Abstract
α1 Protease inhibitor (α1PI) modulates serine protease activity in the lung. Reactive oxygen species inactivate α1PI, and this process has been implicated in the pathogenesis of a variety of forms of lung injury. An imbalance of protease-antiprotease activity is also detected in the airways of patients with cystic fibrosis-associated lung disease who are infected with Pseudomonas aeruginosa. P. aeruginosa secretes pyocyanin, which, through its ability to redox cycle, induces cells to generate reactive oxygen species. We tested the hypothesis that redox cycling of pyocyanin could lead to inactivation of α1PI. When α1PI was exposed to NADH and pyocyanin, a combination that results in superoxide production, α1PI lost its ability to form an inhibitory complex with both porcine pancreatic elastase (PPE) and trypsin. Similarly, addition of pyocyanin to cultures of human airway epithelial cells to which α1PI was also added resulted in a loss of the ability of α1PI to form a complex with PPE or trypsin. Neither superoxide dismutase, catalase, nor dimethylthiourea nor depletion of the media of O2 to prevent formation of reactive oxygen species blocked pyocyanin-mediated inactivation of α1PI. These data raise the possibility that a direct interaction between reduced pyocyanin and α1PI is involved in the process. Consistent with this possibility, pretreatment of α1PI with the reducing agent β-mercaptoethanol also inhibited binding of trypsin to α1PI. These data suggest that pyocyanin could contribute to lung injury in the P. aeruginosa-infected airway of cystic fibrosis patients by decreasing the ability of α1PI to control the local activity of serine proteases.
Tight regulation of local protease activity is critical to the maintenance of the physiologic function of the lung and other tissue sites (15, 40, 48). Serine proteases such as neutrophil elastase are among the proteases found within the human airway (15, 16, 20, 38, 40). Serine protease activity must be tightly regulated in order to protect local tissue from protease-mediated injury. This is accomplished in vivo through the presence of a group of proteins which specifically inhibit serine protease activity (32, 40, 48). Among the key antiproteases in the airway is α1-protease inhibitor (α1PI) (32, 40, 48). α1PI is a 51-kDa protein member of the serpin class of serine protease inhibitors (32, 40, 48). α1PI forms a nearly irreversible enzymatically inactive complex with various serine proteases, including human neutrophil elastase (HNE), porcine pancreatic elastase (PPE), and trypsin (29, 40, 48). The active site of α1PI provides a putative cleavage site for the target enzymes, and it is the methionine 358 within that location which is key for both the protease specificity and inhibitory activity of α1PI. Modifications of this methionine markedly decrease the inhibitory activity of α1PI (29, 40, 48).
A number of laboratories have shown that exposure of α1PI to various oxidant sources, including activated phagocytes (6, 14, 28, 34, 39, 50) and cigarette smoke (17, 41), results in rapid inactivation of the protein due to oxidation of methionine 358 (31). Since the hereditary decrease in α1PI activity leads to early-onset emphysema (10), it has been postulated that oxidant-mediated inactivation of α1PI, resulting in decreased local regulation of serine protease activity, is an important contributor to the pathophysiology of cigarette-associated chronic obstructive lung disease (6, 30).
An imbalance of protease-antiprotease activity is also detected in the airways of patients with cystic fibrosis-associated lung disease (5, 16, 18, 19, 35). Most of this elevated protease activity is due to an increase in HNE activity (23, 43, 45). Studies indicate that the untoward protease imbalance is a result of both elevated amounts of HNE and the presence of functionally inactive α1PI (1, 2, 5, 35, 38, 44). The reason for the presence of inactive α1PI is unclear.
The onset of progressive lung disease in cystic fibrosis usually coincides with the development of persistent colonization and infection of the airway with Pseudomonas aeruginosa. P. aeruginosa secretes a number of potentially cytotoxic factors, including the phenazine derivative pyocyanin (21, 24, 25, 51). Under aerobic conditions, exposure of pyocyanin to NADH or other cell-derived reducing equivalents, leads to redox cycling of the compound with resultant formation of superoxide (O2·−) and hydrogen peroxide (H2O2) (4, 11, 22, 24, 25). Given the ability of pyocyanin to induce oxidant production, we hypothesized that this P. aeruginosa-derived product could contribute to the pathogenesis of the protease-mediated component of cystic fibrosis lung disease by serving as an additional source of oxidant-mediated inactivation of α1PI. The in vitro work reported herein supports the ability of redox cycling of pyocyanin to inactivate α1PI. However, the mechanism may not directly involve oxidant production.
MATERIALS AND METHODS
Reagents.
NADH, CuZn superoxide dismutase (SOD), trypsin, porcine pancreatic elastase (PPE), H2O2, methionine, histidine, catalase, and dimethylthiourea (DMTU) were obtained from Sigma Chemical Co., St. Louis, Mo. α1PI was purchased from Calbiochem, La Jolla, Calif. The PPE preparations contain chymotrypsin-trypsin (25 to 100 U of trypsin activity/mg of protein) as an impurity. 5,5 Dimethyl-N-pyrroline 1-oxide (DMPO) was purchased from the Oklahoma Medical Research Foundation, Oklahoma City, Okla.
Ability of α1PI to form a complex with serine proteases.
