Abstract
Background and Objectives:
Greying of hair is a regular feature of chronological aging that occurs in all regions and races. Premature canities is defined as minimum of five grey hairs in a person less than 20 years in Whites, 25 years in Asians, and 30 years in Africans. Premature canities is a common yet incompletely understood dermatological entity. This study aims at finding any association between premature hair greying (PHG) and parameters like hemoglobin (Hb), ferritin and calcium levels as well as its clinical profile.
Methodology:
This was a hospital-based cross-sectional analytical study conducted in the Department of Dermatology and Venereology, Trivandrum over one and half years. The study population consisted of 40 cases and 40 controls. Severity assessment, calculation of body mass index, and estimation of hemoglobin, serum ferritin, calcium, Random blood sugar, Anti Thyroid Peroxidase antibody, T3, T4, and TSH were done.
Results:
The mean age of the 40 patients was 17.14 years and most patients had onset of greying between 16 and 20 years. The male to female ratio was 1.2:1. A positive family history with a paternal predominance was noted. Vertex was the most common site of onset (42.5%), diffuse pattern was the most common clinical pattern (47.5%) and 60% had involvement of mild grade. Fourteen patients (35%) had abnormal investigations reports, in terms of low ferritin levels in 7 (17.5%), low calcium in 4 (10%) and a low Hb levels in 3 (7.5%) patients. Six (15%) patients had raised Anti TPO values. The association of PHG with low ferritin and raised anti-TPO levels were statistically significant.
Conclusion:
Low serum ferritin and raised Anti TPO levels may have a role in premature hair greying.
KEY WORDS: Anti TPO, calcium, ferritin, hemoglobin, premature hair greying
Introduction
Hair is one of the defining characteristics of mammals that has immense importance in contributing to people's physical appearance and self-perception. Hair color in humans ranges from black, brown, and blonde-to-red.[1] Hair color is derived from the hair follicle pigmentary unit which functions maximally during post-adolescence and early adulthood.[2] The diversity of hair color is attributed to the quantity and ratio of black-brown eumelanin and reddish-brown pheomelanin.[3] This difference in hair color is noted among the three major ethnic populations namely Asians, Africans, and Caucasians.[4]
Hair goes grey (Canities) with chronological aging[5] regardless of gender or race. Aging of hair is comprised of two important components, namely weathering of hair shaft and aging of hair follicle. In weathering, there is degeneration of hair fiber that progresses from the root to the tip and in aging of hair follicle, there is reduced melanocyte function and decreased hair production.[6]
About half the population have a significant amount of grey hair by the age of 50 years.[7] The age of greying varies with race and ethnicity. Typically, white people start going grey in their mid-30s, Asians in their late 30s and African-Americans in their mid-40s.[8] Hair is said to grey prematurely, only if greying occurs before the age of 20 years in Whites, before 25 years in Asians and before 30 years in Africans.[9]
Greying is believed to have a multifactorial etiology which includes genetic component, environmental factors, nutritional factors, oxidative stresses etc. The primary cause of premature hair greying (PHG) is considered to be genetic and other causes include autoimmune disorders such as vitiligo, pernicious anemia and autoimmune thyroid disorders. It is also known to have syndromic associations (e.g. Werner syndrome). Environmental factors such as ultraviolet light and climate, smoking, drugs, deficiencies of trace elements and nutritional deficiencies also play a role in occurrence of PHG.[3] It is also considered that photodamage which plays a major role in skin aging does not hasten greying of hair.[6] But the exact cause or pathogenesis has not been detected yet. This study aims at finding any association between entities like serum ferritin and calcium and hemoglobin levels with PHG.
