Abstract
A genetic locus for a cytolethal distending toxin (CDT) was identified in a polymorphic region of the chromosome of Actinobacillus actinomycetemcomitans, a predominant oral pathogen. The locus was comprised of three open reading frames (ORFs) that had significant amino acid sequence similarity and more than 90% sequence identity to the cdtABC genes of some pathogenic Escherichia coli strains and Haemophilus ducreyi, respectively. Sonic extracts from recombinant E. coli, containing the A. actinomycetemcomitans ORFs, caused the distension and killing of Chinese hamster ovary cells characteristic of a CDT. Monoclonal antibodies made reactive with the CdtA, CdtB, and CdtC proteins of H. ducreyi recognized the corresponding gene products from the recombinant strain. CDT-like activities were no longer expressed by the recombinant strain when an ΩKan-2 interposon was inserted into the cdtA and cdtB genes. Expression of the CDT-like activities in A. actinomycetemcomitans was strain specific. Naturally occurring expression-negative strains had large deletions within the region of the cdt locus. The cdtABC genes were flanked by an ORF (virulence plasmid protein), a partial ORF (integrase), and DNA sequences (bacteriophage integration site) characteristic of virulence-associated regions. These results provide evidence for a functional CDT in a human oral pathogen.
The facultative gram-negative bacterium Actinobacillus actinomycetemcomitans has been the subject of intensive study, because it is considered a predominant pathogen in human periodontal disease (50, 51, 57). Some of the hallmarks of the virulence potential of this species are the abilities of select strains to invade gingival tissues (33) and to express a leukotoxin (5).
Previous studies used restriction fragment length polymorphism (RFLP) analysis to demonstrate that a specific genetic variant (RFLP group II) prevailed in young children who converted from a healthy to a localized juvenile periodontitis (LJP) status (16). RFLP typing was performed with a randomly cloned 4.7-kb fragment of chromosomal DNA from A. actinomycetemcomitans Y4 (14). This hybridization probe recognized 13 distinct RFLP patterns among bacterial isolates from members of 21 families with LJP (15). To determine if the genetic heterogeneity characteristic of this chromosomal region was marked by promiscuous genetic elements, such as insertion sequences (21), transposons, bacteriophages (42, 53), or recombined plasmid DNA (35), the RFLP fragment was sequenced. A consequence of the sequencing experiments was the identification of a locus that encoded a novel cytolethal distending toxin (CDT).
A CDT has been identified in some pathogenic strains of Escherichia coli (24, 26), some Shigella species (25), various Campylobacter species (27), Haemophilus ducreyi (13), and an E. coli F-like virulence plasmid (pVir) (38). The toxin was named for its ability to alter the morphology of cultured eukaryotic cells. Chinese hamster ovary (CHO) cells, HeLa cells, and Vero cells slowly become distended within 48 to 72 h after exposure to the toxin and eventually die. The toxin locus has been cloned and sequenced from the E. coli chromosome (40, 45), pVir from E. coli 1404 (38), Campylobacter jejuni (41), and H. ducreyi (13) and, in all cases, is comprised of an apparent operon of three genes (cdtABC). The functions of the individual cdt genes have not been clearly established, but it appears that all three genes are required for the expression of distension and cytotoxicity.
In this study, we present the complete nucleotide sequence of the RFLP hybridization probe and 2.6 kb of downstream sequence used to group clinical isolates of A. actinomycetemcomitans. We show that the RFLP probe sequence contains homologs of the three cdt genes and provide evidence that CDT-like activities are expressed by this oral pathogen. The putative cdt locus was found adjacent to sequences that are characteristic of virulence-associated regions.
(Parts of this study were presented at the 76th General Session of the International Association for Dental Research, 24 to 27 June 1998 [32a]).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The A. actinomycetemcomitans strains or genetic variants used in this study include FDC Y4, a well-studied member of this species originally isolated at Forsyth Dental Center from a subject with LJP (57), NCTC 9710 (type strain), and a collection of isolates obtained, in our laboratory, from LJP subjects (UP6, -19, -32, -34, -42, -44, and -64 to -71) and healthy subjects (UP54 and UP57) (15). A. actinomycetemcomitans strains were routinely grown in an atmosphere containing 5% CO2 on Trypticase soy agar containing 0.6% yeast extract, as described previously (15). E. coli DH5α [supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1]) and JM109 [recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB)] were grown in Luria-Bertani (LB) medium (44). Ampicillin (100 μg/ml) or kanamycin (30 μg/ml) was added to the medium when required. pCR-Script SK(+) and pBluescript II SK(+) were obtained from a commercial source (Stratagene).
CDT assay.
The CDT screening assay used was based on that originally described by Johnson and Lior (24) and modified by Scott and Kaper (45). Assays were performed with cultured CHO cells. Briefly, CHO cells were grown in Ham’s F-12 medium supplemented with 5% fetal calf serum. The cells were trypsinized and suspended in the same medium containing 1% fetal calf serum at a concentration of 2 × 104 cells/ml. A volume of the adjusted cell suspension (150 μl) was added to each well of 96-well plates.
A. actinomycetemcomitans strains or transformants were grown as described above, washed two times with phosphate-buffered saline (PBS), suspended in Ham’s medium or PBS, and sonicated for 1 min at 4°C (Braun, Norwalk, Conn.). The sonicated bacteria were centrifuged at 12,000 × g for 10 min (Spinco model SS-34 rotor) to remove unbroken cells and sterilized by passage through a 0.22-μm-pore-size filter (Millipore Corp.). Total protein concentrations were determined with the Micro BCA protein assay kit (Pierce). Serial dilutions of each extract were made with sterile Ham’s medium, and 30 μl of each dilution was added, in triplicate, to the freshly plated cells. Sterile medium or PBS (30 μl) was added to those wells that did not receive bacterial extract. Assay plates were incubated for up to 4 days at 37°C in an atmosphere containing 5% CO2. Following the incubation period, the cells were fixed with 10% formaldehyde for 5 min and stained with crystal violet.
A quantitative assay for TD50 determinations was performed with CHO cells treated as described above, except that 3.0 ml of cell suspension was added so that each well of 6-well tissue culture plates received 300 cells. Immediately after the cells were added to the wells, 200 μl of sterile PBS or filter-sterilized sonic extract was added. The plates were incubated for 6 days to allow colonies to appear. Following incubation, the medium was poured off, the cells were fixed and stained in the plates, and the colonies were counted. All samples were run in triplicate. The data were expressed as the percentages of CHO cells surviving after treatment with sonic extract. The average number of CFU in wells lacking sonic extract was taken as representing 100% survival.
DNA techniques and nucleotide sequencing.
Restriction endonuclease digestions were carried out according to the manufacturers’ instructions. Plasmid DNA isolation, ligations, and transformations were performed as described previously (14).
To facilitate DNA sequencing, the inserted DNA fragment from E. coli DH1(pAA2097) was removed by digestion with EcoRI and ligated to the unique EcoRI site in pCR-Script SK(+) (Stratagene) (Table 1). Plasmid DNA was extracted from the resulting transformant, E. coli DH5α(pCRAA-14), with a QIAprep spin kit (Qiagen). Universal primers homologous to the T3 and T7 promoter sites in the pCR-Script vector were used to begin the sequencing of each strand. New sequencing primers were then made as sequence was obtained by walking along both strands. Automated cycle sequencing reactions were conducted by the Genetics Core Facility at the University of Pennsylvania with an Applied Biosystems, Inc., model 373A sequencer with the Stretch upgrade. Sequencing data were compiled and analyzed with the LaserGene suite of programs (DNASTAR, Inc.). The Lipman-Pearson algorithm was used for amino acid sequence alignments (2). The DNA and protein sequence data banks were searched for homologous sequences with the BLAST algorithms (18), accessed through the National Center for Biotechnology Information (34a).