α1PI (10 μg/ml) in H2O was incubated with either PPE (50 to 200 μg/ml) or trypsin (50 to 200 μg/ml) for 15 to 30 min at 37°C. Aliquots of the reaction mixture were then added to Laemmli solubilizing buffer and subjected to standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The results were similar regardless of whether or not the gels were run under reducing conditions. The α1PI-protease complex is well known to remain associated under these conditions (7, 31, 46). Detection of an α1PI-protease complex was assessed by either (i) staining the gel with silver and determining the presence or absence of a new band with an apparent molecular weight consistent with a complex of α1PI and the protease; or (ii) transfer of the SDS-PAGE-separated sample to nitrocellulose. The nitrocellulose was then blocked with 4% bovine serum albumin–Tris-buffered saline for 1 h and then incubated overnight with a 1:100 dilution of rabbit anti-human α1PI (Sigma). The blots were then washed three times, and immunoreactive protein was determined with antirabbit immunoglobulin G linked to alkaline phosphatase. The α1PI-protease complex was manifested as the presence of an α1PI immunoreactive band with an apparent molecular weight higher than that of an α1PI standard.
α1PI activity.
α1PI activity was measured in terms of α1PI’s ability to inhibit PPE-mediated cleavage of succinyl-l-alanyl-l-alanyl-l-alanyl-p-nitroanilide (Sigma) according to the spectrophotometric assay of Travis and Johnson (47).
Cell culture of human lung epithelial cells.
The A549 human lung carcinoma cell line which resembles type II alveolar epithelial cells was maintained in continuous culture with Dulbecco’s modification of Eagle’s medium (DMEM) obtained through the University of Iowa Cancer Center, Iowa City. The culture medium was routinely supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin–streptomycin, and 1% glutamine. HBE cells were cultivated in a similar manner, except that the primary culture medium was 50% DMEM and 50% HAM (hepatocyte medium F12; University of Iowa Cancer Center). For each set of experiments, cells from the stock culture were seeded (2.5 × 104 to 5 × 104 cells/well) into 24- or 48-well plates. Cells were maintained at 37°C in 5% CO2 until they were at least 80% confluent (usually 72 h), at which time they were utilized in the desired experiments.
Purification of pyocyanin.
Pyocyanin was extracted from culture supernatant of P. aeruginosa PAO1 (ATCC 15692; American Type Culture Collection, Rockville, Md.) by serial chloroform extractions followed by sequential extractions with acid and neutral water as previously detailed (9). After the completion of five separation sequences, the pyocyanin was crystallized and dried under vacuum. It was resuspended in water and stored at 4°C until used.
Effect of pyocyanin redox cycling on α1PI-protease complex formation.
Experiments were performed in which α1PI (or, in some cases, the target protease) was exposed to either NADH- or cell-mediated redox cycling of pyocyanin. α1PI (10 μg/ml) was added either to a solution of NADH (6 mM) in H2O or to an A549 or HBE cell monolayer in Hanks’ balanced salt solution after which the desired concentration of pyocyanin was added. The system was then incubated for 30 min at 37°C. At this point, trypsin or PPE (200 μg/ml) was added for an additional 15 min (37°C). The solutions were assessed for the formation of α1PI-protease complex as described above.
EPR and spin trapping.
For spin trapping experiments, the spin trap DMPO (100 mM) and diethylenetriaminepentaacetic acid (DTPA [0.1 mM]) were included in the reaction mixture of interest. After the desired time of incubation, the reaction mixture was transferred to an electron paramagnetic resonance (EPR) quartz flat cell and placed into the cavity of the EPR spectrometer (ES 300; Brüker, Karlsrühe, Germany). EPR spectra were then obtained at 25°C with the following parameters: 4.00 × 109 gain, 335.544-s sweep time, 100-kHz modulation frequency, 0.501-G modulation amplitude, 80-G sweep width, 9.76-GHz frequency, and 20 mW of power. For detection of the pyocyanin radical, 40 μM pyocyanin was added to a 44 μM solution of NADH and 0.1 mM DTPA, which had been sparged with N2 for 20 min. The pyocyanin and NADH were then allowed to react at 25°C in the continuous presence of N2 and subjected to EPR spectroscopy with a Varian E-104A EPR spectrometer (Varian Associates, Inc., Palo Alto, Calif.) with a 1-s time constant, 8-min scan time, 2.5 × 104 gain, 100-kHz modulation frequency, and 20 mW of power.
RESULTS
Pyocyanin-NADH blocks α1PI-protease complex formation.
Several laboratories, including our own, have shown that addition of NADH to pyocyanin leads to the reduction of pyocyanin to the pyocyanin radical (4, 11, 22, 24–26). Under aerobic conditions, the pyocyanin radical will transfer an electron to O2, leading to the formation of O2·− (4, 11, 22, 24–26). Consistent with these earlier data (4, 11, 22, 24–26), when NADH and pyocyanin were incubated under conditions in which O2 was previously depleted from the system by N2 bubbling, an EPR spectrum indicative of the pyocyanin radical was detected (data not shown). When the same reaction was performed under aerobic conditions, the pyocyanin radical was no longer seen, but O2.− production was readily detected by DMPO spin trapping (data not shown).
