ABSTRACT
The purposes of this study are to explore the function and regulatory mechanism of a novel lncRNA MYC-Induced Long non-coding RNA (MINCR) in osteoarthritis (OA). The expression of lncRNA MINCR, miR-146a-5p, and bone morphogenetic protein receptor 2 (BMPR2), Sry-type high-mobility-group box 9 (SOX9), collagen type II alpha 1 (COL2A1), Aggrecan, metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), Matrix metalloproteinase 3 (MMP3), MMP13, COL2A1, and Aggrecan were determined using quantitative real-time PCR (qRT-PCR), western blot, immunohistochemistry (IHC) and immunofluorescence (IF) in vitro and in vivo. And distribution and expression of MINCR were examined by fluorescence in situ hybridization (FISH). Cell proliferation and apoptosis were detected by cell counting kit-8 (CCK-8) assay, 5-Ethynyl-2’-deoxyuridine (EdU) staining, Annexin V-FITC/Propidium Iodide (PI), and Terminal Deoxynucleotidyl transferase-mediated dUTP Nick-End Labeling (TUNEL) staining in vitro and in vivo. The anterior cruciate ligament transection (ACLT) rat model was constructed to analyze the MINCR/miR-146a-5p/BMPR2 axis in vivo. The cartilage degeneration was determined by pathological staining with Hematoxylin and Eosin (H&E) and Safranin O staining. The binding relationship between MINCR and miR-146a-5p, and between miR-146a-5p and BMPR2 were determined by a dual-luciferase reporter gene, RNA Immunoprecipitation (RIP) assay, and RNA-pull down assays. Here, MINCR and BMPR2 were downregulated whereas miR-146a-5p was upregulated in OA cartilage tissues compared with control as well as IL-1β-induced chondrocytes compared with normal chondrocytes. Function experiments indicated that MINCR upregulation promoted cell proliferation and inhibited apoptosis and extracellular matrix (ECM)-degeneration. We also proved the binding relationship between MINCR and miR-146a-5p, and the BMPR2 acted as a target of miR-146a-5p. Mechanism analysis using rescue experiments in vitro and in vivo, MINCR silencing reversed the effects of miR-146a-5p downregulation in OA. Overexpression of miR-146a-5p also reversed the function of BMPR2 overexpression in OA. These data indicated that MINCR prevented OA progression via targeting miR-146a-5p to promote BMPR2 expression.
KEYWORDS: Osteoarthritis, MINCR, miR-146a-5p, BMPR2, inflammation
Introduction
OA is a common and serious degenerative joint disease that affects 240 million people worldwide, OA is approximately twice as common in women as it is in men [1]. It mainly induces pain and disability and reduces the living quality of older adults [2]. The main risk factors, which cause OA development, include age, heredity, epigenetic modification, obesity, metabolic syndrome, estrogen level, previous bone injury, and lifestyle [3,4]. The OA pathology mainly covers articular cartilage-degraded and inflammation activation in the synovial membrane and innate immune system [5]. Besides, numerous cytokines have been demonstrated that function as regulators in the pathology of OA, including tumor necrosis factors (TNFs), interleukin-1β (IL-1β), IL-6, IL-8, increasing proinflammatory factors contribute to catabolism of cartilage matrix [5–7]. The ECM metalloproteinases (MMPs) such as MMP3 and MMP13 and ADAMTSs such as ADAMTS-4 and ADAMTS-5 have been involved in the matrix-degeneration with inflammation [8]. Despite the known pathology of OA, the clinical outcome of OA therapy exhibits dissatisfaction. Therefore, it urgently needs to explore the key molecular and underlying mechanisms in OA.
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs (ncRNAs) with over 200 nucleotides in length. Commonly, lncRNAs function as spongers of microRNAs to regulate their function in multiple physiological and pathological processes of eucaryon [9]. Increasing evidence has demonstrated that lncRNAs act as crucial roles in the initiation and progression of OA. Zhang et al. have reviewed lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) acts as an inducer in OA progression by regulating miR-150-5p/AKT3 axis [10]. LncRNA p50-associated cyclooxygenase-2 extragenic RNA (PACER) has been discovered to downregulation OA and induce chondrocyte apoptosis [11]. Besides, Huang et al. reveal the increase of lncRNA cancer Susceptibility Candidate 2 (CASC2) in OA by promoting IL-17 expression [12]. Interestingly, lncRNAs act different roles in different diseases, for example, lncRNA small nucleolar RNA host gene 7 (SNHG7) plays as an oncogene in colorectal cancer, but functions as a key role in preventing the development of OA [13,14]. XIST also has been reported to function as a tumor suppressor in non-small cell lung cancer and promotes the progression of OA [15,16]. Moreover, MINCR has been demonstrated to increase in multiple tumors, such as nasopharyngeal carcinoma, oral squamous cell carcinoma, hepatocellular carcinoma, and non-small cell lung cancer [17–20]. However, the role and mechanism of MINCR in OA remain unknown.
MicroRNAs (miRNAs) are another group of non-coding RNAs with a small length of about 18–22 nucleotides. Commonly, miRNAs negatively bind with lncRNAs and directly suppress the transcriptional and post-transcriptional expression of target genes to regulate multiple pathological processes [21,22]. miR-146a-5p has been found to increase in serum and cartilage samples of OA patients [23], and high expression of miR-146a-5p is associated with an inflammatory response in LPS-induced articular chondrocytes [24]. miR-146a-5p inhibition may be an efficient therapeutic strategy for OA [25]. However, the interaction between MINCR and miR-146a-5p in OA has been incompletely known.
BMPs belong to the subclass of the transforming growth factor-beta (TGF-β) superfamily, which contributes to chondrogenesis and bone formation [26]. Usually, the BMP effects have been mediated □ by type and type □ receptors, which play as serine- and threonine kinases [27]. For example, miR-1307-3p inhibits chondrogenic differentiation by suppressing BMPR2 [28]. HOTAIRM1-1 downregulation contributes to OA progression via inhibiting BMPR2 [29]. However, miR-146a-5p regulates BMPR2 in OA progression remains unclear.
Taken together, we aimed to explore the function and underlying mechanism of MINCR in OA progression. In this study, we found MINCR and BMPR2 significantly downregulated whereas miR-146a-5p was upregulated in OA cartilage tissues and IL-1β-induced chondrocytes. MINCR acted as a suppressor in OA progression and IL-1β-induced chondrocytes by increasing proliferation and inhibiting apoptosis and ECM-degeneration in vitro and in vivo. Our finding might provide a potential therapeutic target and underlying mechanism for OA treatment.
