Abstract
Use of novel tissue engineering approaches to heal an injured anterior cruciate ligament (ACL) requires suture repair and/or augmentation to provide joint stability. We evaluated the effects of the location of suture augmentation at the femur and tibia in terms of joint stability using a goat model. Eight goat stifle joints were tested with augmentation sutures placed in two femoral tunnel locations: (1) anterior to, or (2) through the ACL footprint, and two tibial tunnel locations: (1) medial to, or (2) medial and lateral to the footprint. Using a robotic/universal force-moment sensor testing system, the anterior tibial translation (ATT) and the corresponding in situ force carried by the sutures were obtained at 30°, 60°, and 90° of flexion in response to external loads. No significant differences were found between augmentation groups due to tunnel location in terms of ATT or the in situ forces carried by the sutures at all flexion angles tested. Similar results were found under 5 N m of varus–valgus torque. Under a 67 N anterior tibial load, the ATT was restored to within 3 mm of the intact joint following suture augmentation (p > 0.05). Suture augmentation, when placed close to the ACL insertion, could be helpful in providing initial joint stability to aid ACL healing in the goat model.
Keywords: anterior cruciate ligament (ACL), suture augmentation, biomechanics, robotic testing system
The anterior cruciate ligament (ACL) is frequently injured and has limited potential to heal.1–3 As such, surgical reconstruction using soft tissue autografts is often performed for active patients.4–6 However, long-term follow-up studies revealed that 20–25% of patients have less than satisfactory results, with pain, loss of range of motion, and other complications associated with donor site morbidity,56 and 20–75%, eventually develop osteoarthritis.7–10 Thus, there exists a need to develop better approaches to treat ACL injuries. Recently, there has been clinical interest11,12 and numerous laboratory studies using functional tissue engineering13–17 to promote healing of an injured ACL. Nevertheless, healing following these new biological approaches has been slow, and suture repair and/or augmentation of the torn ends of the ACL is recommended to achieve initial knee stability and limit loss of function and damage to other tissues, including the medial collateral ligament, medial meniscus, and articular cartilage.18–24
A plethora of procedures exist for suture repair and suture augmentation.17,20,25–28 In repair, the torn ACL ends are either sutured to each other or sutured separately and tensioned by passing the sutures through bone tunnels drilled in the tibia and femur and fixing them against the bone. In suture augmentation, the sutures are passed directly from bone to bone and tied under tension to yield better joint stability. However, the locations of the bone tunnels used in these procedures vary widely.17,20,25–28 On the femoral side, tunnels are placed through the ACL footprint or directly anterior to the footprint (to avoid further injury to the ruptured ACL tissue). On the tibial side, some place a single tunnel, usually through or medial to the footprint, while others use tunnels both medial and lateral to the footprint as an additional support across the broad ACL insertion. Fleming et al.20 stressed the importance of the tibial tunnel location in the sagittal plane. Yet its role in the frontal plane is less well known. The research question becomes whether the location of the tunnels for suture augmentation would impact initial joint stability.
Our objective was to evaluate the locations of the tunnels in restoring joint stability [i.e., multiple degree-of-freedom (DOF) joint kinematics], following complete ACL transection. We also determined the function of the sutures by measuring the in situ force in the augmentation sutures. We hypothesized that sutures placed through the ACL footprint at the femoral origin will better restore kinematics compared to a more anterior femoral tunnel placement because the location better replicates that of the footprint. We also hypothesized that the effect of tibial tunnel location is negligible as the tunnels are placed similarly in the sagittal plane while the slight difference in the frontal plane would result in a minimal shift in the line of action of the sutures.
To accomplish our objective, goat stifle joints were tested on a robotic/universal force-moment sensor (UFS) testing system19,29,30 at 30°, 60°, and 90° of flexion. The remaining five DOF kinematics of the intact, ACL-deficient, and suture augmented joints as well as the in situ forces in the intact ACL and augmentation sutures could be measured and compared. Due to the high position accuracy and repeatability of the robotic/UFS testing system, these measures can be determined in a non-contact manner. Also, as all groups are compared using the same set of joints, there is no interspecimen variability, and repeated measures statistics can be used to compare groups.
METHODS
Eight fresh-frozen stifle joints from male, Boer goats (~30–40 kg) were obtained from a local abattoir. We have published many studies using the goat model for ACL reconstruction.19,29 It offers a large joint for ease of surgery and proper biomechanical testing. The joints were wrapped in saline-soaked gauze, stored in air-tight plastic bags, and kept frozen at −20°C until the day prior to testing.31 After thawing overnight, surrounding tissues (>10 cm proximal and distal to the joint) were dissected. The femur and tibia were potted in epoxy for testing (FiberGlass-Evercoat, Cincinnati, OH). Experiments were performed at room temperature (~21°C) and 40% humidity. The specimens were kept moist during dissection and testing by spraying with 0.9% saline every 15–20 min to the joint covered with gauze. A medial arthrotomy was performed prior to testing to remove any potential influence on the results.
