Abstract
A myosin immunoanalogue was identified in conidia of Aspergillus fumigatus by Western blotting, indirect immunofluorescence assay, and gold immunoelectron microscopy with two different antimyosin antibodies. The distribution pattern of this protein was followed during the early stages of germination. A single 180-kDa polypeptide, detected predominantly in a cell envelope extract, was found to cross-react with monoclonal and polyclonal antibodies raised against vertebrate muscle myosin. Immunoelectron microscopy permitted precise localization of this polypeptide, indicating that myosin analogue was mainly distributed along the plasma membrane of resting and swollen conidia. In germinating conidia, indirect immunofluorescence microscopy revealed myosin analogue at the periphery of germ tubes, whereas actin appeared as dispersed punctate structures in the cytoplasm that were more concentrated at the site of germ tube emergence. A myosin ATPase inhibitor, butanedione monoxime, greatly reduced swelling and blocked germination. In contrast, when conidia were treated with cytochalasin B, an inhibitor of actin polymerization, swelling was not affected and germination was only partially reduced. Butanedione monoxime-treated conidia showed accumulation of cytoplasmic vesicles and did not achieve cell wall reorganization, unlike swollen conidia. Collectively, these results suggest an essential role for this myosin analogue in the deposition of cell wall components during germination of A. fumigatus conidia and therefore in host tissue colonization.
Aspergillus fumigatus, a ubiquitous opportunistic fungus, is a human pathogen infecting mainly immunocompromised hosts. It usually grows and sporulates on decaying organic matter from which conidia are easily dispersed into the air. After inhalation of the airborne conidia, colonization of the airways requires the adherence of conidia to damaged bronchoalveolar surfaces through cell wall receptor-extracellular matrix interactions, followed by swelling, germination, and penetration of the subsequent hyphae into the host tissue (8, 11). The cell wall plays an important role in controlling host tissue adherence and the hyphal growth and therefore the pathogenesis of the fungus. Swelling and germination of conidia, which are the first steps of hyphal extension, are two particular forms of cell growth characteristic of filamentous fungi. They require important rearrangements of the cell wall, with lysis of old materials and synthesis of new components. For example, it has been demonstrated that the reorganization of the cell wall during swelling dramatically influences the expression of surface receptors as well as the physical properties of the cell surface (7, 33, 34).
Cytoskeleton proteins play an important role in morphogenesis for numerous fungi. For example, in Aspergillus nidulans, actin participates in normal apical growth during the germination of conidia (4), in polarized enzyme secretion (31), and in septum formation (16). Studies of the function of myosins have pointed to roles in chitin deposition (8) and in polarized growth and secretion in A. nidulans (24). Myosins are actin-based motor proteins characterized by their highly conserved head domains (9, 10). The best known members of the myosin superfamily are conventional myosins, or class II myosins, which are two-headed molecules with an α-helical tail able to self-associate to form bipolar filaments. Class II myosins are present in muscle and nonmuscle cells. Nonmuscle class II myosins of lower eukaryotes are required for cytokinesis, capping of surface proteins, maintenance of cortical tension, and normal growth (2, 9, 24, 28). The class I myosins, or unconventional myosins, originally included all myosins that are monomeric, do not form filaments, and have a low molecular mass (26). Based on sequence analysis of the amino-terminal head domains, class I myosins can be subdivided into 13 subclasses (10). In protozoon and vertebrate cells, they contribute to pseudopod extension and cell motility, as well as morphogenesis, organelle movement, polarized growth, and secretion (13, 15, 17, 21, 23).
With regard to their crucial role in cell wall extension in many fungi, cytoskeletal proteins may be attractive targets for the development of new antifungal drugs for aspergillosis. Therefore, we have characterized, in A. fumigatus conidial extracts, a myosin immunoanalogue recognized in immunoblotting by both monoclonal and polyclonal antibodies generated against muscle myosin, and we have investigated its distribution during the early stages of germination. Since studies by immunofluorescence are limited by the small size of the conidia, we have localized myosin at the ultrastructural level by immunoelectron microscopy. Finally, to determine whether myosin might be involved in radial and apical growth of conidia, we have quantified the effects of butanedione monoxime (BDM), a myosin ATPase inhibitor, on swelling and germination and studied the cytoplasmic and cell wall organization of treated cells.
MATERIALS AND METHODS
Organisms and culture conditions.
A. fumigatus CBS 113.26 was grown on yeast extract-peptone-dextrose agar at 37°C, and conidia were obtained from 5-day-old cultures as described earlier (33). The conidia were pelleted by centrifugation (1,200 × g for 3 min) and resuspended in distilled water, and the absorbance at 620 nm of the obtained suspension was adjusted to 0.6.
Swollen conidia and germ tubes were obtained by inoculating 1.5 ml of the conidial suspension onto petri dishes containing 15 ml of medium 199 at pH 7.6 as described previously (33). They were then pelleted by centrifugation and resuspended in phosphate-buffered saline (PBS) (0.15 M; pH 7.2).
