Abstract
Enterococci are a common cause of serious infections, especially in newborns, severely immunocompromised patients, and patients requiring intensive care. To characterize enterococcal surface antigens that are targets of opsonic antibodies, rabbits were immunized with various gentamicin-killed Enterococcus faecalis strains, and immune sera were tested in an opsonophagocytic assay against a selection of clinical isolates. Serum raised against one strain killed the homologous strain (12030) at a dilution of 1:5,120 and mediated opsonic killing of 33% of all strains tested. In addition, this serum killed two (28%) of seven vancomycin-resistant Enterococcus faecium strains. Adsorption of sera with the homologous strain eliminated killing activity. The adsorbing antigens were resistant to treatment with proteinase K and to boiling for 1 h, but were susceptible to treatment with sodium periodate, indicating that the antigen inducing opsonic activity is a polysaccharide. Antibodies in immune rabbit sera reacted with a capsule-like structure visualized by electron microscopy both on the homologous E. faecalis strain and on a vancomycin-resistant E. faecium strain. The capsular polysaccharides from E. faecalis 12030 and E. faecium 838970 were purified, and chemical and structural analyses indicated they were identical glycerol teichoic acid-like molecules with a carbohydrate backbone structure of 6-α-d-glucose-1-2 glycerol-3-PO4 with substitution on carbon 2 of the glucose with an α-2-1-d-glucose residue. The purified antigen adsorbed opsonic killing activity from immune rabbit sera and elicited high titers of antibodies (when used to immunize rabbits) that both mediated opsonic killing of bacteria and bound to a capsule-like structure visualized by electron microscopy. These results indicate that approximately one-third of a sample of 15 E. faecalis strains and 7 vancomycin-resistant E. faecium strains possess shared capsular polysaccharides that are targets of opsonophagocytic antibodies and therefore are potential vaccine candidates.
In addition to its importance as a community-acquired pathogen causing endocarditis and urinary tract infections, enterococci are now the third most common nosocomial pathogen isolated from the blood and pulmonary and urinary tracts and are the most common nosocomial pathogen causing surgical site infections (4, 16). Enterococcal infections significantly contribute to mortality as well as to prolonged hospital stay (12). Depending on the patient population, an overall mortality rate of 20 to 68% was reported before vancomycin resistance was observed (6). Since the advent of glycopeptide resistance, crude mortality rates of up to 100% have been reported for patients infected with vancomycin-resistant enterococci (VRE) (6, 14, 15, 24). Mortality has been found to be significantly higher among patients with bloodstream VRE isolates than among those infected with vancomycin-susceptible enterococci (37% versus 16%) (4). However, these are limited data characterizing specific bacterial factors that contribute to enterococcal virulence, so the importance of these pathogens is primarily predicated on clinical findings. The emergence of VRE and the increasing isolation of enterococci from hospitalized patients have driven an inquiry into the virulence mechanisms of this pathogen and the development of alternatives to standard antibiotic treatment.
Many pathogenic bacteria express capsular polysaccharides that are both critical virulence factors and targets for protective antibody. Polysaccharides expressed by enterococci include an antigen designated the type 1 carbohydrate (3), a tetraheteroglycan isolated by Pazur (18) from cell walls that contained a β-d-glucose-1-phosphate component, and the cell wall teichoic acid, initially characterized as the group D streptococcal antigen that is expressed by enterococci (8, 26). An intracellular glycerol-phosphate polymer substituted with kojibiose (an α-1-2 glucose disaccharide) was characterized by Wicken and Baddiley in 1963 (25). More recently Weinstock and colleagues (27, 28) have identified Enterococcus faecalis DNA that encodes proteins putatively involved in synthesis of a polysaccharide antigen that was suggested to be related to the type 1 antigen described by Bleiweis et al. (3). Our minimal knowledge regarding the virulence mechanisms of and the targets for protective immunity against this increasingly important pathogen, the fact that VRE infection is at times considered untreatable, and the genetic plasticity of these organisms prompted us to study whether enterococci possess capsular polysaccharide antigens analogous to those of most important bacterial pathogens that infect humans.
MATERIALS AND METHODS
Bacterial strains.
The clinical strains used in the present study (Table 1) were isolated from patients in various hospitals between 1994 and 1996. Seventeen of these 23 strains have been subtyped by pulsed-field gel electrophoresis and were found to be clonally unrelated according to the criteria of Tenover et al. (22).
TABLE 1.
