Abstract
Background Context:
Individuals living with a spinal cord injury (SCI) are at heightened risk for a number of chronic health conditions such as secondary comorbidities that may develop or be influenced by the injury, the presence of impairment, and/or the process of aging.
Purpose:
The objective of this study was to compare the incidence of and adjusted hazards for cardiovascular and metabolic (cardiometabolic) morbidities among adults following SCI compared to adults without SCIs.
Study Design/Setting:
Longitudinal cohort from a nationwide insurance claims database.
Patient Sample:
Privately-insured beneficiaries were included if they had an ICD-9-CM diagnostic code for traumatic SCI (n=9,081). Adults without SCI were also included (n=1,474,232).
Outcome Measures and Methods:
Incidence estimates of common cardiometabolic morbidities were compared at 4-years of enrollment. Survival models were used to quantify unadjusted and adjusted hazard ratios for incident cardiometabolic morbidities.
Results:
Adults living with traumatic SCIs had a higher 5-year incidence of any cardiometabolic morbidities (56.2% vs. 36.4%) as compared to adults without SCI, and differences were to a clinically meaningful extent. Survival models demonstrated that adults with SCI had a greater hazard for any cardiometabolic morbidity (Hazard Ratio [HR]: 1.67; 95%CI: 1.58, 1.76) and all cardiometabolic disorders; this ranged from HR: 1.45 (1.32, 1.59) for non-alcoholic fatty liver disease to HR: 3.55 (3.36, 3.76) for heart failure.
Conclusions:
Adults with SCIs have a significantly higher incidence of and risk for common cardiometabolic morbidities, as compared to adults without SCIs. Efforts are needed to facilitate the development of improved clinical screening algorithms and early interventions to reduce risk of cardiometabolic disease onset/progression in this vulnerable population.
Keywords: spinal cord injury, cardiovascular disease, diabetes, metabolic disease, hypertension
Introduction
Having a spinal cord injury (SCI) may increase the risk of developing a health condition that is directly linked to the impairment (e.g., neurogenic bladder, etc.) or occurs as an indirect consequence of the impairment itself (e.g., increases in sedentary behaviors that contribute to the conditions such as sarcopenia and osteoporosis1). Through these pathways, living with SCI places individuals at risk of experiencing accelerated age-related chronic health conditions or comorbidities,2–4 and at the population level, represents an extremely high burden of disease.5
As people with SCI age, a wide range of secondary conditions arise, including decreased bone mineral density and osteoporosis,6 increased visceral adiposity,7,8 muscle atrophy and sarcopenia,9,10 impaired glucose tolerance and insulin resistance,11 and decreased physical activity participation and exaggerated sedentary behaviors.12,13 These factors place individuals with SCIs at an accelerated risk for age-related secondary chronic conditions such osteoporosis, diabetes, the metabolic syndrome, and primary cardiovascular disease.6,14,15 Despite the well-established literature pertaining to the interrelationship between age-related cardiometabolic diseases and multimorbidity in the non-SCI population,16–18 the extent to which cardiometabolic conditions arise after SCI has not been studied at the population level. Previous studies have largely been limited to small, cross-sectional clinical studies, and there is thus a lack of information pertaining to the natural history and aging trajectories of chronic cardiometabolic diseases among adults with SCI. Such information is needed not only to facilitate the development of appropriate clinical screening algorithms for this population, but also for the design of targeted, early interventions to reduce risk of disease progression and multimorbidity. Our central hypotheses are that individuals living with traumatic SCI will have higher incidence and shorter time to onset of cardiometabolic morbidities than individuals without SCI.
Methods
Data Source
This is a retrospective cohort study of adults with SCI whose diagnosis could have existed across any patient care setting. This study used a national, private insurance claims database, Clinformatics DataMart Database (OptumInsight, Eden Prairie, MN). This is a de-identified administrative claims database of over 80 million adults and children with commercial insurance representing those on a single, large U.S. private payer who had both medical and pharmacy coverage throughout the enrollment. Enrolled beneficiaries’ emergency department, outpatient, and inpatient encounters are captured. This study was deemed exempt by the Institutional Review Board at the researchers’ institution.
Sample Selection
All individuals 18 years of age and older at the time of their enrollment which could start from 2007 to 2017 were potentially eligible for this analysis. We excluded individuals with less than 12 months of continuous enrollment to require sufficient claim history. All medical claims excluding laboratory and outpatient pharmacy was considered to identify prevalence or treatment for these conditions during the enrollment period.
Identification of Patients with a Spinal Cord Injury
All members with a diagnosis of SCI were identified using International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) (Supplementary Table S1). Members that had SCI prior to 2007 were excluded due to poorer coverage of diagnosis codes during 2001 to 2006 in the database. Members without a diagnosis code in any position when they were 18 years or older during enrollment were excluded. To allow adequate longitudinal follow up for all patients with SCI, only those that had four or more continuous years of enrollment following enrollment following their first SCI diagnosis date within the study period were included.
