2), |
| rps14, rps15, rps16*, rps18, rps19 (×2), | |
| rpl2 (×2)*, rpl14, rpl16b, rpl20, rpl22, rpl23 (× 2), rpl32, rpl33, rpl36 | |
| Conserved open reading frames | ycf1c (×2), ycf2 (× 2), ycf4 |
| Ribosomal RNAs | rrn5 (×2), rrn16 (× 2), rrn23 (× 2) |
| Transfer RNAs |
trnA-UGC (×2)*, trnC-GCA*, trnD-GUC, trnE-UUC (×3)*, trnF-GAA, trnG-GCC, trnH-GUG (× 2), trnK-UUU*, trnL-CAA (× 2), trnL-UAA*, |
| trnL-UAG, trnM-CAU (×4), trnN-GUU (×2), trnP-UGG, trnQ-UUG, | |
|
trnR-ACG(×2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-CGA*, trnS-UGA, trnT-UGU, trnT-GGU, trnV-GAC (× 2), trnW-CCA, trnY-GUA | |
| Other genes | accD*, ccsA, cemA, clpP **, infA, matK |
(×2) indicates the gene sequence is repeated twice. * indicates genes containing one intron; while ** indicates gene containing two introns. a, b and c was contained an intron in A. villosum, A. longipetiolatum and A. maximum respectively
Codon usage and repeat content
Amino acids frequency and codon usage were determined for A. villosum, A. longipetiolatum and A. maximum in CodonW. All the protein-coding genes were composed of 23,037–27,580 codons in the three cp genomes. Just as in most angiosperms [24], leucine was the most abundant amino acid in three Amomum species with a frequency of 10.22–10.26%, followed by isoleucine (8.55–8.61%).
The type of SSRs and its distribution in cp genomes were analyzed by MISA. 152, 113 and 130 SSRs were identified for A. villosum, A. longipetiolatum and A. maximum, respectively (Fig. S1 and Table S1). The majority of SSRs were mono-nucleotide followed by di- and tetra-. Interestingly, mono-, di-, tri-, tetra- and penta-nucleotide repeats were all detected in the three cp genomes, while hexa- nucleotides only occured in A. villosum. Most of the mononucleotide SSRs consisted of A/T motifs that enriching A and T in the cp genomes, and they were the most frequently used base among all SSR types as previous studies confirmed [25, 26]. However, number of A/T repeats differed among three species, ranging from 50 in A. longipetiolatum to 90 in A. villosum (Fig. S2). Other two cp genomes of A. kravanh (MF991963) and A. compactum (MG000589) were downloaded from public database to be analyzed together for the four types of long repeats. The results showed that 99 repeats exist in each of the five cp genomes. Though the number of four types’ long repeats differed among species, the palindrome repeats were found to be the most abundant while complement repeats were the least. The majority of these repeats were between 21 and 30 bp. And it’s found that only repeats in A. maximum were no longer than 50 bp. Moreover, the length of all the complement repeats was less than 30 bp in these cp genomes (Fig. S3).
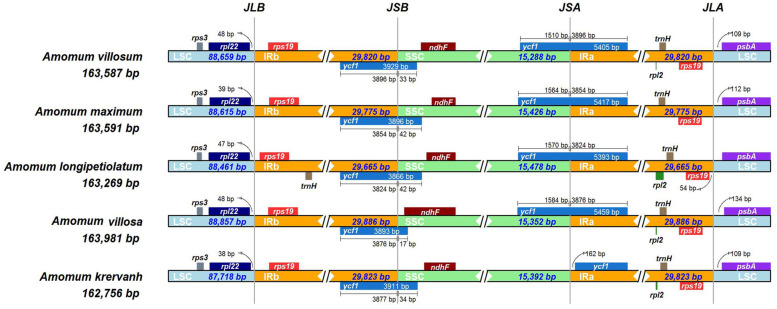
Boundary regions and interspecific comparisons
We compared the contraction and expansion of IRs regions at four junctions between the two IRs (IRa and IRb) and the two single–copy regions (LSC and SSC) among five species of Amomum genus (Fig. 2). After IRscope analysis [27], IR/LSC junction of IRb was found to be located between rpl2 and rps19 with 38-48 bp away from rpl22. Junction of SSC/IRb (JSB) was located in partial ycf1 gene which ranged from 3866 to 3929 bp in IR and the remaining part of ycf1 gene expanded into the SSC regions from 17 to 42 bp. Meanwhile, the rps19 and psbA genes were located on side of the junctions of the IRa/LSC regions in five chloroplast genomes. These data indicated that the contractions and expansions of the IR regions exhibited relatively stable patterns with slight variations in these Amomum cp genomes.
Fig. 2.
Comparison of the borders of the LSC, SSC, and IR regions among five chloroplast genomes of Amomum
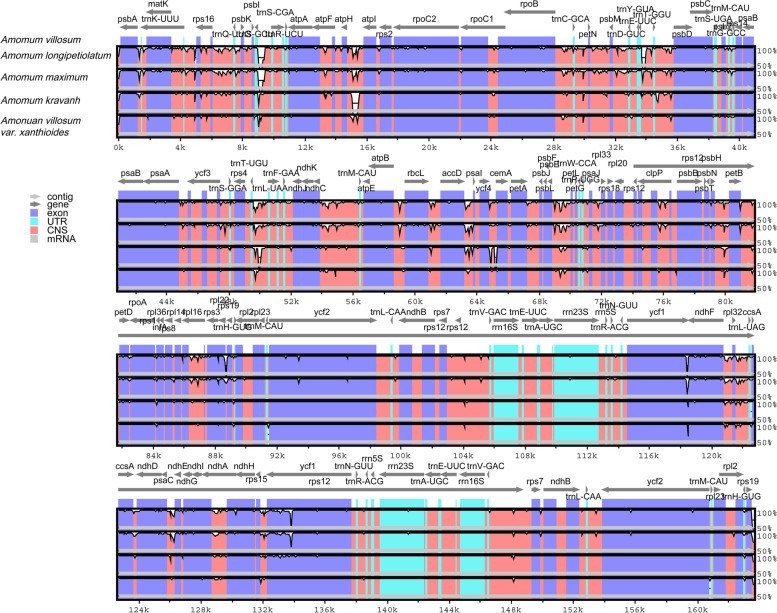
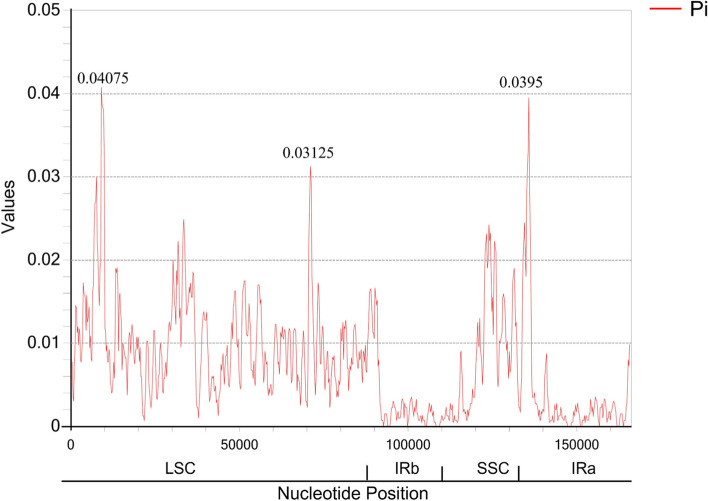
In order to compare the sequence variations within the Amomum genus, complete cp genomes of five Amomum species were aligned using the program mVISTA with A. villosum as a reference (Fig. 3). Among the 5 taxa, A. villosum and A. villosum var. xanthioides showed great similarity with each other in the genetic divergence. More variations were discovered in the LSC and SSC regions than IR regions. The most highly divergent regions among the 5 cp genomes were in intergenic spacers regions. Furthermore, sliding window analysis revealed highly variable regions in the 5 Amomum cp genomes. As shown in Fig. 4, mutational hotspots within these Amomum species were commonly located in the LSC and the SSC regions, which was consistent with the result of mVISTA. The nucleotide diversity (Pi) values were calculated with DnaSP [28] to test divergence level within different regions among the 5 Amomum cp genomes. The average value of nucleotide diversity (Pi) was 0.00702.