TABLE 1.
Plasmids used in this study
| Plasmid | Construction | Application | Source or reference |
|---|---|---|---|
| pAA2097 | 5.4-kb EcoRI fragment of A. actinomycetemcomitans chromosomal DNA ligated to EcoRI site of pBR328 | RFLP hybridization probe | 14 |
| pCRAA-14 | 5.4-kb EcoRI insert fragment from pAA2097 cloned into EcoRI site of pCR-Script SK(+) | DNA sequencing template (RFLP probe) | This study |
| pCDT1 | 2.6-kb SmaI-EcoRI fragment from pCRAA-14 ligated to SmaI and EcoRI sites of pBluescript II SK(+) | Segregation of cdtABC ORFs | This study |
| pCDT1A::ΩKan-2 | Insertion of ΩKan-2 into NheI site of pCDT1 | CDT knockout mutation | This study |
| pCDT1B::ΩKan-2 | Insertion of ΩKan-2 into MluNI site of pCDT1 | CDT knockout mutation | This study |
| pCDT1C::ΩKan-2 | Insertion of ΩKan-2 into PinAI site of pCDT1 | CDT knockout mutation | This study |
| pAA4C-75 | 4.2-kb HindIII fragment of A. actinomycetemcomitans chromosomal DNA ligated to HindIII site of pCR-Script SK(+) | DNA sequencing template (extended sequence 3′ to RFLP probe sequence) | This study |
| pPCDT3 | 3.0-kb PCR product containing the H. ducreyi 35000 cdtABC genes ligated to EcoRI site of pBR322 | Source of H. ducreyi cdtABC genes | 52a |
To extend the DNA sequence beyond the RFLP probe region, chromosomal DNA from strain Y4 was digested with HindIII. The resulting DNA fragments were sized on a 0.7% agarose gel and fragments in the range of 2 to 6 kb were excised. These fragments were ligated to the unique HindIII site in pCR-Script, and the ligation mixture was transformed into competent E. coli JM109. The transformants were transferred to nylon membranes, denatured, and hybridized with a probe, walk1-PCR, homologous to the HindIII (v)-EcoRI (ii) region of pCRAA-14 (Table 2 and Fig. 1). The primers walk1-PCR1 and walk1-PCR2 and template DNA from pCRAA-14 were used in the PCR. The reaction was performed at 94°C (1 min), 52°C (1 min), and 72°C (1 min) for 35 cycles. The PCR product was labeled with digoxigenin (Boehringer Mannheim) for DNA hybridization. Pre- and posthybridizations were performed at 68°C, and positive colonies were detected with the DIG luminescent detection kit (Boehringer Mannheim). Plasmid DNA was extracted from one of the hybridization-positive transformants [E. coli JM109(pAA4C-75)] (Table 1), and the inserted DNA fragment was confirmed by restriction endonuclease mapping. The inserted DNA fragment was obtained by digestion with EcoRI and HindIII and used for DNA sequencing reactions. A primer homologous to the 3′ end of the RFLP probe region sequence was used to begin the sequencing reactions. The remainder of the sequence was obtained by walking along the DNA template.
TABLE 2.
PCR primers used in this study
| Primer | Sequence (5′ to 3′) | Probe | Positions (bp) |
|---|---|---|---|
| walk1-PCR1 | GACGAGGATATAGCTGGAT | walk1-PCR | 3971–3989 |
| walk1-PCR2 | GGCAGATGACTACTATGCGA | 5062–5081 | |
| cdt(A)-PCR1 | GGTTTAGTGGCTTGT | cdt(A)-PCR | 2756–2770 |
| cdt(A)-PCR2 | CACGTAATGGTTCTGTT | 3322–3338 | |
| cdt(C)-PCR1 | GACTTTGACGAGTCATGCA | cdt(C)-PCR | 4309–4327 |
| cdt(C)-PCR2 | CCTGATTTCTCCCCA | 4806–4820 |
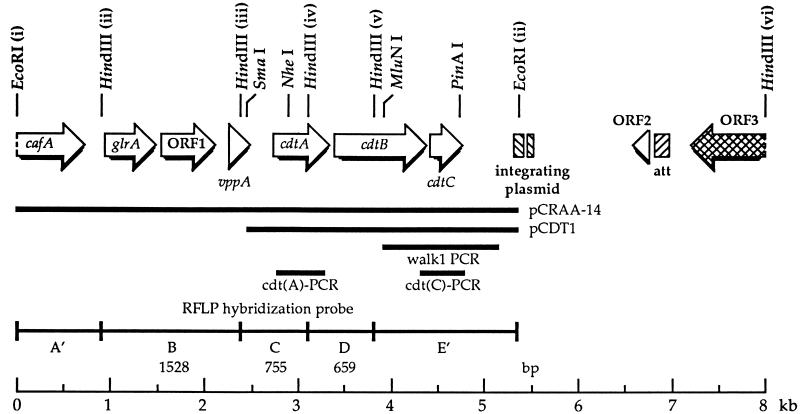
FIG. 1.
Genetic organization of the RFLP probe region from the A. actinomycetemcomitans Y4 chromosome. ORFs and the direction of transcription are designated by the large arrows. Selected restriction endonuclease sites are shown. The HindIII fragments A to E define the various RFLP groups (15). A′ and E′ signify that these end fragments extend to the next upstream and downstream HindIII sites, i and vi, respectively, on the chromosome. The numbers represent the lengths of the HindIII fragments, in base pairs. The hatched boxes indicate sequence identity with an H. influenzae integrating plasmid and bacteriophage HP1 lysogenic insertion site (19, 58). The solid lines show the regions contained in the plasmid constructs pCRAA-14 and pCDT1 and the positions of the DNA probes used for sequence walking (walk1-PCR) and RFLP variant analysis [cdt(A)-PCR and cdt(C)-PCR].
To confirm cdt open reading frame (ORF) deletions in RFLP group variants, the primer pairs (i) cdt(A)-PCR1 and cdt(A)-PCR2 and (ii) cdt(C)-PCR1 and cdt(C)-PCR2 (Table 2) and template DNA from strain Y4 were used to make DNA probes. The first set of primers amplified a sequence within the cdtA ORF that hybridized to RFLP HindIII fragments C and D (Fig. 1). The second set of primers amplified a sequence within the cdtC ORF and recognized HindIII fragment E. PCR was performed at 95°C (1 min), 55°C (1 min), and 72°C (1 min) for 35 cycles. These probes were hybridized to HindIII-digested DNA, from representative members (see above) of each of the RFLP groups, on a Southern blot as described above.
Cloning cdtABC genes.
To segregate the putative cdt ORFs from the others in the RFLP hybridization probe sequence, pCRAA-14 DNA was digested with SmaI and EcoRI. A 2.6-kb DNA fragment, containing the cdtABC ORFs and upstream noncoding region, was ligated to the unique SmaI and EcoRI sites in pBluescript II SK(+) (Stratagene), and the resulting construct was transformed into E. coli. Transformants were selected on LB plates containing isopropyl-β-d-thiogalactopyranoside (IPTG) (400 μg), 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (400 μg), and 100 μg of ampicillin per ml. The final construct was designated E. coli DH5α(pCDT1) (Table 1).