Specimens were then mounted onto the robotic/UFS testing system,19,29,30 which records the six DOF joint motion applied via a robotic manipulator (Puma Model 762; Unimate, Inc., Pittsburgh, PA). With force feedback from the UFS (Model 4015; JR3, Inc., Woodland, CA), the system can apply and measure forces and moments to the stifle joint in 6-DOF.
A summary of the testing protocol is shown in Table 1. To identify the neutral position, the joint was first moved in 1° increments from full extension to 90° of flexion, while the positions, which minimized the forces and moments within the joint, were recorded. These positions served as the reference locations for the remainder of the protocol.
Table 1.
Experimental Protocol and Data Acquired
| Protocol | Data Acquired |
|---|---|
| I. Intact joint | |
| Path of passive flexion-extension | |
| External loading conditions | Intact joint kinematics |
| A. 67 N anterior tibial load | |
| B. 5 N m varus–valgus moment | |
| Transect ACL | |
| Repeat kinematics (I.A, I.B) | In situ forces in ACL |
| II. ACL-deficient joint | |
| Apply loads A and B | ACL-deficient joint kinematics |
| III. Augmented joint | |
| Perform suture augmentation (random selection) | |
| Apply loads A and B | Augmented joint kinematics |
| Release sutures | |
| Repeat kinematics (III.A, III.B) | In situ forces in sutures |
| Repeat III for each augmentation |
The robotic/UFS testing system was then operated in force-control mode to apply external loads at the pre-selected flexion angles, while the resulting five DOF motions [medial–lateral, proximal–distal, anterior–posterior (AP) translations, and internal–external and varus–valgus (VV) rotations] were measured.19,29 Two loads were used: a 67 N AP tibial load and a 5 N m VV torque. For the VV torque, internal–external rotation was constrained using the robotic/UFS testing system because of the large laxity of the goat stifle joint in this rotational plane, which impacts the ability to apply the VV torque. A preliminary study was performed to assess the repeatability of the force measurements. A 67 N AP tibial load was applied to a set of eight intact joints, and the resulting kinematics were measured. For each joint, the same kinematics were repeated without altering the joint, while measuring new forces. The robotic/UFS testing system could repeat the applied load to within 6–9 N.
At each flexion angle, each loading condition was applied five times. The amount of anterior tibial translation (ATT) was monitored to ensure that no significant increases occurred between loading cycles (<0.5 mm). Next, the bone tunnels were created. To determine the influence of the tunnels, the kinematics recorded for the intact joint were repeated by the robotic manipulator while the UFS recorded a new set of forces and moments. These were then compared to the values before the tunnels were made. The difference in forces due to drilling the tunnels (<5 N) were found to be similar to the repeatability of our robotic/UFS testing system (6–9 N).
Then, the ACL was completely transected. By the principle of superposition, the difference in the forces measured before and after cutting the ACL was the in situ force carried by the ACL under the applied loads.29,30 Then, the loading conditions were again applied to determine the kinematics of the ACL-deficient joint.
To determine the effect of tunnel locations for suture augmentation, a two-way factorial design was used. Two femoral tunnels were created and compared: a tunnel anterior to the ACL footprint at the femoral origin (FA) and a tunnel through the footprint at the origin (FT) (Fig. 1A). For the tibia, two groups were compared: a single tunnel medial to the footprint at the tibial insertion (TM) and tunnels medial and lateral to the footprint at the insertion (TLM) (Fig. 1B). Thus, four groups were compared (Table 2). For example, the augmentation using the anterior femoral tunnel and a single tibial tunnel was designated as Group 1 (FA/TM).
Figure 1.
