Abstract
The virulence plasmid-borne genes encoding Yersinia adhesin A (YadA) and several Yersinia secreted proteins (Yops) are involved in the inhibition of phagocytosis and killing of Yersinia enterocolitica by human granulocytes. One of these Yops, YopH, dephosphorylates multiple tyrosine-phosphorylated proteins in eukaryotic cells and is involved in the inhibition of phagocytosis of Y. enterocolitica by human granulocytes. We investigated whether antibody- and complement-opsonized plasmid-bearing (pYV+) Y. enterocolitica inhibits O2− production by human granulocytes in response to various stimuli and whether YopH is involved. Granulocytes were preincubated with mutant strains unable to express YadA or to secrete Yops or YopH. O2− production by granulocytes during stimulation was assessed by measuring the reduction of ferricytochrome c. PYV+ Y. enterocolitica inhibited O2− production by granulocytes incubated with opsonized Y. enterocolitica or N-formyl-Met-Leu-Phe (f-MLP). This inhibitory effect mediated by pYV did not affect receptor-independent O2− production by granulocytes in response to phorbol myristate acetate, indicating that NADPH activity remained unaffected after activation of protein kinase C. The inhibition of f-MLP-induced O2− production by granulocytes depends on the secretion of Yops and not on the expression of YadA. Insertional inactivation of the yopH gene abrogated the inhibition of phagocytosis of antibody- and complement-opsonized Y. enterocolitica by human granulocytes but not of the f-MLP-induced O2− production by granulocytes or tyrosine phosphorylation of granulocyte proteins. These findings suggest that the specific targets for YopH are not present in f-MLP receptor-linked signal transduction and that other Yop-mediated mechanisms are involved.
Human granulocytes are able to kill microorganisms by oxygen-independent and oxygen-dependent mechanisms. Oxygen-independent mechanisms include acidification of the phagosome, deprivation of nutrients, and killing by antimicrobial polypeptides (20). Oxygen-dependent killing involves the production of superoxide anion (O2−) and the subsequent formation of bactericidal reactive oxygen intermediates. The formation of O2− is catalyzed by NADPH oxidase, a membrane-bound enzymatic complex which converts O2 into O2− (14). NADPH oxidase can be activated by receptor-mediated mechanisms, such as opsonized bacteria, C5a, the tripeptide N-formyl-Met-Leu-Phe (f-MLP), and immune complexes, and by receptor-independent mechanisms, including long-chain unsaturated fatty acids and phorbol 12-myristate 13-acetate (PMA). Activation of NADPH oxidase in human granulocytes by receptor-dependent stimuli is accompanied by protein tyrosine phosphorylation (8), suggesting that NADPH oxidase can be switched on by tyrosine kinases. Furthermore, there are indications that tyrosine phosphatases play a role in regulating the magnitude and duration of O2− production by switching off the NADPH oxidase (7).
Virulent strains of Yersinia enterocolitica, a common cause of enterocolitis and mesenteric lymphadenitis in humans, harbor a 70-kb virulence plasmid, called pYV. pYV bears genes that code for the production of the outer membrane protein Yersinia adhesin A (YadA) (12) and several secreted proteins, called Yops (35, 47). YadA and several Yops are involved in the inhibition of phagocytosis and killing of Y. enterocolitica by human granulocytes (15, 33, 42, 50). Yops are synthesized at 37°C and translocated into mammalian cells upon contact (40, 45, 46). One of these Yops, a 51-kDa protein called YopH, dephosphorylates multiple tyrosine-phosphorylated proteins in eukaryotic cells (10, 26) and is involved in the inhibition of uptake of Yersinia by cultured murine macrophages (21, 39) and epithelial cells (38). In preliminary experiments we found that insertional inactivation of the yopH gene by transposon mutagenesis abrogated the inhibition of the phagocytosis of preopsonized Y. enterocolitica by granulocytes. The tyrosine phosphatase activity of YopH may also interfere with the receptor-mediated activation of NADPH oxidase of human granulocytes.
The aim of this study was to determine whether opsonized plasmid-bearing Y. enterocolitica is able to inhibit the activation of the NADPH oxidase of human granulocytes and to determine whether the phosphotyrosine phosphatase activity of the product of plasmid-borne yopH is involved.