Mutagenesis methods.
To create CDT knockout mutations, an ΩKan-2 element that codes for kanamycin resistance (aphA-3) and contains transcriptional and translational stops in all three reading frames (39) was isolated from p100.2 by digestion with SmaI and inserted, in separate reactions, into the unique NheI, MluNI, and PinAI sites present at positions 273, 487, and 534 in the cdtA, cdtB, and cdtC sequences, respectively, of pCDT1. The NheI- and PinAI-digested plasmid DNAs were blunt ended by filling in the 5′ overhang ends with deoxynucleoside triphosphates and the Klenow fragment (44). The resulting plasmids were used to transform E. coli DH5α. Transformants were selected on LB agar medium containing 30 μg of kanamycin per ml. The insertions were confirmed by restriction endonuclease analysis with plasmid DNA that was digested with HindIII from the transformants, and the ΩKan-2 element DNA was used as the hybridization probe.
Immunoblotting.
Whole cell lysates of recombinant E. coli strains were prepared as described previously (52), and proteins in these lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a 12.5% separating gel. After transfer to nitrocellulose, these blots were incubated with the undiluted culture supernatant from hybridomas secreting monoclonal antibodies made reactive with the CdtA, CdtB, and CdtC proteins of H. ducreyi (52a). Affinity-purified and radioiodinated goat anti-mouse immunoglobulin was used to detect mouse monoclonal antibodies. Sonic extracts (approximately 100 μg of protein/slot) from each of the RFLP group variants (see Fig. 3) were applied to nitrocellulose membranes in a slot blot apparatus and treated as the Western blots had been.
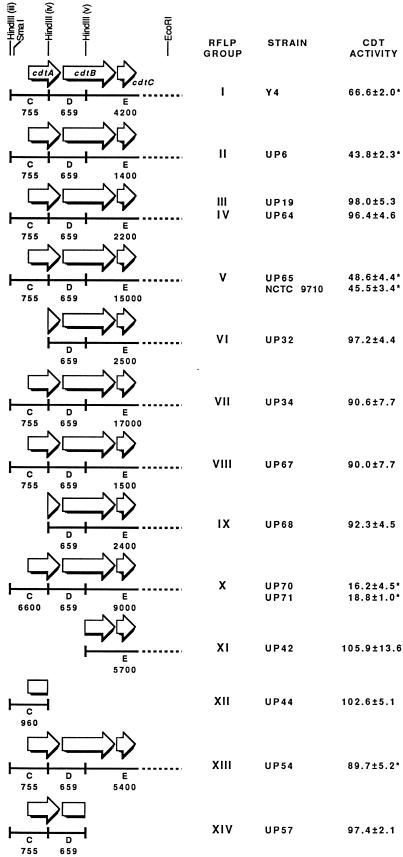
FIG. 3.
Comparison of the cytotoxic effects of sonic extracts from representative members of the RFLP groups of A. actinomycetemcomitans on CHO cells. CHO cells (3 × 103/well) were seeded in 6-well tissue culture plates. Sonic extract (30 μg of protein, except for strains UP6 [14 μg] and UP57 [56 μg]) from each RFLP group variant was added at zero time. The attached cells were stained and counted after 6 days of growth. CFU were expressed as percentages of surviving cells relative to CFU of untreated CHO cell controls. Deletions in the cdt locus were identified by RFLP analyses with the 4.7-kb hybridization probe (15) and Southern blotting with cdtAB and cdtC DNA probes made by PCR (see Materials and Methods). The letters under the solid lines correspond to the RFLP HindIII DNA fragments defined in Fig. 1. Only the region of the cdt locus is shown. The numbers under the solid lines represent the sizes, in base pairs, of these DNA fragments as identified in each genetic variant. RFLP group III and IV variants differ in relation to the size of HindIII DNA fragment B (data not shown). Two genetic variants from RFLP groups V and X were examined. Values marked with an asterisk were statistically significant (P ≤ 0.05).
Statistical analysis.
Student’s t test, with a two-sample equal variance parameter, was used to assess the significance of the CDT-like activities of the genetic variants relative to those with no toxin added.
Nucleotide sequence accession number.
These sequence data were deposited in the DDBJ, EMBL, and GenBank databases as accession no. AF006830.
RESULTS
Identification of a cdt locus in A. actinomycetemcomitans.
The cloned RFLP probe fragment, obtained from E. coli DH5α(pCRAA-14) (Table 1), yielded 5,322 bp of nucleotide sequence. This means that the probe fragment was slightly larger than the previously estimated size of 4.7 kb (14). The DNA sequence contained seven significant contiguous ORFs on the same DNA strand (Fig. 1 and Table 3).
TABLE 3.
Properties of the ORFs identified in the RFLP hybridization probe sequence region from A. actinomycetemcomitans
| ORF | Positions in sequencea | Size (bp) | Size of deduced peptide (kDa) | Comparative homolog | Putative gene product or function | Relevant references |
|---|---|---|---|---|---|---|
| cafA | 1–732 | 732 | Partial | cafA gene of H. influenzae | Cytoplasmic axial filament protein | 17 |
| glrA | 849–1496 | 648 | 24,398 | GrxB of E. coli | Glutaredoxin 2 | 4 |
| ORF1 | 1512–2138 | 627 | 22,862 | Hypothetical E. coli protein | Unknown | |
| vppA | 2238–2504 | 267 | 10,366 | VagC and VapC of H. influenzae Rd | Virulence plasmid protein | 17 |
| VapB of D. nodosus | Virulence-associated protein | 30 | ||||
| VagC of Salmonella dublin | Virulence plasmid protein | 32, 43 | ||||
| VapB of Synechocystis sp. | Virulence-associated protein | 29 | ||||
| ORF of E. coli F factor | Hypothetical ORF in traD-to-traI intergenic region | 10, 23 | ||||
| cdtA | 2723–3391 | 669 | 24,557 | CdtA of E. coli, C. jejuni, and H. ducreyi | CDT complex | 13, 38, 40, 45 |
| cdtB | 3406–4257 | 852 | 31,497 | CdtB of E. coli, C. jejuni, and H. ducreyi | CDT complex | 13, 38, 40, 45 |
| cdtC | 4268–4828 | 561 | 20,710 | CdtC of E. coli, C. jejuni, S. dysenteriae, and H. ducreyi | CDT complex | 13, 37, 38, 40, 45 |
| ORF2b | 6785–6594 | 192 | 6,832 | ORFC of B. burgdorferi circular plasmid | Unknown | 54 |
| ORF3b | 8012–7215 | 795 | Partial | XerC of Lactobacillus leichmannii and Proteus mirabilis | Integrase | 6 |
| Int of bacteriophages DLP12 and HP1 | 22, 34 |
Position 1 in the A. actinomycetemcomitans sequence is the first base of the EcoRI recognition site (GAATTC).
ORF2 and ORF3 are on the antisense strand relative to the other ORFs.
The deduced amino acid sequences of three of the ORFs found in the RFLP probe region were 21 to 48% similar to those of the cdtABC genes responsible for the expression of a cytotoxic protein(s) from certain strains of E. coli (38, 40, 45), a variety of C. jejuni strains (41), and Shigella dysenteriae (37) (Table 4). In addition, the A. actinomycetemcomitans deduced amino acid sequences were 91, 96, and 94% identical, respectively, to the corresponding homologs from H. ducreyi (13).