Material and methods
Specimen
The OA cartilage tissues were separated from 20 OA patients through total knee arthroplasty, and normal separated from 20 patients undergoing traumatic orthopedic surgery in The Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine. All participants were informed and signed the informed consent. All experiments were approved by the Ethical Committee of The Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine. Cartilage tissues were obtained and snap-frozen in liquid nitrogen and stored at −80°C.
Chondrocyte culture and IL-1β-induced chondrocytes
The primary human chondrocytes were separated from normal cartilage tissues, and cultured in DMEM medium (Invitrogen, Carlsbad, CA, USA) supplementary with 10% fetal bovine serum (FBS, Hyclone, South Logan, UT, USA) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA), all cells were incubated at 37°C with 5% CO2. The medium was changed every three days. The third-generation cells were used for a subsequent experiment. Chondrocytes were incubated in DMEM and treated with 10 ng/mL recombinant human IL-1β (Sigma-Aldrich, St. Louis, MO, USA) for 24 h to establish the IL-1β-induced chondrocyte model. The IL-1β-induced chondrocyte model was identified with cytoplasmic shrinks and obvious increasing vacuoles of chondrocytes.
Cell transfection
The sequences of MINCR and BMPR2 were amplified and inserted into pcDNA3.1 plasmid (Invitrogen, Carlsbad, CA, USA), and then MINCR overexpression plasmid, BMPR2 overexpression plasmid, and their negative control pcDNA3.1 plasmid were transfected into cells using Lipofectamine3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. miR-146a-5p overexpression and downregulation were performed using miRNA mimic and inhibitor, respectively. MINCR downregulation was achieved using short hairpin RNA (shRNA) targeting MINCR (sh-MINCR). All oligonucleotides in this study were designed and synthesized by GenePharma (Shanghai, China). These oligonucleotides were transfected into cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instruction.
RNA Extraction and qRT-PCR analysis
Total RNA was isolated from tissues and cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instruction. Then, the cDNA was amplified using First-Strand cDNA Synthesis Kit (TaKaRa, Dalian, China) obeyed the manufacturer’s manual. The qRT-PCR was performed using TB Green® Fast qPCR Mix (TaKaRa, Dalian, China) according to the manufacturer’s protocol. Primers in this study are as follows: MINCR, forward, 5’-GTCTTCCGAACTCTGCTGCT-3’; reverse, 5’-AATGGCAAACAAGGCTCTGC-3’. miR-146a, forward, 5’-ACTGAATTCCATGGGTTGTGTC-3’; reverse, 5’-TGACAGAGATATCCCAGCTGAAG-3’. BMPR2, forward, 5’-CTGCAAATGGCCAAGCATGT-3’; reverse, 5’-ATGGTTGTAGCAGTGCCTCC-3’. U6, forward, 5’-CTCGCTTCGGCAGCACA-3’; reverse, 5’-AACGCTTCACGAATTTGCGT-3’. GAPDH, forward, 5’-GGATTTGGTCGTATTGGGCG-3’; reverse, 5’-TCCCGTTCTCAGCCATGTAG-3’. U6 was used as the internal control of miRNA, and GAPDH was used as the internal control for other genes. The relative expression of the gene was calculated using 2−ΔΔCt methods.
FISH
LncRNA FISH Probe Mix (Genepharma, Shanghai, China) was used to detect the expression and distribution of MINCR. The MINCR probes were designed and purchased from GenePharma (Shanghai, China). Briefly, the frozen section was hybridized with MINCR probes in dark at 37°C overnight. Then, the section was washed and incubated with DAPI (Beyotime, Shanghai, China) for 30 min in dark. And the section was observed and imaged under a fluorescence microscope (Olympus, Tokyo, Japan).
Cell viability analysis
CCK-8 assay (Beyotime, Shanghai, China) was used to determine cell viability according to the manufacturer’s protocol. In brief, chondrocytes were plated on the 96-well plates at a density of 1 × 105 cells. And 10 μL CCK-8 solution was added to each well of the 96-well plate and incubated for 2 h. Furthermore, the absorbance was detected at 450 nm using a spectrophotometric microplate reader. Besides, the cell proliferation was calculated using the EdU kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. Cells were seeded into 96-well plates at a density of 1 × 105 cells, and cultured in the medium with EdU diluent for 2 h.; moreover, cells were stained with Hocheast33258 (Beyotime, Shanghai, China) for 30 min. Cells were observed and photographed under a fluorescence microscope (Olympus, Japan). EdU labeled active cells (red) and Hocheast33258 labeled apoptotic cells (blue).
Apoptosis analysis
Annexin V-FITC Apoptosis Detection Kit (Beyotime, Shanghai, China) was used to assess the cell apoptosis following the manufacturer’s protocol. Briefly, cells were collected and centrifuged at 1000 g for 5 min, then cells were washed with PBS and incubated with 5 μL Annexin V-FITC and 10 μL PI for 20 min in dark. At last, apoptotic cells were counted using flow cytometry. Furthermore, the cell apoptosis in knee joint tissues section using a Colorimetric TUNEL Apoptosis Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s manual. Then, sections were incubated with 50 μL TUNEL solution for 1 h at 37°C in the dark. Lastly, apoptotic cells were observed and counted under an inverted microscope (Leica, Wetzlar, Germany).
Western blotting assay
Protein was separated from tissues and cells using RIPA lysis buffer (Beyotime, Shanghai, China) and the concentration and purity of protein were determined using Bradford Protein Assay Kit (Beyotime, Shanghai, China). Protein was separated by 10% sodium dodecyl sulfate-polyacrylamide (SDS) gel and transferred onto polyvinylidene fluoride (PVDF) members. Moreover, the members were incubated with primary antibodies overnight at 4°C. Then, the members were stained with secondary antibody goat anti-rabbit IgG H&L (1:2000, ab182016) for 1 h at room temperature. And the bands were visualized using an enhanced chemiluminescence (ECL) system (BioRad, CA, USA). The grave values of each band were analyzed using Image Lab software (BioRad, CA, USA). All antibodies were purchased from Abcam (Cambridge, MA, USA), primary antibodies including anti-BMPR2 antibody (1:1000, ab124463), anti-SOX9 antibody (1:1000, ab185966), anti-collagen □ (COL2A1) antibody (1:1000, ab188570), anti-Aggrecan antibody (1:1000, ab3778b), anti-ADAMTS-4 antibody (1:1000, ab185722), anti-MMP3 antibody (1:2000, ab52915), anti-MMP13 antibody (1:3000, ab39012).