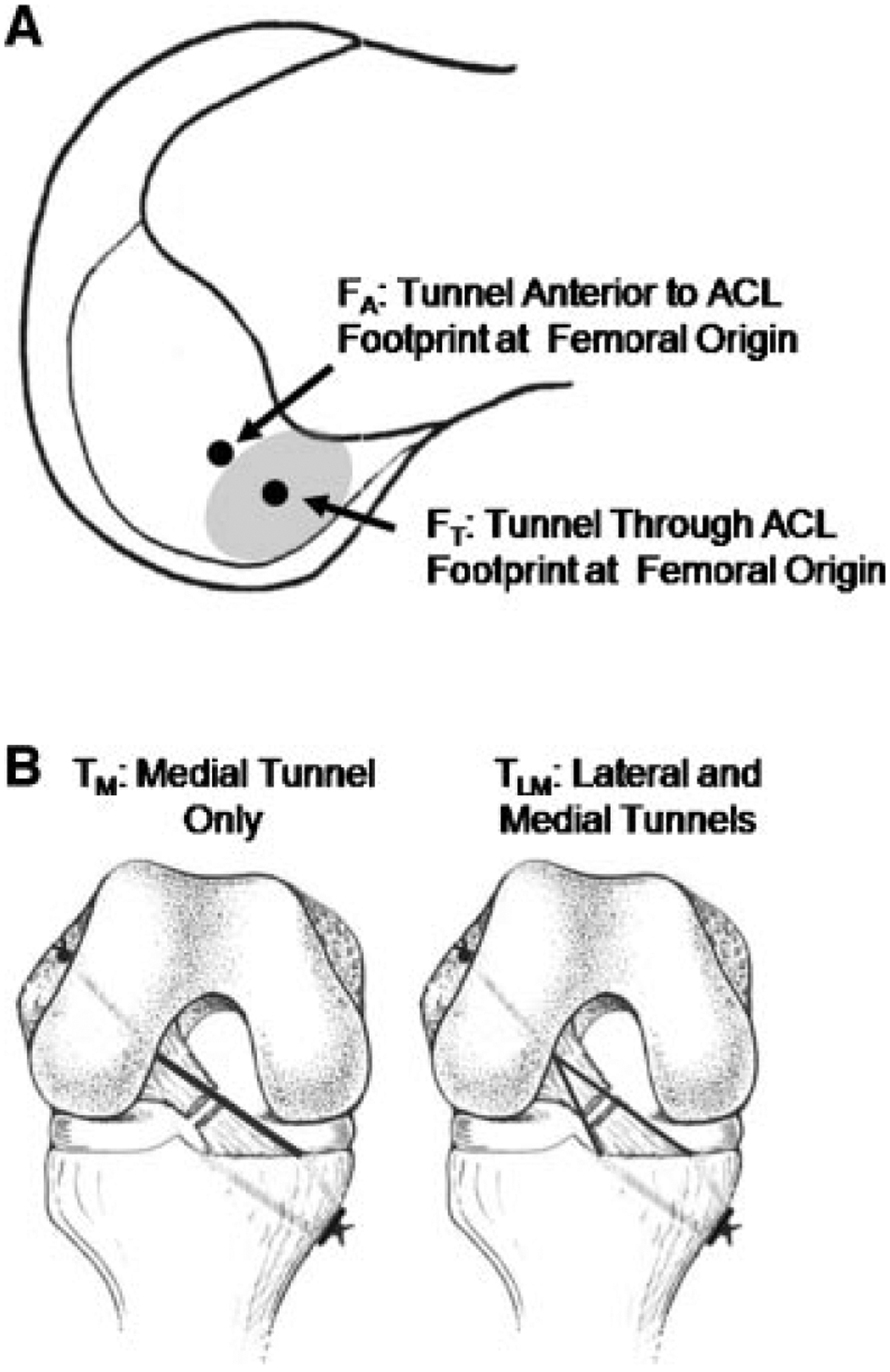
Diagram depicting the tunnel locations for suture augmentation. For FA, the tunnel was located anterior to the ACL footprint at the femoral origin, while for FT, the tunnel was located through the footprint at the origin (A). In the TM and TLM augmentations, tunnels located medial and medial+lateral to the footprint at the tibial insertion were utilized, respectively (B).
Table 2.
Augmentation Procedures, Indicating the Location of the Femoral and Tibial Tunnels
| Femoral Tunnel Location (Relative to ACL Origin) | ||
|---|---|---|
| Anterior (FA) | Through Substance (FT) | |
| Tibial tunnel location (relative to ACL insertion) | ||
| Medial tunnel only (TM) | Group 1 (FA/TM) | Group 2 (FT/TM) |
| Medial and lateral tunnels (TLM) | Group 3 (FA/TLM) | Group 4 (FT/TLM) |
Each augmentation was created by passing two sutures (#2 Fiberwire; Arthrex, Memphis, TN) from the bone tunnels in the femur and tibia. All procedures were done by the same surgeon. For Groups 1 and 2, both sutures were passed through the medial tibial tunnel. For Groups 3 and 4, one suture was passed through the medial tibial tunnel and other through the lateral tibial tunnel. The femoral tunnels were created using a free hand technique and a 1.5 mm diameter drill bit. The tibial tunnels were created using the same drill bit and a Protrac tibial guide (Acufex, Smith & Nephew, Andover, MA). For fixation, a large knot was created in the sutures, which was pulled snugly against the outer femoral cortex, while button fixation (Arthrex) was used on the tibial side at 60° of joint flexion under maximum manual tension. After fixation, the two external loading conditions were applied and the kinematics of the augmented joint recorded. The in situ force carried by the augmentation sutures was obtained by removing the sutures and replaying the kinematics of the augmented joint.29,30 This procedure was repeated for each augmentation. The order of the augmentations was randomized.