Methodology
This was a hospital-based cross-sectional analytical study done over a period of one and half years. The study population consisted of 40, age and gender-matched cases and controls. All consenting male and female patients with age >2 years and </=25 years with premature canities were the cases. Patients with premature greying syndromes have a history of HIV positivity, topical/systemic medications, pregnancy/lactation were excluded. The severity assessment was done clinically using the scale format used in an Indian study by Bhat et al.,[3] as mild, moderate and severe with the presence of <50, 50-100 and >100 grey hairs respectively. Body mass index (BMI) was calculated as per the Asia-Pacific guidelines and was categorized into underweight (<18.5), normal (18.5-22.9), overweight (23-24.9) and obese (>/=25). Estimation of hemoglobin, serum ferritin, calcium, RBS, Anti TPO, T3, T4 and TSH were done. Association between qualitative variables were analyzed by Chi-square test. Data were entered in Microsoft Excel and data analysis was performed using SPSS ver 16.0.
Results
Age of the patients ranged from 2.5 years to 25 years. Most of the forty cases of PHG belonged to the age group of 21-25 years (40%) and the mean age was 17.14 years. Male to female ratio was 1.2:1.
Among the 40 patients [36 (90%)] and controls [36 (90%)] majority were students. 39 (97.5%) and 40 (100%) patients were unmarried among cases and control group respectively. The most common age of onset was 16-20 years (15, 37.5%) [Table 1].
Table 1.
Distribution based on age of onset
| AGE OF ONSET | FREQUENCY | PERCENTAGE |
|---|---|---|
| 1-5 | 4 | 10.0 |
| 6-10 | 5 | 12.5 |
| 11-15 | 9 | 22.5 |
| 16-20 | 15 | 37.5 |
| 21-25 | 7 | 17.5 |
| TOTAL | 40 | 100.0 |
The duration of complaints ranged from 13-24 months in 16 patients (40%), closely followed by 3-12 months in 15 patients (37.5%) [Figure 1].
Figure 1.
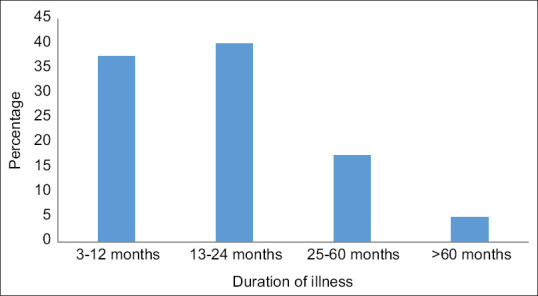
Duration of illness
The site of onset of PHG was most common on the vertex (17, 42.5%) followed by occipital, diffuse, frontal, temporal and parietal areas, in 7 (17.5%), 6 (15%), 5 (12.5%), and (1, 2.5%) patients respectively.
A history of skin/hair diseases were noted among 5 patients (87.5%). 39 patients (97.5%) had no significant past history.
Positive family history of PHG was noted in 17 patients (42.5%). Of these, 7 patients (17.5%) had paternal history of PHG and 6 patients (15%) with a history of PHG among the siblings. A positive maternal history was noted among 4 patients (10%) and two (5%) of them had first degree relative with PHG [Figure 2].
Figure 2.
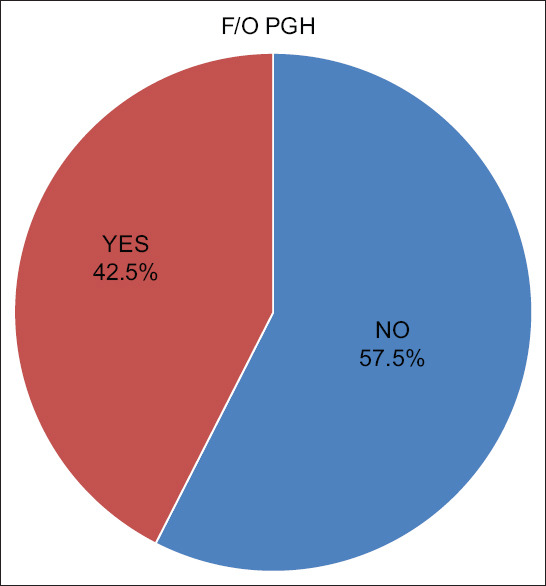
Family history of PHG
Among the 40 cases, 19 patients had a family history of diseases like hypothyroidism (9 patients, 22%), diabetes mellitus (7 patients, 17.5%), and hypertension (3 patients, 7.5%).