A comparison cohort of controls without SCI were also identified using the same aforementioned inclusion criteria. Additional exclusion criteria for identifying the control cohort included removal of any individual with other physically disabling neurological disorders (e.g., non-SCI paraplegia, non-SCI quadriplegia, non-SCI hemiplegia, cerebral palsy, spina bifida, and multiple sclerosis). Among remaining members without SCI, a 20% simple random sample of members was selected to represent the control group. Post-hoc analyses of demographic characteristics were compared between the 20% sample of controls and all controls to ensure no bias in control cohort attributable to random selection. Figure 1 shows the flow of subject inclusion and exclusion for final case and control cohorts.
Figure 1.
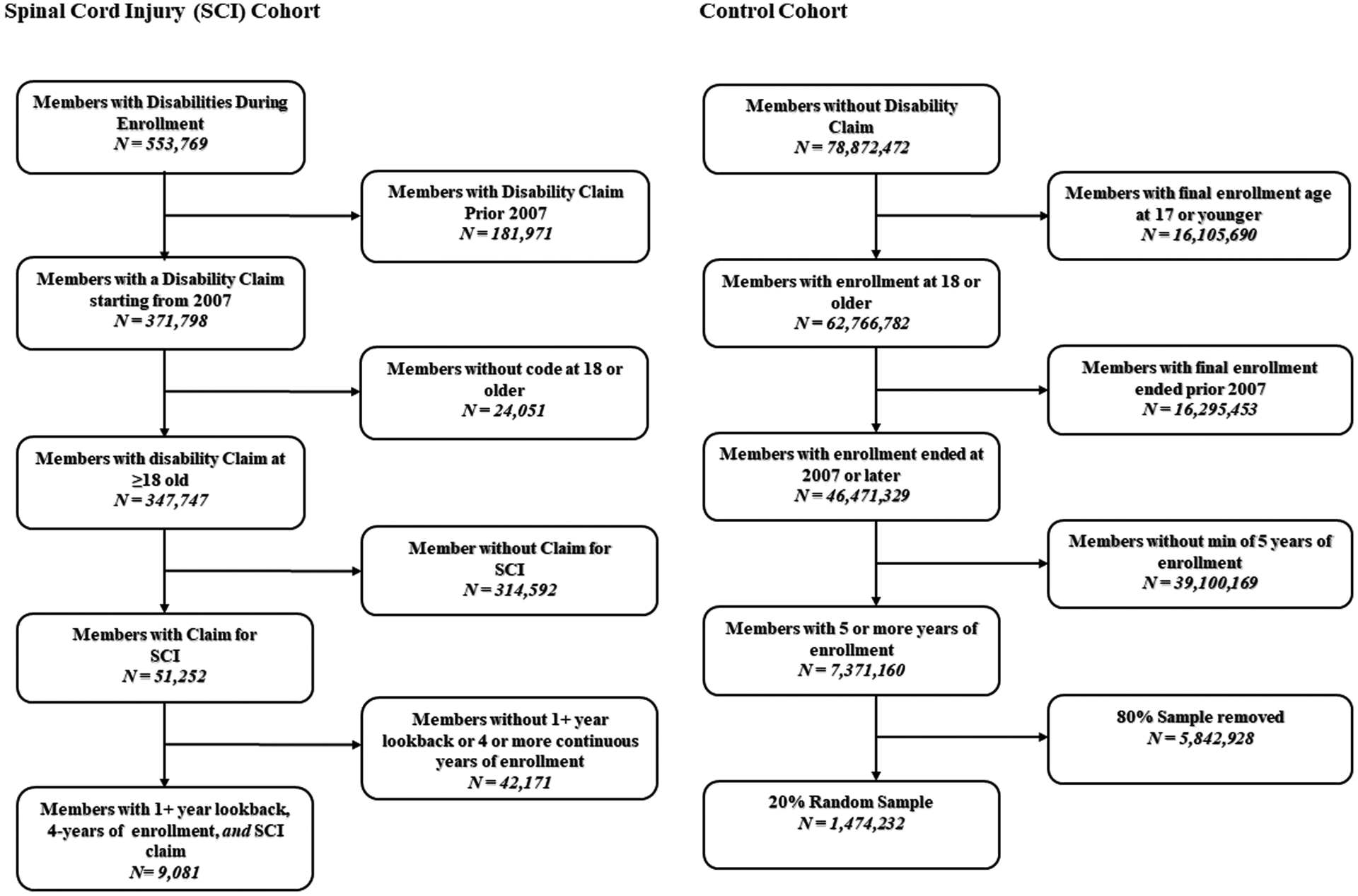
Flow chart of subject inclusion and exclusion for final case and control cohorts.