Susceptibility of clinical isolates of enterococci used in this study to opsonic killing mediated by rabbit antiserum to E. faecalis 12030
| Strain | Sourcea | % of bacteria killed by antisera to strain 12030b |
|---|---|---|
| E. faecalis | ||
| 12030 | Cleveland, Ohio, VA | 96 |
| 12107 | Cleveland, Ohio, VA | 37 |
| 9693 | Cleveland, Ohio, VA | 0 |
| JH2-2 | Laboratory strain | 0 |
| R19.001 | CH | 26 |
| I.006 | CH | 0 |
| 355974L | BIDMC | 47 |
| 334802K | BIDMC | 91 |
| 326578K | BIDMC | 47 |
| 324057B | BIDMC | 0 |
| 175-A | BWH | 79 |
| 10688330A | BWH | 21 |
| 6814 | BWH | 0 |
| 1067761 | BWH | 61 |
| B8597A | BWH | 35 |
| B8610A | BWH | 0 |
| Vancomycin-resistant E. faecium | ||
| 838970 | BWH | 83 |
| 755221 | BWH | 28 |
| 824955 | BWH | 23 |
| B210860 | BWH | 9 |
| 740220 | BWH | 18 |
| 757875 | BWH | 25 |
| 805370 | BWH | 60 |
VA, Veterans Administration Hospital (courtesy of David Shlaes); CH, Children’s Hospital, Boston, Mass.; BIDMC, Beth Israel Deaconess Medical Center, Boston, Mass.; BWH, Brigham and Women’s Hospital, Boston, Mass. Note that BIDMC and BWH isolates were all distinct by pulsed-field gel electrophoresis analysis.
Killing of >45% is significant at P ≤ 0.05 by t test comparing CFU surviving in immune sera with CFU surviving in nonimmune sera (significant results shown in boldface type).
Production of antisera.
Antibodies to whole-cell enterococcal antigens were generated in New Zealand White rabbits in response to immunization with gentamicin-killed bacteria. Because the chemical nature of the putative antigens we were seeking was initially unknown, this method was chosen because it should kill the microorganisms, making them safe for injection, but not disrupt potentially protective epitopes on the bacterial surface. Other methods to prepare bacteria for generation of antisera, such as heat or treatment with reactive aldehydes, could potentially affect important epitopes on the antigens we were seeking to identify. Animals were immunized with three gentamicin-killed strains of E. faecalis (JH2-2, 12030, and 12107) by intravenous injection (three doses per week for 4 weeks).
Antibodies to purified enterococcal polysaccharides were elicited in rabbits by subcutaneous immunization with two 100-μg doses of polysaccharide emulsified in 0.5 ml of complete Freund’s adjuvant followed by three intravenous injections of antigen in saline spaced 3 days apart. After the final infection, animals were bled periodically for determinations of antibody opsonic activity and boosted monthly by intravenous injection of antigen in saline.
Opsonophagocytic assay.
An opsonophagocytic assay was conducted to quantify the killing activity of sera against a selection of clinical isolates of enterococci. Freshly drawn human blood (10 to 30 ml) was mixed with an equal volume of dextran-heparin buffer (4.5 g of dextran; Sigma Chemical, St. Louis, Mo.; 28.4 mg of heparin sodium; Acros Organics USA, Pittsburgh, Pa.; 500 ml of distilled water), and the mixture was incubated at 37°C for 1 h. The upper layer containing the leukocytes was collected by centrifugation, and hypotonic lysis of the remaining erythrocytes was accomplished by suspension of the cell pellet in 1% (wt/vol) NH4Cl. The leukocyte population was subsequently washed in RPMI (BioWhittaker, Walkersville, Md.) with 15% fetal bovine serum. Trypan blue staining and counting in a hemocytometer were used to determine the concentration of live leukocytes, and the final leukocyte concentration was adjusted to 2 × 107 cells per ml. The serum used as a complement source was obtained from healthy humans and absorbed with the target strain of E. faecalis or Enterococcus faecium (108 CFU/ml) at 4°C for 1 h prior to use.
The phagocytosis assay was performed with 100 μl of leukocyte suspension, 100 μl of bacteria (concentration adjusted spectrophotometrically to 2 × 107/ml and confirmed by viable counts), 100 μl of normal or immune rabbit serum, and 100 μl of adsorbed human serum (dilution of 1:10) as a complement source. The reaction mixture was incubated on a rotor rack at 37°C for 90 min; samples were taken at time 0 and after 90 min, diluted in 1% Proteose Peptone (Difco Laboratories, Detroit, Mich.), and plated onto tryptic soy agar plates (Becton Dickinson, Cockeysville, Md.). The mean numbers of CFU surviving in the various samples were then compared, and the overall significance of the differences was determined by a t test (for two-group comparisons) or analysis of variance (ANOVA) (for multigroup comparisons). For multigroup comparisons, the significance of the differences in pairwise comparisons was determined by the probable least-squares differences (PLSD) test (19). The StatView program (Abacus Concepts, Berkeley, Calif.), run on a Macintosh computer, was used for statistical determinations.
Preparation and characterization of capsular polysaccharides.