Stress factor was noted in only one patient (2.5%). There was no history of smoking, drug intake or cosmetic usage among cases or controls [Figure 3].
Figure 3.
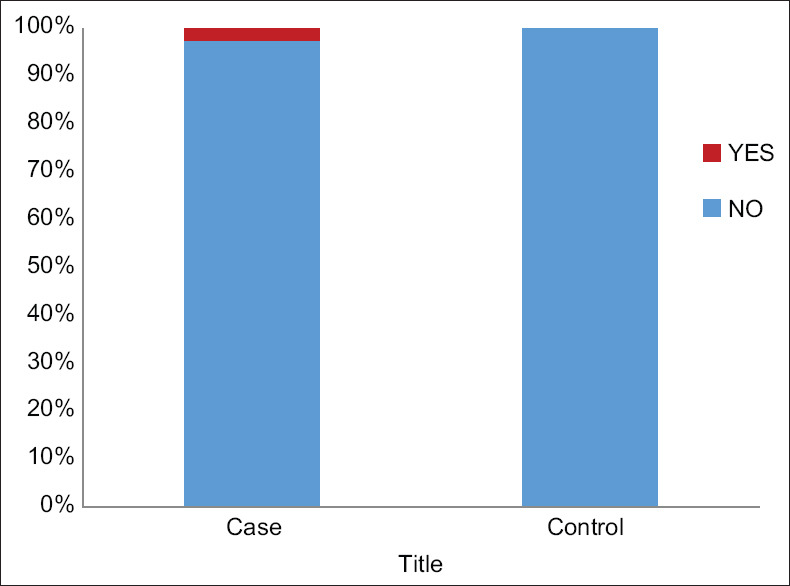
Stress Association
All the patients in case and control groups were moderately built and nourished. None of the cases or controls had pallor, icterus, cyanosis, clubbing, lymphadenopathy or edema. On examination, 26 (65%) patients were having normal BMI. Seven (17.5%) were underweight, 5 (12.5%) were overweight and 2 (5%) were obese.
Diffuse pattern was the most common clinical pattern [19 patients (47.5%)] [Figure 1]. Severity assessment among the patient with PHG showed a predominance of mild grade (60%).
Among the cases, 4 (10%) patients had seborrheic dermatitis and 1 (2.5%) patient had acne vulgaris.
Fourteen patients (35%) had abnormal investigations on evaluation [Table 2].
Table 2.
Investigations: RBS/Hb/Ferritin/Calcium
| CASE (n=40) | CONTROL (n=40) | Students t test | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| MEAN | SD | MEAN | SD | t | P | |
| RBS | 86.30 | 7.28 | 86.03 | 5.93 | 0.185 | 0.854 |
| HB | 12.14 | 1.83 | 11.74 | 0.82 | 1.252 | 0.214 |
| FERRITIN | 38.38 | 19.46 | 47.42 | 7.62 | -2.735 | 0.008 |
| CALCIUM | 9.20 | 0.83 | 9.37 | 0.66 | -1.021 | 0.310 |
| T3 | 1.37 | 0.21 | 1.43 | 0.24 | -1.276 | 0.206 |
| T4 | 9.79 | 1.63 | 10.02 | 1.21 | -0.715 | 0.477 |
Among the 40 cases, seven patients (17.5%) had low ferritin levels. A statistical significance was noted in the ferritin values obtained (P = 0.008). Four (10%) patients had low calcium levels. Low Hb levels were noted in 3 patients (7.5%).
Six patients (15%) had raised Anti TPO values (P < 0.001). [Table 3].
Table 3.