Fig. 3.
The interspecific comparisons of the five Amomum chloroplast genomes in mVISTA. The vertical axis indicates the percentage of identity, ranging from 50 to 100%, while the horizontal axis shows the position within the cp genome. Gray arrows display the gene orientation. Genome regions are color-coded as exon, tRNA/rRNA, CNS and mRNA. A cut-off of 70% identity was used for the plots
Fig. 4.
Sliding window analysis of the five Amomum cp genomes. Window length: 800 bp; step size: 200 bp. X-axis: position of the midpoint of a window. Y-axis: nucleotide diversity of each window
Species identification markers mining
To develop new identification markers of Amomum, 70 shared protein-coding genes and 44 shared intergenic regions of the five Amomum species were extracted for nucleotide diversity (Pi values). The results showed that nucleotide diversity of the Amomum species ranged from 0.00000 to 0.04075, with a mean of 0.00752. 16 CDS and 22 intergenic regions with high Pi values were then aligned and designed with specific primer pairs as candidate markers. 37 leaf samples of 13 Amomum species were collected and used to test the selected markers by PCR and Sanger sequencing. PCR efficiency and species identification resolution through ML tree were used as evaluation indicators. Finally, 11 markers were obtained with high amplification efficiency (89.19-100%) and sequence quality using 37 samples of 13 Amomum species (Table S2). As a result, ccsA showed the strongest discrimination power for Amomum species among these mined markers based on phylogenic analysis, followed by trnC-GCA_petN, rpl20, rps3 and rpoA (Fig. S4-S14).
For comparison, we also amplified and sequenced the plant universal barcodes (ITS/ITS2, matK, rbcL and psbA-trnH) [29] from the 37 samples. Among all the DNA barcodes, matK had the lowest PCR success rates of 27.03% that was not suitable for Amomum species identification (Table 3). From the ML tree, the other two universal markers from cp genomes rbcL and psbA-trnH with higher PCR success rates than matK showed lower species discrimination efficiency (Fig. S15-S19) than most of the mined markers other than rpl33 and ndhB_rps7. Thus, the mined specific markers always performed a better species identification resolution and a higher PCR efficiency than the universal markers matK, rbcL and psbA-trnH from cp genomes. Therefore, these mined markers, especially ccsA, could be considered as the candidate DNA barcodes for Amomum species identification. More importantly, we found that the mined marker trnC-GCA_petN have the potential to distinguish A. villosum from A. villosum var. xanthioides, as sequences of A. villosum var. xanthioides clustered into one clade that apart from those of A. villosum, though the bootstrap value was not that high (Fig. S5). However, we also noted that the nuclear markers ITS2 was more suitable for Amomum species identification than cp markers (Fig. S16 and Table 3). The characteristics of DNA markers were summarized in Table 3.
Table 3.
Efficiency of PCR and sequence characterization of DNA barcodes
| DNA barcodes | No. of sequences | PCR success rates (%) | Aligned length (bp) | Number | Variable sites | K2P | Species identification success rates a (%) |
|---|---|---|---|---|---|---|---|
| Number (%) | |||||||
| ITS2 | 36 | 97.30 | 236 | 87 | 36.86 | 0.000-0.411 | 76.92% |
| ITS | 36 | 97.30 | 682 | 174 | 25.51 | 0.000-0.046 | 38.46% |
| matK | 10 | 27.03 | 725 | 429 | 59.17 | 0.000-1.168 | 15.38% |
| rbcL | 29 | 78.38 | 657 | 15 | 2.28 | 0.000-0.018 | 23.08% |
| psbA-trnH | 33 | 89.19 | 797 | 469 | 58.85 | 0.000-1.124 | 23.08% |
| ndhB_rps7 | 37 | 100 | 297 | 2 | 6.73 | 0.000-0.004 | 0 |
| psaI_ycf4 | 37 | 100 | 342 | 12 | 3.51 | 0.000-0.018 | 23.08% |
| trnC-GCA_petN | 37 | 100 | 507 | 251 | 49.51 | 0.000-0.768 | 30.77% |
| rpl20 | 36 | 97.30 | 297 | 21 | 7.07 | 0.000-0.026 | 30.77% |
| rpl33 | 36 | 97.30 | 148 | 5 | 3.38 | 0.000-0.035 | 0 |
| ccsA | 34 | 91.89 | 939 | 33 | 3.51 | 0.000-0.023 | 38.46% |
| rps3 | 34 | 91.89 | 589 | 83 | 14.09 | 0.000-0.090 | 30.77% |
| rpoA | 33 | 89.19 | 907 | 62 | 6.84 | 0.000-0.021 | 30.77% |
| rps4 | 36 | 97.30 | 599 | 40 | 6.68 | 0.000-0.025 | 23.08% |
| ndhD_1 | 35 | 94.59 | 681 | 18 | 2.64 | 0.000-0.020 | 15.38% |
| ndhD_2 | 34 | 91.89 | 645 | 44 | 6.82 | 0.000-0.025 | 23.08% |
a When the accessions of one species were clustered in one clade in the ML tree, it was considered successfully identified. Species identification success rates were the number of successfully identified species divided by the total number of detected species (13)
Selective pressure analyses
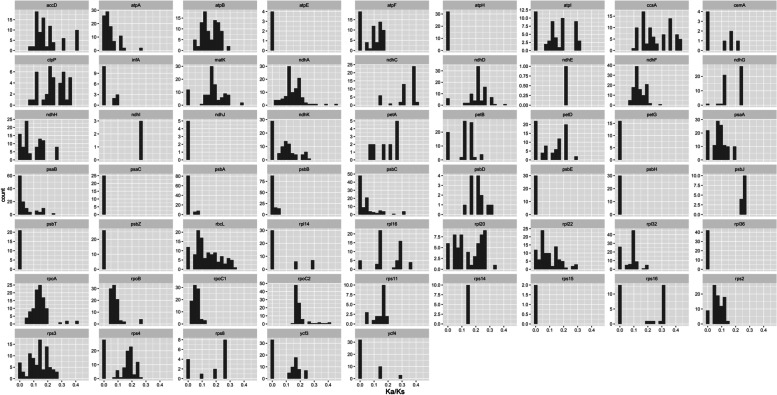
We calculated the nonsynonymous (Ka) and synonymous (Ks) substitution ratios (Ka/Ks) for all the 70 protein coding genes of cp genomes from 12 Amomum as well as 5 Alpinia species respectively with KaKs_calculator by ‘MA’ model and statistically tested by Fisher Exact Test [30] (Table S3). We found all genes with Ka/Ks > 1 that were supposed to be positively-selected genes only have 1 substitution in the multiple sequence alignment (MSA) files for each gene-pair, because those positively-selected results all passed the Fisher Exact Test (Table S4), we further tested whether these mutations could introduce changes in 3D-structure for those genes. In detail, a total of 59 chloroplast genes with 1 substitution were found, wherein, 32 genes with both positive selection and negative selection result, 22 genes with only negative selection and 5 genes with only positive selection result (Table S4). As there are 136 combinations (choose 2 from 17) for each genes, we selected genes ≥13 occurrences with Ka/Ks > 1 (psaI, psbI, psbJ, infA, atpI, cemA, nhdC, psbD, ndhK) and the 5 genes (accD, matK, ndhA, psaI, rps8) with Ka/Ks values all > 1 to inspect their 3D-structures with SWISS-MODEL [31, 32] in order to test whether the mutation is functionally conserved. As a result, the majority of these genes are still conserved in 3D-structure except for psbI, matK, rps8 and psbJ (Fig. S20), indicating the majority of genes evidenced as positively selected may be false positive results. As a result, genes with ≤1 substitution were filtered out in display of Ka/Ks distribution in order to minimize the false positive results. Overall, Ka/Ks values were less than 0.5 for the majority genes, suggesting that CP genes of the Amomum and Alpinia species are conserved and mainly under a purifying selection during the evolution process (Fig. 5), which is reasonable for necessary functions played by the chloroplast genes and is in accordance with previous studies [33].