Cell extracts.
A total of 7 × 108 cells (resting conidia, swollen conidia, or germ tubes) were disrupted in 1 ml of PBS containing protease inhibitors (14 μM pepstatin, 1 mM phenylmethylsulfonyl fluoride, and 1 mM EDTA) in an MSK cell homogenizer (B. Braun, Melsungen, Germany) by using a mix of 1- and 0.25- to 0.30-mm-diameter glass beads and cooling with CO2. Glass beads were removed, and disrupted cells were initially centrifuged at 5,000 × g for 3 min. The pellet corresponding to the insoluble cell envelope fraction (cell wall and plasmalemma) was collected, and the supernatant was then centrifuged at 50,000 × g for 30 min at 4°C. The clear supernatant which corresponded to the soluble cytoplasmic fraction was collected. Both the insoluble and soluble fractions were denatured in Laemmli buffer (22). Total protein contents of the extracts were determined by the Bio-Rad detergent-compatible protein assay (Bio-Rad Laboratories, Hercules, Calif.), and the extracts were stored as aliquots at −20°C until they were used.
Electrophoresis and Western blotting.
Samples (100 μg of protein per lane) were applied to 1.5-mm-thick slab gels of 10% polyacrylamide with 3% polyacrylamide stacking gel and electrophoresed as described by Laemmli (22). The separated proteins were stained with Coomassie brilliant blue or transferred to nitrocellulose membranes for Western blotting. The molecular masses of proteins were determined with high- and low-molecular-mass electrophoresis calibration kits (Pharmacia Biotech, Uppsala, Sweden). In addition, rabbit skeletal muscle myosin (Sigma Chemical Co., St. Louis, Mo.) was electrophoresed as a control.
For immunoblotting, unstained gels were transferred to nitrocellulose membranes at 4°C overnight in 25 mM Tris–192 mM glycine buffer containing 20% methanol (32). The membranes were blocked with 10% nonfat dry milk–0.15% Tween 20 (wt/vol) in PBS for 2 h at 37°C. After three washes in PBS containing 0.05% Tween 20 (PBST), blots were probed for 1 h at 37°C with the following antibodies: a rabbit anti-muscle (skeletal and smooth) myosin antiserum (Sigma M-7648), a monoclonal antibody against pan-myosin antibody (Amersham Life Science, Inc., Cleveland, Ohio), or a rabbit antiactin antibody (anti-C-terminal actin fragment; Sigma A-2066), all at a 1:100 dilution in PBST containing 1% bovine serum albumin (PBST-BSA). They were then washed three times in PBST and incubated for 1 h at 37°C with either peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) F(ab′)2 or peroxidase-conjugated rabbit anti-mouse immunoglobulins (Sigma) at a 1:1000 dilution in PBST-BSA. After the blots were washed with PBST, the peroxidase activity was developed with 0.05% diaminobenzidine in Tris HCl buffer (pH 7.4) and 0.5% H2O2. The reaction was stopped with 5% (vol/vol) acetic acid. The specificity of the reaction was assessed with a nonimmune rabbit serum instead of the primary antibody or the antimyosin antiserum depleted with mammalian myosin as described by Yokota et al. (36). To do this, 500-μl volumes of the rabbit antimyosin antiserum diluted 100-fold with PBS–1% BSA were successively mixed with a nitrocellulose strip containing rabbit skeletal muscle myosin subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and with 2 μl of a solution containing 0.15 mg of rabbit skeletal muscle myosin per ml. Additional controls for specific staining consisted of incubation of nitrocellulose strips with an antiserum containing high-titer antibody against an unrelated protein (rabbit antiactin antiserum) and of Western blots of mammalian myosin probed with the rabbit polyclonal antimyosin antiserum or with the antimyosin antiserum preadsorbed with mammalian myosin.
Immunofluorescence microscopy.
Cells were fixed and processed as described by Harris et al. (16). Briefly, conidia were grown on glass coverslips at 37°C in medium 199. After 7 h of incubation, coverslips with adherent germlings were removed, transferred to 3.7% formaldehyde in PBS containing 5 mM MgSO4 and 25 mM EGTA, and incubated for 45 min at room temperature. For cell wall digestion, coverslips were overlaid for 1 h at room temperature with a 200-μl solution of 20 mg of Novozym 234 (Sigma) per ml of PBS containing 10% egg white albumin. After three washes, coverslips were stored at −20°C until they were used.