A high-molecular-weight carbohydrate-rich fraction was isolated from E. faecalis 12030 and E. faecium 838970 after initial results indicated that these strains elaborated a cross-reactive, heat- and protease-stable, periodate-sensitive antigen that was a target for opsonic antibodies. Bacteria were grown in Columbia broth (Difco Laboratories) with 5% glucose and 0.005% hemin added, and the recovered bacterial cells were suspended in either phosphate-buffered saline (PBS) or sucrose as described previously (17) and digested with mutanolysin and lysozyme (0.1 mg/ml) (Sigma Chemical) for 16 to 18 h at 37°C. Treatment of the cell suspension with nucleases (100 μg/ml) at 37°C for 4 h was followed by addition of proteinase K or pronase (100 μg/ml) and further incubation at 56°C overnight. The remaining insoluble cell wall fragments and cell bodies were removed by centrifugation. The supernatant was collected, filtered, and size fractionated on a Sephacryl S-400 or S-500 column (Pharmacia Biotech, Uppsala, Sweden), with 0.2 M PBS or 0.4 M ammonium carbonate buffers, respectively. Material that eluted in the void volume was pooled, dialyzed against distilled water, and lyophilized.
Samples from E. faecalis cells were further purified by dissolution in 50 mM Tris buffer (pH 8.0) and application to an anion-exchange Q column (Bio-Rad, Richmond, Calif.). Bound antigen was eluted from the column with increasing concentrations of NaCl (0 to 1 M, linear gradient), and fractions containing polysaccharides were identified both by adsorption at 206 nm and by immunodot blots with rabbit antisera to E. faecalis 12030. Fractions were pooled, dialyzed against deionized water, and lyophilized.
Characterization of antigens.
Polysaccharides were analyzed for monosaccharide constituents and structure by both gas chromatography-mass spectroscopy (GC/MS) and nuclear magnetic resonance (NMR), as described elsewhere (23). Stability in response to proteases was determined after treatment of enterococcal cells or purified antigens with proteinase K for 2 to 4 h at 56°C. Stability in response to periodate was determined by treatment of cells or purified antigens with 0.2 M sodium periodate (pH 7.2) overnight at room temperature. Excess periodate was destroyed by the addition of ethylene glycol.
Immunoelectron microscopy.
For the electron microscopic visualization of bacterial cells, immune rabbit sera to either E. faecalis 12030 cells or to purified polysaccharide served as the source of primary antibodies. Bacteria were grown overnight at 37°C in tryptic soy broth; a 1-ml volume was centrifuged, and the bacterial pellet was washed three times with PBS and suspended in 100 μl of PBS. Drops of bacterial solutions (5 μl) were placed on Parafilm, and 200-mesh Formvar-carbon-coated copper grids (Electron Microscopy Sciences, Fort Washington, Mass.) were placed on top of each drop for 1 min. The grids were then blocked by placement on a 5-μl drop of 0.5% fishskin gelatin in PBS with 0.1% Tween 20. Application of the primary antibodies to the grids in a similar fashion (at dilutions of 1:5 to 1:50) was followed by incubation for 20 min at room temperature and washing with 0.1× PBS–Tween. Preimmune rabbit sera were used as controls. Gold-conjugated protein A (20-nm-diameter gold particles) was applied at a dilution of 1:10. After incubation for 20 min at room temperature, the grids were washed four times with deionized water. Further staining of the grids was not necessary, because the bacterial cells were clearly visible at this point in the electron microscope (JEOL 1200 EX). Photographs were taken at magnifications of ×10,000 to ×25,000.
RESULTS
Serum raised against three antibiotic-killed E. faecalis strains were initially evaluated for opsonic killing of the homologous and heterologous enterococcal strains to identify a serum with broadly cross-reactive opsonic killing activity. An antiserum to E. faecalis JH2-2 mediated opsonic killing only of the homologous strain, while an antiserum raised to cells of E. faecalis 12107 mediated opsonic killing of the homologous strain and two other clinical isolates. Serum raised to antibiotic-killed E. faecalis 12030 mediated >50% killing of the homologous bacteria at a dilution of 1:5,120. Preimmune sera had a killing titer of <1:20. When the immune serum was used at a dilution of 1:500, which mediated killing of >90% of the bacteria of the homologous E. faecalis strain, 5 (33%) of 15 additional clinical E. faecalis strains were effectively killed (Table 1). In addition, serum raised against this strain killed two (28%) of seven vancomycin-resistant E. faecium strains (Table 1). Since strain 12030 elicited the most broadly cross-reactive antibodies, it was selected for further studies to identify the surface antigens that were the targets of the opsonic antibodies.
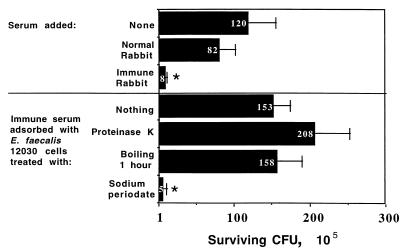
Adsorption of the immune rabbit serum to E. faecalis 12030 with the homologous strain (1 h at 4°C) completely eliminated the opsonic killing activity from this serum. Opsonic killing was also eliminated when the adsorbing bacteria were treated with proteinase K (100 μg/ml) or boiled for 1 h prior to adsorption (Fig. 1). Thus, the epitope that the opsonic antibody bound was heat stable and resistant to proteinase K. Treatment of the bacterial cells with sodium periodate (0.2 M at room temperature for 24 h) destroyed their ability to adsorb out the opsonic killing activity, indicating that a carbohydrate antigen was likely the target of the opsonic antibodies (Fig. 1).