Anti TPO level
| ANTI TPO | Mann-Whitney U test | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| mean | SD | Median | IQR | Z | P | |
| Case | 5.15 | 8.6 | 3.92 | 1.8-5.175 | 4.692 | <0.001 |
| Control | 2.52 | 5.14 | 1.58 | 1.14-2.22 | ||
Comparison of the positive findings are given in [Table 4].
Table 4.
Comparison of positive findings
| ABNORMAL INVESTIGATIONS | FAMILY HISTORY | SITE OF PHG | SEVERITY |
|---|---|---|---|
| Ferritin + Anti TPO | Paternal | Diffuse | Mild |
| Anti TPO | Paternal | Diffuse | Moderate |
| Nil | Maternal | Vertex | Mild |
| Hb + Ferritin | Maternal + Sibling | Diffuse | Mild |
| Calcium | First degree relative | Temporal | Mild |
| Nil | Sibling | Diffuse | Mild |
| Hb + Ferritin | Paternal | Vertex | Mild |
| Anti TPO | First degree relative | Diffuse | Mild |
| Calcium | Paternal | Diffuse | Mild |
| Anti TPO | Paternal | Diffuse | Moderate |
| Hb + Ferritin + Calcium | Maternal + Sibling | Diffuse | Mild |
| Nil | Sibling | Diffuse | Moderate |
| Nil | Sibling | Frontal | Moderate |
| Nil | Sibling | Diffuse | Moderate |
| Nil | Paternal | Diffuse | Severe |
| Nil | Paternal | Diffuse | Severe |
| Nil | Maternal | Diffuse | Severe |
| Ferritin | Nil | Temporal | Mild |
| Ferritin + Calcium | Nil | Diffuse | Severe |
| Ferritin | Nil | Diffuse | Severe |
| Anti TPO | Nil | Vertex | Mild |
| Anti TPO | Nil | Diffuse | Moderate |
The predominant clinical pattern and severity grades noted among patients with abnormal investigations was the diffuse pattern and mild grade respectively. Among them 4 had low ferritin levels, 3 patients each had low Hb values and low calcium levels. Four of them also had raised Anti TPO values.
Severity assessment in the patients with both positive family history and abnormal investigations showed a predominance of mild grade among the patients with low ferritin, low Hb and low calcium levels. And an equal distribution of mild and moderate grade among patients with raised Anti TPO was seen.
Diffuse pattern was the predominant finding among patients with low ferritin, low calcium, low Hb and raised Anti TPO levels who also had a positive family history.
Discussion
The earliest age of onset in this study was 2.5 years. This was similar to the observation by Chakrabarty S et al.,[10] while it was slightly higher (5 years) in the study conducted by Sonthalia S et al.[11]
The mean age among the patients was 17.14 years (SD: 5.78). In the study by Chakrabarty S et al.,[10] the mean age reported was higher (22.8 years). According to Daulatabad D et al.,[12] most of the patients belonged to 11-15 years age group with a mean age (SD) being 11.6 (3.6). According to Bhat et al.,[3] the mean age of the studied cases was 16.8 years and the mean age of onset of premature greying was 15 years.
The male to female ratio noted in this study was 1.2:1. The male to female ratio of 4.2:1 was noted in the study by Chakrabarty S et al.[10] and it was 1.08:1 in the study conducted by Sonthalia S et al.[11]
In this study, 90% of the cases and controls were students. 5% of the cases and 10% of the controls were unemployed. House wives formed only 2.5% of the cases. But, in a study by Sharma N et al.[13] 79. 17% of patients were students, followed by employees (8.33%), housewives (6.67%) and businessmen (5.83%). About 22.50% of patients led sedentary lifestyle as compared to 7.5% in controls, the difference being statistically highly significant (p = 0.001) in the study by Daulatabad D et al.[14]
The duration of complaints noted was 13-24 months in 40% of patients, closely followed by 3-12 months in 37.5%, 25- 60 months in 17.5% and more than 60 months in 5% of cases. According to Daulatabad D et al.,[12] the mean duration of greying at the time of presentation was 39.8 ± 37.3 months (4 months-15 years) whereas it was noted as 47.8+/-32.4 months (3 months – 14 months) by Sonthalia S et al.[11]
In this study, for majority of the patients (42.5%), the site of initial greying of hair noted was around the vertex region, closely followed by occipital, diffuse, frontal distribution in 17.5%, 15% and 12.5% patients respectively. On the contrary, in the study by Daulatabad D et al.,[12] frontal region was noticed to be the first affected area in 25 (48.1%) cases followed by the vertex in 18 (34.6%), occiput in 7 (13.5%) and temporal region in 2 (3.8%). According to Sonthalia S et al.,[11] majority of patients noticed the temporal regions (n = 25; 35.2%) as the first affected site which was followed by frontal region in 13 (18.3%) cases, vertex in 10 (14.1%), occipital in 8 (11.3%) and diffuse involvement in 15 (21.1%) cases [Figure 4].