Cardiometaboic Morbidities
Physician-diagnosed cardiometabolic health disorders were identified based on a single encounter that included at least one of pertinent ICD-9 or ICD-10 codes (in any position) (see Supplementary Table S1). The primary outcome was time in days to any incident cardiometabolic morbidity following enrollment on the plan. Secondary outcomes were component incident cardiometabolic morbidity, including: (1) cardiac dysrhythmias, (2) heart failure, (3) peripheral and visceral atherosclerosis, (4) non-alcoholic fatty liver disease, (5) chronic kidney disease, (6) type 2 diabetes, (7) hypercholesterolemia, and (8) hypertension.
Covariates
Explanatory covariates included age group split into three categories (18–44, 45–64, 65 or older), sex, race, educational attainment, household net worth, and a modified Elixhauser comorbidity index. The Elixhauser comorbidity index was modified to remove nine conditions that would be correlated with incident cardiometabolic morbidity: congestive heart failure, cardiac arrhythmia, valvular disease, peripheral vascular disorders, complicated and uncomplicated hypertension, uncomplicated and complicated diabetes, and renal failure. Therefore, the revised index only considers 22 comorbidities (Supplemental Table S3).
Statistical Analysis
Bivariate analyses of baseline demographic characteristics between patients with SCI and controls were examined. For categorical variables, column percentages were compared between both groups using effect size calculations with Cohen’s h. The Cohen’s h effect size calculation was used since, due to large sample sizes, being statistically overpowered would not provide clinically meaningful differences in proportions between groups. For continuous variables, means and standard deviations as well as medians with upper and lower bounds on interquartile ranges were calculated. Cohen’s d standardized mean differences were calculated for continuous variables to ascertain clinically meaningful differences between groups.
To capture full comorbidity history within the study period, all patients with sufficient continuous enrollment within the study period of five years were retained to enable sufficient follow-up. For the SCI cohort, we used a one year look back period prior to first SCI diagnosis date to capture comorbidity history and to examine if any prevalent musculoskeletal outcomes existed.
To examine disease-free survival of individuals with SCI compared to controls, those patients that had no evidence of composite cardiometabolic morbidity in each group were plotted using Kaplan-Meier product limit survival curves for a three-year period. To establish incidence in claims, we used a one-year lookback period from the index date in each group to obtain evidence of any service utilization with a diagnosis of any cardiometabolic morbidity. These patients were excluded from the product-limit survival curves and other subsequent analyses.
To estimate the unadjusted and adjusted hazard of the composite and each cardiometabolic morbidity, a series of survival models were developed. For each cardiometabolic morbidity, all patients that had evidence of the specific cardiometabolic morbidity were excluded from the model. For example, if depression was being considered as the incident outcome, all patients with prevalent diabetes in the one year prior to the index date would be excluded from the model. Therefore, sample sizes of patients included for each outcome varied based on evidence of prevalent disease in the one year prior to the index date. Survival models were then used to quantify unadjusted and adjusted hazard ratios for each incident cardiometabolic morbidity. Appropriate survival models were based on distributional assumptions that included testing Weibull, lognormal, exponential, gamma, logistic, loglog, and Normal distribution with respect to the follow-up in days by minimizing critical model fit statistics. Critical assessment of Akaiki Information Criterion (AIC) was used as a basis for minimization amongst all candidate distributions. Use of the parametric Weibull regression for incident cardiometabolic outcome was applied stepwise. To examine the effects of incremental adjustment on the exposure variable (SCI), a series of models for each cardiometabolic outcome was evaluated. All patients were right censored if they did not experience the outcome in the follow-up period or disenrolled from the plan. Both unadjusted and all adjusted hazard ratios and 95% confidence intervals for the exposure to SCI were calculated.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Statistical testing was two-tailed with a significance level of 0.05 and effect sizes used a 0.2 meaningful difference cutoff.
Results
The mean time in the plan for eligible enrollees was 10.8 (25th Percentile: 8.0; 75th Percentile: 13.0) and 8.5 (25th Percentile: 6.0; 75th Percentile: 10.3) years for patients with SCI vs controls, respectively (Table 1). Adults living with SCI had a higher 4-year incidence of any cardiometabolic morbidity (56.2% vs. 36.4%) as compared to adults without SCI, and differences were to a clinically meaningful extent. Moreover, adults with SCI had significantly higher incidence of all of the cardiometabolic outcomes, including cardiac dysrhythmias (34.8% vs. 16.5%), heart failure (16.9% vs. 4,9%), peripheral and visceral atherosclerosis (24.7% vs. 8.0%), non-alcoholic fatty liver disease (5.3% vs. 3.6%), chronic kidney disease (16.2% vs. 6.3%), type 2 diabetes (15.9% vs. 9.2%), hypercholesterolemia (25.5% vs. 16.9%), and hypertension (43.7% vs. 24.8%), as compared to adults without SCI (all P<.01 and SMD≥0.2) (Table 2).