Dual-luciferase reporter assay
The wild-type and mutant-type sequences of MINCR/BMPR2 were amplified and inserted into the pGL3 vector (Promega, WI, USA). Then, the pGL3-MINCR/MBPR2-wild/mutant vector and miR-146a-5p mimic or NC mimic were co-transfected into cells and incubated for 48 h. The luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega, WI, USA) following the manufacturer’s instructions. And the relative luciferase activity was calculated according to the ratio of firefly to Renilla activity.
RIP assay
Cells were lysed with RIPA lysis buffer (Beyotime, Shanghai, China), and then the cell lysates were incubated with an anti-Argonaute-2 (AGO2) antibody (1:2000, ab186733, Abcam, Cambridge, MA, USA) or anti-IgG antibody (1:1000, ab133470, Abcam, Cambridge, MA, USA) and magnetic beads overnight at 4°C. Moreover, total RNA was extracted, and the enrichment of RNA was determined by qRT-PCR.
RNA pull-down assay
RNA pull-down assay was performed using Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo, Waltham, MA, USA) following the manual of the manufacturer. In brief, cells were incubated with biotinylated NC mimic or miR-146a-5p mimic for 48 h. Cell lysates were incubated with streptavidin-conjugated magnetic beads. Then, total RNA was extracted, and the enrichment of lncRNA MINCR was evaluated by qRT-PCR.
IF assay
Cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.2% Triton X-100 for 5 min, subsequently blocked with 2% bovine serum albumin (BSA) for 30 min. Next, the cells were incubated with primary antibodies including, anti-COL2A1 (1:50, sc52625, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-Aggrecan (1:1000, ab3778b, Abcam, Cambridge, MA, USA), anti-ADAMTA-4 (1:200, ab185722, Abcam, Cambridge, MA, USA), anti-MMP13 (1:2000, ab219620, Abcam) overnight at 4°C. Then, cells were rinsed with PBS and incubated with secondary antibodies for 1 h at room temperature. Lastly, cells were stained with DAPI for 15 min and observed under a confocal-scanning microscope (Leica, Wetzlar, Germany).
IHC assay
The histology of the knee joint was analyzed by Safranin O-fast green staining and H&E staining assay following the previous report [30]. The degeneration of the articular cartilage of the medial and lateral tibial plateau joint was assessed by OARIS score according to the previously described [31]. And the ratio of HC and CC was evaluated following the previously described [32]. The 4-μm paraffin-embedded slices were dewaxed with xylene, dehydrated with alcohol, and retrieved with antigen. 4-μm sections were stained with hematoxylin solution for 10 min and then stained with eosin for 1 min according to the protocol of the H&E staining kit (Beyotime, Shanghai, China). In addition, the cartilage tissues were detected using Safranin O-fast green staining (Solarbio, Beijing, China) following the manufacturer’s protocol. The cartilage tissues were observed with red color, and osteoblasts were observed with green or blue color. For IHC, the slices were incubated with primary antibody anti-BMPR2 body (1:500, ab124463, Abcam, Cambridge, MA, USA) overnight at 4°C. And the slices were subsequently incubated with secondary antibody goat anti-rabbit IgG H&L (HRP) at room temperature for 1 h. Lastly, the slides were counterstained with hematoxylin for 10 at 37°C. Then, slices were observed and photographed under a reversed microscope (Leica, Wetzlar, Germany).
Construction of OA rat model
The ACLT model was constructed according to the description in the previous report [33]. All animal experiments were approved by the Ethics Committee of the Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine and performed according to the Institutional Animal Care and Use Committee Guide in The Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine. Twenty-four eight-week-old male Sprague-Dawley (SD) rats were used for OA model establishment. The rats were randomly divided into four groups with six rats in each group (Normal group, OA group, OA+OE-NC group, OA+OE-MINCR group). The normal group (n = 6) was established by only laterally dislocating the patella after the rat was anesthetized with isoflurane. For OA model establishment, each rat was anesthetized with isoflurane and the right knee joint was exposed, the lateral patella was dislocated and the knee was compressed then ACL transection. The OE-MINCR and OE-NC were injected into the joint, respectively. Eight-week later, the rats were sacrificed, and knee joints were separated for subsequent experiments.
Statistics analyses
Statistics analyses in this study were performed using GraphPad Prism 9.0. All value was represented as mean ± standard deviation (SD) from triple experiments. Comparison of multiple groups was performed using student’s t-test or one-way ANOVA. P-value < 0.05 was considered the statistical significance.
Results
Downregulation of MINCR in OA Tissues and IL-1β-Induced Chondrocytes
We firstly examined the subcellular distribution and expression of MINCR in cartilage tissues from health and OA patients. The FISH results indicated that MINCR was mostly distributed in the cytoplasm and downregulated in OA cartilage tissues (Figure 1(a)). In addition, qRT-PCR results also supported the previous finding, that MINCR was reduced in OA patients compared with control (Figure 1(b)). In vitro, chondrocytes were stimulated with 10 ng/mL IL-1β to mimic the OA condition. As expected, MINCR was significantly reduced in IL-1β-induced chondrocytes, compared with control (Figure 1(c)). These findings revealed that MINCR was negatively associated with OA progression.
Figure 1.

Downregulation of MINCR in OA tissues and IL-1β-induced chondrocytes. (a) FISH was utilized to examine the expression and subcellular distribution of MINCR in cartilage tissues from OA patients and normal. (b)-(c) QRT-PCR was used to detect the mRNA expression of MINCR in OA cartilage tissues compared with normal as well as IL-1β-induced chondrocytes compared with normal chondrocytes. **P < 0.01, ***P < 0.001.
Upregulation of MINCR promotes Cell proliferation whereas suppresses apoptosis and ECM-degeneration in IL-1β-induced chondrocytes
Next, we investigated the function of MINCR in OA progression in vitro by the gain of function experiments. MINCR was successfully overexpressed in normal chondrocytes (Figure 2(a)). Then, we detected the function of MINCR in OA. CCK-8 results indicated that overexpression of MINCR restored the cell viability of IL-1β-induced chondrocytes (Figure 2(b)). Edu/Hocheast33258 double staining results also proved that overexpression of MINCR promoted cell proliferation both in normal and IL-1β-induced chondrocytes (Figure 2(c,d)). Furthermore, the apoptosis of IL-1β-induced chondrocytes was observed whereas reduced by MINCR overexpression (Figure 2(e)). Furthermore, we examined the mRNA and protein expression of ECM-contribution genes (SOX9, COL2A1, and Aggrecan) and the ECM-degeneration genes (ADAMTS-4, MMP3, and MMP13). As shown in figure 2(f,h), SOX9, COL2A1, and Aggrecan remarkably reduced whereas SOX9, COL2A1, and Aggrecan increased in IL-1β-induced chondrocytes, however, previous phenotypes in IL-1β-induced chondrocytes were restored by MINCR overexpression. IF staining results also supported previous findings (Figure 2(i,j)). Our finding revealed that MINCR function as a suppressor in OA by promoting chondrocyte proliferation, whereas inhibited apoptosis and ECM-degeneration.