An a priori power analysis performed using G*Power software indicated that eight joints would be needed to detect a mean difference of 3 mm in ATT and 10 N of in situ force among groups with α = 0.05 and a power of 0.8.32 The difference in ATT was based on the clinical literature in which successful reconstruction following ACL injury could restore translation to within 2–3 mm at the time of surgery.4–6 The difference in in situ force was chosen based on the repeatability of our system (6–9 N). For statistical analysis (SPSS software, Version 14.0; SPSS, Inc., Chicago, IL), the ATT recorded for the augmentation techniques were compared to each other and to the intact and ACL-deficient conditions. The forces in the augmentation sutures were compared among procedures and to the intact ACL. The data were confirmed to be normally distributed by a Kolmogorov–Smirnov test. As all groups were done on the same joints, a repeated measures ANOVA was used. To determine statistical differences among groups, Bonferroni post hoc tests were done. Significance was set at p < 0.05.
RESULTS
For all experimental conditions, values for ATT were higher at 30° and 60° in response to a 67 N anterior tibial load and then decreased at 90° (Table 3). For the intact joint, mean ATT ranged from 1.9 to 2.5 mm for the flexion angles tested. Corresponding values for the ACL-deficient joint were 12.8–15.5 mm, representing a large six- to sevenfold increase (p < 0.05). Following suture augmentation, mean ATT was reduced to 2.5–5.2 mm and were 10.3–11.2 mm lower than the ACL-deficient joint for all three flexion angles (p < 0.05). More importantly, no significant difference was found from those of the intact joint (maximum difference of means of 2.9, 2.4, and 1.3 mm at 30°, 60°, and 90°, respectively, p > 0.05). No significant differences occurred among the four augmentation groups at the three flexion angles; the largest difference was 0.8 mm (p > 0.05).
Table 3.
Anterior Tibial Translation of the Joint (A) and In Situ Forces Carried by the Intact ACL and Augmentation Sutures (B) in Response to a 67 N Anterior Tibial Load at 30°, 60°, and 90° of Flexion (Mean ± SD)
| A. Anterior Tibial Translation (mm) | B. In situ Force of ACL/Sutures (N) | |||||
|---|---|---|---|---|---|---|
| 30° | 60° | 90° | 30° | 60° | 90° | |
| Intact joint | 2.3 ± 0.5 | 2.5 ± 0.5 | 1.9 ± 0.6 | 69 ± 6 | 63 ± 4 | 56 ± 7 |
| ACL-deficient joint | 15.3 ± 2.2* | 15.5 ± 3.0* | 12.8 ± 3.3* | NA | NA | NA |
| Suture augmented joint | ||||||
| Group 1 (FA/TM) | 5.2 ± 1.9 | 4.9 ± 1.9 | 2.8 ± 1.8 | 60 ± 4** | 57 ± 6 | 51 ± 6 |
| Group 2 (FT/TM) | 4.8 ± 1.8 | 4.5 ± 1.7 | 2.7 ± 1.7 | 58 ± 7** | 62 ± 5 | 52 ± 8 |
| Group 3 (FA/TLM) | 4.4 ± 1.5 | 4.3 ± 1.4 | 2.5 ± 1.5 | 61 ± 5 | 60 ± 5 | 52 ± 10 |
| Group 4 (FT/TLM) | 4.7 ± 1.7 | 4.6 ± 1.6 | 3.2 ± 1.6 | 62 ± 5 | 60 ± 6 | 47 ± 13 |
p < 0.05 compared with all other joint conditions at the same flexion angle.
p < 0.05 compared to intact joint at the same flexion angle.
The intact ACL carried mean forces ranging from 56 to 69 N in response to the 67 N anterior tibial load (Table 3). Similar values were found for the sutures in all four augmentation groups. The maximum difference in means was only 11, 6, and 9 N from the intact ACL at 30°, 60°, and 90°, respectively (p > 0.05). The only two exceptions were that forces for Groups 1 (FA/TM) and 2 (FT/TM) were significantly lower than those for the intact ACL at 30° (p < 0.05). When compared among the four groups, the findings were the same as for ATT, with no significant differences in the in situ forces (p > 0.05).