MATERIALS AND METHODS
Media.
The medium to lyse erythrocytes consisted of 0.18 M NH4Cl, 9.99 mM KHCO3, and 8.76 μM EDTA in distilled H2O, adjusted to pH 7.36. Phosphate-buffered saline (PBS) was supplemented with 0.9 M CaCl2·2H2O, 0.5 M MgCl2·6H2O, and 0.55 M glucose (PBS-Ca2+-Mg2+-glucose). Ca2+ medium contained 138 mM NaCl, 6 mM KCl, 1.1 mM CaCl2 · H2O, 1 mM MgSO4 · 7H2O, 1 mM NaH2PO4 · H2O, 5.5 mM glucose, 0.1 mM EGTA, 20 mM HEPES, and 0.1% (vol/vol) bovine serum albumin (BSA). Hanks’ balanced salt solution was supplemented with 0.1% (wt/vol) gelatin and 10 mM HEPES. Lipopolysaccharide-free RPMI 1640 medium containing 25 mM HEPES and 2.05 mM l-glutamine (Gibco BRL, Life Technologies Ltd., Paisley, Scotland) was divided into aliquots of 50 ml and stored at 4°C until use.
Granulocytes.
Granulocytes were isolated from heparinized buffy coats (Red Cross Blood Bank, Leiden, The Netherlands) from adult healthy donors by Ficoll-amidotrizoate (ρ = 1.077 g/ml) gradient centrifugation (13). Erythrocytes present in the granulocyte-rich pellet were removed by NH4Cl lysis for 10 min at 4°C, followed by centrifugation and three washes with PBS containing 0.5 U of heparin per ml. A cell suspension of 107 granulocytes per ml was prepared in PBS-Ca2+-Mg2+-glucose and maintained in this medium at room temperature until use. The viability of the granulocytes, as determined by trypan blue dye exclusion, before and after each experiment, exceeded 95%.
Microorganisms.
Studies were carried out with the virulent (pYV+) strain, the isogenic plasmid-cured avirulent (pYV−) strain, and various mutant strains of Y. enterocolitica W22703 (kindly provided by G. Cornelis, Microbial Pathogenesis Unit, Universtité Catholique de Louvain, Brussels, Belgium), summarized in Table 1. Bacteria were prepared as described previously (50). The expression of the Yop regulon was induced by incubating bacteria in brain heart infusion broth (Oxoid Ltd., Basingstoke, United Kingdom) supplemented with 20 mM sodium oxalate with shaking for 180 min at 37°C (31). The microorganisms were harvested by centrifugation, washed twice with PBS, and resuspended in PBS-Ca2+-Mg2+-glucose at various concentrations, as estimated by measuring the optical density at 600 nm by spectrophotometry. The actual numbers of viable bacteria were determined by plating serial dilutions of the suspension onto blood agar, which was incubated for 18 h at 25°C.
TABLE 1.
Y. enterocolitica W22703 mutants used
Analysis of YadA expression and YopH secretion.
Bacteria were prepared as described above. The presence of YadA on the outer membranes of bacteria of the various strains was checked by immunofluorescence microscopy (50). The bacterial suspension was first incubated with rabbit anti-YadA antiserum (kindly provided by J. Heesemann, Institut für Max von Pettenkoffer-Institut für Hygiene und Medizinische Mikrobiologie, Munich, Germany) (30, 43) and next with fluorescein isothiocyanate (FITC)-labelled anti-rabbit immunoglobulin G (IgG) (Nordic Immunological Laboratories, Tilburg, The Netherlands).