Figure 2.
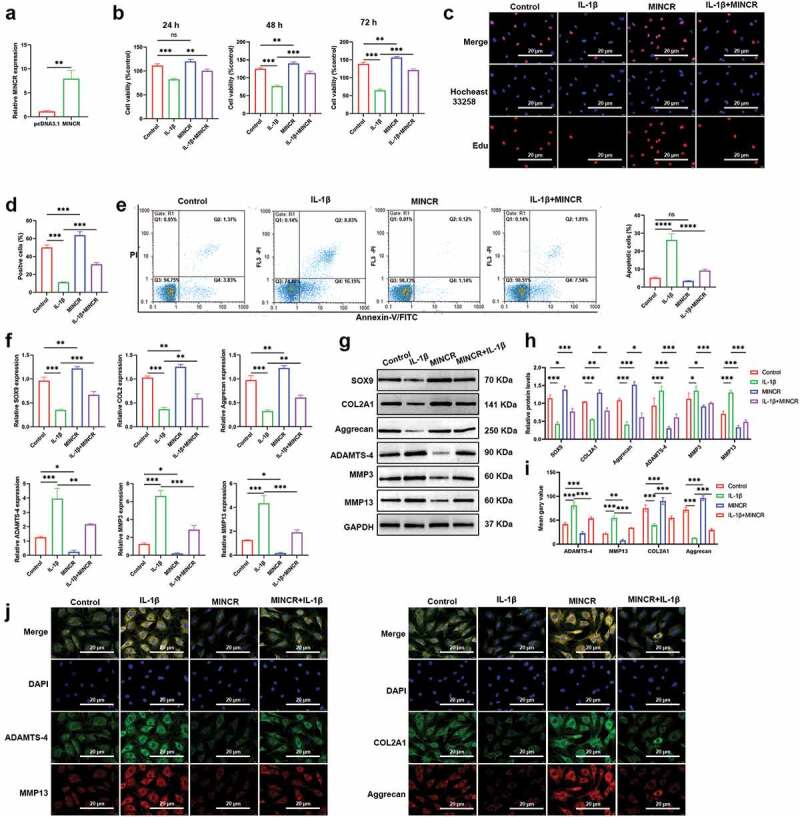
Upregulation of MINCR promotes cell proliferation whereas suppresses apoptosis and ECM-degeneration in IL-1β-induced chondrocytes. (a) QRT-PCR was used to detect the mRNA expression of MINCR after pcDNA3.1-mediated MICNR overexpression. (b) CCK-8 assay was used to examine cell viability after IL-1β stimulation, MINCR overexpression, and IL-1β stimulation + MINCR overexpression. (c)-(d) EdU/Hocheast33258 double staining was used to detect the living cells and apoptotic cells after IL-1β stimulation, MINCR overexpression, and IL-1β stimulation + MINCR overexpression. (e) Annexin V-FITC/PI was used to analyze apoptotic cells after IL-1β stimulation, MINCR overexpression, and IL-1β stimulation + MINCR overexpression. (f)-(h) QRT-PCR and western blot were used to examine mRNA and protein levels of SOX9, COL2A1, Aggrecan, ADAMTS-4, MMP3, and MMP13 after IL-1β stimulation, MINCR overexpression, and IL-1β stimulation + MINCR overexpression. (i)-(j) IF staining was used to detect the levels of ADAMTS-4, MMP13, COL2A1, and Aggrecan after IL-1β stimulation, MINCR overexpression, and IL-1β stimulation + MINCR overexpression. *P < 0.05, **P < 0.01, ***P < 0.001.
miR-146a-5p serves as a target for MINCR
To further predicate the downstream of MINCR based on Starbase (http://starbase.sysu.edu.cn/). As shown in Figure 3(a), the AGUUCUC sequences of MINCR directly bind with 3ʹUTR of miR-146a-5p on UCAAGAG. We artificially mutated AGUUCUC into UCAAGAG and inserted it into the luciferase reporter gene to investigate the relationship between MINCR and miR-146a-5p. We found that the luciferase activity was significantly reduced by co-transfecting with wild type of MINCR and miR-146a-5p mimic into cells, compared with others (Figure 3(b)). Besides, RNA-pull down and RIP results also demonstrated the binding relationship between MINCR and miR-146a-5p (Figure 3(c,d)). qRT-PCR results indicated that the expression of miR-146a-5p was inhibited by MINCR overexpression (Figure 3(e)). Moreover, we also found that the expression of miR-146a-5p significantly increased in OA tissues compared with control as well as IL-1β-induced chondrocytes compared with normal DCs (Figure 3(f,g)). Our results suggested that MINCR was directly bound with miR-146a-5p and negatively regulated its expression.
Figure 3.
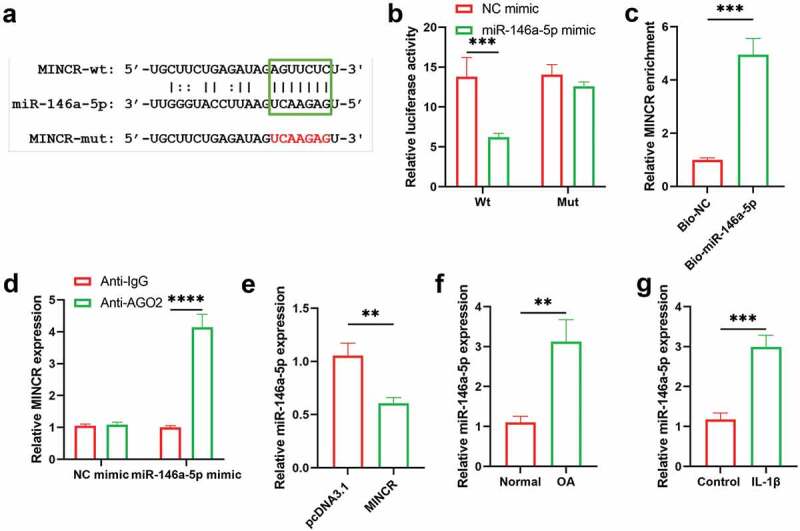
miR-146a-5p Serves as A Target for MINCR. (a) The binding site sequences of MINCR on miR-146a-5p. (b) Dual-luciferase reporter gene assay was used to detect the binding relationship between MINCR and miR-146a-5p after wild/mutant type of MINCR-recombination reporter gene co-transfection with miRNA mimics into cells. (c) The MINCR enrichment was detected by qRT-PCR after RNA-pull down performing. (d) The MINCR expression was analyzed by qRT-PCR after RNA samples were bound to Ago2 antibody by RIP assay. (e) The miR-146a-5p expression was detected by qRT-PCR after overexpression of MINCR. (f)-(g) The miR-146a-5p expression was examined by qRT-PCR in OA cartilage tissues compared with normal as well as IL-1β-induced chondrocytes compared to normal chondrocytes. **P < 0.01, ***P < 0.001.