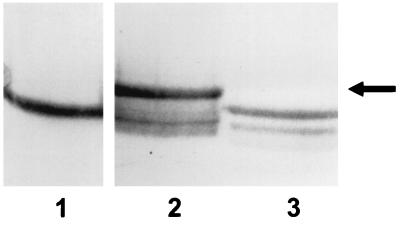
Given the susceptibility of α1PI to inactivation by oxidant species (6, 17, 41, 52), the ability of NADH-mediated redox cycling of pyocyanin to decrease α1PI activity was assessed. As shown in Fig. 1 and 2, the presence of NADH and pyocyanin decreased the ability of α1PI to form a stable complex with either trypsin or PPE. Concentrations of pyocyanin required to completely prevent detectable complex formation varied from experiment to experiment, ranging from 25 to 125 μM. In the PPE experiments, a major protease-α1PI complex (top) and a somewhat fainter protease-α1PI complex (bottom) were routinely observed. The lower bands are likely due to the presence of chymotrypsin and/or trypsin, which routinely contaminate commercial PPE preparations and also form a complex with α1PI. Neither NADH nor pyocyanin alone prevented formation of the α1PI-protease complex (data not shown), indicating that reduction of pyocyanin was required. These results were similar, regardless of whether the α1PI-protease complex was detected by silver staining or immunoblot analysis.
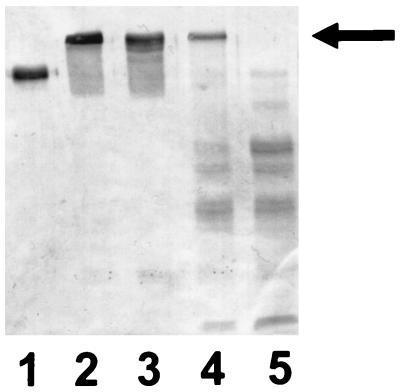
FIG. 1.
Immunoblot with antisera to α1PI in which gel samples were comprised of 10 μg of α1PI alone (lane 1); α1PI which had been incubated with trypsin for 30 min at 37°C (lane 2); or α1PI and 200 μg of trypsin along with 6 mM NADH and pyocyanin at a concentration of 12.5 μM (lane 3), 50 μM (lane 4), and 100 μM (lane 5). The arrow designates the location of the complex formed by α1PI and trypsin. The results are representative of 10 experiments.
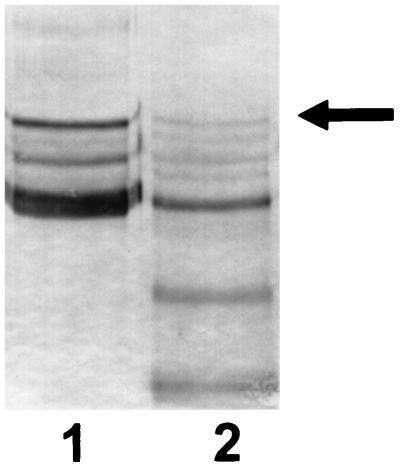
FIG. 2.
Immunoblot with antisera to α1PI. The gel sample in lane 1 consisted of α1PI which had been incubated for 30 min at 37°C with 200 μg of PPE. Lane 2 shows results obtained under the same conditions as lane 1, except that 6 mM NADH and 100 μM pyocyanin were added prior to the addition of PPE to the reaction mixture. The results are representative of five separate experiments.
An additional feature frequently noted when the NADH-pyocyanin combination was present was a decrease in the apparent quantity of α1PI detectable on the gel and evidence of proteolytic degradation products of α1PI (Fig. 1 and 2). The fact that these were detected by anti-α1PI immunoblotting indicates they indeed reflect degradation of α1PI. Lower-molecular-weight bands reactive with anti-α1PI were not observed with NADH-pyocyanin-treated α1PI unless an active protease was also added to the reaction mixture (data not shown [n = 3]). These data are consistent with the hypothesis that NADH-pyocyanin exposure leads to a modification of α1PI, such that the antiprotease is converted to a legitimate substrate for PPE or trypsin.
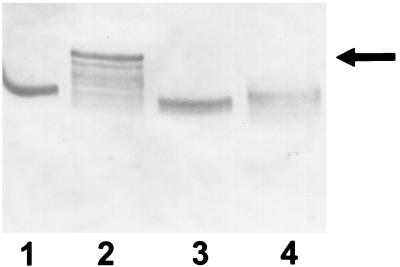
Since the incubation conditions described above resulted in exposure of trypsin and PPE to NADH-pyocyanin as well as α1PI, experiments were performed so that we could be certain that the effect of NADH-pyocyanin was on α1PI and not the protease. α1PI-protease complex formation was compared under conditions in which α1PI or the protease was first exposed to NADH-pyocyanin for 30 min, after which, the protease or α1PI was then added to the system, respectively, and α1PI-protease complex formation was assessed (Fig. 3). When α1PI was first incubated with NADH-pyocyanin and then PPE or trypsin was added, no α1PI-protease complex was generated (Fig. 3). In contrast, when trypsin or PPE was the component incubated initially with NADH-pyocyanin, after which α1PI was added, the same magnitude of α1PI-protease complex was observed as in the non-NADH-pyocyanin-treated control (Fig. 3). These results indicate that the effect of NADH-pyocyanin is on α1PI rather than the protease.
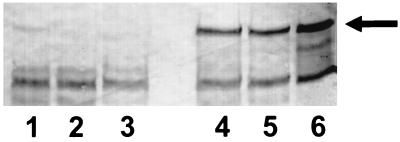
FIG. 3.