The presence of YopH in culture supernatants and lysates of pYV− or pYV+ Y. enterocolitica or Y. enterocolitica W22703(pGC1152) was analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and immunoblotting. Proteins were isolated from equal numbers of bacteria as described previously (23) and suspended in SDS sample buffer (10% [wt/vol] SDS, 0.1 M dithioerythreitol, 10% [vol/vol] 2-mercaptoethanol, 20% [vol/vol] glycerol, 8 mM EDTA, and 0.01% [wt/vol] bromophenol blue in 20 mM Tris buffer [pH 6.8]). Samples (10 μl) were electrophoresed at a constant current of 30 mA with a 0.75-mm-thick slab gel of 10% acrylamide as the running gel and 3.5% acrylamide as the stacking gel. YopH obtained from Y. enterocolitica (1.25 μg; Biomol Research Laboratories, Plymouth Meeting, Pa.) was used as the positive control. The protein bands were transferred to nitrocellulose paper (Whatmann International Ltd., Maidstone, United Kingdom) for immunoblotting. The presence of YopH was detected by enhanced chemiluminescence immunodetection (ECL Western blotting; Amersham International plc, Little Chalfont, Buckinghamshire, United Kingdom) with rabbit anti-YopH antibodies (final dilution of 1:1,000, kindly provided by J. Heesemann) and horseradish peroxidase (HRP)-labelled swine anti-rabbit antibodies (final dilution of 1:10,000; DAKO, Glostrup, Denmark). The light emission resulting from the HRP-hydrogen peroxide-catalyzed oxidation of luminol was detected by autoradiography (Fuji medical X-ray film). The detection limit of this assay was 1 ng of YopH.
Preopsonization of Y. enterocolitica.
Preopsonization with Yersinia antibodies and complement was performed by incubating Y. enterocolitica with 10% (vol/vol) fresh rabbit immune serum (50) with rotation (4 rpm) for 30 min at 37°C, followed by centrifugation at 1,200 × g for 10 min. The bacteria were washed twice with PBS and suspended in PBS-Ca2+-Mg2+-glucose. Rabbit immune serum is not bactericidal to the Yersinia strains used in this study and promotes opsonization of Y. enterocolitica in human granulocytes.
Measurement of O2− production.
The activity of the NADPH oxidase of granulocytes was assessed by measuring O2− production with the superoxide dismutase-inhibitable reduction of ferricytochrome c (type IV, horse heart; Sigma Chemical Co., St. Louis, Mo.) as described previously (2). In short, 106 granulocytes and 1 mmol of ferricytochrome c were incubated together in 1 ml of PBS-Ca2+-Mg2+-glucose in the presence of various numbers of pYV− or pYV+ Y. enterocolitica organisms in polypropylene tubes under rotation (4 rpm) at 37°C. After 30 min of incubation, the reaction was stopped by placing the tubes into crushed ice, and the amount of reduced ferricytochrome c in the supernatant was determined by measuring the extinction at 550 nm with a spectrophotometer. Results are expressed as nanomoles of O2− per 106 granulocytes per milliliter per 30 min.
To study the effect of Y. enterocolitica on receptor-dependent or receptor-independent activation of NADPH oxidase, granulocytes were preincubated with preopsonized bacteria of the various strains of Y. enterocolitica at a ratio of 10 bacteria to 1 granulocyte in PBS-Ca2+-Mg2+-glucose under rotation (4 rpm) for 30 min at 37°C. After the nonadherent bacteria were removed by differential centrifugation and two washes, the pellet was resuspended in PBS-Ca2+-Mg2+-glucose supplemented with 10 μg of cytochalasin E (Sigma Chemical Co.) per ml. Cytochalasin E did not affect the viability of granulocytes, as determined by trypan blue dye exclusion, or the viability of bacteria, as determined by a microbiological assay (50).
f-MLP (100 nM; Sigma Chemical Co.) was used as a receptor-dependent stimulus for NADPH oxidase. For optimal stimulation of O2− production, granulocytes were incubated with 10 μg of cytochalasin E per ml for 5 min at 37°C prior to the addition of f-MLP (32, 52); 25 ng of PMA (Consolidated Midlands, Brewster, N.J.) per ml was used as a receptor-independent stimulus for NADPH oxidase (50).
Determination of binding of N-formyl peptides to their receptors on granulocytes.