Fig. 5.
Pairwise Ka/Ks ratios for sharednon-redundant genes from Amomum and Alpinia, genes with ≥1 substitutions and significant different Ka and Ks values, examined by Fisher Exact Test by KaKs calculator were plotted. Genes like ndhJ only have 3 Ka/Ks values indicating most of them among different species are too conserved to calculate out the Ka/Ks values
Phylogenetic analysis
To discuss the phylogenetic and evolutionary relationships of Amomum species, phylogenetic trees of 37 complete cp genome sequences from Alpinieae, the largest tribes of Zingiberaecae [21], were aligned by MAFFT and then constructed the phylogenetic trees by different methods including Bayesian inference (BI), maximum likelihood (ML) and neighbor joining (NJ) with the software RAxML-ng [34]. Two species from genus Zingiber were set up as the out-group. The results showed that most nodes in the phylogenetic trees were strongly supported (post probability or bootstrap values > 90%) except for a few ones (Fig. 6 and S21). All the BI, ML and NJ trees constructed by different methods were showing consistent topologic structures: species from Amomum and Alpinia were clustered into a paraphyletic branch. The cp genomes of A. longipetiolatum and A. maximum were used in phylogenetic analysis for the first time and they clustered together, showing a very close kinship with each other. These two species were then clustered with Alpinia nigra and Alpinia galanga as a sister group to the rest species forming another group. Next, the two sequences of Lanxangia tsaoko split off into another branch, and the remaining Alpinia and Amomum species split off into a large branch. In the large branch, Alpinia and Amomum species clustered into two branches, respectively. For the Amomum branch, A. kravanh and A. compactum first formed one clade and all the accessions of A. villosum, A. villosum var. xanthioides and A. longiligulare clustered into another clade, indicating that these three authentic plant sources of Amomi Fructus were indeed closely related. In general, our result proved that Amomum is paraphyletic.
Fig. 6.
Phylogenetic trees constructed by ML (a) and BI (b) based on complete chloroplast genome. Numbers under the nodes indicate bootstrap probabilities (%) in the ML tree and post probabilities (%) in the BI tree. Our sequenced samples are marked in red. Species from different genuses are marked in different color on the nodes
ITS2 analysis
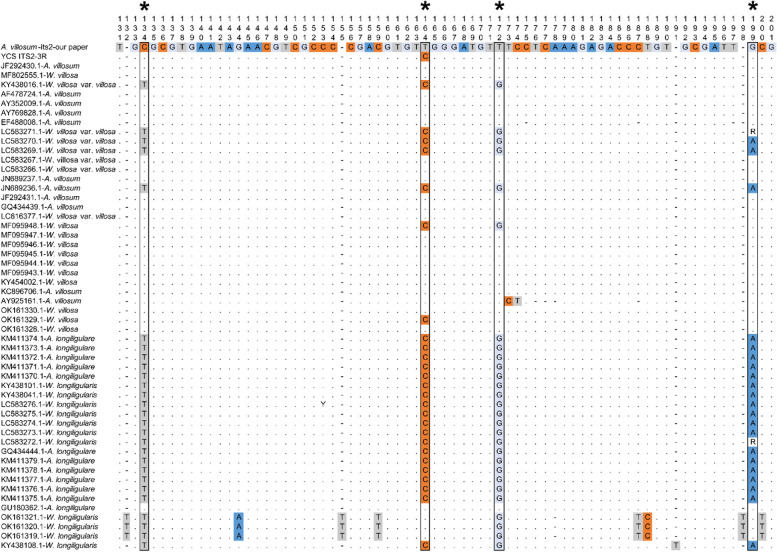
Unusually, we noticed that our sample of A. villosum clustered with samples of A. longiligulare into a clade, which was separated with other samples of A. villosum in the phylogenetic analysis. We inferred that the collected sample of A. villosum in our study is possibly a hybrid with the female parent of A. longiligulare and the male parent of A. villosum, as the cp genome is always maternal inherited. We investigated the gene fragment of nuclear genome for more evidence. We aligned the sequencing reads of NGS data to the ITS2 sequence of A. villosum with Bowtie2 [35]. Consequently, four nucleotide sites were found SNP sites though IGV [36, 37]. Further, we downloaded all the Sanger sequencing data of ITS2 sequences of A. villosum and A. longiligulare from NCBI and aligned the sequences with MEGA. Then the ITS2 haplotypes of A. villosum and A. longiligulare were summarized based on the four sites (Fig. 7 and Table S5). As a result, some species-specific loci of these two species both appeared among ITS2 reads in the NGS data of our A. villosum (Fig. 8 and Table S6), providing another hybrid evidence of this sample.
Fig. 7.
Part of ITS2 alignment of A. villosum and A. longiligulare from Sanger sequencing. Nucleotides the same as the reference are marked in dots (.) and gaps are marked in dashes (−)
Fig. 8.
ITS2 haplotype compositions of next-generation sequencing data of A. villosum and A. longiligulare. This histogram shows the haplotype composition of ITS2 reads. The former two samples are non-hybrids and the latter three samples are hybrids. When the ITS2 reads cannot match the haplotypes of A. villosum or A. longiligulare in the Sanger sequencing analysis, they are classified as other types
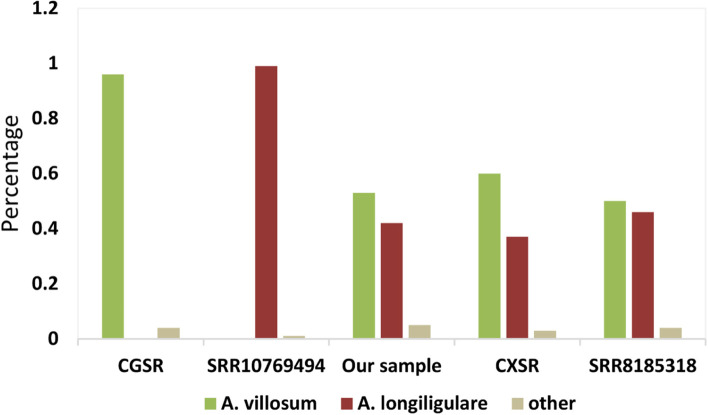
For validation, we also analyzed other NGS data of A. villosum and A. longiligulare. Finally, we found species-specific loci in each of the A. villosum (CGSR) and A. longiligulare (SRR10769494) sample, whereas, similar to our sample, there were species-specific loci of both two species in the artificially hybridized sample of A. villosum (CXSR, Fig. 8 and Table S6) [38]. Interestingly, we found another sample of A. villosum (SRR8185318) hybridized through our ITS2 analysis (Fig. 8 and Table S6) and the published phylogenetic analysis [39], which further proved the reliability of the method. To our knowledge, this is a novel method for hybridized species identification and the first report of natural hybridizations of Amomum.