For immunolabeling, the coverslips were incubated for 5 min in an extraction solution containing 0.1% Nonidet P-40, washed in PBS, and immersed in absolute ethanol at −20°C for 10 min. After being washed several times in PBS, the coverslips were incubated for 1 h at room temperature with the rabbit antimyosin antiserum at a 1:100 dilution in PBST-BSA or with the rabbit antiactin antiserum at a 1:20 dilution in PBST-BSA. Coverslips were then washed three times with PBS and stained for 1 h with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antibodies (Sigma) at a 1:100 dilution in PBST-BSA. Finally, the coverslips were washed with PBS, mounted on glass slides in glycerol-PBS (9:1 [vol/vol]), and examined under a Leitz microscope equipped for epifluorescence. For control experiments, coverslips were treated with PBS or nonimmune rabbit serum instead of the rabbit antimyosin or antiactin antiserum. For localization of myosin, additional controls were performed by using primary antibodies depleted, as described above, with rabbit skeletal muscle myosin.
Drug treatment.
Conidia were suspended in medium 199 alone or in medium 199 containing 50 mM BDM (Sigma) or 50 μM cytochalasin B (CB) (Sigma). The level of swelling was determined by measuring the diameters of at least 100 conidia after 4 h of incubation with an imager analysis system (Leica model Q 500 MC). Experiments were repeated three times, and the results were expressed as mean volumes of conidia ± standard deviations. For quantification of germination, at least 100 conidia were scored and the percentage of conidia bearing germ tubes was determined after a 7-h incubation in medium 199. Experiments were repeated three times, and data were expressed as the mean percentages of germination ± standard deviations. For reversibility experiments, cells were washed, resuspended in fresh medium, and incubated overnight at 37°C.
Electron microscopy.
Fungal suspensions containing resting and swollen conidia were fixed for 1 h with freshly prepared fixative (0.1% glutaraldehyde–2% paraformaldehyde) buffered at pH 7.4 with 0.1 M sodium cacodylate buffer. After being washed in cacodylate buffer, samples were treated for 30 min with 50 mM ammonium chloride prepared in the same buffer, washed again, and treated for 30 min with a 0.5% uranyl acetate solution. Samples were dehydrated at −18°C with 70% ethanol for 1 h, with 95% ethanol for 2 h, and twice with 100% ethanol for 2 h each time. Dehydration was followed by treatment at −18°C with mixtures of LR white (Sigma)-ethanol (1:2) overnight, LR white-ethanol (1:1) for 12 h, and fresh LR white overnight. The samples were then placed in fresh resin and polymerized for 2 days at −18°C.
For immunolabeling, ultrathin sections collected on nickel grids with carbon-coated Formvar film were incubated at room temperature on drops of rabbit antimyosin antiserum (Sigma M-7648) at a 1:100 dilution in PBS–1% BSA for 30 min, washed three times (5 min for each wash) in PBS–1% BSA, and treated with protein A-gold (20 nm; 1:100 dilution in PBS; Sigma). Two washes (5 min each) were done in distilled water after protein A treatment. Thin sections were contrasted with uranyl acetate and examined on a model 100 CX JEOL electron microscope. The specificity of the immunostaining procedures was established with the following controls: (i) omission of the primary antibody and (ii) use of a nonimmune rabbit serum instead of the rabbit antimyosin immune serum.
To visualize the effects of BDM treatment on cell morphology, samples were fixed for 1 h in 2.5% glutaraldehyde buffered at pH 7.4 with 0.1 M sodium cacodylate. After being washed, cells were postfixed in 1% OsO4 buffered with sodium cacodylate (pH 7.4), dehydrated in ethanol, and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate.
RESULTS
Identification of myosin immunoanalogue in A. fumigatus.
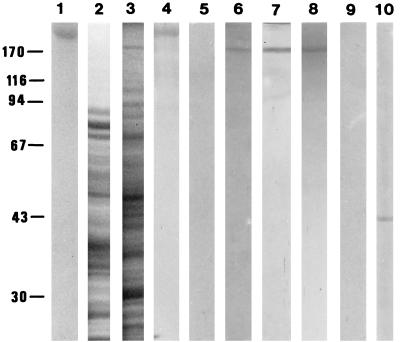
Representative profiles of rabbit skeletal muscle myosin and fungal extracts (the cytosolic extract and the cell envelope extract), analyzed by SDS-PAGE under reducing conditions on a 10% polyacrylamide slab gel and stained with Coomassie blue, are shown in Fig. 1 (lanes 1 to 3). The identification of A. fumigatus myosin analogue was performed by Western blotting with the monoclonal anti-pan-myosin antibody and the rabbit polyclonal antimyosin antiserum. In the two cases, a dominant immunoreactive polypeptide of approximately 180 kDa was detected in both extracts from resting conidia (Fig. 1, lanes 6 to 8) but was predominantly associated with the cell envelope fraction. The presence of the 180-kDa myosin was not restricted to resting conidia, since myosin was also detectable in the other stages studied, swollen conidia and germ tubes (data not shown). Control experiments performed with a nonimmune rabbit serum (data not shown) or the rabbit polyclonal antimyosin antiserum previously depleted with mammalian myosin (Fig. 1, lane 9) as the primary antibody gave negative results. When tested with the rabbit antiactin antiserum, immunoblots revealed a single band of 43 kDa, in both the cell envelope (Fig. 1, lane 10) and the cytosolic extracts (data not shown). Rabbit skeletal muscle myosin stained with the rabbit antimyosin antiserum is also shown (Fig. 1, lane 4). As a control for specific staining a Western blot of mammalian myosin was probed with the antimyosin antiserum depleted with mammalian myosin (Fig. 1, lane 5). No immunological staining was observed.