FIG. 1.
Opsonic killing of E. faecalis 12030 by antibodies in a 1:500 dilution of antisera raised to antibiotic-killed cells of this organism and adsorption of opsonic killing activity by homologous cells treated in the manner indicated on the y axis. Black bars and white numbers indicate mean CFU surviving, and error bars show standard deviations. P = 0.001 (ANOVA) for overall significance. Asterisks indicate survival significantly (P < 0.001) lower than that in normal rabbit serum according to PLSD comparisons.
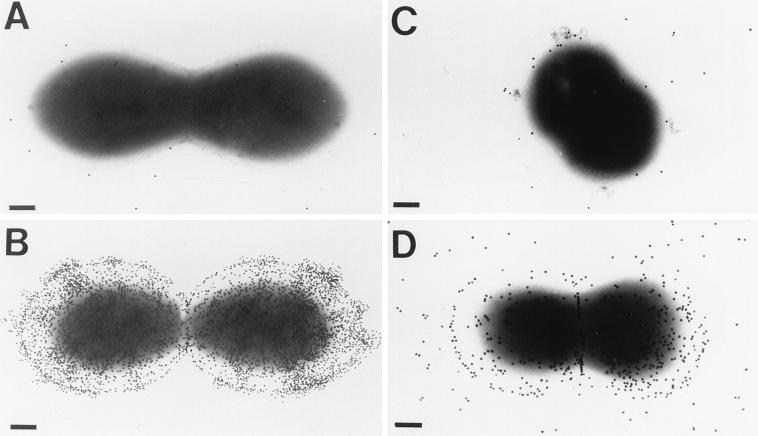
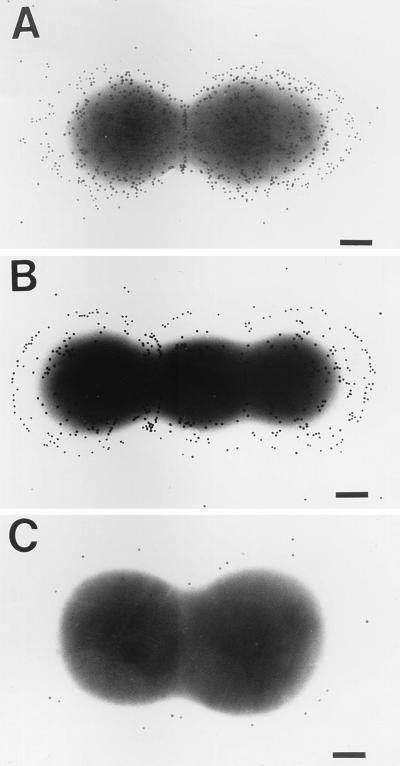
Electron microscopic visualization of the bacterial cells revealed a capsule-like structure in both the homologous strain and vancomycin-resistant E. faecium strain 838970 (Fig. 2), which could be killed by rabbit antibodies to E. faecalis 12030 (Table 1). Only low levels of binding of gold-labeled protein A were observed when normal rabbit serum was used (Fig. 2). Treatment of bacterial cells with trypsin (100 μg/ml for 1 h at 37°C) did not eliminate antibody binding (data not shown). These results indicate that a protease- and heat-stable, periodate-sensitive antigenic target for opsonic killing is present on the surface of these enterococcal strains.
FIG. 2.
Electron microscopic visualization of capsule-like material in E. faecalis 12030 and vancomycin-resistant E. faecium strain 838970. (A) E. faecalis 12030 treated with normal rabbit serum. (B) E. faecalis 12030 treated with rabbit antiserum raised to antibiotic-killed strain 12030. (C) E. faecium 838970 treated with normal rabbit serum. (D) E. faecium 838970 treated with rabbit antiserum raised to antibiotic-killed strain 12030. Bars, 200 nm. Gold particles measure 20 nm in diameter.
We next determined that mutanolysin-lysozyme treatment of E. faecalis 12030 released the putative carbohydrate antigen from the cell wall, because this crude extract inhibited opsonic killing activity. The mutanolysin-lysozyme-released cellular extracts were further treated with nucleases and then with proteinase K and were size fractionated on a Sephacryl S-500 column. The resulting high-molecular-weight (i.e., void volume) material also inhibited opsonic killing activity of immune rabbit sera against strains of both E. faecalis and E. faecium (data not shown). Furthermore, the high-molecular-weight material lost its ability to adsorb out opsonic killing activity if it was first treated with sodium periodate (data not shown). However, this material was not pure, so it was subjected to ion-exchange chromatography.