Figure 4.
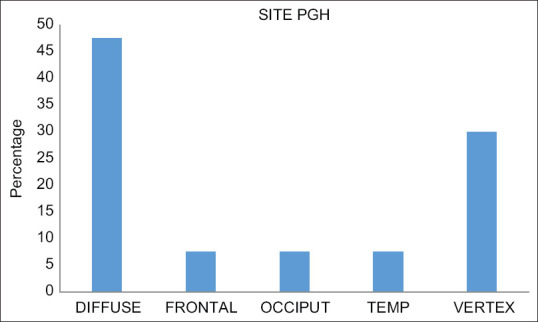
Sites of PHG
No other hairy areas had PHG in this study. In contrast, in the study by Sonthalia S et al.,[11] history of premature greying of eyebrows was reported by 1 woman and of the beard in 3 men.
History of atopic diathesis could be elicited in a significantly higher proportion of cases as compared to controls (19 [36.5%] vs 5 [9.6%]); odds ratio of 3.8 in the study by Daulatabad D et al.,[12] whereas only one (2.5%) among the 40 patients in this study had given a history of atopy [Figure 5].
Figure 5.
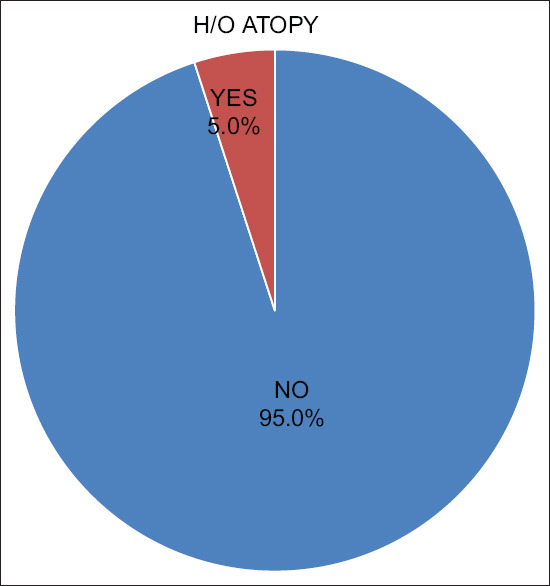
Association with Atopy
Positive family history of PHG was noted in 17 patients (42.5%), among which, 7 (17.5%of total cases) had paternal history of PHG and 6 patients (15%) had siblings with PGH and 4 patients (10%) had maternal history of PHG. Two patients (5%) gave a history of PHG in first degree relative and both maternal and sibling having history of PHG were seen in 2 (5%) patients. This was not in concordance with the study by Daulatabad D et al.,[12] where a positive family history of premature canities was obtained in 39 (75%) of the cases. Paternal and maternal relatives were equally affected with 13 (25%) cases each.