FIG. 1.
SDS-PAGE and Western blot analysis. Lanes 1 to 3 show SDS–10% PAGE gels of rabbit skeletal muscle myosin (lane 1), cytosolic extract (lane 2), and insoluble cell envelope extract (lane 3) of A. fumigatus resting conidia stained with Coomassie brilliant blue. Lanes 4 to 10 show nitrocellulose blots of SDS–10% PAGE gels containing rabbit skeletal muscle myosin (lanes 4 and 5), cytosolic extract (lane 6), and cell envelope extract (lanes 7 to 10). Lanes 6 and 7 were probed with the polyclonal anti-pan-myosin antibodies, and lane 8 was probed with the rabbit antimyosin antiserum. Note that a polypeptide of about 180 kDa in the two extracts is labeled with both the monoclonal and polyclonal antibodies. As controls, lane 4 was probed with the rabbit antimyosin antiserum, lanes 5 and 9 were probed with the rabbit antimyosin antiserum depleted with rabbit skeletal muscle myosin, and lane 10 was probed with the rabbit antiactin antiserum showing a band cross-reacting with actin at 43 kDa. No reactive band was detected at 180 kDa in the controls.
Localization of myosin immunoanalogue in A. fumigatus.
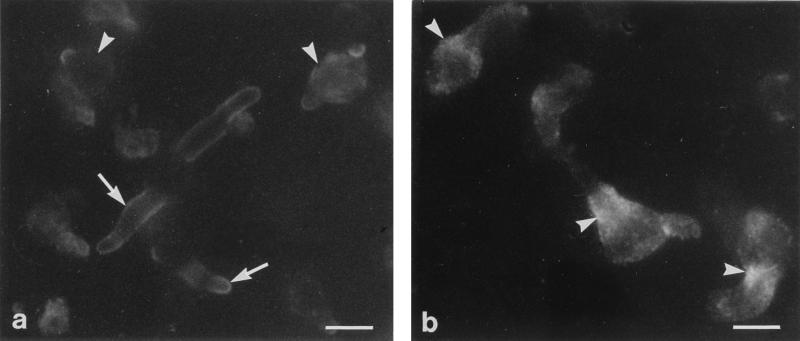
Indirect immunofluorescence indicated that myosin immunoanalogue was concentrated mainly in the cell envelopes of conidia. After incubation of conidia with the antimyosin immune serum, a bright fluorescence was seen at the cell periphery. In contrast, the cytoplasm exhibited only a faint fluorescence. In germinated cells, an intense staining was detected at the periphery of germ tubes, whereas no labeling was seen in the mother cell of elongated germ tubes (Fig. 2a). No staining was detected in control experiments (data not shown). Actin localization was also analyzed by immunofluorescence in growing germ tubes. Because actin-containing structures in A. fumigatus cells were not stained with fluorochrome-conjugated phalloidin, we used a polyclonal antiactin antibody known to stain F-actin in mammalian cells. Although filamentous structures were occasionally observed, actin was predominantly detected as dots. The organization of the actin cytoskeleton appeared clearly different from the arrangement of myosin, since intense punctate staining was obvious predominantly at sites of germ tube emergence (Fig. 2b). Actin granules were also dispersed throughout the cytoplasm.
FIG. 2.
Localization of myosin analogue and actin in A. fumigatus germ tubes by indirect immunofluorescence microscopy. (a) Myosin was concentrated at the periphery and at the apex of the germ tube (arrows). In new germlings, the mother cell showed diffuse staining (arrowheads), whereas no labeling was seen in the mother cell of elongated germ tubes. (b) Actin appeared predominantly as patches concentrated at sites of germ tube emergence (arrowheads). Bars, 5 μm.