TABLE 4.
Pairwise matches between deduced amino acid sequences of the A. actinomycetemcomitans ORFs and those of homologs from other bacterial genera, plasmids, or bacteriophages
| Bacterial species, plasmid, or bacteriophage | Protein | Identity relative to corresponding A. actinomycetemcomitans homologa
|
Extent of sequence match (no. of amino acids) | No. of gaps | |
|---|---|---|---|---|---|
| ORF | Similarity index (%) | ||||
| H. influenzae Rd | CafA | cafA | 72.0 | 243 | 0 |
| E. coli | GrxB | glrA | 48.4 | 215 | 0 |
| E. coli | ORF o279 | ORF1 | 47.7 | 191 | 4 |
| H. influenzae Rd | VagC | vppA | 41.2 | 51 | 0 |
| VapC | 31.0 | 67 | 3 | ||
| D. nodosus | VapB | 35.0 | 59 | 0 | |
| S. dublin plasmid | VagC | 28.9 | 70 | 3 | |
| Synechocystis sp. | VapB | 27.4 | 77 | 5 | |
| E. coli F factor | 26.4 | 48 | 2 | ||
| E. coli E6468/62 | CdtA | cdtA | 42.1 | 143 | 2 |
| E. coli 9142-88 | 24.3 | 221 | 5 | ||
| E. coli plasmid pVir | 23.5 | 211 | 5 | ||
| C. jejuni | 33.8 | 156 | 4 | ||
| H. ducreyi | 91.1 | 223 | 1 | ||
| E. coli E6468/62 | CdtB | cdtB | 47.9 | 276 | 4 |
| E. coli 9142-88 | 46.1 | 265 | 4 | ||
| E. coli plasmid pVir | 47.8 | 264 | 5 | ||
| C. jejuni | 47.5 | 274 | 6 | ||
| H. ducreyi | 96.1 | 283 | 0 | ||
| E. coli E6468/62 | CdtC | cdtC | 25.5 | 152 | 9 |
| E. coli 9142-88 | 20.7 | 146 | 4 | ||
| E. coli plasmid pVir | 31.8 | 21 | 0 | ||
| C. jejuni | 42.9 | 21 | 0 | ||
| S. dysenteriae | 26.1 | 151 | 9 | ||
| H. ducreyi | 93.5 | 186 | 0 | ||
Alignments were based on the Lipman-Pearson algorithm (2). The percent values for alignments for H. ducreyi are for amino acid sequence identity.
Characterization of the A. actinomycetemcomitans cdt gene region.
Each of the three ORFs had an apparent ribosomal binding site (AGGAG) 5 to 7 bases from the putative start codon. The proteins encoded by the three ORFs had calculated molecular masses of 24,557, 31,497, and 20,710 Da, respectively. The predicted sizes were consistent with those of the Cdt proteins from the other bacterial species. Each of the proteins had a basic amino terminus followed by a hydrophobic region indicative of a leader sequence. The first ORF contained the consensus sequence (LXACX) for the signal peptidase II cleavage site found in lipoproteins (56). Based on the characteristics of these ORFs, they were named cdtA, cdtB, and cdtC (Fig. 1).
Four ORFs were present immediately upstream from the cdtABC genes in the RFLP probe region (Fig. 1 and Table 3). The ORFs designated cafA, glrA, and ORF1 had deduced amino acid sequence similarity to hypothetical or characterized proteins from Haemophilus influenzae or E. coli (Tables 3 and 4). However, these matches had no obvious significance relative to the virulence potential of A. actinomycetemcomitans.
The deduced amino acid sequence of the ORF designated vppA was 27 to 41% similar to sequences of a class of proteins (Vap-Vag) termed virulence plasmids or virulence-associated proteins (Table 4). The sequence similarities extended throughout the entire lengths of these proteins, which ranged in mass from 8.5 to 11.5 kDa. The deduced amino acid sequence of vppA was also similar to that of a putative protein in the traD-to-traI region of the F plasmid of E. coli (24).
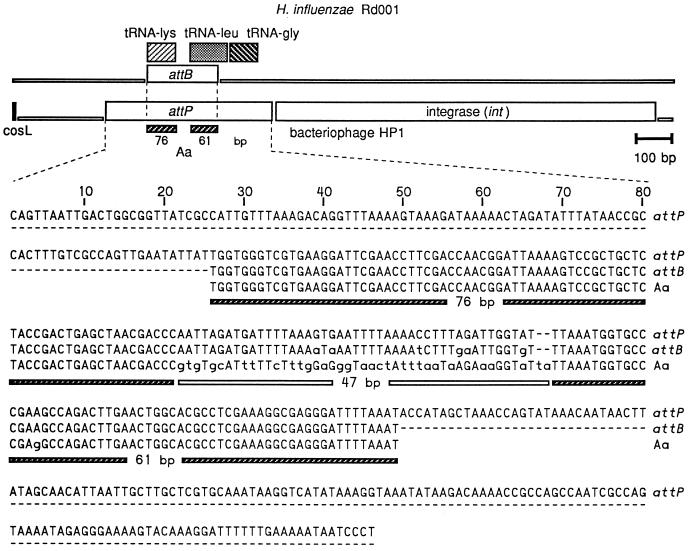
An additional 2.6 kb of nucleotide sequence downstream from the 3′ end of the existing 5.4-kb RFLP probe sequence was obtained by walking along the chromosome. One 194-bp region within the additional sequence had 80% identity to a DNA sequence from an unpublished H. influenzae integrating plasmid (GenBank accession no. U68467). A region further downstream had sequences of 76 and 61 bp that were 100 and 98% identical to two regions in the attachment site (attP and attB) for the lysogenic insertion of H. influenzae bacteriophages HP1 (58) and S2 (49) (Fig. 2). The 76- and 61-bp sequences resided in the H. influenzae Rd001 tRNA-Lys and tRNA-Leu genes, respectively (20). The two regions of homology were separated by 47 bp in the A. actinomycetemcomitans sequence. This 47-bp sequence corresponded to the 45-bp intergenic region between the H. influenzae tRNA-Lys and tRNA-Leu genes. However, there was no homology between these A. actinomycetemcomitans and H. influenzae sequences.
FIG. 2.
Relationship between the lysogenic integration site and tRNA gene sequences of H. influenzae and the homologous sequence from A. actinomycetemcomitans. Bases in lowercase differ from the attP sequence. The relative location of the A. actinomycetemcomitans sequence is shown in Fig. 1. The attB site is within the tRNA genes for lysine and leucine. Only a portion of the chromosome of H. influenzae Rd001 and the left arm of bacteriophage HP1 are shown. attP, bacteriophage HP1 attachment site sequence (19, 58); attB, H. influenzae Rd001 attachment site sequence (19, 20); Aa, A. actinomycetemcomitans sequence marked “att” in Fig. 1.
Two ORFs (ORF2 and ORF3) were found on the antisense strand downstream from the cdt ORFs (Fig. 1 and Table 3). The deduced amino acid sequence of ORF2 was 46.2% similar to that of a portion of orfC present on a Borrelia burgdorferi circular plasmid (54). The deduced amino acid sequence of the partial ORF3 (3′ end) was 20 to 27% similar to those of numerous bacterial and bacteriophage integrase proteins. Although sequence homology was limited, the three catalytic basic amino acid residues (Arg, His, and Arg) and active-site tyrosine, conserved in members of the Eubacteria integrase family (22), were present in the A. actinomycetemcomitans sequence.