Immunoblot with antisera to α1PI in which gel samples were comprised of 10 μg of α1PI plus 200 μg of trypsin which had been incubated for 30 min at 37°C. Lanes 1 to 3 show the results obtained when α1PI was previously exposed to 6 mM NADH and 100 μM pyocyanin alone (lane 1), or with SOD (300 U/ml; lane 2) or catalase (5,000 U/ml; lane 3) added, for 20 min prior to the addition of trypsin to the reaction mixture. Samples in lanes 4 to 6 were obtained under the same conditions as those of lanes 1 to 3, except that the trypsin rather than the α1PI was previously exposed to 6 mM NADH and 100 μM pyocyanin alone (lane 4), or with SOD (300 U/ml; lane 5) or catalase (5,000 U/ml; lane 6) added, for 30 min prior to its interaction with α1PI. The results are representative of three experiments.
Biochemical evidence of NADH-pyocyanin inactivation of α1PI.
As confirmation that exposure to NADH-pyocyanin leads to loss of α1PI functional activity, the ability of NADH-pyocyanin-treated α1PI to inhibit PPE enzymatic activity was assessed. α1PI exposed to NADH-pyocyanin exhibited an impaired ability to inhibit PPE cleavage of succinyl-l-alanyl-l-alanyl-l-alanyl-p-nitroanilide. In the absence of α1PI, cleavage of succinyl-l-alanyl-l-alanyl-l-alanyl-p-nitroanilide by PPE yielded a change in A410 of 0.132 ± 0.012 U/min (mean ± standard error [SE]; n = 10). Addition of α1PI decreased this to 0.040 ± 0.005 U/min (mean ± SE; n = 10). When α1PI was first exposed to NADH-pyocyanin for 10 min, its ability to inhibit PPE activity was significantly decreased (P < 0.001 by analysis of variance) because A410 was 0.076 ± 0.009 U/min (mean ± SE; n = 10). NADH-pyocyanin had no effect on PPE activity as assessed in this assay (data not shown).
Epithelial cell-mediated redox cycling of pyocyanin inactivates α1PI.
In vivo redox cycling of pyocyanin in the P. aeruginosa-infected airway would likely occur via epithelial cell-mediated reduction of the compound rather via its interaction with extracellular NADH or NADPH. We have previously shown that incubation of monolayers of A549 (3, 12, 37) or HBE cells (12) with pyocyanin results in the formation of O2·−, as detected by spin trapping. Since initial reduction of pyocyanin would likely occur intracellularly, it was unclear whether cell-mediated redox cycling of pyocyanin could inactivate extracellular α1PI. In order to assess this, A549 (or HBE) monolayers were incubated with α1PI (10 μg/ml) ± pyocyanin (10 to 200 μM) for 30 min. PPE or trypsin was then added, and after 15 min of additional incubation, formation of α1PI-protease complex in the extracellular milieu was assessed by immunoblot analysis. As shown in Fig. 4 and 5, the presence of pyocyanin resulted in a marked decrease in the subsequent ability of α1PI to form a complex with either trypsin or PPE. This was dependent on the concentration of pyocyanin present. As with NADH-pyocyanin, supernatants from pyocyanin-treated, but not control, epithelial cells often exhibited evidence of α1PI proteolysis (Fig. 4).
FIG. 4.
Immunoblot with antisera to α1PI in which gel samples were comprised of supernatants removed from monolayers of HBE cells 30 min following the addition of α1PI (lane 1), α1PI plus 50 μM pyocyanin (lane 2), and α1PI plus 200 μM pyocyanin (lane 3), which were then mixed with trypsin for 30 min at 37°C. Each sample was subjected to SDS-PAGE and immunoblot analysis as described in Materials and Methods. The results are representative of three experiments.
FIG. 5.
Immunoblot with antisera to α1PI in which gel samples were comprised of supernatants removed from monolayers of A549 cells 30 min following the addition of α1PI (lane 2) or α1PI plus 100 μM pyocyanin (lane 3), which were then mixed with trypsin for 30 min at 37°C. The samples were then subjected to SDS-PAGE and immunoblot analysis as described in Materials and Methods. Lane 1 contains only α1PI, which was not incubated with trypsin and is included as a reference. The results are representative of three experiments.
Role of reactive oxygen intermediates in pyocyanin-associated inactivation of α1PI.
The ability of neutrophils to inactivate α1PI is linked to oxidation of the protein by reactive oxygen species, particularly those arising from the reaction of H2O2 and myeloperoxidase (MPO) (6, 14, 34, 52). Accordingly, we postulated that oxidant species resulting from pyocyanin-induced O2·− formation were responsible for NADH-pyocyanin-mediated inhibition of α1PI activity. In order to test this, we determined if the presence of SOD, catalase, or the H2O2-hydroxyl radical scavenger DMTU blocked NADH-pyocyanin-mediated inactivation of α1PI. Surprisingly, none of these agents alone nor the combination of SOD and catalase exhibited any ability to block the effect of NADH-pyocyanin (Fig. 3). SOD and catalase are reasonably large proteins, raising the possibility that they were unable to adequately reach the site of α1PI which required protection. However, we found that methionine, which blocks MPO-mediated inactivation of α1PI (6) via its ability to spare methionine 358 within the active site of α1PI (6, 29, 40, 48), did not protect α1PI from the effects of NADH-pyocyanin (data not shown). In addition, since O2·− is not generated as a consequence of pyocyanin reduction under anaerobic conditions, we assessed the ability of O2 depletion on the process by bubbling the experimental system with N2 prior to the addition of pyocyanin. NADH-pyocyanin retained its ability to inactivate α1PI under O2-depleted conditions (Fig. 6). That bubbling of N2 effectively depleted the system of O2 was confirmed with the anaerobic indicator resazurin (33).