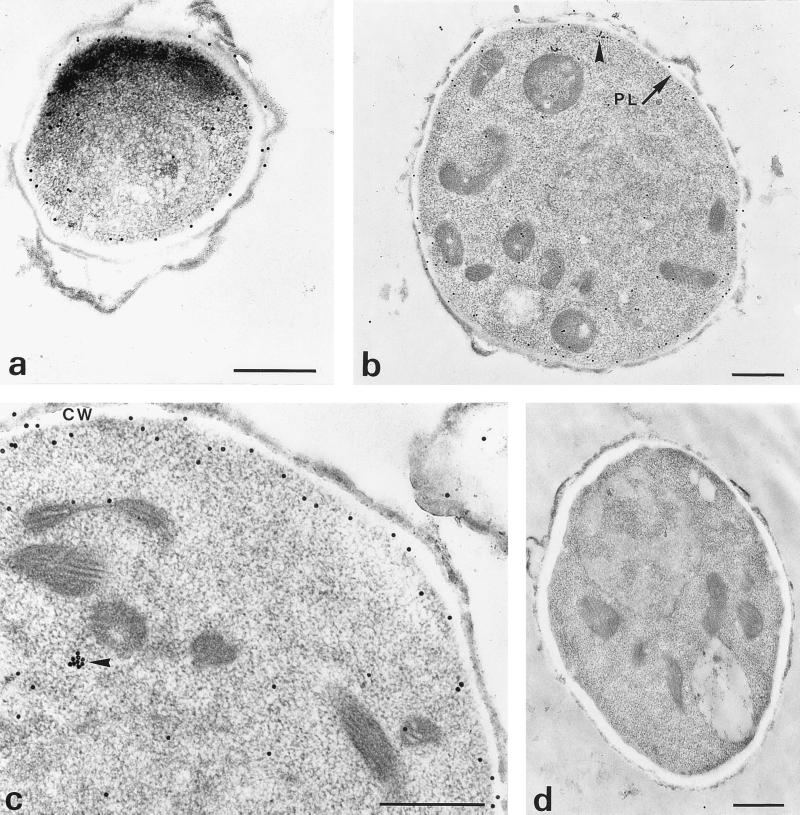
To clarify the connections between the myosin immunoanalogue and the cell envelope, we used immunoelectron microscopy. The results obtained were in good agreement with those of immunofluorescence assays. In resting conidia, gold was detected as single particles along the plasmalemma and in the cytoplasm (Fig. 3a). Similar myosin distribution was observed in swollen conidia. Gold particles were distributed mainly near their plasmalemmas, and a few gold particles were found dispersed in their cytoplasms (Fig. 3b and c). In some cases, clusters of particles were also detected near the plasmalemma (Fig. 3b) or in the cytoplasm (Fig. 3c).
FIG. 3.
Detection of myosin immunoanalogue with the polyclonal antimyosin antiserum by immunoelectron microscopy on sections of resting (a) and swollen (b and c) conidia. Gold particles were distributed mainly under the plasmalemma (PL). Note the clusters of particles (arrowhead) beneath the plasmalemma and in the cytoplasm. A few gold particles were also found dispersed in the cytoplasm. No labeling was observed in the control section treated with a nonimmune rabbit serum instead of the rabbit antimyosin antiserum (d). CW, cell wall. Bars, 0.5 μm.
Control sections with nonimmune rabbit serum as the primary antibody or that were realized by omission of the primary antibody were free of labeling (Fig. 3d).
Effects of myosin and actin inhibitors on swelling and germination.
To determine whether myosin and actin contribute to swelling and germination, we tested the abilities of BDM and CB to inhibit the radial and apical growth of conidia. Since the first germ tubes appeared after 5 h of incubation in medium 199, swelling was evaluated by measuring the diameters of the cells after 4 h of incubation, and germination was quantified after 7 h of incubation in the presence of BDM (50 mM) or CB (50 μM). BDM treatment drastically reduced swelling and abolished germination (Table 1). These effects were not the result of a decrease in viability, since swelling and germination occurred when conidia were resuspended in fresh medium without drugs and cultivated overnight. Finally, CB treatment had no effect on swelling but partially inhibited the germination of conidia (Table 1).
TABLE 1.
Effects of BDM and CB on swelling and germination of A. fumigatus conidiaa
| State of A. fumigatus | Mean volume (μm3) | % Germination |
|---|---|---|
| Resting conidia | 17.1 ± 1.6 | 0 |
| Incubation in medium 199 | 47.7 ± 9.3 | 49 ± 5 |
| Incubation in medium 199 with BDM | 24.3 ± 4.3 | 0 |
| Incubation in medium 199 with CB | 48.2 ± 8.8 | 23.3 ± 3 |
Swelling was quantified by measuring the mean volumes of resting conidia (in μm3) before and after a 4-h incubation in medium 199. The percentage of germination was scored after an incubation time of 7 h in medium 199. BDM and CB were added at the beginning of the incubation. Results are expressed as means (± standard deviations) of results from three independent experiments.