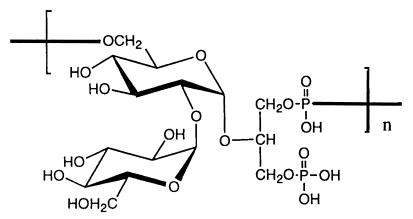
Two distinct polysaccharide fractions were obtained from the ion-exchange column, the first of which eluted at about 200 mM NaCl, and the second of which eluted at 400 mM NaCl. Analysis of these materials by NMR spectroscopy indicated that they were of sufficient purity to be amenable to compositional analysis by gas chromatography and to structural analysis by NMR. The earlier-eluting material contained four distinct monosaccharides, with signals suggesting the likely presence of amino sugars and deoxyhexoses. However, this material was poorly reactive with immune sera raised to whole cells and did not inhibit opsonic killing of enterococci by whole-cell antisera. The later-eluting polysaccharide was strongly reactive with whole-cell immune serum, had a purity of >90% as determined by GC/MS analysis, and inhibited opsonic killing of the homologous enterococcal strain when added to the immune serum. Its structure was determined to be a glycerol teichoic acid-like molecule (Fig. 3) by GC/MS and NMR analysis. The repeat unit contained one glycerol molecule and two glucose molecules. The carbohydrate backbone contained glycerol and one glucose residue, which were linked via the no. 2 carbon (C-2) of the glycerol and C-1 of the glucose; C-6 of this glucose was linked via a phosphodiester bridge to the next glycerol. The glucose residue in the backbone was substituted α-2-1 to a second glucose residue.
FIG. 3.
Structure of the capsular polysaccharide of E. faecalis 12030, as determined by NMR and GC/MS.
When methodology comparable to that used to obtain the purified glycerol-glucose polymer from E. faecalis 12030 was used to isolate an antigenic fraction from E. faecium 838970, an identical polymer was obtained, as indicated by chemical and spectroscopic analyses. The antigenic preparation from E. faecium did not contain the tetraheteroglycan found in polysaccharide preparations from E. faecalis after molecular sieve chromatography. Thus, the composition of the E. faecium glycerol-glucose polymer could be determined without further use of ion-exchange chromatography. Details of the spectroscopic analyses used to determine these structures are being prepared for publication.
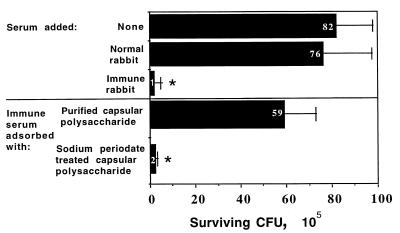
Immunization of rabbits with the purified glycerol-glucose polymer resulted in specific high-titered antibodies that mediated bacterial killing in an opsonophagocytic assay. The homologous E. faecalis strain, 12030, was killed at levels comparable to those achieved with serum raised to whole bacteria (i.e., 85 to 95% killing by a 1:500 serum dilution compared to normal rabbit sera), and this killing activity could be abolished by adsorption of the immune rabbit sera with the purified glycerol-glucose polymer (100 μg/ml of serum) (Fig. 4). Pretreatment of the purified capsular polysaccharide with sodium periodate prior to adsorption destroyed the ability of the polysaccharide to eliminate the killing activity (Fig. 4). Antibodies to the purified capsule also mediated killing of the same enterococcal strains (Table 1) killed by whole-cell 12030 antiserum, including E. faecium 838970 (data not shown). Immunogold electron microscopy experiments showed that the antibodies raised to the purified glycerol-glucose polymer bound to a capsular material surrounding cells of E. faecalis 12030 and E. faecium 838970, but not to E. faecalis 12107, which was not susceptible to opsonic killing by these antibodies (Fig. 5). These results confirmed that the carbohydrate antigen was a surface-exposed capsular polysaccharide on the enterococcal strains tested.
FIG. 4.
Opsonic killing of E. faecalis 12030 by antibodies in sera raised to purified capsular polysaccharide and inhibition of the killing by purified or periodate-treated capsular polysaccharide. Black bars and white numbers indicate mean 105 CFU surviving, and error bars show standard deviations. P = 0.0001 (ANOVA) for overall significance. Asterisks indicate survival significantly (P < 0.001) lower than that in normal rabbit serum according to PLSD comparisons.
FIG. 5.
Visualization of capsular polysaccharide of enterococcal strains in immunogold electron microscopy experiments with antibodies raised to purified glucose-glycerol polysaccharide antigen. Cells treated with preimmune rabbit sera appeared identical to those shown in Fig. 2 for normal rabbit serum. (A) E. faecalis 12030. (B) E. faecium 838970. (C) Negative control E. faecalis strain 12107. Bars, 200 nm. Gold particles measure 20 nm in diameter.