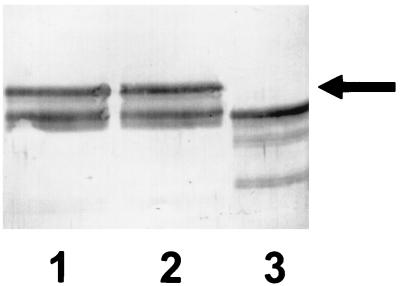
FIG. 6.
Immunoblot with antisera to α1PI in which gel samples were comprised of 10 μg of α1PI alone (lane 1) or 10 μg of α1PI alone plus 200 μg of trypsin (lane 2), which had been incubated for 30 min at 37°C. Lanes 3 and 4 show results obtained under the same conditions as lane 2, except that 6 mM NADH and 100 μM pyocyanin were added prior to the addition of trypsin to the reaction mixture. The incubation in lane 3 was performed under standard aerobic conditions, whereas lane 4 reflects results obtained under O2-depleted conditions in which the reaction mixture was bubbled with N2 for 20 min prior to the addition of pyocyanin. The results are representative of three separate experiments.
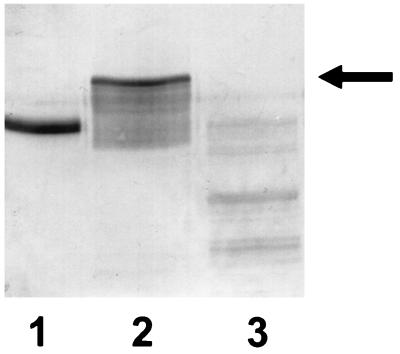
These data do not suggest a role for O2·−, or subsequently generated oxidants such as H2O2, in the ability of reduced pyocyanin to inactivate α1PI. Instead, they raise the possibility that pyocyanin radical, a reducing species formed by the initial electron transfer from NADH to pyocyanin, could be inactivating α1PI via a reducing rather than an oxidizing reaction. In support of this, exposure of α1PI to the reducing agent β-mercaptoethanol resulted in an inability of the α1PI to subsequently bind trypsin (Fig. 7). This is in contrast to reports in the literature (7, 31, 46) and or own experience (data not shown) that, once formed, the α1PI-trypsin complex does not dissociate upon exposure to β-mercaptoethanol.
FIG. 7.
Immunoblot with antisera to α1PI in which gel samples were comprised of 10 μg of α1PI alone (lane 1) or 10 μg of α1PI alone plus 200 μg of trypsin (lane 2), which had been incubated for 30 min at 37°C. The reaction in lane 3 was performed under the same conditions as those of lane 2, except that the α1PI had been exposed to 14 mM β-mercaptoethanol prior to the addition of the trypsin. Although not shown in this figure, addition of β-mercaptoethanol after the completion of the incubation of trypsin and α1PI has no effect on complex formation.
DISCUSSION
An elevated ratio of protease to antiprotease activity has been detected in the P. aeruginosa-infected airways of cystic fibrosis patients, and this is felt to contribute to the pathogenesis of cystic fibrosis lung disease (23, 43, 45). This imbalance is likely due to the presence of markedly elevated levels of the serine protease HNE as well as a higher than expected frequency of inactive α1PI (1, 2, 5, 35, 38, 44). Given the known susceptibility of α1PI to oxidant-mediated inactivation (6, 14, 17, 34, 41, 52), the ability of the redox-active P. aeruginosa secretory product pyocyanin to inactivate α1PI was assessed. Such a process could contribute to protease-mediated lung injury in cystic fibrosis, as well as potentially necrotizing acute nosocomial P. aeruginosa. Nearly all strains of P. aeruginosa are capable of producing pyocyanin.
In support of this hypothesis, we found that the reaction of NADH with concentrations of pyocyanin reported to be present in sputum sol of P. aeruginosa-infected cystic fibrosis patients (51), in excess of 100 μM, was able to alter α1PI such that it was no longer able to bind to and inhibit the enzymatic activity of the serine proteases PPE and trypsin (binding studies only). The concentration of pyocyanin present in the lung in the setting of P. aeruginosa pneumonia in the non-cystic fibrosis-hospitalized patient is not known. Our studies were performed with concentrations of α1PI and serine proteases very close to the mean values of 12 and 88 μg of α1PI and human neutrophil elastase, respectively, per ml reported to be present in the sputum of P. aeruginosa-infected cystic fibrosis patients (38). Although we would expect a similar effect of pyocyanin exposure on α1PI binding to HNE, this was not assessed due to the cost-prohibitive requirement of HNE for such studies. α1PI binding to all serine proteases occurs via the same basic process (32, 40, 48).