Discussion
Chloroplast genome evolution of Amomum
In this study, we sequenced and annotated complete cp genomes of A. villosum, A. longipetiolatum and A. maximum. The three cp genomes exhibited typical quadripartite structures. The features of these three cp genomes were very conserved among Amomum species [8, 40, 41] and even species from family Zingiberaceae [42, 43]. The higher GC content of the IR region than the LSC and SSC regions could be due to a reduced number of duplicated AT nucleotides in the ribosomal RNA (rRNA) genes (Fig. 1) [44] and this might be one of the causes for the IR region to be more conserved than LSC and SSC regions [45]. This phenomenon is also evident in many other angiosperms [46, 47]. There are 79 protein-coding genes in the three Amomum plastomes, meanwhile, 70 up to 88 such genes are reported present in angiosperm plastomes [48]. Similar to other cp genomes, the A/T appearance at the third codon position was higher than that at the first and second positions in the protein-coding regions. And this bias can be used to discriminate cp DNA from nuclear and mitochondrial DNA [8]. SSRs are important and widely developed as molecular genetic markers for species identification [49]. Long repeat structures could promote the rearrangement of the cp genome and increase the population’s genetic diversity [50]. Hexa- nucleotides only occured in A. villosum among the three sequenced cp genomes, which indicates that A. villosum might have a more flexible cp genomes than A. maximum and A. longipetiolatum. Introns have important roles in the regulation of gene expression and can accumulate more mutations than exons; thus, they maybe contain older gene function information that has lost during evolution [51]. There were 14 genes containing one intron of Amomum cp genomes, meanwhile, ycf3 and clpP containing two introns that might have intron splicing events and lead to the low Ka/Ks [52].
Most of the protein-coding genes of Amomum species were found to be under purifying selection by Ka/Ks analysis, which was conservative in plastid genomes of most angiosperms [33]. Several CP genes under positive selection were also identified while only 1 substitution was found during the Ka/Ks calculation process for those genes, so a 3D-structure analysis was conducted to minimize the false positive results. as a result, mutations in psbI, matK, rps8 and psbJ were found to result typical structure alteration, and the hypothesis that those genes might be undergone positive selection during evolution could not be rejected. However, whether the function is actually affected still need further validation by molecular biology techniques. It seems that 3D-structures for the majority of genes with Ka/Ks > 1 are still conserved indicating most of the positively-selected genes tested with Ka/Ks > 1 by KaKs_calculator are false negative results. Additionally, the positively-selected genes with 3D-structure conserved also have been reported by other researches, such as cemA (Envelope membrane protein), ndhC (NADH-dehydrogenase) and rps15 (Small subunit of ribosome). ndhC was found undergo positive selection in other species from Zingiberaceae, such as Amomum kravanh [41], Alpinia oxyphylla [43] and Curcuma species [52]. Genes under positive selection might play vital roles in obtaining higher fitness to diverse environment. The ndh gene retains photosynthetic ability due to the non-functional role and the activity of the NDH complex seems to be of particular importance for adaptation of the photosynthetic machinery to stress conditions [53]. For these typical shade-loving plants whose natural habitats are shade forests of South China, the phototrophic component of NADH-dehydrogenase (ndhC) must be critical in adapting to light or other stress conditions that related to the adaptation of photosynthetic machinery at the chloroplast level [43]. In addition, our phylogenetic analysis confirmed that the paraphyletic status of species in Amomum and further validate the phylogenetic relationships in the Zingiberaceae reported in previous studies [1, 11].
Species identification based on cp genomes
DNA barcoding is an efficient tool for species identification [54]; however, it doesn’t work for some closely related taxa such as A. villosum and A. villosum var. xanthioides as demonstrated here (Fig. S4-S19) and in previous studies [9]. Thus, the complete chloroplast genomes containing large-scale information sites are used as super barcodes or to screen more variable regions for resolution enhancement [55, 56]. In this study, we sequenced and annotated three cp genomes of Amomum species and conducted a comparative analysis with the published cp genome data of this genus. We tested and verified the highly variable regions of these cp genomes through experiments on 37 samples from 13 species in Amomum. Finally, 8 markers were mined with a better species resolution than the universal markers matK, rbcL and psbA-trnH from cp genomes in Amomum species identification. We proved that specific barcodes derived from the comparison among cp genomes targeted at specific taxa could be more efficient in specific species identification instead of using a universal barcode. Compared with those highly variable sites identified by Cui et al. [8], we found different ones possibly because we used different Amomum species in our analyses. And adding more species might be more accurate for developing highly variable markers.
However, we found the nuclear barcodes ITS2 perform better in Amomum species identification than all the cpDNA barcodes including specific barcodes in this study (Fig. S4-S19 and Table 3), which is consistent with the previous study [10]. This is possibly due to the uniparental inheritance of cpDNA with low nucleotide substitution mutation rates [12]. Therefore, the use of cpDNA in species with frequent gene exchange has certain limitations. We confirmed ITS2 is an excellent DNA barcode with short fragment length, high amplification efficiency and the strongest species resolving power in our study. Our team has also constructed a reference ITS2 barcode library with 1276 sequences for Southern Chinese Medicine for its quality monitoring and control [54].
Though nuclear marker ITS2 had the strongest species identification resolution among all the assessed markers, it did not distinguish every species in Amomum. Instead, individuals from one species always clustered together based on the whole cp genomes (Fig. 6 and S20), indicating a better species identification trend than ITS2, yet there needs more cp genome data to support. Meanwhile, the advantage of the chloroplast genome as a super barcode for species identification was then validated. As the 3rd-generation sequencing technology represented by PacBio platform and Oxford Nanopore are becoming more and more mature, obtaining the cp genomes must be much easier and more convenient. We believe that the cp genomes will play a more important role in species identification in the future.
Interspecific hybrids identification by ITS2 reads analysis and cp genomes
Sexual hybridization of plants belonging to different species is a naturally occurring phenomenon [57]; ITS2 could also be used to identify hybrid strains [58]. As a part fragment of rDNA with multiple copies of variation [59], holotypes of ITS2 could be fully revealed by NGS sequencing than Sanger method. In this study, ITS2 analysis was performed on the NGS data of five A. villosum and A. longiligulare samples (Fig. 8 and Table S6). The results showed that more than 94% of the reads in all samples matched the haplotype analysis by sanger sequencing. In the next-generation data, there were a few reads that could not be matched to the sanger sequencing results, which possibly be caused by the base exchange of hybrids, PCR errors, etc. For the non-hybrid samples, only the haplotype reads corresponding to the species appeared in A. villosum and A. longiligulare; for the hybrid samples, both the haplotype reads of male and female parent were present and accounted for the main proportion in each NGS data, which might also reflect the hybridization. For example, for the artificially hybridized A. villosum sample (CXSR), it’s possible that the hybrid progeny had undergone some hybridizations, resulting in the ratio of the ITS2 haplotypes of A. villosum and A. longiligulare in the NGS data to 1.62 while the ratio was nearly 1 in the sample SRR8185318, indicating that only one hybridization event might have occurred. Hybridization was detected simultaneously in the samples of this study and the other study (SRR8185318), suggesting that this phenomenon may be relatively common between A. villosum and A. longiligulare in nature.