Kinematics for the 5 N m valgus torque are shown in Table 4. For the intact joint, the highest and lowest values occurred at 60° and 30°, respectively. The trends were different for the ACL-deficient and suture augmented conditions. Mean values for ATT for the intact joint ranged from −0.3 to 0.2 mm for the three flexion angles. With ACL-deficiency, the range of mean values was 2.5–5.0 mm, although these increases were not significantly different (p > 0.05). Following suture augmentation, mean ATT was reduced to −0.4–1.3 mm. There was no significant difference from those of the intact joint (maximum difference in means of 1.6, 0.4, and 0.4 mm at 30°, 60°, and 90°, respectively, p > 0.05). No significant differences occurred among the four groups, with the largest difference reaching only 0.3 mm (p > 0.05).
Table 4.
Anterior Tibial Translation of the Joint (A) and In Situ Forces Carried by the Intact ACL and Augmentation Sutures (B) in Response to a 5 N m Valgus Torque at 30°, 60°, and 90° of Flexion (Mean ± SD)
| A. Anterior Tibial Translation (mm) | B. In situ Force of ACL/Sutures (N) | |||||
|---|---|---|---|---|---|---|
| 30° | 60° | 90° | 30° | 60° | 90° | |
| Intact joint | −0.3 ± 0.4 | 0.2 ± 0.7 | 0.0 ± 1.2 | 31 ± 13 | 22 ± 10 | 19 ± 8 |
| ACL-deficient joint | 4.7 ± 4.5 | 5.0 ± 6.1 | 2.5 ± 4.8 | NA | NA | NA |
| Suture augmented joint | ||||||
| Group 1 (FA/TM) | 1.0 ± 1.3 | 0.4 ± 1.2 | −0.4 ± 1.3 | 13 ± 8 | 13 ± 6 | 16 ± 12 |
| Group 2 (FT/TM) | 0.9 ± 1.1 | 0.4 ± 1.0 | −0.3 ± 1.3 | 14 ± 7 | 15 ± 9 | 15 ± 10 |
| Group 3 (FA/TLM) | 1.2 ± 1.5 | 0.6 ± 1.5 | −0.4 ± 1.3 | 15 ± 6 | 13 ± 5 | 16 ± 7 |
| Group 4 (FT/TLM) | 1.3 ± 1.5 | 0.6 ± 1.4 | −0.1 ± 1.5 | 14 ± 8 | 15 ± 8 | 13 ± 9 |
The intact ACL carried mean forces ranging from 19 N at 90° to 31 N at 30° under the 5 N m valgus torque (Table 4). Corresponding mean values for the augmentation sutures were not significantly different from the intact ACL and ranged from 13 to 16 N (p > 0.05). When compared among the four groups, no significant differences were found in the in situ forces (p > 0.05).
Kinematics in response to a 5 N m varus torque are shown in Table 5. Similar to the valgus torque, the intact joint had higher values at 90° and lower values at 30°, while the opposite trends were observed for the ACL-deficient and suture augmented conditions. Mean ATT for the intact joint ranged from −0.2 to 0.5 mm for the three flexion angles. With ACL-deficiency, the corresponding range was 11.7–8.0 mm, which was an increase of 7.5–11.9 mm (p < 0.05). After suture augmentation, the mean ATT was reduced to 1.4–4.2 mm. At 30°, the mean ATT for all augmentation groups was about 4.0 mm greater than that of the intact joint (p < 0.05). At 60°, mean ATT for all groups was within 3.0 mm of that for the intact joint, with the only significant difference between the intact joint and Group 3 (FA/T2; p < 0.05). At 90°, all augmentation groups restored mean ATT to within 1.6 mm, and were not significantly different from the intact joint (p > 0.05). At all three angles, the values after augmentation were significantly lower than the ACL-deficient joint (p < 0.05). No significant differences were found between the augmentations, with a maximum difference in means of only 0.7 mm at 90° (p > 0.05).
Table 5.