Table 1.
Descriptive characteristics among adults with SCI (case) or without SCI (control).
| Case | Control | |
|---|---|---|
| Overall | 9,081 (100%) | 1,474,232 (100%) |
| Full Enrollment Length | ||
| Mean (SD) | 10.8 (3.4) | 8.5 (3.2) |
| Median (Q1-Q3) | 10.3 (8.0–13.0) | 7.6 (6.0–10.3) |
| Years Post Eligibility Start Date † | ||
| Mean (SD) | 6.0 (1.7) | 5.3 (1.5) |
| Median (Q1-Q3) | 5.6 (4.7–7.0) | 4.7 (4.2–5.8) |
| Age Group | ||
| 18–44 | 1384 (15.2%) | 542106 (36.8%) |
| 45–64 | 2547 (28.0%) | 512676 (34.8%) |
| 65 or Older | 5150 (56.7%) | 419450 (28.5%) |
| Gender | ||
| Female | 5252 (57.8%) | 774282 (52.5%) |
| Male | 3829 (42.2%) | 699950 (47.5%) |
| Race | ||
| Asian | 263 (2.9%) | 56134 (3.8%) |
| Black | 637 (7.0%) | 117545 (8.0%) |
| Hispanic | 718 (7.9%) | 129689 (8.8%) |
| Unknown | 1758 (19.4%) | 285255 (19.3%) |
| White | 5705 (62.8%) | 885609 (60.1%) |
| Education | ||
| <High School Diploma | 58 (0.6%) | 8418 (0.6%) |
| High School Diploma | 2386 (26.3%) | 354512 (24.0%) |
| <Bachelor Degree | 4999 (55.0%) | 784246 (53.2%) |
| Bachelor Degree | 1451 (16.0%) | 284927 (19.3%) |
| Unknown/Missing | 187 (2.1%) | 42129 (2.9%) |
| Net Worth | ||
| Unknown | 1554 (17.1%) | 250582 (17.0%) |
| <$25K | 1479 (16.3%) | 222644 (15.1%) |
| $25K-$149K | 1622 (17.9%) | 258445 (17.5%) |
| $150K-$249K | 886 (9.8%) | 151485 (10.3%) |
| $250K-$499K | 1376 (15.2%) | 245106 (16.6%) |
| ≥$500K | 2164 (23.8%) | 345970 (23.5%) |
All adults with SCI have their Index Date set the same as start of eligibility date (start of 2007, year when turned 18, or enrollment start date, whichever was the latest)
Table 2.
Incidence of any and all cardiometabolic morbidities among adults with and without SCI with one-year clean enrollment period.
| †No Outcome at Baseline | ||
|---|---|---|
| Case/Denominator | Control/Denominator | |
| Any Cardiometabolic Morbidity | 1527/2718 (56.2%)* | 328752/904321 (36.4%) |
| Cardiac dysrhythmias | 2477/7112 (34.8%)* | 225021/1365803 (16.5%) |
| Heart Failure | 1393/8259 (16.9%)* | 70573/1444810 (4.9%) |
| Peripheral and visceral atherosclerosis | 2001/8098 (24.7%)* | 113708/1429279 (8.0%) |
| Non-Alcoholic Fatty Liver Disease | 472/8938 (5.3%)* | 52000/1462013 (3.6%) |
| Chronic kidney disease | 1341/8269 (16.2%)* | 90704/1433320 (6.3%) |
| Type 2 Diabetes | 1115/7032 (15.9%)* | 120202/1305982 (9.2%) |
| Hypercholesterolemia | 1824/7148 (25.5%)* | 219222/1300144 (16.9%) |
| Hypertension | 1636/3741 (43.7%)* | 254523/1028251 (24.8%) |
P<.01 and standard mean difference (SMD) ≥0.2
Denominators for both cases and controls reflect a one-year clean period during their enrollment for the specific condition. For instance, among cases (SCI), there exist 7,112 patients whose first year of enrollment had no evidence of Cardiac dysrhythmias; therefore, inferred incident Cardiac dysrhythmias could be estimated for this subset of the full SCI cohort. As a result, all patient cohorts’ denominators dynamically change conditional on the incident outcome being measured to ensure a clean period in the first year of enrollment.