In addition, it should be noted that there is the same haplotype (C-T-T-G) between A. villosum and A. longiligulare based on the 4 hybridization sites when analyzing ITS2 sequences with Sanger sequencing. But ITS2 sequences with this haplotype exhibit two extremes in the proportion of two species—the largest proportions in A. villosum while the least proportion in A. longiligulare (Table S5). Therefore, we classified the reads with this haplotype as A. villosum during our analysis. We are not yet able to determine whether this haplotype in A. longiligulare is caused by misidentification (such as a hybrid) or not. Taking a cautious approach, we recommend using the dominant haplotype sequences of A. villosum and A. longiligulare to identify hybrids, that is, when the majority of ITS2 reads from NGS data are C-T-T-G (A. villosum) and T-C-G-A (A. longiligulare), the sample could be determined as a hybrid. When primarily one of these two types, it is a purebred.
This study demonstrates that high-throughput sequencing-based ITS2 analysis could be an efficient tool for interspecific hybrid identification and with the help of the chloroplast genome, the hybrid parents can be also determined.
Conclusions
We sequenced and annotated the complete chloroplast genomes of A. villosum, A. maximum and A. longipetiolatum by high-throughput sequencing. The comparative analysis was conducted for a better understanding of cp genome structures and evolutionary history of Amomum species. We found most of the protein-coding genes of Amomum species were under purifying selection. Furthermore, most of the mined genetic markers performed better than universal DNA barcodes from cp genomes in Amomum species identification. Phylogenetic analysis proved that Amomum is paraphyletic, and with the ITS2 analysis, we found the sequenced sample of A. villosum to be a hybrid, becoming the first report of natural hybridization of this genus. This study provides insights into species identification and evolutionary relationships of Amomum.
Materials and methods
Plant materials, DNA extraction and sequencing
Fresh and healthy leaf samples of three species A. villosum, A. longipetiolatum and A. maximum were collected for cp genomes sequencing. When the leaves were removed from the plants, they were stored in the liquid nitrogen in the field and were transferred to the − 80 °C refrigerator (Eppendorf, Hamburg, Germany) right back to the laboratory. 37 samples of dry leaves were collected from 13 Amomum species for species identification verification. All the samples were obtained from South China Botanical Garden, Chinese Academy of Sciences and identified by Ye Yushi, the engineer in SCBG. And all the voucher specimens were deposited in the Second Clinical College of Guangzhou University of Chinese Medicine (Table S2). No permission was required to collect the above samples and we confirm that all methods were performed in accordance with IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. We extracted total genomic DNA using a Qiagen DNeasy Plant Mini kit (Qiagen Co., Hilden, Gemany). The quality and quantity of extracted DNA was determined with 1% gel electrophoresis and Nanodrop2000C (ThermoScientific, Delaware, USA). Pure DNA from dry leaves was used in amplification of DNA markers and sanger sequencing. Pure DNA from fresh leaves was used in the cp genomes sequencing by Illumina NovaSeq (Illumina Inc., CA, USA) platform at Shanghai Majorbio Pharmaceutical Technology Co., Ltd. that generating the whole genome shotgun by Paired end library of 150 bp.
Chloroplast genome assembly and annotation
Many strategies are available for chloroplast genome assembly [60, 61], here we referred to the method of Zhou et al. [62]. The quality of sequencing raw data was evaluated by FastQC [41] and trimmed using Trimmomatic software [63]. To extract cp-like reads from clean reads, we used BLAST searches in a reference database constructed by all cp genomes retrieved from NCBI. The extracted reads were then assembled into contigs by SOAPdenovo [22]. SSPACE [64] and Gap Filler package [65] were used to extend sequences to scaffolds and fill gaps. The complete cp genome was firstly annotated by CPGAVAS2 [23] with default parameters. For the predicted protein-coding genes, we used BLAST searches against Swiss-Prot database and checked manually in Apollo software for a more precise annotation. Then the original CPGAVAS2’s prediction was updated by the latest GFF3 file. Subsequently, tRNAs were identified by tRNAscan-SE [66]. A circular cp genome map was drawn with OGDRAW v1.3.1 (Organellar Genome DRAW) [67]. The GC content of the cp genome was calculated by GC function planted in seqinr package [68].
Analysis of codon usage and repeat content
Relative synonymous codon usage (RSCU) in protein coding sequences of A. villosum, A. longipetiolatum and A. maximum was determined in CodonW. Simple sequence repeats (SSRs) of three Amomum species were determined by MISA [69]. For long-repeat, we downloaded other two cp genomes of A. kravanh (MF991963) and A. compactum (MG000589) from NCBI by mVISTA [70] to analyze together. Forward (F), reverse (R), complement (C) and palindromic (P) repeat types in the cp genomes were identified by the online tool REPuter [71] with default settings.
Boundary regions and interspecific comparisons
Five cp genomes of A. villosum, A. longipetiolatum, A. maximum, A. kravanh (MF991963) and A. compactum (MG000589) were analyzed. The contraction and expansion of IR regions were visualized with IRScope [27] package between the four main parts of cp genomes (LSC/IRb/SSC/IRa). For interspecific comparisons, the five cp genomes were aligned using MAFFT [72]. Nucleotide diversity was calculated through sliding window analysis with DnaSP [28] based on alignment results. The step size and window length were set to 200 bp and 800 bp respectively for DnaSP.
Species identification markers mining
The shared protein-coding genes and intergenic regions for the five Amomum species were extracted and calculated with Pi values. Specific primer pairs were designed at the conserved regions of gene fragments with high Pi values and amplified as the candidate markers for species identification. For comparison, the universal plant DNA barcodes including ITS, ITS2, matK, rbcL and psbA-trnH were also amplified. A total of 37 dry leaf samples of 13 Amomum species were used to test and verify. The PCR reaction system of the DNA markers contained 2xTaq PCR Mix 12.5 μL, forward primer (10.0 μM) 1.0 μL, reverse primer (2.5 μM) 1.0 μL, genomic DNA 2.0 μL, and added up to 25 μL with ddH2O. The primers and conditions for PCR were listed in Table S7. All the PCR products were sent to Sangon Biotech Guangzhou branch office for sequencing. The bi-directionally sequenced peaks of DNA markers were assembled using the CondonCode Aligner v8.0.1 software. Maximum Likelihood (ML) trees were constructed with 1000 bootstrap replicates for each marker in MEGA version 6.0 [73] to evaluate its discrimination power [substitution model], using 50% as a cut-off value for the condensed tree. Sequence length, variable sites and K2P distances were statistics by MEGA.
Selective pressure estimation and phylogenetic analysis
We extracted shared non-redundant gene CDS among 20 cp genomes of 12 species from Amomum and Alpinia, each gene’s CDS-pair of one-by-one species’ combination were extracted and were aligned by MAFFT. The rates of synonymous substitutions (Ks) and non-synonymous substitutions (Ka) and Ka/Ks were then calculated by ParaAT2.0 [74] which is planted in KaKs_Calculator2.0 with ‘MA’ model [30], the command we applied in this study is as follows: “ParaAT.pl -c 11 -h homologs.txt -n CDS -a PEP -p proc -o OUT -k -f axt -m mafft -v”. The 37 cp genome sequences from 20 species were aligned by MAFFT. And the alignment results were used to construct Bayesian, maximum likelihood (ML) and neighbor joining (NJ) trees with MrBayes3.2 [75], RAxML-ng [34] with and MEGA [76] respectively. The parameters for MrBayes. Default parameters of MrBayes were used in this study; The parameters for RAxML-ng were set as follows: GTR with 4 free rates heterogeneity but with ML estimate of the base frequencies model (GTR + R4 + FO) was choosed, default tree searching strategy was choosed using 10 random and 10 parsimony-based starting trees (tree = pars, rand), Transfer Bootstrap Expectation (TBE) and standard booststrap support metric (Felsenstein’s bootsrap, FBP) were applied (bs-metric = fbp, tbe) [77]. 100 starting trees (50 random and 50 parsimony-based) were used to pick the best-scoring topology, bootstrap replicates were set to 1000. Parameters for MEGA were set as follows: bootstrap value to 10,000, Kimura 2-parameter model were choosed for nucleotide substitution, Neighbor-joining method were applied to construct NJ trees.