DISCUSSION
The emergence of enterococci as prominent nosocomial pathogens and the occurrence of enterococcal infections not treatable with currently available antibiotics are events that clearly justify intensive exploration of pathogenic factors as targets for immunotherapeutic interventions. Surprisingly little is known about either bacterial virulence factors or host defense mechanisms operative in enterococcal infections. The list of definite and potential enterococcal virulence factors is short (11). Our results show that some strains of both E. faecalis and vancomycin-resistant E. faecium express extracellular capsular polysaccharides that are targets for opsonic antibodies. Such polysaccharides could be important both in the virulence of these organisms and in immunity to them.
Little is known so far about the chemical nature, composition, and structure of enterococcal cell wall carbohydrates. Bleiweis et al. (3) identified glucose, glucosamine, galactosamine, rhamnose, ribitol, phosphorus, and residual peptidoglycan as the components of the type 1 carbohydrate of what was then called group D streptococci, an antigen obtained from an autolytic digest of bacterial cells. Pazur (18) identified a tetraheteroglycan from the cell wall of a strain of Streptococcus (Enterococcus) faecalis that contained glucose, galactose, rhamnose, N-acetylgalactosamine, and phosphate. This composition is similar to that of the polysaccharide that eluted from ion-exchange columns with 200 mM NaCl in the present study. However, we found no antibodies reactive with this antigen in serum raised to whole E. faecalis cells, and this tetraheteroglycan did not inhibit phagocytic killing of enterococci by this whole-cell antiserum. Sood et al. (21) identified a similar carbohydrate that was present in E. faecalis, but not in E. faecium, and further claimed that this antigen, which they designated capsular polysaccharide type 1, elicited opsonic antibody to a majority of E. faecalis strains when conjugated to diphtheria toxoid and used for immunization of rabbits. In their abstract, there was no demonstration by electron microscopy that their carbohydrate antigen was actually a capsule. The streptococcal group D antigen expressed by enterococci differs from the cell wall carbohydrates characterizing other streptococcal serogroups in that it is a glycerophosphate polymer (8) with alanine ester residues that essentially represent the cell’s teichoic acid antigen (26). However, additional cell wall or surface carbohydrates have previously been recognized as potential type-specific antigens (7), although the technology available at the time did not allow definitive identification of any of these structures as capsules.
In 1963, Wicken and Baddiley (25) determined the structure of what was thought to be an intracellular teichoic acid from S. faecalis 39 that is similar to the glycerol-glucose capsular polysaccharide we have identified in E. faecalis 12030. They found the same glucose disaccharide in an α-1-2 linkage (kojibiose) that we found, but they reported that this disaccharide was linked to a glycerol phosphate polymer backbone. Our glycerol-glucose polymer differs in that it has a backbone of glucose linked α-1-2 to glycerol and the no. 6 carbon of the glucose is linked to the glycerol in the next repeat unit via a phosphodiester bond. A second glucose residue is linked to the no. 2 carbon of the backbone glucose via an α-2-1 bond. It is possible that Wicken and Baddiley (25) obtained the same material as we did, but due to the technology available 36 years ago, could not completely characterize the glycosidic bonds in their material. We are also unable to make comparisons with their material due to the time that has lapsed and the lack of availability of antigen or strains for comparison. Nonetheless, it is likely that we have obtained a previously uncharacterized enterococcal antigen, given the intracellular location of the antigen described by Wicken and Baddiley and the structural differences between their molecule and ours.
Several investigators have attempted to visualize capsules or capsule-like structures in enterococci (1, 13). Using electron microscopy of enterococcal strains stabilized with specific antiserum and ruthenium red staining, Arduino et al. (1) found small, electron-dense clumps consistent with capsular material adjacent to cells. Other techniques used by these workers (including light microscopy, staining by the Hiss method [20], and a modified quellung reaction), failed to demonstrate a capsule in E. faecium or E. faecalis (1). Additional methods commonly employed for identifying capsules—e.g., India ink, binding of calcifluor, and use of serum soft agar—have failed to identify enterococcal capsules in our research (unpublished observation). However, by electron microscopy with antisera raised to the purified glycerol-glucose polymer, we demonstrated the extracellular location of this antigen and also showed its ability to serve as a target for opsonic killing antibodies. These findings are consistent with the glycerol-glucose polymer being a capsular polysaccharide.
Recent studies have begun to explore in more detail some aspects of host immunity to enterococcal infection. Harvey and colleagues (10) studied the contribution of complement and immunoglobulin to neutrophil-mediated killing of enterococci and found that hyperimmune rabbit serum promoted a significantly greater killing of enterococci than normal rabbit serum. However, their results suggested that complement was the major serum factor mediating killing, in that neutrophil bactericidal activity was as efficient in hypogammaglobulinemic serum and agammaglobulinemic serum as it was in pooled normal human serum and pooled newborn human serum. The conclusion of these authors—that complement in the absence of antibody promotes efficient bacterial killing—was based on the activity of hypogammaglobulinemic and agammaglobulinemic sera as complement sources along with normal human polymorphonuclear neutrophils (PMNs). Since some of these sera do have low immunoglobulin G levels, a contribution of antibody to opsonic killing could not be ruled out entirely. Indeed, Gaglani et al. (9) showed that adsorption of hypogammaglobulinemic serum with enterococcal cells eliminated opsonic killing, which was restored when human immunoglobulins were added back to the adsorbed serum. These findings indicate that antibodies along with complement are needed for opsonic killing of enterococci.