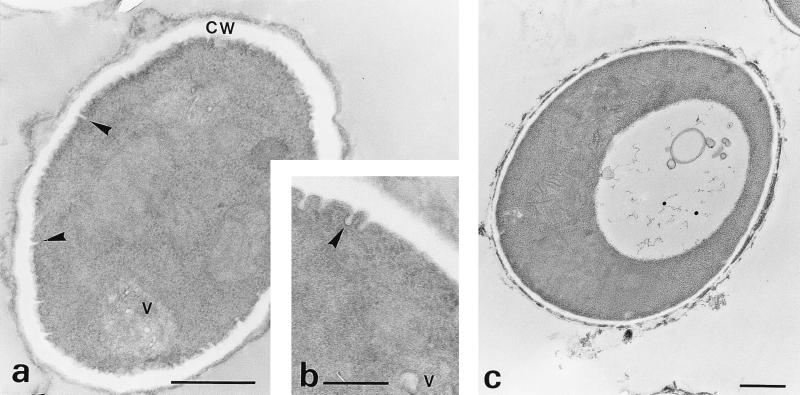
The effects of BDM treatment of the conidia on cell wall morphology were further investigated by thin-section electron microscopy. The ultrastructure of the cell walls of BDM-treated conidia was similar to that observed in resting conidia, with a thin electron-transparent inner layer covered by an electron-dense outer layer (Fig. 4a). Unlike swollen conidia (Fig. 4c), BDM-treated cells appeared to be unable to shed their outer cell wall layers. However, their plasma membranes showed numerous prominent invaginations. The accumulation of cytoplasmic vesicles which often formed aggregates and fusion between vesicles and plasma membrane invaginations were also observed (Fig. 4a and b).
FIG. 4.
Effects of BDM on swelling and germination of conidia. Conidia were incubated for 4 h in medium 199 containing 50 mM BDM before being processed for electron microscopy. (a) In BDM-treated cells, the cell wall (CW) showed a thick inner layer and the presence of an intact electron-dense outer layer. Accumulation of cytoplasmic vesicles (V) and invaginations of the plasma membrane (arrowheads) were also observed. (b) Vesicles fusing with invaginations of the plasma membrane (arrowhead). (c) As a control, a swollen conidium after 4 h of incubation in medium 199 alone shows the outer cell wall layer being shed. Bars in panels a and c, 0.5 μm; bar in panel b, 0.2 μm.
DISCUSSION
In the present study, we provide evidence that A. fumigatus conidia express a myosin immunoanalogue mainly associated with their plasma membranes and required for swelling and germination. A 180-kDa polypeptide was found to cross-react with the anti-pan-myosin antibody, as well as with the anti-skeletal and smooth muscle myosin antiserum. These antibodies recognize epitopes on the heavy chain of muscle and nonmuscle class II myosins (14, 15, 29, 30). This could explain why smaller molecules like class I myosins, for instance, an analogue of Myo A of A. nidulans (25), were not detected in A. fumigatus and suggests that this 180-kDa polypeptide and the heavy chain of muscle myosins share antigenic determinants. Interestingly myosin heavy chains with similar molecular masses have also been identified in plant cells (20, 29, 30, 35) and unicellular eukaryotes, including protozoa and fungi (15, 18, 24, 26).
The 180-kDa myosin immunoanalogue of A. fumigatus was detected in all the stages studied (resting conidia, swollen conidia, and germ tubes). As for Myo A of A. nidulans (25), the fact that this myosin immunoanalogue was found in resting conidia suggests that it is required during the earliest event of germination, i.e., swelling. Western blotting showed that the myosin immunoanalogue of A. fumigatus was concentrated mainly in the cell envelope fractions (cell walls and plasmalemmas) of conidia. Based on the pattern of immunofluorescence and immunogold labelings, it seems obvious that this polypeptide is localized mainly under the plasmalemmas of conidia, although staining was also seen throughout their cytoplasms. As germination occurs, this immunoreactive material progressively appeared to accumulate under the plasmalemmas of germ tubes. Nevertheless, an improved immunoelectron microscopy technique is necessary to clarify the interconnections between this myosin analogue and the plasma membrane. Several other studies showed that myosins are frequently associated with cell membranes. In protozoa, myosin appears tightly bound to the cortical membrane components of Gregarina blaberae (15) and was concentrated beneath the plasma membrane of Toxoplasma gondii (13). Likewise, Acanthamoeba (12) and Dictyostelium class I myosins (10) are associated with the plasma and vacuole membranes and interact in vitro with membrane lipids (1). In fungi, cell fractionation experiments indicated that Myo A is associated with the plasma membrane of A. nidulans (25), and a 110-kDa myosin of Candida albicans appeared associated with the cell envelopes of blastoconidia (14).
Myosin is considered the motor protein involved in cell locomotion and intracellular transport of organelles and molecules during morphogenesis (9, 18). The considerable cell wall extension of A. fumigatus conidia during swelling and germination involves the shedding of the outer cell wall layer and the assembly of a new plasmalemma and cell wall material. In Saccharomyces cerevisiae, it has been demonstrated that Myo1p, which is similar to conventional class II myosins, is implicated in the deposition of chitin and cell wall components (27), whereas in A. nidulans, Myo A was required for secretion and polarized growth (25). Therefore, the presence of myosin under the plasmalemmas of resting conidia may account for cell wall-plasmalemma extension during swelling of A. fumigatus cells.