A Kaplan-Meier curve for the unadjusted disease-free survival for any cardiometabolic morbidity in adults with SCI and controls are demonstrated in Figure 2. Unadjusted survival models demonstrated a robust increased hazard ratio (HR) for each of the incident cardiometabolic morbidities among adults with SCI, and ranged from HR: 1.45 (1.32, 1.59) for non-Alcoholic fatty liver disease to HR: 3.55 (3.36, 3.76) for heart failure (all p<0.001). Fully adjusted survival models demonstrated that adults with SCI had a greater hazard for any cardiometabolic morbidity (HR: 1.29; 95%CI: 1.22–1.36) (Supplemental Table S3), and all but three cardiometabolic disorders (chronic kidney disease, type 2 diabetes, and hypercholesterolemia), and ranged from HR: 1.23 (1.17, 1.30) for hypertension to HR: 1.37 (1.31, 1.44) for peripheral and visceral atherosclerosis (Table 3).
Figure 2.
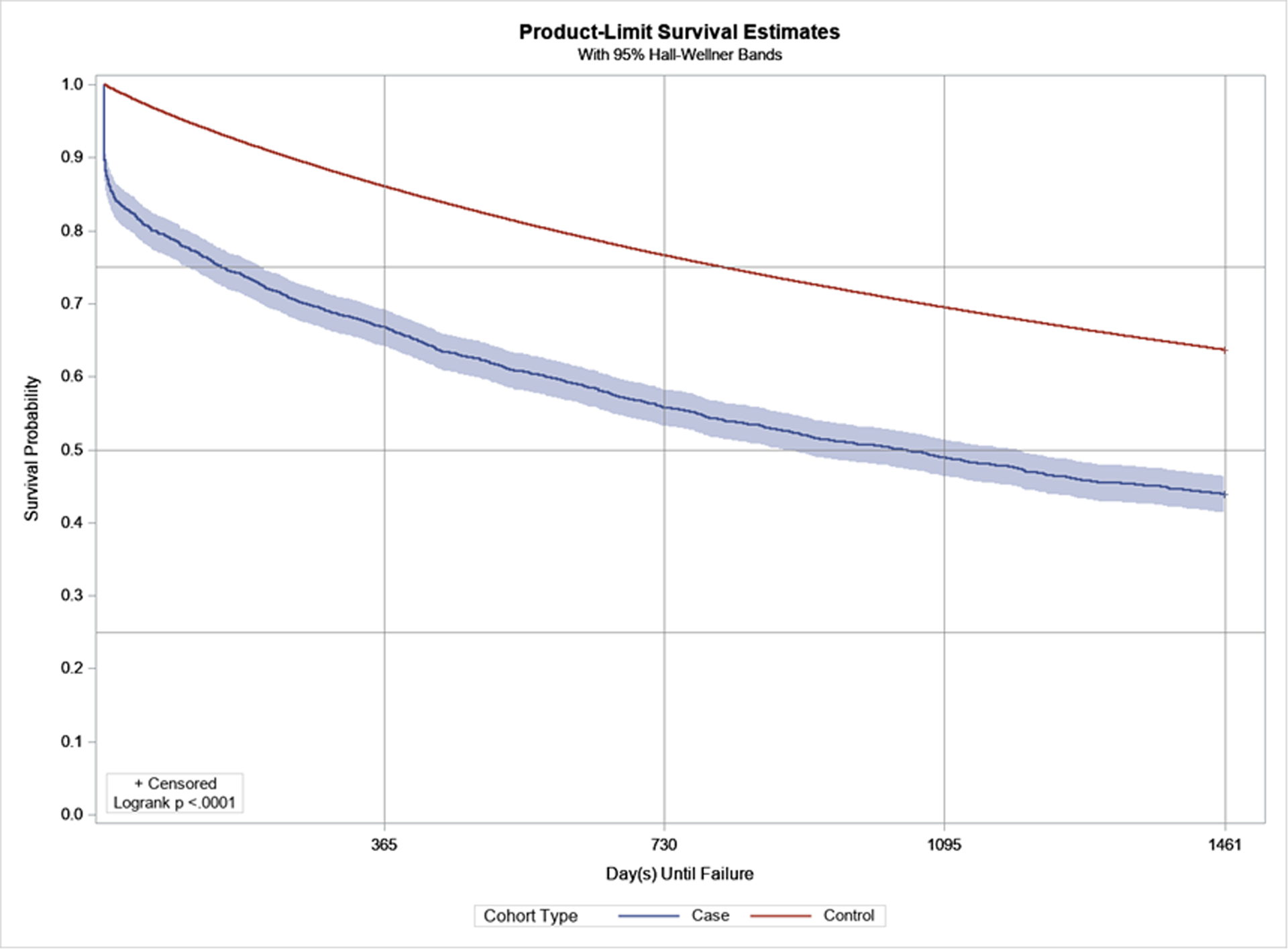
Disease-free survival and Kaplan-Meier product-limit survival curves (3-year) for adults with SCI (blue) and without SCI (red), for any cardiometabolic morbidity.