Similar to the results with NADH-pyocyanin, in studies in which human lung epithelial cell monolayers were exposed to pyocyanin, α1PI was inactivated when the protein was present in the extracellular milieu. Thus, in spite of the intracellular site at which pyocyanin reduction likely occurs, there was sufficient interaction between reduced pyocyanin and/or its oxidant products to inactivate α1PI. Previous spin trapping studies with endothelial cells support the concept that these pyocyanin-derived products do have access to the extracellular space (4).
Regardless of whether NADH or epithelial cells were employed as the means of reducing pyocyanin, incubation of pyocyanin-treated α1PI with either trypsin or PPE resulted in the formation of proteolytic degradation products of α1PI. This suggests that redox cycling of pyocyanin leads to modifications of α1PI which allow it to be cleaved by the protease rather than resulting in the formation of an irreversible complex between α1PI and the protease. Oxidant-mediated inactivation of another airway protease inhibitor, secretory leukocyte protease inhibitor (SLPI), also renders it more susceptible to proteolysis (49).
Consistent with our previous work (3, 4, 37) and that of others (4, 8, 11, 22, 24–26, 42), we found by using EPR techniques that the addition of NADH to pyocyanin leads to the initial formation of pyocyanin radical, which in the presence of O2 transfers that electron to O2 to form O2·−. O2·− production was also observed upon the addition of pyocyanin to human airway epithelial cells. Although not specifically measured, production of O2·− would lead in turn to the formation of H2O2 via the dismutation reaction of O2·− with itself (36). In view of previous observations that oxidation of methionine 358 at the α1PI active site is the mechanism whereby MPO-H2O2 inactivates α1PI (29, 40, 48), we suspected a similar process was involved in the inactivation of α1PI by pyocyanin. However, several pieces of data argue against such a mechanism. Free methionine failed to protect α1PI from the effect of pyocyanin, in contrast to its reported ability to protect the protein from inactivation by the MPO-H2O2 system (6). Neither SOD, catalase, DMTU, nor depletion of O2 from the system altered the ability of NADH-pyocyanin to inactivate α1PI. These data argue strongly against a role for pyocyanin-derived O2·− and/or H2O2 in the ability of this P. aeruginosa-derived compound to inactivate α1PI. Thus, pyocyanin-mediated inactivation of α1PI inhibition does not likely occur via oxidation of methionine at the α1PI active site.
These data raise the possibility that a direct interaction between α1PI and the pyocyanin radical itself, rather than reactive oxidant species generated from the interaction of reduced pyocyanin and O2, modifies α1PI such that it loses its ability to bind serine proteases. Under this hypothesis, the pyocyanin radical would directly modify α1PI by the transfer of an electron (reduction) to an amino acid constituent of the protein resulting in either direct modification of the active site or alternatively a conformational change in the molecule that decreases its ability to irreversibly bind serine proteases. This is consistent with the fact that the pyocyanin radical is predominantly a reducing radical. Supporting this hypothesis, exposure to the reducing agent β-mercaptoethanol also resulted in a loss of the ability of α1PI to bind trypsin. We are unaware of studies investigating the potential for reduction rather than oxidation of α1PI to decrease its ability to bind serine proteases. What amino acid(s) would be the target of such reduction is difficult to predict. This hypothesis will need to be further examined with previously generated site-specific mutations of α1PI as well as by molecular analysis of the pyocyanin-modified protein.
Although the exact mechanism remains to be definitively defined, our data are consistent with the potential for pyocyanin present in the P. aeruginosa-infected airway to cause a local inhibition of α1PI activity, thereby contributing to protease-mediated tissue injury. It is likely that the pathophysiology responsible for the protease-antiprotease imbalance which marks the airway of cystic fibrosis patients is multifactorial. There is an increased burden of HNE due to the large neutrophilic infiltrate that marks this disease state. Oxidants produced by the same neutrophils, particularly those derived from MPO-H2O2, also likely serve to inactivate α1PI. Interestingly, recent data suggest that pyocyanin, through its ability to induce interleukin 8 release from airway epithelial cells, may contribute to this neutrophilic infiltration of the airway (13). Besides α1PI, the airway contains other antiproteases, such as (SLPI), capable of inhibiting HNE (49). SLPI is also susceptible to oxidant-mediated inactivation (27, 49) due to the presence of a methionine residue at its active site. Whether SLPI can also be inactivated by pyocyanin is unknown and is worthy of investigation.
In summary, the present work demonstrates that, once reduced, pyocyanin can in turn interact with α1PI in such a way that there is a loss of the protein’s ability to form an irreversible complex with and inhibit the enzymatic activity of serine proteases. Although the exact mechanism requires further delineation, occurrence of these events in vivo could contribute to the pathogenesis of serine protease-mediated injury in P. aeruginosa-infected lungs of patients with cystic fibrosis chronic lung disease and/or acute nosocomial P. aeruginosa pneumonia.
ACKNOWLEDGMENTS
This work was supported in part by research grants from the Office of Research and Development, Medical Research Service, Department of Veteran Affairs, and the Public Health Service (HL44275 and AI34954), as well as the Cystic Fibrosis Foundation, and was performed during the tenure of B.E.B. as an Established Investigator of the American Heart Association.
We acknowledge and thank George Rasmussen for performing the spin trapping studies and Gerene Denning for helpful discussions.