To determine if myosin immunoanalogue was involved in cell wall rearrangement during swelling and germination, conidia were incubated with BDM, a potent general inhibitor of myosin ATPase. BDM has been described as blocking processes mediated by myosin, like vesicle recruitment during exocytosis in sea urchin embryonic cells (6) and gliding motility and cell invasion in T. gondii (13). In our study, BDM markedly affected swelling and abolished germination of A. fumigatus conidia. As evidenced by electron microscopy, cells treated with BDM showed a cell wall organization similar to that of resting conidia, with intact outer cell wall layers and thick inner layers. In addition, cells accumulated cytoplasmic vesicles, and their plasma membranes showed prominent invaginations, suggesting that the effect of BDM was not due to the inhibition of vesicle fusion with the plasma membrane. Usually, vesicles are rapidly transported to the area of growth, where they fuse with the plasma membrane. Therefore, the accumulation of vesicles in the cytoplasm and the morphology of the cell wall after BDM treatment indicate defects in vesicle transport of essential components to the growing cell wall. Nevertheless, whether BDM reduces swelling and inhibits germination in A. fumigatus by direct inhibition of the myosin ATPase or by another mechanism remains to be determined.
It has been demonstrated that actin has a primary role in the movement of secretory vesicles and in the cell growth of fungi (17, 31). In A. nidulans, actin appears to be concentrated at the growing tips of hyphae, where it colocalizes with myosin, and at the sites of developing septa (16, 25). In our study, as in other aspergilli (16, 31), actin filaments were occasionally visible and actin patches were concentrated in areas of germ tube emergence. Our antibody is known to stain filamentous actin in mammalian cells. Therefore, these spots may represent an aggregation of filamentous actin or an artifactual depolymerization during material preparation. Since the first septation events occur at the emergence site of the extending germ tube, these findings are consistent with the fact that actin participates in septum formation. Furthermore, it is well known that CB prevents the polymerization of G-actin to F-actin and inhibits germination (4). In A. fumigatus, CB reduced germination but failed to inhibit swelling. This might imply that actin is required for apical growth and septum formation, as previously described for A. nidulans (16, 25), rather than for swelling of conidia and suggests a differential role for myosin and actin in the initial events of conidial germination.
Microtubule motors are other potential candidates for morphogenesis of A. fumigatus conidia. On the other hand, it has been demonstrated that some filamentous fungi contain an integrin homologue that functions in cytoplasm-cell wall attachment like focal adhesions in animal cells (3, 19). Studies of connections between cytoskeleton proteins and the cell envelope may further our understanding of swelling and germination in A. fumigatus conidia. In addition, the 180-kDa myosin may be an attractive target for a new approach in antifungal chemotherapy.
ACKNOWLEDGMENT
We thank G. Daniault (EA 1720, Laboratoire de Parasitologie et Mycologie Médicale, Centre Hospitalier Universitaire La Milétrie, Poitiers) for her technical assistance.
REFERENCES
- 1.Adams R J, Pollar T D. Binding of myosin I to membrane lipids. Nature. 1986;340:565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- 2.Arhets P, Gounon P, Sansonetti P, Guillén N. Myosin II is involved in capping and uroid formation in the human pathogen Entamoeba histolytica. Infect Immun. 1995;63:4358–4367. doi: 10.1128/iai.63.11.4358-4367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachewich C L, Heath I B. Differential cytoplasm-plasma membrane-cell wall adhesion patterns and their relationships to tip growth and organelle motility. Protoplasma. 1997;200:71–86. [Google Scholar]
- 4.Barja F, Chappuis M L, Turian G. Differential effects of anticytoskeletal compounds on the localization and chemical patterns of actin in germinating conidia of Neurospora crassa. FEMS Microbiol Lett. 1993;107:261–266. doi: 10.1111/j.1574-6968.1993.tb06040.x. [DOI] [PubMed] [Google Scholar]
- 5.Bement W M, Mooseker M S. Keeping out the rain. Nature. 1993;365:785–786. doi: 10.1038/365785a0. [DOI] [PubMed] [Google Scholar]
- 6.Bi G Q, Morris R L, Liao G, Alderton J M, Scholey J M, Steinhardt R A. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+-regulated exocytosis. J Cell Biol. 1997;138:999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchara, J.-P. Unpublished data.