ITS2 analysis
We downloaded all the ITS2 sequences of A. villosum and A. longiligulare from NCBI. The sequences were aligned with MEGA version 6.0 [73]. For the ITS2 analysis of NGS data, Bowtie2 [35] was used to align the sequencing reads to ITS2 of A. villosum. Then the files were sorted and formatted by Samtools [78], and observed by IGV. The variable sites were counted by the written JavaScript. The reads that not fully covered the four sites were neglected. For validation, we also analyzed the downloaded NGS data of A. villosum (SRR8185318) and A. longiligulare (SRR10769494) from NCBI, and the received NGS data from Xiasheng Zheng (A. villosum, CGSR), Danyan Zhang and Shijie Li (artificially hybridized sample of A. villosum, CXSR).
Supplementary Information
Additional file 1: Table S2.. Genbank accession numbers of DNA barcodes. Table S4. Results for one-vs-one calculation of Ka/Ks generated by KaKs_Calculator.
Additional file 2: Table S1.. Number of different SSR types detected in three Amomum species. Table S3. Species information of Ka/Ks. Table S5. ITS2 haplotypes of A. villosum and A. longiligulare from Sanger sequencing. Table S6. ITS2 reads of A. villosum and A. longiligulare from NGS data. Table S7. The primers and conditions for PCR.
Additional file 3: Fig. S1. Analysis of simple sequence repeats (SSRs) in the cp genomes of three Amomum species. Fig. S2. Frequency of identified SSR motifs in different repeat class types. Fig. S3. Repeat sequences of three Amomum species. F, P, R, and C indicate the repeat types F (forward), P (palindrome), R (reverse), and C (complement), respectively. Fig. S4. ML tree based on ccsA sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S5. ML tree based on trnC-GCA_petN sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S6. ML tree based on ndhB_rps7 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S7. ML tree based on psaI_ycf4 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S8. ML tree based on rpl20 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S9. ML tree based on rpl33 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S10. ML tree based on rps3 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S11. ML tree based on rpoA sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S12. ML tree based on rps4 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S13. ML tree based on ndhD_1 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S14. ML tree based on ndhD_2 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S15. ML tree based on ITS sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S16. ML tree based on ITS2 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S17. ML tree based on psbA-trnH sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S18. ML tree based on matK sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S19. ML tree based on rbcL sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S20. 3D-structure for the represented positively-selected genes with 1 substitution. Fig. S21. Phylogenetic tree constructed by NJ based on complete chloroplast genome. Numbers under the nodes indicate bootstrap probabilities. Our sequenced samples are marked in red. Species from different genuses are marked in different color on the nodes.
Acknowledgements
We sincerely thank professor Yushi Ye, South China Botanical Garden, Chinese Academy of Sciences, for the wonderful identification work. Dr. Jiang Xu, Baosheng Liao from Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences gave us important instructions and guidance. Thank you very much for their help.
Abbreviations
- Cp
Chloroplast
- LSC
Large single-copy region
- IR
Inverted repeat region
- SSC
Small single-copy region
- CDS
Coding DNA sequence
- tRNAs
Transport RNAs
- rRNAs
Ribosomal RNAs
- SSRs
Simple sequence repeats
- Pi
Nucleotide diversity
- Ka/Ks
The rate of non-synonymous substitutions to the rate of synonymous substitutions
- RSCU
Relative synonymous codon usage
Authors’ contributions
HZH and SH contributed to the conception and planning of the research. GL, DXX and GW contributed to the preparation of the manuscript. SH, GL, DXX, ZDC, ZJ and BJQ analyzed the data. DXX, ZDC, GW and HJ did the experiments. ZJ, BJQ, XW and QXH helped with the plant collection. ZXS, ZDY and LSJ provided the NGS data of two samples of A. villosum (CGSR and CXSR). All authors have read and approved the manuscript.
Funding
This work was supported by grants from Guangdong Provincial Medical Products Administration of China (002009/2019KT1261/2020ZDB25) and the special foundation of Guangzhou Key Laboratory (NO.202002010004).
Availability of data and materials
All original data is available from the NCBI database. The accession numbers of chloroplast genome sequences of A. villosum, A. longipetiolatum and A. maximum are MW970344, MW995975 and MW995976. The accession numbers of sequences amplified from cp genomes with universal and specific primers are listed in Table S2.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lu Gong and Xiaoxia Ding contributed equally to this work.
Contributor Information
Zhihai Huang, Email: zhhuang7308@163.com.
He Su, Email: suhe@gzucm.edu.cn.
References
- 1.Boer HD, Newman M, Poulsen AD, Droop AJ, Leong-Korniková J. Electronic supplement to: convergent morphology in Alpinieae (Zingiberaceae): Recircumscribing Amomum as a monophyletic genus. Taxon. 2018;67(1).
- 2.Yang L, Feng C, Cai M-M, Chen J-H, Ding P. Complete chloroplast genome sequence of Amomum villosum and comparative analysis with other Zingiberaceae plants. Chin Herb Med. 2020;12(04):375–383. doi: 10.1016/j.chmed.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Board E. Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China [M]. Beijing: Science Press. 1981;16(2):110. http://www.iplant.cn/info/Amomum?t=z.
- 4.Commission CP. The pharmacopoeia of the People’s republic of China. 2020 Edition. Beijing: The Medicine Science and Technology Press of China; 2020. [Google Scholar]
- 5.Cai R, Yue X, Wang Y, Yang Y, Sun D, Li H, Chen L. Chemistry and bioactivity of plants from the genus Amomum. J Ethnopharmacol. 2021;281:114563. doi: 10.1016/j.jep.2021.114563. [DOI] [PubMed] [Google Scholar]
- 6.Qin Q, Jiang CL, Jiang ML, Zhang LH, Jiang XH. Compare on the content of Bronyl acetate and Total volatile oil in Fructus Amomi from different producing area. Guid J Tradit Chin Med Pharm. 2017;23:70–73. [Google Scholar]
- 7.Lu CL, Wang LN, Li YJ, Fan QF, Chen JJ. Anti-hyperglycaemic effect of labdane diterpenes isolated from the rhizome of Amomum maximum Roxb., an edible plant in Southwest China. Nat Prod Res. 2021:1–5. [DOI] [PubMed]
- 8.Cui Y, Chen X, Nie L, Sun W, Hu H, Lin Y, et al. Comparison and phylogenetic analysis of chloroplast genomes of three medicinal and edible Amomum species. Int J Mol Sci. 2019;20(16). [DOI] [PMC free article] [PubMed]
- 9.Doh EJ, Kim JH, Lee G. Identification and monitoring of Amomi Fructus and its adulterants based on DNA barcoding analysis and designed DNA markers. Molecules. 2019;24(22). [DOI] [PMC free article] [PubMed]
- 10.Shi L, Song J, Chen S, Hui Y, Han J. Identification of Amomum (Zingiberaceae) through DNA barcodes. World J Tradit Chin Med. 2010;13:473–478. [Google Scholar]
- 11.Xia YM, Kress WJ, Prince LM. Phylogenetic analyses of Amomum (Alpinioideae: Zingiberaceae) using ITS and matK DNA sequence data. Syst Bot. 2004;29:334–344. [Google Scholar]
- 12.Palmer JD. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- 13.Nock CJ, Waters DL, Edwards MA, Bowen SG, Rice N, Cordeiro GM, Henry RJ. Chloroplast genome sequences from total DNA for plant identification. Plant Biotechnol J. 2011;9(3):328–333. doi: 10.1111/j.1467-7652.2010.00558.x. [DOI] [PubMed] [Google Scholar]