To investigate whether Y. enterocolitica affected the binding of N-formyl peptides to their receptors on the surface of a granulocyte, the binding of a fluoresceinated N-formyl hexapeptide, N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys-FITC (f-NLPNYK-FITC, Molecular Probes Inc., Eugene, Oreg.), to human granulocytes was analyzed by flow cytometry (44). In short, granulocytes were preincubated with preopsonized bacteria of the various strains of Y. enterocolitica at a ratio of 10 bacteria to 1 granulocyte in PBS-Ca2+-Mg2+-glucose under rotation (4 rpm) for 30 min at 37°C. After the nonadherent bacteria were removed by differential centrifugation and two washes, the pellet was resuspended in PBS supplemented with 1% (vol/vol) BSA. Next, granulocytes were incubated with 10 nM f-NLPNYK-FITC at 4°C (44). Internalization of the ligand-receptor complex does not occur at 4°C (44). After 60 min of incubation, granulocytes were fixed by adding an equal volume of 2% (vol/vol) paraformaldehyde in saline for 15 min at 4°C. After one wash, granulocytes were resuspended in PBS supplemented with 1% (vol/vol) BSA. The specificity of the binding of f-NLPNYK-FITC to the N-formyl peptide receptor was determined by measuring the residual binding of f-NLPNYK-FITC after preincubation of the granulocytes with 10 μM f-MLP for 5 min at 4°C. In each sample 104 cells were analyzed by flow cytometry on a FACStar (Becton Dickinson, Mountain View, Calif.) equipped with an argon-ion laser (excitation wavelength, 488 nm; laser power, 300 mW) and a 530-nm-long band pass filter (width, 20 nm). Results are expressed as the means of the values determined for fluorescence intensity.
Assessment of tyrosine-specific protein phosphorylation.
Tyrosine phosphorylation of proteins in granulocytes during stimulation with f-MLP was detected according to the method of Connelly et al. (17) with modifications. Granulocytes were preincubated with preopsonized Y. enterocolitica (ratio of 20 bacteria to 1 granulocyte) in RPMI 1640 under rotation (4 rpm) for 90 min at 37°C. The nonadherent bacteria were removed by differential centrifugation and two washes. Next, the bacterium- and granulocyte-rich pellet (108 per ml) was resuspended in RPMI 1640 and stimulated with 1 μM f-MLP for 30 s at 37°C. The reaction was stopped by mixing 55 μl of the cell suspension with 50 μl of SDS sample buffer at 95°C, followed by heating at 95°C for 5 min. Samples (10 μl) of the cell lysates were electrophoresed at a constant current of 60 mA with a 0.75-mm-thick slab gel of 7.5% acrylamide as the running gel and 3.5% acrylamide as the stacking gel for 90 min at 20°C. The protein bands were electrophoretically transferred to nitrocellulose paper (Whatmann International Ltd.) for immunoblotting. Tyrosine-phosphorylated proteins were detected by enhanced chemiluminescence immunodetection (ECL Western blotting; Amersham International plc) according to the instructions of the manufacturer with 1 μg of antiphosphotyrosine monoclonal antibody 4G10 (IgG2bk; Upstate Biotechnology Inc., Lake Placid, N.Y.) per ml of PBS supplemented with 0.1% (vol/vol) Tween 20, 1% (wt/vol) milk powder, and HRP-labelled rabbit anti-mouse antibodies (final dilution of 1:10,000; DAKO).
F-actin content.
The content of filamentous actin (F-actin) of granulocytes during stimulation with f-MLP was analyzed by staining with bodipy-phallacidin as described previously (5, 6). In short, granulocytes were preincubated with preopsonized Y. enterocolitica (ratio of 10 bacteria to 1 granulocyte) in PBS-Ca2+-Mg2+-glucose under rotation (4 rpm) at 37°C. After 30 min of incubation, nonadherent bacteria were removed and the pellet (107 bacteria per ml) was resuspended in Ca2+ medium and stimulated with 100 nM f-MLP for the time intervals indicated in the figures at 37°C. The reaction was stopped by mixing 100 μl of the bacterium-cell suspension with 250 μl of 3.2% (vol/vol) paraformaldehyde, followed by incubation for 30 min at 4°C. After two washes, granulocytes were permeabilized with 75 μg of l-α-lysophosphatidylcholine (Sigma) per ml, and stained with 0.3 μg of bodipy-phallacidin (Bodipy FL phallacidin; Molecular Probes Inc.) per ml of Ca2+ medium at 4°C. After 30 min of incubation, the suspension was washed and resuspended in Ca2+ medium for flow cytometry. In each sample 104 cells were analyzed as described in a previous paragraph. Results are expressed as the means of the values determined for fluorescence intensity.