Arduino and coworkers have also proposed that complement in normal human sera mediates most of the phagocytic killing of enterococci (2). They found that pooled normal human serum was as efficient at PMN-mediated phagocytic killing as serum pooled from patients recovering from serious enterococcal infections. These researchers also found that some heat-inactivated sera from infected patients supported killing by PMNs, a result indicating the potential role for complement-independent, antibody-mediated PMN killing. It is interesting that, when comparing the various sera as sources of antibody, these workers used 5% intact pooled normal human serum as their complement source. Since they failed to adsorb the complement source to remove endogenous antibodies, it is likely that the resident antibodies also contributed to phagocytic killing, perhaps obscuring differences in antibody activity between sera from normal and infected patients. In developing the opsonic killing assay used in the present study to identify the glycerol-glucose capsular polysaccharide, we found that normal rabbit sera and human sera contained sufficient levels of endogenous antibodies to mediate opsonic killing when used in high concentrations (5 to 10%). However, adsorption of the normal human sera that were used as sources of complement for opsonizing enterococcal strains removed all detectable opsonic killing activity while leaving complement function intact. This finding indicated that opsonic killing of enterococci in normal human serum requires both antibody and complement, a finding also reported by Gaglani et al. (9).
Arduino and coworkers have also proposed that some E. faecium strains are more resistant to PMN-mediated killing than E. faecalis strains (1). Exposure of bacterial cells to pronase, trypsin, or phospholipase C did not affect either species’ resistance to phagocytosis, while treatment with periodate (10 mM for 20 min at 4°C) eliminated this resistance in E. faecium. The authors concluded that a carbohydrate structure was responsible for the resistance to phagocytic killing, although they did not isolate and chemically characterize a specific factor. By electron microscopy, they identified small, electron-dense clumps consistent with capsular material on E. faecium, as well as on E. faecalis (1). The finding of capsule-like material is consistent with identification of E. faecalis DNA by Weinstock and colleagues (27, 28) that encodes proteins putatively involved in synthesis of a polysaccharide antigen reactive with antibodies in convalescent-phase sera from infected patients.
In summary, the emergence of VRE and the increasing isolation of enterococci from nosocomial infections emphasize the importance of understanding the virulence determinants of these pathogens and potential targets for immunoprophylaxis and therapy. In the study reported here, one E. faecium strain and one E. faecalis strain were found to produce a capsular polysaccharide composed of glucose and glycerol phosphate that served as a target for opsonic antibodies. About one-third of an additional 20 enterococcal isolates were also killed by antibodies to this capsular polysaccharide, indicating that a similar cross-reactive structure is present on the surface of these strains. Further studies of the role of antibodies to the glycerol-glucose teichoic acid capsule in mediating protection against enterococcal infection are under way, as are attempts to isolate serologically distinct capsular polysaccharides from other strains of enterococci. If a sufficient number of clinical isolates of enterococci prove to be encapsulated, then the development of active and passive therapies against these antigens—an approach that has been the basis for successful vaccines against numerous bacterial pathogens—will be a realistic goal.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 23335 (G.B.P.), AI 38424 (L.C.M.), AI23339, and AI75326 (D.L.K.) and a grant from the Hood Foundation (J.H.).