- 14.He L, Qian J, Li X, Sun Z, Xu X, Chen S. Complete chloroplast genome of medicinal plant Lonicera japonica: genome rearrangement, intron gain and loss, and implications for phylogenetic studies. Molecules. 2017;22(2). [DOI] [PMC free article] [PubMed]
- 15.Li Z, Long H, Zhang L, Liu Z, Cao H, Shi M, Tan X. The complete chloroplast genome sequence of tung tree (Vernicia fordii): organization and phylogenetic relationships with other angiosperms. Sci Rep. 2017;7(1):1869. doi: 10.1038/s41598-017-02076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao B, Hu H, Xiao S, Zhou G, Sun W, Chu Y, et al. Global pharmacopoeia genome database is an integrated and mineable genomic database for traditional medicines derived from eight international pharmacopoeias. Sci China Life Sci. 2021:1–9. [DOI] [PMC free article] [PubMed]
- 17.Li W, Liu Y, Yang Y, Xie X, Lu Y, Yang Z, Jin X, Dong W, Suo Z. Interspecific chloroplast genome sequence diversity and genomic resources in Diospyros. BMC Plant Biol. 2018;18(1):210. doi: 10.1186/s12870-018-1421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teske D, Peters A, Möllers A, Fischer M. Genomic profiling: the strengths and limitations of chloroplast genome-based plant variety authentication. J Agric Food Chem. 2020;68(49):14323–14333. doi: 10.1021/acs.jafc.0c03001. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Zheng Y, Huang P. Molecular markers from the chloroplast genome of rose provide a complementary tool for variety discrimination and profiling. Sci Rep. 2020;10(1):12188. doi: 10.1038/s41598-020-68092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Vázquez L, Chen X, Li H, Zhang H, Liu Z, Zhao G. Development of chloroplast and nuclear DNA markers for Chinese oaks (Quercus subgenus Quercus) and assessment of their utility as DNA barcodes. Front Plant Sci. 2017;8:816. doi: 10.3389/fpls.2017.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Song MF, Li Y, Sun HF, Tang DY, Xu AS, Yin CY, Zhang ZL, Zhang LX. Complete chloroplast genome analysis of two important medicinal Alpinia species: Alpinia galanga and Alpinia kwangsiensis. Front Plant Sci. 2021;12:705892. doi: 10.3389/fpls.2021.705892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Complete chloroplast DNA sequences of Zanduri wheat (Triticum spp.). Genet Resour Crop Evol. 2015;62(8):1269–77.
- 23.Liu C, Shi L, Zhu Y, Chen H, Zhang J, Lin X, Guan X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genomics. 2012;13(1):715–715. doi: 10.1186/1471-2164-13-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maheswari P, Kunhikannan C, Yasodha R. Chloroplast genome analysis of angiosperms and phylogenetic relationships among Lamiaceae members with particular reference to teak (Tectona grandis L.f). J Biosci. 2021;46. [PubMed]
- 25.Li DM, Zhao CY, Liu XF. Complete chloroplast genome sequences of Kaempferia Galanga and Kaempferia Elegans: molecular structures and comparative analysis. Molecules. 2019;24(3). [DOI] [PMC free article] [PubMed]
- 26.Gao C, Deng Y, Wang J. The complete chloroplast genomes of Echinacanthus species (Acanthaceae): phylogenetic relationships, adaptive evolution, and screening of molecular markers. Front Plant Sci. 1989;2018:9. doi: 10.3389/fpls.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiryousefi A, Hyvönen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34(17):3030–3031. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 28.Librado R. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics (Oxford, England) 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 29.Li DZ, Gao LM, Li HT, Wang H, Ge XJ, Liu JQ, Chen ZD, Zhou SL, Chen SL, Yang JB, et al. Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci U S A. 2011;108(49):19641–19646. doi: 10.1073/pnas.1104551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Li J, Zhao XQ, Wang J, Wong KS, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genom Proteom Bioinform. 2006;4(4):259–263. doi: 10.1016/S1672-0229(07)60007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 32.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Li X, Li M, Xu W, Schwarzacher T, Heslop-Harrison JS. Comparative chloroplast genome analyses of Avena: insights into evolutionary dynamics and phylogeny. BMC Plant Biol. 2020;20(1):406. doi: 10.1186/s12870-020-02621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35(21):4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14(2):178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson J. Thorvaldsdóttir, Helga, Winckler, Wendy, Guttman, Mitchell. Lander: Integrative genomics viewer. Nature Biotechnology; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Xiong Q, Li S, Li X, Chen J, Cao M, Lu L, Wan M, Zhang D. A comparison study on polysaccharides from novel hybrids of Amomum villosum and its female parent. Int J Biol Macromol. 2015;81:396–399. doi: 10.1016/j.ijbiomac.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Li DM, Li J, Wang DR, Xu YC, Zhu GF. Molecular evolution of chloroplast genomes in subfamily Zingiberoideae (Zingiberaceae) BMC Plant Biol. 2021;21(1):558. doi: 10.1186/s12870-021-03315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu ML, Li Q, Xu J, Li XW. Complete chloroplast genome of the medicinal plant Amomum compactum: gene organization, comparative analysis, and phylogenetic relationships within Zingiberales. Chin Med. 2018;13:10. doi: 10.1186/s13020-018-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Li Q, Hu Z, Li X, Chen S. The complete Amomum kravanh chloroplast genome sequence and phylogenetic analysis of the Commelinids. Molecules. 2017;22(11). [DOI] [PMC free article] [PubMed]
- 42.Li, Yang C, Feng MM, Cai JH, Chen P, Ding: Complete chloroplast genome sequence of Amomum villosum and comparative analysis with other Zingiberaceae plants. Chin Herb Med 2020, 12(04):33-41. [DOI] [PMC free article] [PubMed]
- 43.Bingmiao G, Lin Y, Tianle T, Jie H, Kun P. The complete chloroplast genome sequence of Alpinia oxyphylla Miq. And comparison analysis within the Zingiberaceae family. PLoS One. 2019;14(6):e0218817. doi: 10.1371/journal.pone.0218817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang D, Zhao Z, Zhang T, Zhong W, Liu C, Yuan Q, et al. The chloroplast genome sequence of Scutellaria baicalensis provides insight into intraspecific and interspecific chloroplast genome diversity in Scutellaria. Genes (Basel). 2017;8(9). [DOI] [PMC free article] [PubMed]
- 45.Yang Y, Dang Y, Li Q, Lu J, Li X, Wang Y. Complete chloroplast genome sequence of poisonous and medicinal plant Datura stramonium: organizations and implications for genetic engineering. PLoS One. 2014;9(11):e110656. doi: 10.1371/journal.pone.0110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi C, Han K, Li L, Seim I, Lee SM, Xu X, Yang H, Fan G, Liu X. Complete chloroplast genomes of 14 mangroves: phylogenetic and comparative genomic analyses. Biomed Res Int. 2020;2020:8731857. doi: 10.1155/2020/8731857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Z, Liao R, Yang T, Dong X, Lan D, Qin R, Liu H. Analysis of six chloroplast genomes provides insight into the evolution of Chrysosplenium (Saxifragaceae) BMC Genomics. 2020;21(1):621. doi: 10.1186/s12864-020-07045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol. 2011;76(3-5):273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song SL, Lim PE, Phang SM, Lee WW, Hong DD, Prathep A. Development of chloroplast simple sequence repeats (cpSSRs) for the intraspecific study of Gracilaria tenuistipitata (Gracilariales, Rhodophyta) from different populations. BMC Res Notes. 2014;7:77. doi: 10.1186/1756-0500-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavalier-Smith T. Chloroplast evolution: secondary symbiogenesis and multiple losses. Curr Biol. 2002;12(2):R62–R64. doi: 10.1016/s0960-9822(01)00675-3. [DOI] [PubMed] [Google Scholar]