Anterior Tibial Translation of the Joint (A) and In Situ Forces Carried by the Intact ACL and Augmentation Sutures (B) in Response to a 5 N m Varus Torque at 30°, 60°, and 90° of Flexion (Mean ± SD)
| A. Anterior Tibial Translation (mm) | B. In situ Force of ACL/Sutures (N) | |||||
|---|---|---|---|---|---|---|
| 30° | 60° | 90° | 30° | 60° | 90° | |
| Intact joint | −0.2 ± 0.3 | 0.3 ± 0.4 | 0.5 ± 0.5 | 59 ± 7 | 61 ± 13 | 65 ± 16 |
| ACL-deficient joint | 11.7 ± 2.0* | 11.2 ± 1.6* | 8.0 ± 1.4* | NA | NA | NA |
| Suture augmented joint | ||||||
| Group 1 (FA/TM) | 4.0 ± 2.0** | 3.1 ± 2.2 | 1.4 ± 1.4 | 43 ± 4** | 51 ± 17 | 55 ± 10 |
| Group 2 (FT/TM) | 3.9 ± 1.8** | 3.0 ± 2.1 | 1.5 ± 1.2 | 44 ± 7** | 54 ± 14 | 56 ± 8 |
| Group 3 (FA/TLM) | 3.9 ± 1.5** | 3.0 ± 1.6** | 1.7 ± 1.1 | 47 ± 10** | 52 ± 15 | 59 ± 14 |
| Group 4 (FT/TLM) | 4.2 ± 2.4** | 3.4 ± 2.5 | 2.1 ± 1.6 | 43 ± 6** | 52 ± 10 | 52 ± 11 |
p < 0.05 compared with all other joint conditions at the same flexion angle.
p < 0.05 compared to intact joint at the same flexion angle.
The intact ACL carried mean forces ranging from 59 at 30° to 65 N at 90° under the 5 N m varus torque (Table 5). At 30°, the mean forces of all augmentations were 12–16 N lower than those for the intact ACL (p < 0.05); however, similar values were observed at 60° and 90° compared to those for the intact ACL (p > 0.05). When comparing among the four groups, no significant differences occurred in the in situ forces carried by the sutures (p > 0.05).
DISCUSSION
Use of the robotic/UFS testing system allowed data on the multiple DOF kinematics of the intact, ACL-deficient, and suture augmented joints and the in situ forces carried by the ACL and augmentation sutures in response to externally applied loads to be compared in the same set of goat stifle joints. Following ACL injury, suture augmentation was important to restore initial joint stability versus the ACL-deficient joint. However, the location of bone tunnels had only minimal impact on joint stability. Most importantly, the largest differences among the augmentation groups were only 0.8 mm of ATT and 8 N of force in the sutures. Contrary to our hypotheses, femoral and tibial tunnel locations for the sutures had no significant effect of joint stability or the force carried by the sutures.
Further, all four suture augmentation groups restored joint kinematics to those of the joint with an intact ACL with a few exceptions when the joint was subjected to a 67 N anterior tibial load or a 5 N m valgus torque. Also, the in situ forces carried by the sutures were similar to those for the normal ACL. For the 5 N m varus torque at 60° and 90° of joint flexion, all augmentation groups could restore ATT and the in situ force carried by the ACL; the only exception was that ATT for Group 3 was significantly different from the intact joint at 60°. At 30°, none of the augmentations could restore ATT or the in situ force carried by the ACL. It should be noted that the use of repeated measures statistics provided increased power. Thus, small and consistent differences can become significant, even if the difference in the values of the means (~10 N) was small and on the same order of magnitude of the repeatability of our testing system.
Our data on tunnel location for suture augmentation are consistent with those in the literature, where the effect of femoral tunnel position has been extensively studied in the human knee joint.7,33–37 Having a graft placed within the ACL footprint at the femoral origin of the ACL is important as it better restores knee stability. In our study, the tunnels used for suture augmentation were much smaller than those used for ACL reconstruction. This allowed the FA tunnel to be placed immediately anterior to the footprint at the femoral origin, such that the location of the FA and FT tunnels differed only by a few millimeters, which may explain why no differences due to femoral tunnel location were discernable under either the anterior tibial load or the varus–valgus torque.
For tibial tunnel location, an augmentation suture passing within the anterior or middle portion of the ACL footprint at the tibial insertion in the sagittal plane restores kinematics for ACL reconstruction using a soft tissue graft7,33,34 or suture augmentation.20 However, in the frontal plane, ACL grafts are usually placed within the footprint at the tibial insertion.7,33,34,38 In using biological stimulation to heal an ACL, it becomes necessary to place the tunnels adjacent to the footprint to avoid further damage to the injured ACL. In our study, sutures placed both medial and lateral to the footprint showed no biomechanical advantage when compared to placing the augmentation sutures medial to the footprint at the tibial insertion alone. Thus, the location of the tibial tunnels in the frontal plane had no influence on joint stability.