Downregulation of MINCR reverses effects of miR-146a-5p silencing on IL-1β-induced chondrocytes
Furthermore, we analyzed the regulatory mechanism of MINCR and miR-146a-5p in OA progression by rescue experiments in vitro. MINCR was stably inhibited using the shRNA (Figure 4(a)), then, the regulatory mechanism was investigated. As shown in Figure 4(b-d), cell proliferation was promoted by miR-146a-5p inhibition whereas inhibited by MINCR silencing in IL-1β-induced chondrocytes. Apoptotic rates increased by miR-146a-5p inhibition but were suppressed by MINCR silencing in IL-1β-induced chondrocytes (Figure 4(e)). After that, ECM-formation and degeneration genes were examined, and the results indicated that expression of SOX9, COL2A1, and Aggrecan was increased whereas expression of ADAMTS-4, MMP3, and MMP13 was reduced by miR-146a-5p inhibition, but the effects of miR-146a-5p inhibition were reversed by MINCR silencing (Figure 4(f-h)). The above results revealed that MINCR regulated the OA progression by inhibiting miR-146a-5p.
Figure 4.

Downregulation of MINCR reverses effects of miR-146a-5p silencing on IL-1β-induced chondrocytes. (a) The efficiency of knockdown of MINCR using shRNA was detected by qRT-PCR. After IL-1β stimulation, IL-1β stimulation + miR-146a-5p inhibitor, and IL-1β stimulation + miR-146a-5p inhibitor + MINCR silencing, (b) CCK-8 assay was used to examine cell viability. (c)-(d) EdU/Hocheast33258 double staining was used to detect the living cells and apoptotic cells. (e) Annexin V-FITC/PI was used to analyze apoptotic cells. (f)-(g) QRT-PCR and western blot were used to examine mRNA and protein levels of SOX9, COL2A1, Aggrecan, ADAMTS-4, MMP3, and MMP13. (h) IF staining was used to detect the levels of ADAMTS-4, MMP13, COL2A1, and Aggrecan. *P < 0.05, **P < 0.01, ***P < 0.001.
BMPR2 acts as a potential target of miR-146a-5p, and MINCR promotes BMPR2 via inhibiting miR-146a-5p
Then, we also found that BMPR2 acted as a target of miR-146a-5p based on http://starbase.sysu.edu.cn/, BMPR2 directly binds with 3’-UTR of miR-146a-5p (Figure 5(a)). The binding relationship between miR-146a-5p and BMPR2 was demonstrated by luciferase reporter gene assay (Figure 5(b)). qRT-PCR and western blot results also revealed that both mRNA and protein expression of BMPR2 were inhibited by miR-146a-5p overexpression, whereas promoted by miR-146a-5p downregulation, respectively (Figure 5(c,d)). Additionally, qRT-PCR and IHC results indicated that expression of BMPR2 was reduced in OA cartilage compared with control as well as IL-1β-induced chondrocytes compared with normal chondrocytes (Figure 5(e-g)). We next investigated whether MINCR targeted miR-146a-5p to regulate BMPR2 expression, and we found that both mRNA and protein expression of BMPR2 were inhibited by miR-146a-5p upregulation whereas reversed by MINCR overexpression (Figure 5(h,i)). These findings suggested that BMPR2 acted as a target of miR-146a-5p, and MINCR promoted BMPR2 via targeting miR-146a-5p.
Figure 5.
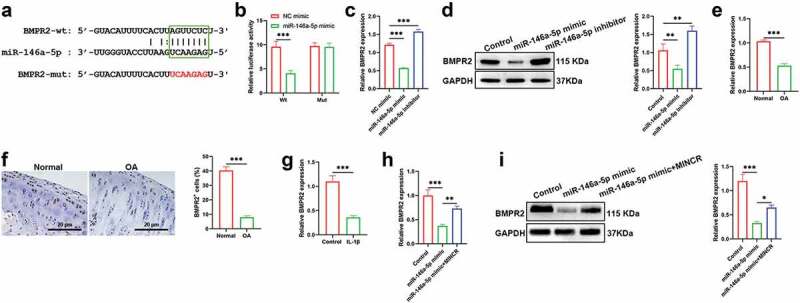
BMPR2 Acts as A Potential Target of miR-146a-5p, And MINCR Promotes BMPR2 via Inhibiting miR-146a-5p. (a) The binding sites of BMPR2 on miR-146a-5p. (b) The luciferase activity was performed after recombination reporter genes which contained wild/mutant type of BMPR2 and miRNA mimics transfected into cells. (c)-(d) The mRNA and protein expression of BMPR2 were detected by qRT-PCR and western blot after being transfected with miR-146a-5p mimic/inhibitor. (e)-(g) The expression of BMPR2 in OA cartilage tissues compared with normal as well as IL-1β-induced chondrocytes compared with normal chondrocytes. (H)-(I) The mRNA and protein expression of BMPR2 were detected by qRT-PCR and western blot after being transfected with miR-146a-5p mimic and miR-146a-5p mimic + BMPR2. *P<0.05, **P<0.01, ***P<0.001.