According to Sonthalia S et al.,[11] a positive family history of PHG was obtained in 64 (90.1%) out of the 71 cases. 14 (21.9%) cases reported PHG in both the parents and 50 cases (78.1%) reported it in one of the parents (22 in maternal and 28 in paternal). Further 17 of these 64 patients (26.6%) reported at least one of their siblings being affected with PHG. Paternal history of PHG were obtained in 14.2% of patients studied by Bhat et al.[3]
BMI values of our patients were almost similar to the study by Sharma N et al.[13] where normal BMI was found in 65% (in this study) Vs 65%(in study by Sharma et al.), underweight in 17.5% Vs 20%, overweight in 12.5% Vs 12.5% and obese in 5% Vs 2.5% cases.
The majority of the patients (60%) in the present study had mild grade canities, moderate in 22.5% and severe in 17.5% unlike study by Bhat et al.,[3] where he reported 65.7% with a moderate severity, 23% with severe greying and only 11.5% cases with mild grade of PHG [Figure 6].
Figure 6.
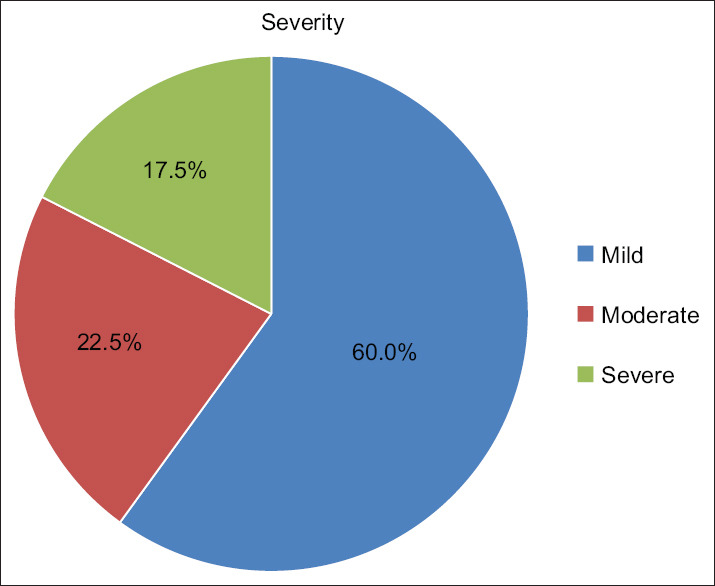
Severity of PHG
Fourteen (35%) patients in this study were found to have abnormal investigations. Among these, 7 had low ferritin levels (17.5%). The mean values of serum ferritin noted among the patients and healthy subjects were 38.38 (SD: 19.46) and 47.42 (SD: 7.62) respectively. A statistical significance was noted in the ferritin values obtained (P = 0.008) which might indicate the role of low serum ferritin as an etiological factor in PHG. The study done by Chakrabarty S et al.,[10] also showed a low serum ferritin levels in individuals with PHG as compared to the controls (P < 0.001). Serum ferritin levels were also found to be low compared to the controls in the study by Bhat et al.[3]
No significant difference was noted in the calcium levels (P = 0.310). According to Bhat et al.,[3] calcium levels were found to be lower in cases compared to the controls.
Three patients (7.5%) out of the 14 who had abnormal investigations, showed anemia. No significant differences were noted among cases and controls (P = 0.214). But in the study by Daulatabad D et al.,[14] only 5.8% of patients had a hemoglobin level of <10 mg/dl suggestive of anemia, whereas none of the controls had anemia.
It was found that 6 patients had raised Anti TPO values (15%). A significant difference was noted (P < 0.001). There are no previous studies comparing PHG and Anti TPO values.
No statistical differences were noted in T3 (P = 0.206), T4 (P = 0.477) and TSH (P = 0.066) values. The mean RBS values noted in this study was 86.30 (SD: 7.28) among the cases and in the healthy subjects it was 86.03 (SD: 5.93). No statistical significance was noted this study (P = 0.854%). Almost similar results were noted in the study done by Daulatabad D et al.,[12] were the mean blood glucose levels (86.6 ± 9.9 mg/dl in cases vs. 86.2 ± 7.8 mg/dl in controls; P = 0.85), TSH, T3 and T4 were all within normal limits and comparable in both cases and controls.