Table 3.
Survival models with parametric Weibull regression was completed stepwise for each incident cardiometabolic outcome to examine the effects of incremental adjustment on the exposure variable (SCI).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Any Cardiometabolic Morbidity | 1.67 (1.58, 1.76)*** | 1.49 (1.41, 1.58)*** | 1.31 (1.23, 1.38)*** | 1.29 (1.22, 1.36)*** |
| Cardiac dysrhythmias | 2.24 (2.15, 2.34)*** | 1.64 (1.57, 1.71)*** | 1.36 (1.30, 1.41)*** | 1.35 (1.29, 1.41)*** |
| Heart Failure | 3.55 (3.36, 3.76)*** | 1.80 (1.70, 1.90)*** | 1.34 (1.27, 1.42)*** | 1.35 (1.28, 1.43)*** |
| Peripheral and visceral atherosclerosis | 3.38 (3.23, 3.54)*** | 1.74 (1.66, 1.82)*** | 1.37 (1.31, 1.43)*** | 1.37 (1.31, 1.44)*** |
| Non-Alcoholic fatty liver disease | 1.45 (1.32, 1.59)*** | 1.22 (1.11, 1.33)*** | 0.84 (0.77, 0.92)*** | 0.84 (0.77, 0.92)*** |
| Chronic kidney disease | 2.64 (2.50, 2.79)*** | 1.24 (1.17, 1.31)*** | 0.99 (0.93, 1.04) | 1.00 (0.94, 1.05) |
| Type 2 Diabetes | 1.72 (1.62, 1.83)*** | 1.16 (1.09, 1.24)*** | 0.99 (0.94, 1.06) | 1.00 (0.94, 1.06) |
| Hypercholesterolemia | 1.53 (1.46, 1.61)*** | 1.08 (1.03, 1.13)** | 0.97 (0.93, 1.02) | 0.97 (0.92, 1.02) |
| Hypertension | 1.82 (1.73, 1.92)*** | 1.39 (1.32, 1.47)*** | 1.25 (1.19, 1.32)*** | 1.23 (1.17, 1.30)*** |
Model 1: Unadjusted
Model 2: Model 1 + Demographic variables (age, sex, race, geographic region).
Model 3: Model 1 + Model 2 + Modified Elixhauser Comorbidity Index
Model 4: Model 1 + Model 2 + Model 3 + Education + Income
As with incidence estimates (Table 2), all survival models used cases (SCI) and control cohorts consistent with Table 2, which required a one-year clean period with no evidence of the condition being measured
P-value < 0.001
Discussion
The principal finding of this study is that adults living with traumatic SCI had a higher incidence and adjusted hazard and of any and all cardiometabolic morbidities than adults without traumatic SCI. Specifically, adults with traumatic SCI have an elevated risk of developing a variety of cardiometabolic morbidities after injury, as compared to the general adult population of privately insured beneficiaries without SCI. Most large scale observational studies tend to use databases that are uniquely tied to the Department of Veterans Affairs or limited to the National SCI Database (NSCID) which only includes patients who received initial SCI rehabilitation in one of SCI Model Systems in the United States.19 This cohort study includes the largest longitudinal sample of adults with traumatic SCI outside the VA and the NSCID data. Moreover, most large administrative claims databases do not contain socioeconomic indicators such as net worth, race, and geographical location making it challenging to gather data on individuals with SCIs. Herein, we provide adjusted hazards and incidence estimates for cardiometabolic morbidity while considering several sociodemographic variables. Lastly, although clinical trials may be considered the “gold standard” in clinical research, cohort studies include broader patient populations, are less costly and are more efficient. In fact, there is little evidence to support the superiority of clinical trials over observational studies.20
Unlike neurogenic bowel and bladder, secondary complications in SCI such as cardiometabolic disease typically develop without overt symptoms and may not be apparent until it is too late to intervene. Younger persons with SCI are a moderate to high risk for long-term hard cardiac events with cited obesity, dyslipidemia and carbohydrate metabolism dysfunction.21 Moreover these cardiac events preferentially affect those with cervical neurological level of injury, indicating a need for lifelong chronic impairment risk assessment that is also semi- specific.21 Although the Consortium of SCI Clinical practice guidelines for cardiometabolic disease management in patients with SCI advocates for early interventions and screening in common cardiometabolic diseases, most of the information is newer as it was released in 2018. Future efforts should better qualify the economic healthcare burden associated with poor screening methods and to advocate for the development of appropriate clinical screening algorithms with implementations of early behavioral interventions and treatments that reduce risk of cardiometabolic morbidities22–24 and to improve health care outcomes. Due to the nature of their disability, individuals living with SCI have much higher levels of sedentary behavior and lower levels of physical activity as compared to the general population. The 2018 Physical Activity Guidelines (PAG) for Americans provides recommendations on amount and intensity of physical activity for the general population to decrease risk for cardiovascular disease. The PAG recommendations for individuals living with chronic disease or disability are similar, with suggestions to adjust to the individual’s ability.25
Limitations and Strengths