ORFs related to the cdt genes, heterologous plasmids, and integrases and sequences similar to bacteriophage integration sites could be indicative of a previous recombinational event. Except for the cdtB ORF (41% G+C), the percentages of G+C contents of the A. actinomycetemcomitans ORFs (31.7 to 39.7%) were more similar to those of H. influenzae (39.0%) and H. ducreyi (38.0%) than to that of the parent species (42.7%) (31). The percentages of G+C content of the putative bacteriophage integration site sequences (51.0%) was closest to those of E. coli (48.0 to 52.0%) and Salmonella species (49.0 to 53.0%) genomes.
Expression of CDT-like activity by A. actinomycetemcomitans.
To determine if the cdtABC genes expressed an active CDT-like toxin, dilutions of sonic extracts from A. actinomycetemcomitans Y4 (RFLP group I) and representative members of each of the remaining 13 previously characterized RFLP groups (15) were examined for the ability to cause the distension and killing of CHO cells (Fig. 3). Genetic variants representing RFLP groups I (Y4), II (UP6), V (UP65), and X (UP70 and UP71) were highly cytotoxic for CHO cells (P < 0.01). Extracts from variants from RFLP group XIII (UP54) had relatively low levels of CDT-like activity (P = 0.05). Alternatively, extracts from variants from RFLP groups III (UP19), IV (UP64), VI (UP32), VII (UP34), VIII (UP67), IX (UP68), XI (UP42), XII (UP44), and XIV (UP57) appeared to lack killing activity (P > 0.05). The extract from the group XIV variant did not affect CHO cells when up to 56 μg of protein was used. The CDT-like activities in sonic extracts from members of the same RFLP group were comparable (see RFLP groups V and X in Fig. 3). Genetic variants that appeared to have deletions in the cdt locus, based on the results of RFLP analysis (15) and Southern blots with PCR-generated probes for the specific cdt ORFs (Fig. 3), did not express CDT-like activity. These variants were considered to be naturally occurring expression-negative mutants. There was no evidence of deletions in the cdt locus of RFLP group III, IV, VII, and VIII variants. However, these genetic variants did not express statistically significant levels of CDT-like activity.
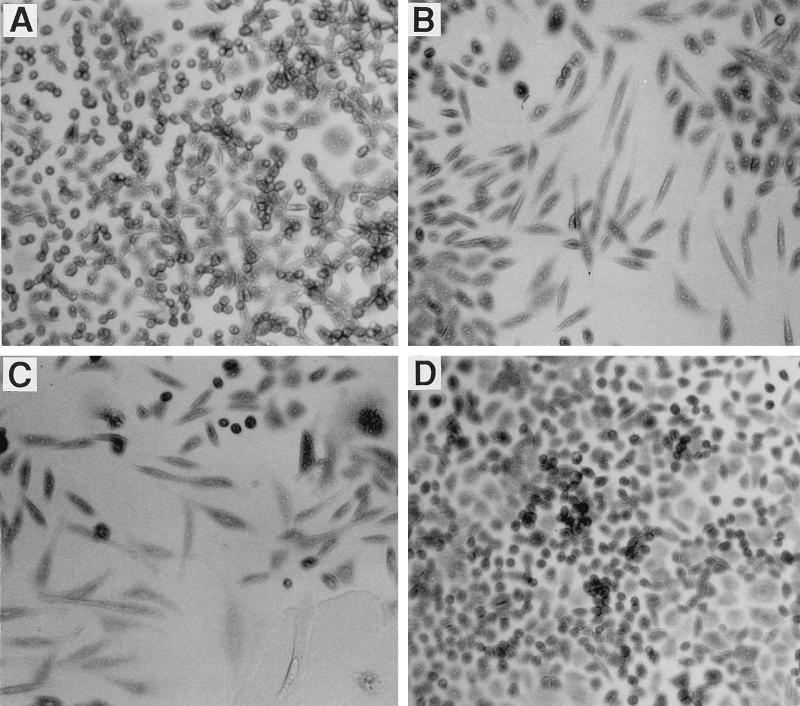
Cellular distension was routinely observed within 2 to 3 days following the exposure of CHO cells to A. actinomycetemcomitans Y4 protein extracts (Fig. 4B). In contrast, protein extracts from the RFLP group XIV expression-negative variant did not have an effect on the morphology of CHO cells (Fig. 4D). Distension was observed only with the sonic extracts from those genetic variants that resulted in a loss of cell viability (data not shown).
FIG. 4.
Microscopic examination of CHO cells following treatment with sonic extracts from A. actinomycetemcomitans. CHO cells (3 × 103/well) and serial dilutions of the fractions were added to 96-well microtiter plates. The cells were stained and photographed after 3 days of growth. Shown are untreated CHO cells (A) and CHO cells treated with extract from strain Y4 (2.0 μg of protein/well) (B), an RFLP group II variant (UP6; 350 ng of protein/well) (C), and an RFLP group XIV variant (UP57; 18.7 μg of protein/well) (D).
The A. actinomycetemcomitans cdt gene locus codes for the CDT-like activities.
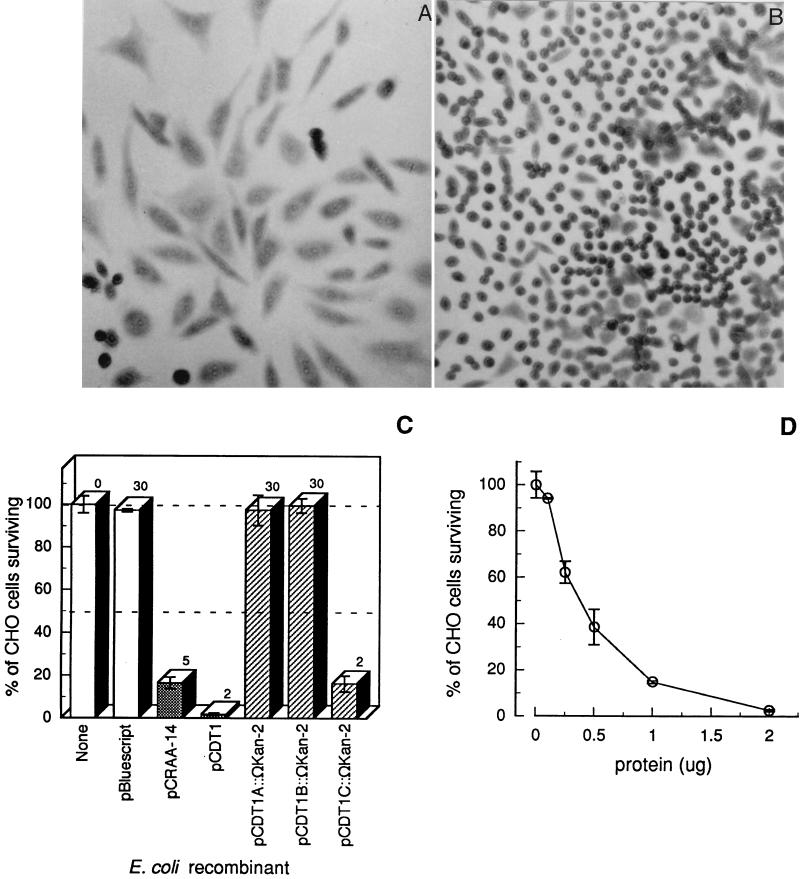
To establish that the cdt ORFs from A. actinomycetemcomitans were responsible for the observed distension and cytotoxic activities, CHO cells were treated with sonic extracts from E. coli transformants containing the entire RFLP probe region or only the cdtABC ORFs. Sonic extracts from E. coli DH5α(pCRAA-14) and E. coli DH5α(pCDT1) (Fig. 5A) caused the distension and killing (Fig. 5C) of CHO cells. The cytotoxicity exhibited by E. coli DH5α(pCDT1) was dose dependent (Fig. 5D). A TD50 was obtained with approximately 400 ng of sonic extract protein from this recombinant clone. Extracts from the isogenic insertion mutants E. coli DH5α(pCDT1A::ΩKan-2) and E. coli DH5α(pCDT1B::ΩKan-2) had no affect on the morphology (Fig. 5B) or viability (Fig. 5C) of CHO cells. However, the sonic extract from E. coli DH5α(pCDT1C::ΩKan-2) was cytotoxic. The interposon insertion site was only 24 nucleotides from the 3′ end of the gene. Sonic extracts from E. coli DH5α[pCR-Script SK(+)] and E. coli DH5α[pBluescriptII SK(+)] were negative for distension and cytotoxicity.