- 8.Bromley I M J, Donalson K. Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: relevance to asthmatic lung. Thorax. 1996;51:1203–1209. doi: 10.1136/thx.51.12.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheney R E, Riley M A, Mooseker M S. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskelet. 1993;24:215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- 10.Coluccio L M. Myosin I. Am J Physiol. 1997;273:C347–C359. doi: 10.1152/ajpcell.1997.273.2.C347. [DOI] [PubMed] [Google Scholar]
- 11.Dehart D J, Agwu D E, Julian N C, Washburn R G. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infect Dis. 1997;175:146–150. doi: 10.1093/infdis/175.1.146. [DOI] [PubMed] [Google Scholar]
- 12.Doberstein S K, Baines I C, Wiegand G, Korn E D, Pollard T D. Inhibition of contractile vacuole function in vivo by antibodies against myosin-I. Nature. 1993;365:841–843. doi: 10.1038/365841a0. [DOI] [PubMed] [Google Scholar]
- 13.Dobrowolski J M, Carruthers V B, Sibley L D. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghazali M, Rodier M L, El Moudni B, Quellard N, Jacquemin J L. Detection of myosin immunoanalogue in the yeast Candida albicans. Exp Mycol. 1995;19:101–110. doi: 10.1006/emyc.1995.1012. [DOI] [PubMed] [Google Scholar]
- 15.Ghazali M, Schrevel J. Myosin-like protein (Mr 175,000) in Gregarina blaberae. J Eukaryot Microbiol. 1993;40:345–354. doi: 10.1111/j.1550-7408.1993.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 16.Harris S D, Morrell J L, Hamer J E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics. 1994;136:517–535. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill K L, Catlett N L, Weissman L S. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston G C, Prendergast J A, Singer R A. The Saccharomyces cerevisiae MYO 2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminskyj S G, Heath I B. Integrin and spectrin homologues, and cytoplasm-wall adhesion in tip growth. J Cell Sci. 1995;108:849–856. doi: 10.1242/jcs.108.2.849. [DOI] [PubMed] [Google Scholar]
- 20.Kohno T, Ishikawa R, Nagata T, Kohama K, Shimmen T. Partial purification of myosin from lily pollen tubes by monitoring with in vitro motility assay. Protoplasma. 1992;170:77–85. [Google Scholar]
- 21.Korn E D. Acanthamoeba myosin I: past, present and future. Curr Top Membr Transp. 1991;17:23–25. [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lillie S H, Brown S S. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May K M, Watts F Z, Jones N, Hyams J S. Type II myosin involved in cytokinesis in the fission yeast, Schizosaccharomyces pombe. Cell Motil Cytoskelet. 1997;38:385–396. doi: 10.1002/(SICI)1097-0169(1997)38:4<385::AID-CM8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.McGoldrick C A, Gruver C, May G S. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J Cell Biol. 1995;128:577–587. doi: 10.1083/jcb.128.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard T D, Doberstein S K, Zot H G. Myosin-I. Annu Rev Physiol. 1991;53:653–681. doi: 10.1146/annurev.ph.53.030191.003253. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J R, Paterson B M. Yeast myosin heavy chain: maintenance of the cell type specific budding and the deposition of chitin and cell wall components requires an intact myosin heavy chain gene. Cell Motil Cytoskelet. 1990;17:301–308. doi: 10.1002/cm.970170405. [DOI] [PubMed] [Google Scholar]
- 28.Shelden E, Knecht D A. Dictyostelium cell shape generation requires myosin II. Cell Motil Cytoskelet. 1996;35:59–67. doi: 10.1002/(SICI)1097-0169(1996)35:1<59::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Tang X, Hepler P K, Scordilis S P. Immunochemical and immunocytochemical identification of a myosin heavy chain polypeptide in Nicotiana tubes. J Cell Sci. 1989;92:569–574. doi: 10.1242/jcs.92.4.569. [DOI] [PubMed] [Google Scholar]
- 30.Tirlapur U K, Cai G, Faleri C, Moscatelli A, Scali M, Del Casino C, Tiezzi A, Cresti M. Confocal imaging and immunogold electron microscopy of changes in distribution of myosin during pollen hydration, germination and pollen tube growth in Nicotiana tabacum L. Eur J Cell Biol. 1995;67:209–217. [PubMed] [Google Scholar]
- 31.Torralba S, Raudaskoski M, Pedregosa A M, Laborda F. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in A. nidulans. Microbiology. 1998;144:45–53. doi: 10.1099/00221287-144-1-45. [DOI] [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4530–4534. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tronchin G, Bouchara J P, Ferron M, Larcher G, Chabasse D. Cell surface properties of Aspergillus fumigatus conidia: correlation between adherence, agglutination and rearrangements of the cell wall. Can J Microbiol. 1995;41:714–721. doi: 10.1139/m95-098. [DOI] [PubMed] [Google Scholar]
- 34.Tronchin G, Esnault K, Renier G, Filmon R, Chabasse D, Bouchara J P. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect Immun. 1997;65:9–15. doi: 10.1128/iai.65.1.9-15.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokota E, Shimmen T. Isolation and characterization of plant myosin from pollen tubes of lily. Protoplasma. 1994;177:153–162. [Google Scholar]
- 36.Yokota E, McDonald A R, Liu B, Shimmen T, Palevitz B A. Localization of a 170 kDa myosin heavy chain in plant cells. Protoplasma. 1995;185:178–187. [Google Scholar]