Our study has several limitations. First, the sample size was chosen using a power analysis based on the clinical literature and experimental limitations. The changes in ATT and in situ force that would be important for joint function is unknown and may be lower than the power of this study. Second, the results for suture augmentation were for the goat model and cannot be extrapolated to the human knee. A study using human cadaveric knees is warranted. Third, we used externally applied loads similar to those used in a clinical examination for ACL function, rather than attempting to mimic those during in vivo activities.39,40 Recently, our collaborators and others have developed biplanar fluoroscopy imaging systems to record in vivo joint kinematics.41–43 These kinematics data could be repeated using our robotic/UFS system such that the in situ forces in the ACL and the augmentation sutures could be determined. Such studies will be important to characterize the function of the augmentation to enhance ACL healing.
As no significant change in anterior joint stability occurred among these four suture augmentation procedures, our findings suggest that sutures be placed anterior to the ACL footprint of the femoral origin and medial to the footprint of the tibial insertion. Such an approach allows for a simple surgical procedure, and the augmentation would avoid further injury to the ACL. Future studies should include examination of the effects of additional suture strands, alternative suture material, and the effects of repetitive cyclic loading to optimize the procedure. For tissue engineering, the goal of suture augmentation is to temporarily maintain joint stability during early healing. Ideally, as the ACL heals, it would bear more load over time to allow successful healing. In vivo animal studies to evaluate the effectiveness of suture repair and augmentation to restore joint stability over time will be needed to examine the progress of healing of an injured ACL. Nevertheless, we believe our data can serve as a basis for guiding those studies and eventually lead to the successful use of functional tissue engineering to heal an injured ACL.
ACKNOWLEDGMENTS
Financial support from the Commonwealth of Pennsylvania and the NSF Engineering Research Center for Revolutionizing Metallic Biomaterials (Award 0812348) are gratefully acknowledged. Fiberwire sutures and fixation buttons were kindly donated by Arthrex, Inc.
REFERENCES
- 1.Murray MM, Martin SD, Martin TL, et al. 2000. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am 82-A:1387–1397. [DOI] [PubMed] [Google Scholar]
- 2.Andersson C, Odensten M, Good L, et al. 1989. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am 71:965–974. [PubMed] [Google Scholar]
- 3.Kannus P, Jarvinen M. 1987. Conservatively treated tears of the anterior cruciate ligament. Long-term results. J Bone Joint Surg Am 69:1007–1012. [PubMed] [Google Scholar]
- 4.Aglietti P, Buzzi R, Zaccherotti G, et al. 1994. Patellar tendon versus doubled semitendinosus and gracilis tendons for anterior cruciate ligament reconstruction. Am J Sports Med 22:211–217; discussion 217–218. [DOI] [PubMed] [Google Scholar]
- 5.Freedman KB, D’Amato MJ, Nedeff DD, et al. 2003. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med 31:2–11. [DOI] [PubMed] [Google Scholar]
- 6.Spindler KP, Kuhn JE, Freedman KB, et al. 2004. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med 32:1986–1995. [DOI] [PubMed] [Google Scholar]
- 7.Aglietti P, Buzzi R, Giron F, et al. 1997. Arthroscopic-assisted anterior cruciate ligament reconstruction with the central third patellar tendon. A 5–8-year follow-up. Knee Surg Sports Traumatol Arthrosc 5:138–144. [DOI] [PubMed] [Google Scholar]
- 8.Jomha NM, Borton DC, Clingeleffer AJ, et al. 1999. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res 358:188–193. [PubMed] [Google Scholar]
- 9.Drogset JO, Grontvedt T, Robak OR, et al. 2006. A sixteen-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Joint Surg Am 88:944–952. [DOI] [PubMed] [Google Scholar]
- 10.Von Porat AREM, Roos H. 2004. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Br J Sports Med 38:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobbi A, Bathan L, Boldrini L. 2009. Primary repair combined with bone marrow stimulation in acute anterior cruciate ligament lesions: results in a group of athletes. Am J Sports Med 37:571–578. [DOI] [PubMed] [Google Scholar]
- 12.Steadman JR, Cameron-Donaldson ML, Briggs KK, et al. 2006. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg 19:8–13. [DOI] [PubMed] [Google Scholar]
- 13.Murray MM, Palmer M, Abreu E, et al. 2009. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res 27:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anitua E, Sanchez M, Orive G, et al. 2007. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 28:4551–4560. [DOI] [PubMed] [Google Scholar]
- 15.Agung M, Ochi M, Yanada S, et al. 2006. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc 14:1307–1314. [DOI] [PubMed] [Google Scholar]