REFERENCES
- 1.Allen E D. Opportunities for the use of aerosolized α1-antitrypsin for the treatment of cystic fibrosis. Chest. 1996;110(Suppl.):256S–260S. doi: 10.1378/chest.110.6_supplement.256s. [DOI] [PubMed] [Google Scholar]
- 2.Birrer P, McElvaney N G, Rüdeberg A, Wirz Sommer C, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal R G. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–213. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 3.Britigan B E, Rasmussen G T, Cox C D. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect Immun. 1997;65:1071–1076. doi: 10.1128/iai.65.3.1071-1076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britigan B E, Roeder T L, Rasmussen G T, Shasby D M, McCormick M L, Cox C D. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells: implications for pseudomonas-associated tissue injury. J Clin Investig. 1992;90:2187–2196. doi: 10.1172/JCI116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce M C, Poncz L, Klinger J D, Stern R C, Tomashefski J F, Jr, Dearborn D G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985;132:529–535. doi: 10.1164/arrd.1985.132.3.529. [DOI] [PubMed] [Google Scholar]
- 6.Carp H, Janoff A. In vitro suppression of serum-elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Investig. 1979;63:793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen S, Valnickova Z, Thogersen I B, Pizzo S V, Nielsen H R, Roepstorff P, Enghild J J. Sodium dodecyl sulfate-stable complexes between serpins and active or inactive proteinases contain the region COOH-terminal to the reactive site loop. J Biol Chem. 1995;270:14859–14862. doi: 10.1074/jbc.270.25.14859. [DOI] [PubMed] [Google Scholar]
- 8.Cohen M S, Charniga L M, Stutts M J, Yankaskas J R, Hassett D J, Krochmal E, Gatzy J T, Boucher R C. Program and abstracts of the 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1990. Effects of Pseudomonas pyocyanin on cystic fibrosis epithelial cells, abstr. 44; p. 94. [Google Scholar]
- 9.Cox C D. Role of pyocyanin in the acquisition of iron from transferrin. Infect Immun. 1986;52:263–270. doi: 10.1128/iai.52.1.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crystal R G. α-1-Antitrypsin deficiency, emphysema, and liver disease: genetic basis and strategies for therapy. J Clin Investig. 1990;85:1342–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis G, Thornalley P J. Free radical production from the aerobic oxidation of reduced pyridine nucleotides catalyzed by phenazine derivatives. Biochim Biophys Acta. 1983;724:456–464. doi: 10.1016/0005-2728(83)90106-8. [DOI] [PubMed] [Google Scholar]
- 12.Denning G M, Railsback M A, Rasmussen G T, Cox C D, Britigan B E. Pseudomonas pyocyanine alters calcium signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 1998;274:L893–L900. doi: 10.1152/ajplung.1998.274.6.L893. [DOI] [PubMed] [Google Scholar]
- 13.Denning G M, Wollenweber L A, Railsback M A, Cox C D, Stoll L L, Britigan B E. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect Immun. 1998;66:5777–5784. doi: 10.1128/iai.66.12.5777-5784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desrochers P E, Mookhtiar K, Van Wart H E, Hasty K A, Weiss S J. Proteolytic inactivation of α1-proteinase inhibitor and α1-antichymotrypsin by oxidatively activated human neutrophil metalloproteinases. J Biol Chem. 1992;267:5005–5012. [PubMed] [Google Scholar]
- 15.Döring G. The role of neutrophil elastase in chronic inflammation. Am J Respir Crit Care Med. 1994;150(Suppl.):S114–S117. doi: 10.1164/ajrccm/150.6_Pt_2.S114. [DOI] [PubMed] [Google Scholar]
- 16.Döring G, Goldstein W, Röll A, Schiøtz P O, Høiby N, Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985;49:557–562. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans M D, Church D F, Pryor W A. Aqueous cigarette tar extracts damage human alpha-1-proteinase inhibitor. Chem Biol Interact. 1991;79:151–164. doi: 10.1016/0009-2797(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 18.Fick R B., Jr Pathogenesis of the Pseudomonas lung lesion in cystic fibrosis. Chest. 1989;96:158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- 19.Fick R B, Jr, Hata J S. Pathogenetic mechanisms in lung disease caused by Pseudomonas aeruginosa. Chest. 1989;95:206S–213S. [Google Scholar]
- 20.Fick R B, Jr, Naegel G P, Squier S U, Wood R E, Gee J B L, Reynolds H Y. Proteins of the cystic fibrosis respiratory tract: fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Investig. 1984;74:236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank L H, DeMoss R D. On the biosynthesis of pyocyanine. J Bacteriol. 1959;77:776–782. doi: 10.1128/jb.77.6.776-782.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner P R. Superoxide production by the mycobacterial and pseudomonad quinoid pigments phthiocol and pyocyanine in human lung cells. Arch Biochem Biophys. 1996;333:267–274. doi: 10.1006/abbi.1996.0390. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein W, Doring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Hassan H M, Fridovich I. Intracellular production of superoxide radical and hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979;196:385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- 25.Hassan H M, Fridovich I. Mechanism of the antibiotic action of pyocyanine. J Bacteriol. 1980;141:156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassett D J, Charniga L, Bean K, Ohman D E, Cohen M S. Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect Immun. 1992;60:328–336. doi: 10.1128/iai.60.2.328-336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel-Wieland R, Steffens G J, Flohé L. Inhibitory characteristics and oxidation resistance of site-specific variants of recombinant human antileukoproteinase. Biomed Biochim Acta. 1990;50:677–681. [PubMed] [Google Scholar]