This study has several limitations that should be acknowledged. First, the ICD-9 and ICD 10 codes for the traumatic SCI diagnosis do not differentiate between a Complete and Incomplete SCI as defined by the International Standards for Neurological Classification of Spinal Cord injury. This is significant because the completeness of SCI has a pathophysiological difference relating to the functioning balance of the cardiovascular and endocrine system.26,27 Being able to code for completeness of injury and then utilizing only the incomplete SCI codes against the control cohort may be of more accurate comparison for the hazard ratios for each of the incident cardiometabolic morbidities, as an incomplete SCI may be less effected by autonomic dysfunction. Secondly, we cannot rule out time-varying confounding effects since baseline measurements of all covariates were included in the final models. Thus, whether having SCI “causes” an elevated risk for earlier-onset cardiometabolic disease, or if changes in other health parameters (being less active and sedentary a known predictor of cardiometabolic disease risk) themselves, are the cause of worse cardiometabolic health is not apparent. Thus, we were unable to determine if other unmeasured confounding risk factors or competing risks like worsening functional baseline influenced the observed findings.
The Clinformatics DataMart Database only provided private health insurance claim data for study extraction. Patients with SCI lose employer based health coverage due to their disability if presenting at a lower functional level and may be more likely to be on a publically funded health insurance program (e.g., Medicare). Therefore, the data claims sample may be more reflective of a higher functioning, and potentially more affluent segment of the SCI population, as they had to be enrolled in private insurance by purchasing their own insurance through employment or through another eligible family member relationship. Furthermore, the uninsured population waiting for retroactive start dates for their health insurance to become active were not included. Another limitation of this database is the lack of information regarding time since injury for this population with traumatic SCI. The effect of length or duration of SCI on cardiometabolic disease is thus unknown in this cohort of patients.
Administrative claims data may be prone to inaccurate coding of medical diagnoses in two major ways which may have an effect on our incidence estimates. Validation studies have shown that using more than one claim for a medical condition improves the ability to identify beneficiaries with that medical condition,28 but because the medical condition of SCI is unique, it is important to note that the same billing codes were utilized to mean the same outcome for both groups, yet the diagnostic criteria of cardiometabolic in the SCI population differ from that of the able bodied population. For example, the American Heart Association defines hypertension as elevated blood pressure greater than 130/85 mmHg or use of medication for hypertension as two ways to meet the criteria for diagnosis. Commonly used anti-spasticity medications like Clonidine and/or Tizanidine are anti-hypertensive medications on label with the FDA and may inadvertently decrease a patient’s blood pressure to a normal range, masking the clinical diagnostic ability of the provider.
However, the accuracy of identifying medical conditions depends on the number of years for the study period and the medical condition examined. A strength in the study design is the length of the study period. All patients with sufficient continuous enrollment within the period of four years were retained. This period length is important because it ensures that the SCI cohort is not a mix of acute and chronic SCIs, but only chronic SCIs. Chronic SCI patients reach a homeostasis near the 12–16 month mark regarding recovery and therefore motor, sensory and autonomic dysfunction changes plateau.29 The SCI cohort included a one year look back period as well which ensured the chronic SCI definition is met so as to not confound the outcomes.
Conclusion
In conclusion, adults with SCI have an elevated risk of developing a variety of cardiometabolic morbidities compared to the general adult population of privately insured beneficiaries without SCI. Individuals with SCI frequently utilize healthcare services as part of their routine clinical care. Therefore, increasing clinical awareness of the secondary health disorders experienced and risks among adults with SCI, improving clinical screening strategies, and developing efficient referral resources for coordinated care may help reduce the burden of cardiovascular and metabolic health disorders in this high need population.
Supplementary Material
Footnotes
Supplemental Table S1. Diagnostic codes for SCI, and all cardiometabolic morbidities using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Supplemental Table S2. Modified Elixhauser Index.
Supplemental Table S3. Fully adjusted survival model for any cardiometabolic morbidity.