FIG. 5.
Expression of CDT-like activity by recombinant E. coli strains containing the cdt ORFs. The CHO assay was performed as described in the legend to Fig. 4. (A) CHO cells treated with 169 ng of protein/well of sonic extract from E. coli DH5α(pCDT1). (B) CHO cells treated with 12 μg of protein/well of sonic extract from E. coli DH5α(pCDT1A::ΩKan-2). (C) Quantitative estimation of cytotoxicity with sonic extracts from E. coli DH5α[pBluescript II SK(+)] (30 μg of protein/well), E. coli DH5α(pCRAA-14) (5 μg of protein/well), E. coli DH5α(pCDT1) (2 μg of protein/well), E. coli DH5α(pCDT1A::ΩKan-2) (30 μg of protein/well), E. coli DH5α(pCDT1B::ΩKan-2) (30 μg of protein/well), and E. coli DH5α(pCDT1C::ΩKan-2) (2 μg of protein/well). (D) Dose-response curve. Various amounts of sonic extract (in micrograms) from E. coli DH5α(pCDT1) were added to 300 CHO cells/well and processed as described in the legend to Fig. 3. Error bars indicate standard deviations.
Expression of A. actinomycetemcomitans cdt gene products.
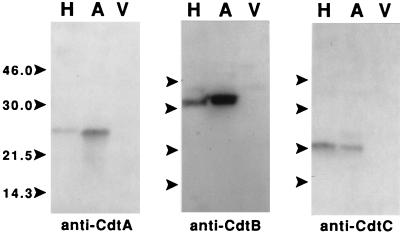
Sonic extracts from strain Y4, E. coli DH5α(pCDT1), and the insertion mutants were examined on Western blots with monoclonal antibodies made reactive with the CdtA, CdtB, and CdtC proteins of H. ducreyi. Distinct products, having the same apparent molecular weights as the H. ducreyi Cdt proteins, were observed, with the Cdt antibodies only in the extract from the recombinant strain E. coli DH5α(pCDT1) (Fig. 6). No reaction was obtained with equivalent extracts from E. coli DH5α(pBluescript II SK). Sonic extracts from each of the RFLP group variants used in Fig. 3 were examined on slot blots with the Cdt antibodies. A reactive product was observed only with the sonic extract from the recombinant E. coli DH5α(pCDT1) control. Only the CdtC antibody inhibited the distension and cytotoxic activities in the CHO cell assays.
FIG. 6.
Immunoblot-based detection of recombinant Cdt proteins with monoclonal antibodies raised against the H. ducreyi Cdt proteins. Proteins present in whole cell lysates from E. coli DH5α(pPCDT3) (lanes H), E. coli DH5α(pCDT1) (lanes A), and E. coli DH5α(pBluescript II SK) (lanes V) were incubated with monoclonal antibodies reactive with the CdtA, CdtB, and CdtC proteins of H. ducreyi. The positions of molecular size markers are shown by the arrows on the left side of each blot. The sizes of the markers are shown in kilodaltons and were the same for each blot.
DISCUSSION
A genetic locus for the synthesis of a novel cytotoxin that causes the distension and death of eukaryotic cells was discovered in A. actinomycetemcomitans during the sequencing of a RFLP hybridization probe routinely used for the identification of genetic variants associated with LJP. The three ORFs that comprised this locus had significant amino acid sequence similarity to the deduced amino acid sequences of the CDT genes (cdtABC) of E. coli (38, 40, 45) and C. jejuni (41). The finding that the deduced amino acid sequences of these ORFs from A. actinomycetemcomitans were more than 90% identical to those of the cdtABC genes from H. ducreyi (13) provided more convincing evidence that a genetic locus for a CDT was present in the periodontal pathogen.
Six additional ORFs were found in the region of the cdt locus. Comparisons of the deduced amino acid sequences to those of proteins in the public databases showed that this region of the A. actinomycetemcomitans genome contained sequences characteristic of virulence-associated regions (vap and vag) found in the genomes or on plasmids of other pathogenic bacterial species (12, 17, 29, 30, 32, 43). The significance of the sequence match to a hypothetical ORF from the F factor of E. coli is less clear. This ORF is located on the antisense strand in the intergenic region (traD to traI) of the F plasmid (10, 23). The presence of a piece of the F plasmid in an oral pathogen may be an indication of the extent of horizontal gene transfer among bacterial species. The recent discovery of homologous cdt genes in an F-like virulence plasmid (pVir) from E. coli (38) appears to provide genetic evidence for the spread of the CDT.
The implied relationship between the cdt genes and virulence-associated regions in A. actinomycetemcomitans is strengthened by the finding that sequences immediately downstream from the cdt locus were identical to a portion of the bacteriophage lysogenic integration site of the H. influenzae bacteriophage HP1 (20, 58). HP1 is a temperate bacteriophage that inserts itself into a defined site in the H. influenzae Rd chromosome by a site-specific recombination event. The bacteriophage has a 500-bp attachment site (attP) that contains a 182-bp sequence that duplicates the host attachment site (attB) and part of the host operon of tRNA genes (19, 20). The 76- and 61-bp portions of the A. actinomycetemcomitans sequence that match the attachment site also match the corresponding sequence within the H. influenzae tRNA-Lys and tRNA-Leu genes, respectively. However, there was no evidence that the cdt locus resides in an A. actinomycetemcomitans tRNA gene region, because these operons have not yet been located on the chromosome.
A putative integrase ORF was located immediately downstream from the lysogenic integration site sequence (att). The partial deduced amino acid sequence from this ORF matched a multitude of integrase sequences from bacteria and bacteriophages. However, the extent of the similarity was not strikingly great, suggesting that the ORF may have originated from an A. actinomycetemcomitans bacteriophage or plasmid rather than from a Haemophilus source. Regardless of the source of ORF3, the presence of the highly conserved catalytic amino acids and active-site tyrosine is good evidence that this ORF codes for an integrase (22).