Upregulation of BMPR2 Inverts the Effects of miR-146a-5p on Cell Proliferation, Apoptosis, and ECM-Degeneration in IL-1β-Induced Chondrocytes
We also demonstrated whether miR-146a-5p promoted the OA progression via inhibiting BMPR2. Cell viability analysis indicated that overexpression of BMPR2 promoted cell proliferation, but was inhibited by miR-146a-5p upregulation (Figure 6(a-c)). Besides, the inhibitory effects of BMPR2 overexpression on cell apoptosis were reversed by miR-146a-5p upregulation (Figure 6(d)). Moreover, the expression of SOX9, COL2A1, and Aggrecan was increased whereas expression of ADAMTS-4, MMP3, and MMP13 were suppressed by overexpression of BMPR2, however, the effects of overexpression of BMPR2 on ECM were reversed by miR-146a-5p upregulation (Figure 6(e-i)).
Figure 6.

Upregulation of BMPR2 inverts the effects of miR-146a-5p on cell proliferation, apoptosis, and ECM-degeneration in IL-1β-induced chondrocytes. After IL-1β stimulation, IL-1β stimulation + BMPR2, and IL-1β stimulation + miR-146a-5p mimic + MINCR, (a) CCK-8 assay was used to examine cell viability. (b)-(c) EdU/Hocheast33258 double staining was used to detect the living cells and apoptotic cells. (d) Annexin V-FITC/PI was used to analyze apoptotic cells. (e)-(g) QRT-PCR and western blot were used to examine mRNA and protein levels of SOX9, COL2A1, Aggrecan, ADAMTS-4, MMP3, and MMP13. (h)-(i) IF staining was used to detect the levels of ADAMTS-4, MMP13, COL2A1, and Aggrecan. *P < 0.05, **P < 0.01, ***P < 0.001.
Upregulation of MINCR Prevents OA progression in vivo
To determine the function of MINCR in vivo based on the construction of the ACLT rat model. QRT-PCR results indicated that MINCR and BMPR2 significantly decreased whereas miR-146a-5p increased in OA mice; however, the expression of MINCR, BMPR2, and miR-146a-5p were reversely regulated by MINCR overexpression in OA (Figure 7(a-c,f)). The morphological structure of the distal femur was analyzed by H & E and Safranin O staining, and the severe destruction of cartilage was observed in OA rats, but the destruction of cartilage was alleviated by MINCR overexpression (Figure 7(d, f)). The TUNEL staining also revealed increasing apoptotic chondrocytes in OA; however, apoptosis was inhibited by MINCR overexpression (Figure 7(e-g)). ECM formation/degeneration genes were analyzed, and the results as shown in Figure 8, the expression of SOX9, COL2A1, and Aggrecan was inhibited whereas expression of ADAMTS-4, MMP3, and MMP13 was upregulated in OA, but reversed by MINCR overexpression. These results indicated that MINCR played as a suppressor in OA progression via regulating miR-146a-5p/BMPR2 axis.
Figure 7.
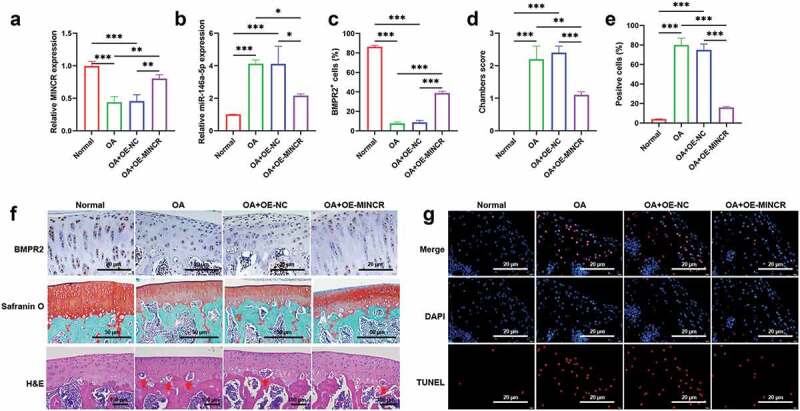
Upregulation of MINCR Prevents pathological alterations in ACLT rat model. (a)-(b) The expression of MINCR and miR-146a-5p was detected by qRT-PCR in normal rats (normal), OA rats (OA), pcDNA3.1-transfected OA rats (OA+OE-NC), MINCR-overexpressed OA rats (OA+OE-MINCR). (d) Column charts of the statistical results of BMPR2 positive cells in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. (e) Column charts of the statistical results of TUNEL positive cells s in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. (f) IHC, Safranin O, and H&E staining were respectively used to examine BMPR2 levels, collagen-containing, and cartilage-degeneration s in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. (g) TUNEL staining was used to examine apoptotic cells in cartilage tissues s in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 8.

Upregulation of MINCR Prevents ECM-degeneration in ACLT rat model. (a)-(b) The mRNA and protein expression of SOX9, COL2A1, Aggrecan, ADAMTS-4, MMP3, and MMP13 were analyzed by qRT-PCR and western blot in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. (c)-(e) IF staining was utilized to detect the levels of ADAMTS-4, MMP13, COL2A1, and Aggrecan in the normal group, OA group, OA+OE-NC group, and OA+OE-MINCR group. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
OA is a global aggressive chronic joint disease among older adults, which characteristics of cartilage degeneration and inflammation of the joint [34]. Emerging evidence has revealed that ncRNAs, including lncRNAs, circular RNAs (circRNAs), and miRNAs are closely related to the occurrence of several diseases [35]. Abundant studies have demonstrated that lncRNAs act vital roles in OA progression via regulating chondrocyte proliferation, apoptosis, autophagy, ECM formation, and inflammatory activity and response [36–38]. However, there is a large number of lncRNAs and their regulatory mechanism are still unknown in OA.
In the present study, we found MINCR and BMPR2 downregulated whereas miR-146a-5p increased in OA cartilage tissues compared with control as well as IL-1β-induced chondrocytes compared with normal chondrocytes. MINCR is a novel lncRNA, and little research is about the role and mechanism analysis of MINCR. MINCR has been found that exerts itself as an oncogene in multiple solid cancers to promote tumor initiation and development, for instance, MINCR promotes nasopharyngeal carcinoma irradiation resistance via inhibiting miR-223 to increase ZEB1 expression [17]. MINCR contributes to tumor growth by regulating the miR-126/SLC7A5 axis in non-small cell lung cancer [20]. MINCR accelerates the progression of gallbladder cancer via stimulating EZH2 [39]. MINCR is also associated with tumorous cell aggressive behaviors and poor prognosis in human hepatocellular carcinoma [40]. However, there are rare reports about the role of MINCR in other diseases.