REFERENCES
- 1.Arduino R C, Jacques-Palaz K, Murray B E, Rakita R M. Resistance of Enterococcus faecium to neutrophil-mediated phagocytosis. Infect Immun. 1994;62:5587–5594. doi: 10.1128/iai.62.12.5587-5594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arduino R C, Murray B E, Rakita R M. Roles of antibodies and complement in phagocytic killing of enterococci. Infect Immun. 1994;62:987–993. doi: 10.1128/iai.62.3.987-993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleiweis A S, Young F E, Krause R M. Cell walls of group D streptococci. II. Chemical studies on the type 1 antigen purified from the autolytic digest of cell walls. J Bacteriol. 1967;94:1381–1387. doi: 10.1128/jb.94.5.1381-1387.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 5.Chenoweth C E, Schaberg D R. Enterococcus species. In: Mayall G, editor. Hospital epidemiology and infection control. Baltimore, Md: Williams and Wilkins; 1996. pp. 334–345. [Google Scholar]
- 6.Edmond M B, Ober J F, Dawson J D, Weinbaum D L, Wenzel R P. Vancomycin-resistant enterococcal bacteremia: natural history and attributable mortality. Clin Infect Dis. 1996;23:1234–1239. doi: 10.1093/clinids/23.6.1234. [DOI] [PubMed] [Google Scholar]
- 7.Elliott S D. Group and type-specific polysaccharides of group D streptococci. Nature. 1959;184:1342. doi: 10.1038/1841342a0. [DOI] [PubMed] [Google Scholar]
- 8.Elliott S D. Teichoic acid and the group antigen of group D streptococci. Nature. 1962;193:1105–1106. doi: 10.1038/1931105a0. [DOI] [PubMed] [Google Scholar]
- 9.Gaglani M J, Baker C J, Edwards M S. Contribution of antibody to neutrophil-mediated killing of Enterococcus faecalis. J Clin Immunol. 1997;17:478–484. doi: 10.1023/a:1027371727225. [DOI] [PubMed] [Google Scholar]
- 10.Harvey B S, Baker C J, Edwards M S. Contributions of complement and immunoglobulin to neutrophil-mediated killing of enterococci. Infect Immun. 1992;60:3635–3640. doi: 10.1128/iai.60.9.3635-3640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landry S L, Kaiser D L, Wenzel R P. Hospital stay and mortality attributed to nosocomial enterococcal bacteremia: a controlled study. Am J Infect Control. 1989;17:323–329. doi: 10.1016/0196-6553(89)90001-1. [DOI] [PubMed] [Google Scholar]
- 13.Matthews K R, Oliver S P. Encapsulation of streptococci isolated from bovine milk. Zentralbl Veterinarmed. 1993;40:597–602. doi: 10.1111/j.1439-0450.1993.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 14.Montecalvo M A, Horowitz H, Gedris C, Carbonaro C, Tenover F C, Issah A, Cook P, Wormser G P. Outbreak of vancomycin-, ampicillin-, and aminoglycoside-resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrob Agents Chemother. 1994;38:1363–1367. doi: 10.1128/aac.38.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris J G, Jr, Shay D K, Hebden J N, McCarter R J, Jr, Perdue B E, Jarvis W, Johnson J A, Dowling T C, Polish L B, Schwalbe R S. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med. 1995;123:250–259. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance. National Nosocomial Infections Surveillance (NNIS) Report, data summary from October 1986–April 1997, issued May 1997. Am J Infect Control. 1997;25:477–487. [PubMed] [Google Scholar]
- 17.Paoletti L C, Kasper D L, Michon F, DiFabio J, Jennings H J, Tosteson T D, Wessels M R. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Investig. 1992;89:203–209. doi: 10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pazur J H. β-D-Glucose 1-phosphate. A structural unit and an immunological determinant of a glycan from streptococcal cell walls. J Biol Chem. 1982;257:589–591. [PubMed] [Google Scholar]
- 19.Rosner B. Fundamentals of biostatistics. Boston, Mass: Duxbury Press; 1990. pp. 474–525. [Google Scholar]
- 20.Sonnenwirth A C. Stains and staining procedures. In: Sonnenwirth A C, Jaret L, editors. Gradwohl’s clinical laboratory methods and diagnosis. Vol. 2. St. Louis, Mo: C. V. Mosby Co.; 1980. pp. 1378–1390. [Google Scholar]
- 21.Sood R K, Poth M, Shepherd S, Patel A, Naso R, Fattom A. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Capsular serotyping of Enterococcus faecalis: isolation, characterization and immunogenicity of capsular polysaccharide isolated from E. faecalis type 1, abstr. E-19; p. 238. [Google Scholar]
- 22.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Hollingsworth R I. The structure of the O-antigenic chain of the lipopolysaccharide of Rhizobium trifolii 4s. Carbohydr Res. 1994;260:305–317. doi: 10.1016/0008-6215(94)84048-2. [DOI] [PubMed] [Google Scholar]
- 24.Wells C L, Juni B A, Cameron S B, Mason K R, Dunn D L, Ferrieri P, Rhame F S. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin Infect Dis. 1995;21:45–50. doi: 10.1093/clinids/21.1.45. [DOI] [PubMed] [Google Scholar]
- 25.Wicken A J, Baddiley J. Structure of intracellular teichoic acids from group D streptococci. Biochem J. 1963;87:54–62. doi: 10.1042/bj0870054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicken A J, Elliott S D, Baddiley J. The identity of streptococcal group D antigen with teichoic acid. J Gen Microbiol. 1963;31:231–239. doi: 10.1099/00221287-31-2-231. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Jiang L, Murray B E, Weinstock G M. Enterococcus faecalis antigens in human infections. Infect Immun. 1997;65:4207–4215. doi: 10.1128/iai.65.10.4207-4215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Murray B E, Weinstock G M. A cluster of genes involved in polysaccharide biosynthesis from Enterococcus faecalis OG1RF. Infect Immun. 1998;66:4313–4323. doi: 10.1128/iai.66.9.4313-4323.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]