- 28.Hubbard R C, Ogushi F, Fells G A, Cantin A M, Jallat S, Courtney M, Crystal R G. Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of α1-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. J Clin Investig. 1987;80:1289–1295. doi: 10.1172/JCI113204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber R, Carrell R W. Implications of the three-dimensional structure of α1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- 30.Janoff A, Carp H, Laurent P, Raju L. The role of oxidative processes in emphysema. Am Rev Respir Dis. 1983;127:S31–S38. doi: 10.1164/arrd.1983.127.2P2.S31. [DOI] [PubMed] [Google Scholar]
- 31.Johnson D, Travis J. The oxidative inactivation of human α-1-proteinase inhibitor: further evidence for methionine at the reactive center. J Biol Chem. 1979;254:4022–4026. [PubMed] [Google Scholar]
- 32.Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- 33.Mandell G L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974;9:337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matheson N R, Wong P S, Travis J. Enzymatic inactivation of human α-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979;88:402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- 35.Meyer K C, Lewandoski J R, Zimmerman J J, Nunley D, Calhoun W J, Dopico G A. Human neutrophil elastase and elastase/alpha1-antiprotease complex in cystic fibrosis: comparison with interstitial lung disease and evaluation of the effect of intravenously administered antibiotic therapy. Am Rev Respir Dis. 1991;144:580–585. doi: 10.1164/ajrccm/144.3_Pt_1.580. [DOI] [PubMed] [Google Scholar]
- 36.Miller R A, Britigan B E. The formation and biologic significance of phagocyte-derived oxidants. J Investig Med. 1995;43:39–49. [PubMed] [Google Scholar]
- 37.Miller R A, Rasmussen G T, Cox C D, Britigan B E. Protease cleavage of iron-transferrin augments pyocyanin-mediated endothelial cell injury via promotion of hydroxyl radical formation. Infect Immun. 1996;64:182–188. doi: 10.1128/iai.64.1.182-188.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor C M, Gaffney K, Keane J, Southey A, Byrne N, O’Mahoney S, Fitzgerald M X. α1-Proteinase inhibitor, elastase activity, and lung disease severity in cystic fibrosis. Am Rev Respir Dis. 1993;148:1665–1670. doi: 10.1164/ajrccm/148.6_Pt_1.1665. [DOI] [PubMed] [Google Scholar]
- 39.Ossanna P J, Test S T, Matheson N R, Regiani S, Weiss S J. Oxidative regulation of neutrophil elastase-alpha-a-proteinase inhibitor interactions. J Clin Investig. 1986;77:1939–1951. doi: 10.1172/JCI112523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- 41.Pryor W A, Dooley M M, Church D F. The inactivation of α-1-proteinase inhibitor by gas-phase cigarette smoke: protection by antioxidants and reducing species. Chem Biol Interact. 1986;57:271–283. doi: 10.1016/0009-2797(86)90002-5. [DOI] [PubMed] [Google Scholar]
- 42.Schellhorn H E, Pou S, Moody C, Hassan H M. An electron spin resonance study of oxyradical generation in superoxide dismutase- and catalase-deficient mutants of Escherichia coli K-12. Arch Biochem Biophys. 1989;271:323–331. doi: 10.1016/0003-9861(89)90282-8. [DOI] [PubMed] [Google Scholar]
- 43.Suter S. The imbalance between granulocyte neutral proteases and antiproteases in bronchial secretions from patients with cystic fibrosis. Antibiot Chemother. 1989;42:158–168. doi: 10.1159/000417616. [DOI] [PubMed] [Google Scholar]
- 44.Suter S. The role of bacterial proteases in the pathogenesis of cystic fibrosis. Am J Respir Crit Care Med. 1994;150(Suppl.):S118–S122. doi: 10.1164/ajrccm/150.6_Pt_2.S118. [DOI] [PubMed] [Google Scholar]
- 45.Suter S, Schaad O B, Roux L, Nydegger U E, Waldvogel F A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984;149:523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- 46.Thomas R M, Nauseef W M, Iyer S S, Peterson M W, Stone P J, Clark R A. A cytosolic inhibitor of human neutrophil elastase and cathepsin G. J Leukocyte Biol. 1991;50:568–579. doi: 10.1002/jlb.50.6.568. [DOI] [PubMed] [Google Scholar]
- 47.Travis J, Johnson D. Human α1-proteinase inhibitor. Methods Enzymol. 1981;80:754–765. [Google Scholar]
- 48.Travis J, Salvesen G S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 49.Vogelmeier C, Hubbard R C, Fells G A, Schnebli H-P, Thompson R C, Fritz H, Crystal R G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Investig. 1991;87:482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss S J, Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Investig. 1984;73:1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson R, Sykes D A, Watson D, Rutman A, Taylor G W, Cole P J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaslow M C, Clark R A, Stone P J, Calore J D, Snider G L, Franzblau C. Human neutrophil elastase does not bind alpha1-protease inhibitor that has been exposed to activated human neutrophils. Am Rev Respir Dis. 1983;128:434–439. doi: 10.1164/arrd.1983.128.3.434. [DOI] [PubMed] [Google Scholar]