MYC is a transcription factor that controls cell proliferation, differentiation, apoptosis, and embryogenesis [41]. A previous study has indicated that MYC expression promotes chondrocyte active proliferation and type □ collagen synthesis [42]. Moreover, recent research also found that c-MYC converts fibroblasts to chondrocyte-like cells and contributes to chondrocyte proliferation, differentiation, maturation, and bone formation [43]. Despite the controversy surrounding whether MINCR is an MYC-induced lncRNA [44,45], of interest, our results demonstrated upregulation of MINCR contributed to chondrocyte proliferation and upregulated the ECM-formation proteins such as SOX9, COL2A1, and Aggrecan. To sum up, our finding likely supports that MINCR is an MYC-induced lncRNA. There is the first demonstration that the role of MINCR in nonneoplastic disease, which acted as a restorer in OA by increasing chondrocyte proliferation and inhibiting apoptosis and ECM-degeneration.
For mechanism analysis, the FISH results indicated that the MINCR is mainly distributed in the cytoplasm and binds with miR-146a-5p. It is widely known that lncRNA commonly exerts as a competing endogenous (ce) RNA to sponge miRNA while it distributes in the cytoplasm [46]. The cytoplasmic lncRNAs usually regulate cellular structure and functions via forming nuclear lncRNA-associated ribonucleoprotein complexes (lncRNPs) to control transcripts’ stability and translation [47]. Increasing studies highlight the key roles of lncRNAs for regulating the processes of OA via interacting with miRNAs, such as TUG1 accelerates OA progression via inhibiting miR-195 to promote MMP13 expression [48]. SMHG16 has been demonstrated to promote OA occurrence via sponging miR-373-3p [49]. In the present study, we found that MINCR contributed to OA inhibition via targeting miR-146a-5p to promote BMPR2 expression. Therefore, lncRNAs may be used as the therapeutic targets in OA.
Most of lncRNAs influence OA via regulating chondrocytes and ECM, and inflammatory activation and inhibition. We deeply explored how MINCR/miR-146a-5p/BMPR2 axis regulated the OA progression and found that MINCR inhibited OA-degeneration via increasing proliferation while decreasing apoptosis of chondrocytes and prevented ECM-degeneration. It is known to us that the cartilage structure contains the chondrocytes and ECM, and its development includes chondrocytes differentiation from mesenchymal stem cells (MSCs), chondrocyte differentiation and hypertrophy, and cartilage matrix calcification and degeneration [50]. BMPs and their receptors (BMPRs) are present in chondrocytes and adjacent perichondrium that act in vital roles in skeletal development [51]. The previous study indicates that the BMP-Smad4 signaling pathway exerts a vital role to evoke chondrocyte differentiation, moreover, BMPs exert their function via forming a signal-transducing complex by binding to BMPR1 and BMPR2 [52]. Although previous studies have found that BMPR2 induces cartilage formation in vivo and mostly distributes in chondrocyte-like cells in the cartilaginous matrix of human osteochondromas [53,54], we speculated that BMPR2 exerts a critical role promotes chondrocyte differentiation.
Taken together, our findings demonstrated that MINCR promoted proliferation and inhibited apoptosis of chondrocytes, and prevented ECM-degeneration via targeting miR-146a-5p to promote BMPR2 expression. Our results may provide a novel therapeutic target and new insight for OA treatment.
Acknowledgments
We thank Yunnan Labreal biotechnology co., LTD for technical support during this work.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81860833), and Health Scientific Research Project of Kunming Municipal Health Committee (2021-0310-002).
Data availability statement
All data generated from this study are available. The raw data could be obtained by contecting to correspoding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics statement
Our research has been supported by the Ethics Committee of the Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, the clinical samples were collected from the Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine, and all participants have known the purpose of this research and signed the informed consent. Animal experiments were approved by the Ethics Committee of the Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine and performed according to the Institutional Animal Care and Use Committee of the Third Affiliated Hospital of Yunnan University of Traditional Chinese Medicine.
Author contribution
Shunkui Gang conceived and designed the experiments. Xiaoying Wang, Tengda Yi, Lirui Feng, and Mingxing Zhang performed the experiments. Yongsheng He and Mingxing Zhang supplied reagents, materials, and analysis tools. Dongyun Li and Xiaoying Wang wrote the original draft. Shunkui Gang and Yongsheng He contributed to the review and editing.
References
- [1].Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. [DOI] [PubMed] [Google Scholar]
- [3].Griffin TM, Scanzello CR. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37 Suppl 120(5):57–63. [PMC free article] [PubMed] [Google Scholar]
- [4].Xu X, Li X, Liang Y, et al. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med Sci Monit. 2019;25:3146–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Glyn-Jones S, J R Palmer A, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991): 376–387 [DOI] [PubMed] [Google Scholar]
- [6].Luo P, Jiang C, Ji P, et al. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. [DOI] [PubMed] [Google Scholar]
- [8].Moussa M, Lajeunesse D, Hilal G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146–156. [DOI] [PubMed] [Google Scholar]
- [9].Zhang X, Hamblin MH, Yin KJ. The long noncoding RNA Malat1: its physiological and pathophysiological functions. RNA Biol. 2017;14(12):1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang Y, Wang F, Chen G, et al. LncRNA MALAT1 promotes osteoarthritis by modulating miR-150-5p/AKT3 axis. Cell Biosci. 2019;9(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jiang M, Liu J, Luo T, et al. LncRNA PACER is down-regulated in osteoarthritis and regulates chondrocyte apoptosis and lncRNA HOTAIR expression. Biosci Rep. 2019;39(6). DOI: 10.1042/BSR20190404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang T, Wang J, Zhou Y, et al. LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci Rep. 2019;39(5). DOI: 10.1042/BSR20182454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shan Y, Ma J, Pan Y, et al. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis. 2018;9(7):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tian F, Junhu W, Zhanhua Z, et al. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol Res. 2020;53(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu J, Yao L, Zhang M, et al. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY). 2019;11(18):7830–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang T, Liu Y, Wang Y, et al. Long non-coding RNA XIST promotes extracellular matrix degradation by functioning as a competing endogenous RNA of miR-1277-5p in osteoarthritis. Int J Mol Med. 2019;44(2):630–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhong Q, Chen Y, Chen Z. LncRNA MINCR regulates irradiation resistance in nasopharyngeal carcinoma cells via the microRNA-223/ZEB1 axis. Cell Cycle. 2020;19(1):53–66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [18].Lyu Q, Jin L, Yang X, et al. LncRNA MINCR activates Wnt/β-catenin signals to promote cell proliferation and migration in oral squamous cell carcinoma. Pathol Res Pract. 2019;215(5):924–930. [DOI] [PubMed] [Google Scholar]