There have been reports confirming the existence of temperate bacteriophages (53) and integrated plasmid sequences (35) in A. actinomycetemcomitans. It has been shown that fragments of the large A. actinomycetemcomitans plasmid pVT745 (35) are present in the chromosome of strain Y4 (15). However, plasmid DNA from pVT745 did not hybridize to the 8-kb region sequenced in this study.
The genetic organization of the virulence-associated region in Dichelobacter nodosus provides a model for the organization of the cdt region in A. actinomycetemcomitans (12). Characteristic features of the virulence-associated region are duplicate att sequences which flank the region, an integrase sequence, and the presence of duplicated vap genes. All of these features have not yet been identified in the A. actinomycetemcomitans cdt region. However, there are a number of striking similarities. Both the D. nodosus and A. actinomycetemcomitans regions have an integrase ORF adjacent to an att site. However, the putative integrase ORF in A. actinomycetemcomitans is only a partial sequence at the present time. There is at least one vap gene that is homologous between the two species and has homology to the same F plasmid sequence. In each case, there are vap gene sequence homologies to cyanobacteria species (Synechococcus and Synechocystis). Finally, both regions contain heterologous plasmid DNA sequences. It is possible that the DNA sequence obtained to date from A. actinomycetemcomitans may represent only one end of an extensive virulence-associated region or pathogenicity island. The insertion of large fragments of foreign DNA, containing genes that are functionally related to virulence factors, into tRNA genes is a hallmark of pathogenicity islands (7, 8). However, there is no direct evidence at the present time that this is the case in A. actinomycetemcomitans. Additional DNA sequence both upstream and downstream of the cdt locus will be required to make a full determination.
In total, there were six sites within the 8 kb of sequenced A. actinomycetemcomitans DNA that appeared to be related to Haemophilus sequences. These included the partial cafA ORF, vppA, cdtABC, ORF3, and the two short stretches of sequence downstream from the putative cdt locus that were similar to plasmid and bacteriophage integration elements. The deduced amino acid sequence of ORF2 appeared to be related to that of an ORF on a circular B. burgdorferi plasmid (54), but the significance of this similarity is not clear.
It may not be coincidental that the cdt locus was discovered as a result of targeting heterogeneous regions of the A. actinomycetemcomitans chromosome by RFLP analysis. Chromosomal sequences of heterologous DNA sequences that arise by the acquisition of pathogenicity islands, lysogenic insertion, and conjugative or transpositional events can be unstable (8). It is also possible that the putative integrase gene may be functional in A. actinomycetemcomitans. The related integrase from H. influenzae bacteriophage HP1 functions in both integration and excision (22). Therefore, it makes sense that this region would denote genetic polymorphism due to the inherent instability of the heterologous DNA.
Our observations further dispel an earlier supposition that the CDT was specifically associated with enteric bacteria that cause diarrheal disease (1, 3, 9, 36). The first indication that this hypothesis may not be valid was the discovery of a cdt locus in H. ducreyi, a bacterium that causes genital ulcers (13). Our findings further demonstrated that the CDT is more widespread among prokaryotes than previously thought.
Experiments employing sonic extracts from representative members of the RFLP groups of A. actinomycetemcomitans showed that the expression of the CDT-like activities was strain dependent. These results were expected, since earlier studies had shown that members of some RFLP groups had apparent deletions in the region encompassing the cdt ORFs (15). Naturally occurring expression-negative mutants can be found with reasonable frequencies in human subjects. Members of some RFLP groups appeared to contain a complete cdt locus but lacked CDT-like activity. One explanation is that these variants contain point mutations in one or more of the cdt ORFs.
Sonic extracts prepared from a recombinant clone, E. coli DH5α(pCDT1), containing only the three A. actinomycetemcomitans cdt ORFs and the noncoding upstream region between vppA and cdtA, also caused the classical distension and cytotoxicity of CHO cells. As expected, Cdt monoclonal antibodies made reactive with the H. ducreyi proteins recognized recombinant products on Western blots. This was not surprising due to the high identity between the CdtA, CdtB, and CdtC proteins from these two species.
Two of the insertional inactivation mutants, E. coli DH5α(pCDT1A::ΩKan-2) and E. coli DH5α(pCDT1B::ΩKan-2), did not express the CDT-like activities, providing proof that the A. actinomycetemcomitans cdt ORFs were biologically functional and confirming that they coded for the CDT-like activities. The interposon insertion in the cdtC ORF was very close to the 3′ end of the gene. Since this mutant expressed CDT-like activity it appears that the last eight amino acids are not required to obtain an active gene product. However, this change appears to have altered recognition by the CdtC antibody.
Other investigators have reported heat-labile cytostatic and cytolytic activities in extracts from A. actinomycetemcomitans (28, 46, 47). Since the description of the genetic and physical properties of these factors was limited, it is difficult to make detailed comparisons. However, it was reported that the immunosuppressive factor was purified to apparent homogeneity as a 60-kDa protein (48). This protein is significantly larger than any of the three cdt gene products. It was also reported that a microscopic examination of cultures treated with the factor that inhibits fibroblast proliferation did not reveal the presence of detached or nonadherent cells or demonstrate evidence of cytopathic effects (46). It was concluded that the immunosuppressive factor and fibroblast inhibitory factor were distinct biologically active mediators. Based on the published data it appears that the activities of some of these other factors may not be attributable to the expression of the cdt genes.
During the completion of this paper a similar study of the A. actinomycetemcomitans CDT was published by Sugai et al. (55).
ACKNOWLEDGMENTS
Thomas Stamato and Sharon DiRienzo of the Lankanau Hospital Research Center are gratefully acknowledged for their assistance with the CHO cell assays.
Marcia Mayer was supported by a grant from the Fundação de Auxilio a Pesquisa do Estado de São Paulo (FAPESP). This study was supported by a University of Pennsylvania Research Foundation award and National Institutes of Health grants DE/OD10891 to J.M.D. and AI32011 to E.J.H.