References
- 1.Rodriguez G, Berri M, Lin P, Kamdar N, Mahmoudi E, Peterson MD. Musculoskeletal morbidity following spinal cord injury: A longitudinal cohort study of privately-insured beneficiaries. Bone. 2020;142:115700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen MP, Molton IR, Groah SL, et al. Secondary health conditions in individuals aging with SCI: terminology, concepts and analytic approaches. Spinal Cord. 2012;50(5):373–378. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoudi E, Lin P, Peterson MD, Meade MA, Tate DG, Kamdar N. Traumatic Spinal Cord Injury and Risk of Early and Late Onset Alzheimer’s Disease and Related Dementia: Large Longitudinal Study. Arch Phys Med Rehabil. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson MD, Kamdar N, Chiodo A, Tate DG. Psychological Morbidity and Chronic Disease Among Adults With Traumatic Spinal Cord Injuries: A Longitudinal Cohort Study of Privately Insured Beneficiaries. Mayo Clin Proc. 2020;95(5):920–928. [DOI] [PubMed] [Google Scholar]
- 5.Hall OT, McGrath RP, Peterson MD, et al. The Burden of Traumatic Spinal Cord Injury in the United States: Disability-Adjusted Life Years. Arch Phys Med Rehabil. 2019;100(1):95–100. [DOI] [PubMed] [Google Scholar]
- 6.Gifre L, Vidal J, Carrasco JL, et al. Risk factors for the development of osteoporosis after spinal cord injury. A 12-month follow-up study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(9):2273–2280. [DOI] [PubMed] [Google Scholar]
- 7.Cirnigliaro CM, LaFountaine MF, Dengel DR, et al. Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring). 2015;23(9):1811–1817. [DOI] [PubMed] [Google Scholar]
- 8.Gorgey AS, Mather KJ, Gater DR. Central adiposity associations to carbohydrate and lipid metabolism in individuals with complete motor spinal cord injury. Metabolism. 2011;60(6):843–851. [DOI] [PubMed] [Google Scholar]
- 9.Castro MJ, Apple DF, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol O. 1999;80(4):373–378. [DOI] [PubMed] [Google Scholar]
- 10.Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. Journal of applied physiology. 2004;96(2):561–565. [DOI] [PubMed] [Google Scholar]
- 11.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24(4):266–277. [DOI] [PubMed] [Google Scholar]
- 12.Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part II: activity types, intensities, and durations. Arch Phys Med Rehabil. 2010;91(5):729–733. [DOI] [PubMed] [Google Scholar]
- 13.Ginis KA, Latimer AE, Arbour-Nicitopoulos KP, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part I: demographic and injury-related correlates. Arch Phys Med Rehabil. 2010;91(5):722–728. [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86(2):142–152. [DOI] [PubMed] [Google Scholar]
- 15.Gater DR Jr., Farkas, Berg AS, Castillo C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2019;42(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajan KB, Arvanitakis Z, Lynch EB, et al. Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology. 2016;87(16):1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes care. 2001;24(6):1069–1078. [DOI] [PubMed] [Google Scholar]
- 18.Kimbro LB, Mangione CM, Steers WN, et al. Depression and all-cause mortality in persons with diabetes mellitus: are older adults at higher risk? Results from the Translating Research Into Action for Diabetes Study. Journal of the American Geriatrics Society. 2014;62(6):1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith B, Evans C, Ullrich P, et al. Using VA Data for Research in Persons With Spinal Cord Injuries and Disorders. J Rehabil Res Dev. 2010;47(8):679–688. [DOI] [PubMed] [Google Scholar]
- 20.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–1886. [DOI] [PubMed] [Google Scholar]
- 21.Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80. [DOI] [PubMed] [Google Scholar]
- 22.Nash MS, Groah SL, Gater DR, et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury. J Spinal Cord Med. 2019;42(5):643–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash MS, Groah SL, Gater DR Jr., et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. Top Spinal Cord Inj Rehabil. 2018;24(4):379–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey DP, Withers TM, Goosey-Tolfrey VL, et al. Acute effects of breaking up prolonged sedentary time on cardiovascular disease risk markers in adults with paraplegia. Scand J Med Sci Sports. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claus-Walker J, Halstead LS. Metabolic and endocrine changes in spinal cord injury: II (section 2). Partial decentralization of the autonomic nervous system. Arch Phys Med Rehabil. 1982;63(11):576–580. [PubMed] [Google Scholar]
- 27.Claus-Walker J, Halstead LS. Metabolic and endocrine changes in spinal cord injury: II (section 1). Consequences of partial decentralization of the autonomic nervous system. Arch Phys Med Rehabil. 1982;63(11):569–575. [PubMed] [Google Scholar]
- 28.Reeves S, Garcia E, Kleyn M, et al. Identifying Sickle Cell Disease Cases Using Administrative Claims. Acad Pediatr. 2014;14((5 Suppl)):S61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Consortium for Spinal Cord M. Outcomes following traumatic spinal cord injury: clinical practice guidelines for health-care professionals. J Spinal Cord Med. 2000;23(4):289–316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