- 16.Wiig ME, Amiel D, VandeBerg J, et al. 1990. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res 8:425–434. [DOI] [PubMed] [Google Scholar]
- 17.Murray MM, Spindler KP, Abreu E, et al. 2007. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 25: 81–91. [DOI] [PubMed] [Google Scholar]
- 18.Murray MM, Palmer M, Abreu E, et al. 2009. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res 27:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramowitch SD, Papageorgiou CD, Withrow JD, et al. 2003. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J Orthop Res 21:708–715. [DOI] [PubMed] [Google Scholar]
- 20.Fleming BC, Carey JL, Spindler KP, et al. 2008. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res 26: 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakane M, Livesay GA, Fox RJ, et al. 1999. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surg Sports Traumatol Arthrosc 7:93–97. [DOI] [PubMed] [Google Scholar]
- 22.Abramowitch SD, Yagi M, Tsuda E, et al. 2003. The healing medial collateral ligament following a combined anterior cruciate and medial collateral ligament injury – a biomechanical study in a goat model. J Orthop Res 21:1124–1130. [DOI] [PubMed] [Google Scholar]
- 23.Papageorgiou CD, Gil JE, Kanamori A, et al. 2001. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med 29:226–231. [DOI] [PubMed] [Google Scholar]
- 24.Jackson DW, Schreck P, Jacobson S, et al. 1999. Reduced anterior tibial translation associated with adaptive changes in the anterior cruciate ligament-deficient joint: goat model. J Orthop Res 17:810–816. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan N, Wickiewicz TL, Warren RF. 1990. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med 18:354–358. [DOI] [PubMed] [Google Scholar]
- 26.O’Donoghue DH, Rockwood CA Jr., Frank GR, et al. 1966. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am 48:503–519. [PubMed] [Google Scholar]
- 27.Feagin JA Jr., Curl WW. 1976. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med 4:95–100. [DOI] [PubMed] [Google Scholar]
- 28.Seitz H, Menth-Chiari WA, Lang S, et al. 2008. Histological evaluation of the healing potential of the anterior cruciate ligament by means of augmented and non-augmented repair: an in vivo animal study. Knee Surg Sports Traumatol Arthrosc 16:1087–1093. [DOI] [PubMed] [Google Scholar]
- 29.Woo SL-Y, Abramowitch SD, Kilger R, et al. 2006. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech 39:1–20. [DOI] [PubMed] [Google Scholar]
- 30.Fujie H, Livesay GA, Woo SL-Y, et al. 1995. The use of a universal force-moment sensor to determine in-situ forces in ligaments: a new methodology. J Biomech Eng 117:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Woo SL-Y, Orlando CA, Camp JF, et al. 1986. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech 19:399–404. [DOI] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Lang AG, et al. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. [DOI] [PubMed] [Google Scholar]
- 33.Howell SM, Gittins ME, Gottlieb JE, et al. 2001. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med 29:567–574. [DOI] [PubMed] [Google Scholar]
- 34.Khalfayan EE, Sharkey PF, Alexander AH, et al. 1996. The relationship between tunnel placement and clinical results after anterior cruciate ligament reconstruction. Am J Sports Med 24:335–341. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Hsu WH, Woo SL-Y, et al. 2004. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med 32:1825–1832. [DOI] [PubMed] [Google Scholar]
- 36.Loh JC, Fukuda Y, Tsuda E, et al. 2003. Knee stability and graft function following anterior cruciate ligament reconstruction: comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy 19:297–304. [DOI] [PubMed] [Google Scholar]
- 37.Sommer C, Friederich NF, Muller W. 2000. Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc 8:207–213. [DOI] [PubMed] [Google Scholar]
- 38.Hefzy MS, Grood ES. 1986. Sensitivity of insertion locations on length patterns of anterior cruciate ligament fibers. J Biomech Eng 108:73–82. [DOI] [PubMed] [Google Scholar]
- 39.Holden JP, Grood ES, Korvick DL, et al. 1994. In vivo forces in the anterior cruciate ligament: direct measurements during walking and trotting in a quadruped. J Biomech 27:517–526. [DOI] [PubMed] [Google Scholar]
- 40.Darcy SP, Rosvold JM, Beveridge JE, et al. 2008. A comparison of passive flexion-extension to normal gait in the ovine stifle joint. J Biomech 41:854–860. [DOI] [PubMed] [Google Scholar]
- 41.Tashman S, Kolowich P, Collon D, et al. 2007. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res 454:66–73. [DOI] [PubMed] [Google Scholar]
- 42.Li G, Van de Velde SK, Bingham JT. 2008. Validation of a noninvasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J Biomech 41:1616–1622. [DOI] [PubMed] [Google Scholar]
- 43.Giphart JE, Shelburne KB, Anstett K, et al. Measurement of 3D in vivo knee motion using biplane fluoroscopy: investigation of non-contact ACL injuries. XVIth International Conference on Mechanics in Medicine and Biology. Pittsburgh, PA, 2008. [Google Scholar]