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S G, Bettelheim K A, Neogi P K B, Bhuiyan N A, Kaper J B. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J Clin Microbiol. 1996;34:717–719. doi: 10.1128/jcm.34.3.717-719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J D, MacNab A J, Gransden W R, Damm S A M, Johnson W M, Lior H. Gastroenteritis and encephalopathy associated with a strain of Escherichia coli O55:K59:H4 that produced a cytolethal distending toxin. Pediatr Infect Dis J. 1987;6:1135–1136. [PubMed] [Google Scholar]
- 4.Aslund F, Ehn B, Miranda-Vizuete A, Pueyo C, Holmgren A. Two additional glutaredoxins exist in Escherichia coli: glutaredoxin 3 is a hydrogen donor for ribonucleotide reductase in a thioredoxin/glutaredoxin 1 double mutant. Proc Natl Acad Sci USA. 1994;91:9813–9817. doi: 10.1073/pnas.91.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baehni P C, Tsai C-C, McArthur W P, Hammond B F, Taichman N S. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233–243. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker J, Brendel M. Molecular characterization of the xerC gene of Lactobacillus leichmannii encoding a site-specific recombinase and two adjacent heat shock genes. Curr Microbiol. 1996;32:232–236. doi: 10.1007/s002849900042. [DOI] [PubMed] [Google Scholar]
- 7.Bloch C A, Rode C K. Pathogenicity island evaluation in Escherichia coli K1 by crossing with laboratory strain K-12. Infect Immun. 1996;64:3218–3223. doi: 10.1128/iai.64.8.3218-3223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands form tRNA-specific loci in the chromosome of an Escherichia coli wild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouzari S, Varghese A. Cytolethal distending toxin (CLDT) production by enteropathogenic Escherichia coli (EPEC) FEMS Microbiol Lett. 1990;71:193–198. doi: 10.1016/0378-1097(90)90055-u. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw H D, Jr, Traxler B A, Minkley E G, Jr, Nester E W, Gordon M P. Nucleotide sequence of the traI (helicase I) gene from the sex factor F. J Bacteriol. 1990;172:4127–4131. doi: 10.1128/jb.172.7.4127-4131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno L C, Mayer M P A, DiRienzo J M. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J Periodontol. 1998;69:998–1007. doi: 10.1902/jop.1998.69.9.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheetham B F, Katz M E. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol Microbiol. 1995;18:201–208. doi: 10.1111/j.1365-2958.1995.mmi_18020201.x. [DOI] [PubMed] [Google Scholar]
- 13.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiRienzo J M, Cornell S, Kazoroski L, Slots J. Probe-specific DNA fingerprinting applied to the epidemiology of localized juvenile periodontitis. Oral Microbiol Immunol. 1990;5:49–56. doi: 10.1111/j.1399-302x.1990.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 15.DiRienzo J M, McKay T L. Identification and characterization of genetic cluster groups of Actinobacillus actinomycetemcomitans isolated from the human oral cavity. J Clin Microbiol. 1994;32:75–81. doi: 10.1128/jcm.32.1.75-81.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiRienzo J M, Slots J, Sixou M, Sol M-A, Harmon R, McKay T L. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 18.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 19.Hauser M, Scocca J J. Location of the host attachment site for phage HP1 within a cluster of Haemophilus influenzae tRNA genes. Nucleic Acids Res. 1990;18:5305. doi: 10.1093/nar/18.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser M, Scocca J J. Site-specific integration of the Haemophilus influenzae bacteriophage HP1: identification of the points of recombinational strand exchange and the limits of the host attachment site. J Biol Chem. 1992;267:6859–6864. [PubMed] [Google Scholar]
- 21.Hayashida H, Hotokezaka H, Ohara N, Kimura M, Takagi O, Yamada T. Molecular analysis of a new insertion sequence from Actinobacillus (Haemophilus) actinomycetemcomitans FDC Y4. Microbiology. 1996;142:2449–2452. doi: 10.1099/00221287-142-9-2449. [DOI] [PubMed] [Google Scholar]
- 22.Hickman A B, Waninger S, Scocca J J, Dyda F. Molecular organization in site-specific recombination: the catalytic domain of bacteriophage HP1 integrase at 2.7 A resolution. Cell. 1997;89:227–237. doi: 10.1016/s0092-8674(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 23.Jalajakumari M B, Manning P A. Nucleotide sequence of the traD region in the Escherichia coli F sex factor. Gene. 1989;81:195–202. doi: 10.1016/0378-1119(89)90179-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson W M, Lior H. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987;43:19–23. [Google Scholar]
- 25.Johnson W M, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella spp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 26.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 27.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 28.Kamen P R. Inhibition of keratinocyte proliferation by extracts of Actinobacillus actinomycetemcomitans. Infect Immun. 1983;42:1191–1194. doi: 10.1128/iai.42.3.1191-1194.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 30.Katz M E, Strugnell R A, Rood J I. Molecular characterization of a genomic region associated with virulence in Dichelobacter nodosus. Infect Immun. 1992;60:4586–4592. doi: 10.1128/iai.60.11.4586-4592.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. [Google Scholar]
- 32.Lax A J, Pullinger G D, Baird G D, Williamson C M. The virulence plasmid of Salmonella dublin: detailed restriction map and analysis by transposon mutagenesis. J Gen Microbiol. 1990;136:1117–1123. doi: 10.1099/00221287-136-6-1117. [DOI] [PubMed] [Google Scholar]
- 32a.Mayer M P A, Bueno L C, DiRienzo J M. Abstr. 1107. J Dent Res. 1998;77B:770. [Google Scholar]
- 33.Meyer D H, Sreenivasan P K, Fives-Taylor P. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muramatsu S, Mizuno T. Nucleotide sequence of the region encompassing the int gene of a cryptic prophage and the dnaY gene flanked by a curved DNA sequence of Escherichia coli K12. Mol Gen Genet. 1990;220:325–328. doi: 10.1007/BF00260503. [DOI] [PubMed] [Google Scholar]
- 34a.National Center for Biotechnology Information. 11 December 1998, copyright date. [Online.] http://www.ncbi.nlm.nih.gov/. [11 December 1998, last date accessed.]
- 35.Novak K F, Lee L N, LeBlanc D J. Functional analysis of pVT745, a plasmid from Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1998;13:124–128. doi: 10.1111/j.1399-302x.1998.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 36.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 38.Pérès S Y, Marchès O, Daigle F, Nougayrède P, Hérault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preus H R, Olsen I, Namork E. Association between bacteriophage-infected Actinobacillus actinomycetemcomitans and rapid periodontal destruction. J Clin Periodontol. 1987;14:245–247. doi: 10.1111/j.1600-051x.1987.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 43.Pullinger G D, Lax A J. A Salmonella dublin virulence plasmid locus that affects bacterial growth under nutrient-limited conditions. Mol Microbiol. 1992;6:1631–1643. doi: 10.1111/j.1365-2958.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenker B J, Kushner M E, Tsai C-C. Inhibition of fibroblast proliferation by Actinobacillus actinomycetemcomitans. Infect Immun. 1982;38:986–992. doi: 10.1128/iai.38.3.986-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenker B J, McArthur W P, Tsai C-C. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128:148–154. [PubMed] [Google Scholar]
- 48.Shenker B J, Vitale L A, Welham D A. Immune suppression induced by Actinobacillus actinomycetemcomitans: effects on immunoglobulin production by human B cells. Infect Immun. 1990;58:3856–3862. doi: 10.1128/iai.58.12.3856-3862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skowronek K, Piekarowicz A. Determination of the cos sequence of the mature genome of S2/HP1 type B bacteriophage of Haemophilus influenzae. Gene. 1986;2:71–73. doi: 10.1016/0378-1119(96)00196-5. [DOI] [PubMed] [Google Scholar]
- 50.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 51.Slots J, Zambon J J, Rosling B G, Reynolds H S, Christersson L A, Genco R J. Actinobacillus actinomycetemcomitans in human periodontal disease: association, serology, leukotoxicity, and treatment. J Periodontal Res. 1982;17:447–455. doi: 10.1111/j.1600-0765.1982.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 52.Stevens M K, Porcella S, Lumbley S, Klesney-Tait J, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Stevens, M. K., and E. J. Hansen. Unpublished data.
- 53.Stevens R H, Hammond B F, Lai C H. Characterization of an inducible bacteriophage from a leukotoxic strain of Actinobacillus actinomycetemcomitans. Infect Immun. 1982;35:343–349. doi: 10.1128/iai.35.1.343-349.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugai M, Kawamoto T, Pérès S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanner A C R, Haffer C, Brathall G T, Visconti R A, Socransky S S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979;6:278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 58.Waldman A S, Goodman S D, Scocca J J. Nucleotide sequences and properties of the sites involved in lysogenic insertion of the bacteriophage HP1c1 genome into the Haemophilus influenzae chromosome. J Bacteriol. 1987;169:238–246. doi: 10.1128/jb.169.1.238-246.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]