All the 3 patients (100%) with low Hb had mild grade of PHG whereas 75% (3 out of 4 patients) of those with low calcium and 71% (5 out of 7 patients) of those with low ferritin had predominantly mild PHG. 50% of those with Anti TPO also had only mild PHG.
Among the 17 patients with positive family history, 4 had low ferritin levels (23.5%), 3 patients each (17.6%) had low Hb values and low calcium levels. Four of them (23.5%) also had raised Anti TPO values.
Severity assessment in the patients with both positive family history and abnormal investigations showed a predominance of mild grade among the patients with low ferritin, low Hb and low calcium levels. And an equal distribution of mild and moderate grade of PHG were seen among patients with raised Anti TPO.
Patients with low ferritin, calcium and Hb as well as those with raised Anti TPO levels in this study had predominantly diffuse pattern of PHG.
Conclusion
Even though multiple etiological factors have been proposed for PHG, the exact cause is still to be unraveled. A few of such proposed factors studied here revealed a significant association between PHG and low serum ferritin levels and raised Anti TPO values. So timely replenishment of the ferritin storages and correction of the thyroid dysfunction in patients may help in reversing the distressing symptom of PHG.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ito S, Wakamatsu K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J Eur Acad Dermatol Venereol. 2011;25:1369–80. doi: 10.1111/j.1468-3083.2011.04278.x. [DOI] [PubMed] [Google Scholar]
- 2.Pandhi D, Khanna D. Premature greying of hair. Indian J Dermatol Venereol Leprol. 2013;79:641–53. doi: 10.4103/0378-6323.116733. [DOI] [PubMed] [Google Scholar]
- 3.Bhat RM, Sharma R, Pinto AC, Dandekeri S, Martis J. Epidemiological and investigative study of premature greying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17–21. doi: 10.4103/0974-7753.114706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trueb RM. Oxidative stress in ageing of hair. Int J Trichology. 2009;1:6–14. doi: 10.4103/0974-7753.51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trueb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–9. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin DJ. Aging of hair follicle pigmentation system. Int J Trichology. 2009;1:83–93. doi: 10.4103/0974-7753.58550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koegh EV, Walsh RJ. Rate of greying of human hair. Nature. 1965;207:877–8. doi: 10.1038/207877a0. [DOI] [PubMed] [Google Scholar]
- 8.Panhard S, Lozano I, Loussouarn G. Greying of the hair: A worldwide survey, revisiting the '50' rule of thumb. Br J Dermatol. 2012;167:865–73. doi: 10.1111/j.1365-2133.2012.11095.x. [DOI] [PubMed] [Google Scholar]
- 9.Tobin DJ, Paus R. Gerontobiology of the hair follicle pigmentary unit. Gerentology. 2001;36:29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarty S, Krishnappa AG, Gowda DG, Hiremath J. Int J Trichology. 2016;8:11–4. doi: 10.4103/0974-7753.179384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonthalia S, Priya A, Tobin DJ. Demographic characteristics and association of serum Vitamin B12, ferritin and thyroid function with premature canities in Indian patients from an urban skin clinic of North India: A retrospective analysis of 71 cases. Indian J Dermatol. 2017;62:304–8. doi: 10.4103/ijd.IJD_221_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daulatabad D, Singal A, Grover C, Chhillar N. Profile of Indian patients with premature canities. Indian J Dermatol Venereol Leprol. 2016;82:169–72. doi: 10.4103/0378-6323.168911. [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Dogra D. Association of epidemiologicaland biochemical factors with premature graying of hair: A case–control study. Int J Trichology. 2018;10:211–7. doi: 10.4103/ijt.ijt_39_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daulatabad D, Singal A, Grover C, Chhillar N. Prospective analytical controlled study evaluating serum biotin, vitamin B12 and folic acid in patients with premature canities. Int J Trichology. 2017;9:19–24. doi: 10.4103/ijt.ijt_79_16. [DOI] [PMC free article] [PubMed] [Google Scholar]