- 51.Parenteau J, Abou Elela S. Introns: good day junk is bad day treasure. Trends Genet. 2019;35(12):923–934. doi: 10.1016/j.tig.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Liang H, Zhang Y, Deng J, Gao G, Ding C, Zhang L, Yang R. The complete chloroplast genome sequences of 14 Curcuma species: insights into genome evolution and phylogenetic relationships within Zingiberales. Front Genet. 2020;11:802. doi: 10.3389/fgene.2020.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suorsa M, Sirpiö S, Aro EM. Towards characterization of the chloroplast NAD(P) H dehydrogenase complex. Mol Plant. 2009;2(6):1127–1140. doi: 10.1093/mp/ssp052. [DOI] [PubMed] [Google Scholar]
- 54.Gong L, Qiu XH, Huang J, Xu W, Bai JQ, Zhang J, Su H, Xu CM, Huang ZH. Constructing a DNA barcode reference library for southern herbs in China: a resource for authentication of southern Chinese medicine. PLoS One. 2018;13(7):e0201240. doi: 10.1371/journal.pone.0201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Zhou J, Cui Y, Wang Y, Duan B, Yao H. Identification of Ligularia herbs using the complete chloroplast genome as a super-barcode. Front Pharmacol. 2018;9:695. doi: 10.3389/fphar.2018.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Yang Y, Henry RJ, Rossetto M, Wang Y, Chen S. Plant DNA barcoding: from gene to genome. Biol Rev Camb Philos Soc. 2015;90(1):157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 57.Kuligowska K, Lütken H, Müller R. Towards development of new ornamental plants: status and progress in wide hybridization. Planta. 2016;244(1):1–17. doi: 10.1007/s00425-016-2493-7. [DOI] [PubMed] [Google Scholar]
- 58.Zou P, Ng WL, Wu W, Dai S, Ning Z, Wang S, et al. Similar Morphologies but Different Origins: Hybrid Status of Two More Semi-creeping Taxa of Melastoma. Front Plant Sci. 2017;8:673. [DOI] [PMC free article] [PubMed]
- 59.Symonová R. Integrative rDNAomics-importance of the oldest repetitive fraction of the eukaryote genome. Genes (Basel). 2019;10(5). [DOI] [PMC free article] [PubMed]
- 60.Freudenthal JA, Pfaff S, Terhoeven N, Korte A, Frster F. A systematic comparison of chloroplast genome assembly tools. Genome Biol. 2020;21(1):254. doi: 10.1186/s13059-020-02153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Schalamun M, Morales-Suarez A, Kainer D, Schwessinger B, Lanfear R. Assembly of chloroplast genomes with long- and short-read data: a comparison of approaches using Eucalyptus pauciflora as a test case. BMC Genomics. 2018;19(1). [DOI] [PMC free article] [PubMed]
- 62.Zhou J, Hui Y, Chen X, Ying L, Song J. Molecular structure and phylogenetic analyses of complete chloroplast genomes of two Aristolochia medicinal species. Int J Mol Sci. 2017;18(9):1839. doi: 10.3390/ijms18091839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolger AM, Marc L, Bjoern U. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;15:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–579. doi: 10.1093/bioinformatics/btq683. [DOI] [PubMed] [Google Scholar]
- 65.Acemel RD, Tena JJ, Irastorza-Azcarate I, Marlétaz F, Gómez-Marín C, Elisa CM, et al. A single three-dimensional chromatin compartment in amphioxus indicates a stepwise evolution of vertebrate Hox bimodal regulation. Nat Genet. 2016. [DOI] [PubMed]
- 66.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:686–689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007;52(5-6):267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 68.Charif D, Thioulouse J, Lobry JR, Perrière G. Online synonymous codon usage analyses with the ade4 and seqinR packages. Bioinformatics. 2005;21(4):545–547. doi: 10.1093/bioinformatics/bti037. [DOI] [PubMed] [Google Scholar]
- 69.Yang XM, Sun JT, Xue XF, Zhu WC, Hong XY. Development and characterization of 18 novel EST-SSRs from the Western flower Thrips, Frankliniella occidentalis (Pergande). Int J Mol Sci. 2012;13(3). [DOI] [PMC free article] [PubMed]
- 70.Frazer KA, Lior P, Alexander P, Rubin EM, Inna D. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32(Web Server issue):W273. [DOI] [PMC free article] [PubMed]
- 71.Stefan K, Choudhuri JV, Enno O, Chris S, Jens S, Robert G. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;22:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2017. [DOI] [PMC free article] [PubMed]
- 73.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Xiao J, Wu J, Zhang H, Liu G, Wang X, Dai L. ParaAT: a parallel tool for constructing multiple protein-coding DNA alignments. Biochem Biophys Res Commun. 2012;419(4):779–781. doi: 10.1016/j.bbrc.2012.02.101. [DOI] [PubMed] [Google Scholar]
- 75.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Norgate M, Chamings J, Pavlova A, Bull JK, Murray ND, Sunnucks P. Mitochondrial DNA indicates late pleistocene divergence of populations of Heteronympha merope, an emerging model in environmental change biology. PLoS One. 2009;4(11):e7950. doi: 10.1371/journal.pone.0007950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10(2). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S2.. Genbank accession numbers of DNA barcodes. Table S4. Results for one-vs-one calculation of Ka/Ks generated by KaKs_Calculator.
Additional file 2: Table S1.. Number of different SSR types detected in three Amomum species. Table S3. Species information of Ka/Ks. Table S5. ITS2 haplotypes of A. villosum and A. longiligulare from Sanger sequencing. Table S6. ITS2 reads of A. villosum and A. longiligulare from NGS data. Table S7. The primers and conditions for PCR.
Additional file 3: Fig. S1. Analysis of simple sequence repeats (SSRs) in the cp genomes of three Amomum species. Fig. S2. Frequency of identified SSR motifs in different repeat class types. Fig. S3. Repeat sequences of three Amomum species. F, P, R, and C indicate the repeat types F (forward), P (palindrome), R (reverse), and C (complement), respectively. Fig. S4. ML tree based on ccsA sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S5. ML tree based on trnC-GCA_petN sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S6. ML tree based on ndhB_rps7 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S7. ML tree based on psaI_ycf4 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S8. ML tree based on rpl20 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S9. ML tree based on rpl33 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S10. ML tree based on rps3 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S11. ML tree based on rpoA sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S12. ML tree based on rps4 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S13. ML tree based on ndhD_1 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S14. ML tree based on ndhD_2 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S15. ML tree based on ITS sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S16. ML tree based on ITS2 sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S17. ML tree based on psbA-trnH sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S18. ML tree based on matK sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S19. ML tree based on rbcL sequences of Amomum species. This Bootstrap consensus tree was constructed by K2P model with 1000 bootstrap replicates. Numbers under the nodes indicate bootstrap probabilities. The cut off value for the condensed tree was 50%. Fig. S20. 3D-structure for the represented positively-selected genes with 1 substitution. Fig. S21. Phylogenetic tree constructed by NJ based on complete chloroplast genome. Numbers under the nodes indicate bootstrap probabilities. Our sequenced samples are marked in red. Species from different genuses are marked in different color on the nodes.
Data Availability Statement
All original data is available from the NCBI database. The accession numbers of chloroplast genome sequences of A. villosum, A. longipetiolatum and A. maximum are MW970344, MW995975 and MW995976. The accession numbers of sequences amplified from cp genomes with universal and specific primers are listed in Table S2.