- [19].Cao J, Zhang D, Zeng L, et al. Long noncoding RNA MINCR regulates cellular proliferation, migration, and invasion in hepatocellular carcinoma. Biomed Pharmacother. 2018;102:102–106. [DOI] [PubMed] [Google Scholar]
- [20].Wang J, Ding M, Zhu H, et al. Up-regulation of long noncoding RNA MINCR promotes non-small cell of lung cancer growth by negatively regulating miR-126/SLC7A5 axis. Biochem Biophys Res Commun. 2019;508(3):780–784. [DOI] [PubMed] [Google Scholar]
- [21].Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wieczorek E, Reszka E. mRNA, microRNA and lncRNA as novel bladder tumor markers. Clin Chim Acta. 2018;477:141–153. [DOI] [PubMed] [Google Scholar]
- [23].Skrzypa M, Szala D, Gablo N, et al. miRNA-146a-5p is upregulated in serum and cartilage samples of patients with osteoarthritis. Pol Przegl Chir. 2019;91(3):1–5. [DOI] [PubMed] [Google Scholar]
- [24].Sun T, Li X, Song H, et al. MiR-146a aggravates LPS-induced inflammatory injury by targeting CXCR4 in the articular chondrocytes. Cell Physiol Biochem. 2017;44(4):1282–1294. [DOI] [PubMed] [Google Scholar]
- [25].Zhang X, Wang C, Zhao J, et al. miR-146a facilitates osteoarthritis by regulating cartilage homeostasis via targeting Camk2d and Ppp3r2. Cell Death Dis. 2017;8(4):e2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schmal H, Mehlhorn AT, Pilz IH, et al. Immunohistological localization of BMP-2, BMP-7, and their receptors in knee joints with focal cartilage lesions. ScientificWorldJournal. 2012;2012:467892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–241. [DOI] [PubMed] [Google Scholar]
- [28].Yang Z, Li R, Ao J, et al. miR-1307-3p suppresses the chondrogenic differentiation of human adipose-derived stem cells by targeting BMPR2. Int J Mol Med. 2018;42(6):3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xiao Y, Yan X, Yang Y, et al. Downregulation of long noncoding RNA HOTAIRM1 variant 1 contributes to osteoarthritis via regulating miR-125b/BMPR2 axis and activating JNK/MAPK/ERK pathway. Biomed Pharmacother. 2019;109:1569–1577. [DOI] [PubMed] [Google Scholar]
- [30].Lin C, Liangliang L, Chun Z, et al. Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12. Bone Res. 2019;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(3):S17–23. [DOI] [PubMed] [Google Scholar]
- [32].Hu J, Jinyi Z, Jinting W, et al. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J Ethnopharmacol. 2020;247:112261. [DOI] [PubMed] [Google Scholar]
- [33].Hayami T, Pickarski M, Zhuo Y, et al. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. [DOI] [PubMed] [Google Scholar]
- [34].Barnett R. Osteoarthritis. Lancet. 2018;391(10134):1985. [DOI] [PubMed] [Google Scholar]
- [35].Wang J, Zhu S, Meng N, et al. ncRNA-encoded peptides or proteins and cancer. Mol Ther. 2019;27(10):1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen K, Hao Z, Min-Qian Z, et al. LncRNA MEG3 inhibits the degradation of the extracellular matrix of chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 axis. Cartilage. 2019;13:1947603519855759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].He B, Jiang D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. 2020;44(2):524–535. [DOI] [PubMed] [Google Scholar]
- [38].Zheng J, Li Q. Methylene blue regulates inflammatory response in osteoarthritis by noncoding long chain RNA CILinc02. J Cell Biochem. 2019;120(3):3331–3338. [DOI] [PubMed] [Google Scholar]
- [39].Wang SH, Yang Y, Wu X-C, et al. Long non-coding RNA MINCR promotes gallbladder cancer progression through stimulating EZH2 expression. Cancer Lett. 2016;380(1):122–133. [DOI] [PubMed] [Google Scholar]
- [40].Jin XL, Lian JR, Guan YH. Overexpression of long non-coding RNA MINCR contributes to progressive clinicopathological features and poor prognosis of human hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(23):8197–8202. [DOI] [PubMed] [Google Scholar]
- [41].Henriksson M, Lüscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109–182. [DOI] [PubMed] [Google Scholar]
- [42].Quarto R, Dozin B, Tacchetti C, et al. Constitutive myc expression impairs hypertrophy and calcification in cartilage. Dev Biol. 1992;149(1):168–176. [DOI] [PubMed] [Google Scholar]
- [43].Shi JW, Zhang -T-T, Liu W, et al. Direct conversion of pig fibroblasts to chondrocyte-like cells by c-Myc. Cell Death Discov. 2019;5(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hart JR, Weinberg MS, Morris KV, et al. MINCR is not a MYC-induced lncRNA. Proc Natl Acad Sci U S A. 2016;113(5):E496–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Doose G, Hoffmann S, Iaccarino I. Reply to Hart et al.: MINCR and MYC: more than expression correlation. Proc Natl Acad Sci U S A. 2016;113(5):E498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol. 2016;1402:271–286. [DOI] [PubMed] [Google Scholar]
- [47].Noh JH, Kim KM, McClusky WG, et al. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip Rev RNA. 2018;9(3):e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tang LP, Ding JB, Liu ZH, et al. LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev Med Pharmacol Sci. 2018;22(24):8574–8581. [DOI] [PubMed] [Google Scholar]
- [49].Fan H, Ding L, and Yang Y. lncRNA SNHG16 promotes the occurrence of osteoarthritis by sponging miR‑373‑3p. Mol Med Rep. 2021;23(2):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhu J, Yu W, Wang Y, et al. lncRNAs: function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res Ther. 2019;10(1):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kobayashi T, Lyons KM, McMahon AP, et al. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102(50):18023–18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Reddi AH. Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN. Arthritis Res. 2001;3(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nakase T, Myoui A, Shimada K, et al. Involvement of BMP-2 signaling in a cartilage cap in osteochondroma. J Orthop Res. 2001;19(6):1085–1088. [DOI] [PubMed] [Google Scholar]
- [54].Cuellar A, Inui A, James MA, et al. Immunohistochemical localization of bone morphogenetic proteins (BMPs) and their receptors in solitary and multiple human osteochondromas. J Histochem Cytochem. 2014;62(7):488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated from this study are available. The raw data could be obtained by contecting to correspoding author.