Statistical analysis.
All data are means ± standard errors of results from at least three independent experiments. Statistical analysis was performed with the Student t test and the Systat software package for the comparison of mean values.
RESULTS
Production of O2− during incubation of human granulocytes with preopsonized Y. enterocolitica.
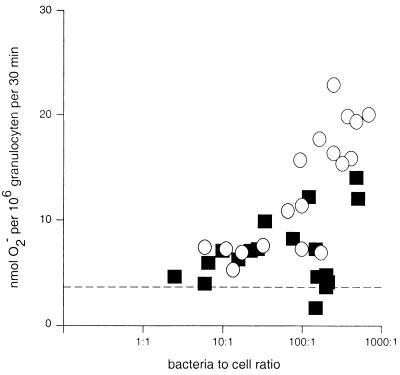
O2− production during incubation of granulocytes with preopsonized Y. enterocolitica at various bacteria-to-cell ratios showed a clear dose-response relationship between the numbers of pYV− Y. enterocolitica organisms and the amount of O2− produced (Fig. 1). Incubation of granulocytes with increasing numbers of pYV+ Y. enterocolitica organisms resulted in only a slight increase in O2− production. Cytospin preparation of the bacterium-granulocyte suspension did not show evident agglutination of pYV+ Y. enterocolitica that could account for the observed differences between pYV− and pYV+ Y. enterocolitica (data not shown).
FIG. 1.
O2− production by human granulocytes during incubation with Y. enterocolitica. Granulocytes were incubated together with various numbers of pYV− Y. enterocolitica (○) or pYV+ Y. enterocolitica (■) organisms for 30 min. O2− production was determined by measuring the superoxide dismutase-inhibitable reduction of ferricytochrome c. The mean (± standard error) O2− production by granulocytes in the absence of bacteria amounted to 3.64 (±0.28) nmol of O2− per 106 granulocytes per ml per 30 min and is indicated by the dashed line.
Production of O2− in response to receptor-dependent or receptor-independent stimulation.
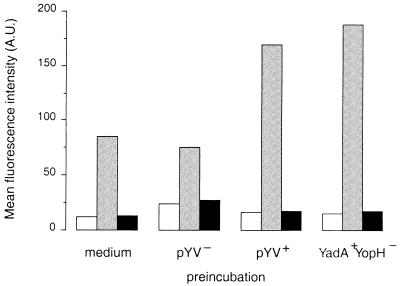
To determine whether Y. enterocolitica inhibits receptor-dependent O2− production, granulocytes were preincubated with preopsonized Y. enterocolitica and next stimulated with f-MLP or preopsonized Y. enterocolitica. PYV+ Y. enterocolitica-preincubated granulocytes produced less O2− during stimulation with f-MLP than granulocytes preincubated with the pYV− strain (Table 2). Control experiments showed that the binding of N-formyl peptides to their receptors on granulocytes was not inhibited by preincubation with pYV+ Y. enterocolitica (Fig. 2). Granulocytes that were preincubated with preopsonized pYV+ Y. enterocolitica and next stimulated with preopsonized Y. enterocolitica (ratio of 50 bacteria to 1 granulocyte) produced less O2− than granulocytes preincubated with the pYV− strain (Table 2). Preincubation of granulocytes with either strain did not inhibit receptor-independent O2− production during stimulation with PMA (Table 2). Together, these results indicate that preopsonized pYV+ Y. enterocolitica inhibits receptor-dependent, but not receptor-independent, O2− production by human granulocytes.
TABLE 2.
Effects of various mutant strains of Y. enterocolitica on O2− production by granulocytes in response to different stimulia
| Preincubation substance | O2− production by granulocytes during stimulation with:
|
||||
|---|---|---|---|---|---|
| Medium | PMA | f-MLP | pYV− | pYV+ | |
| Medium | 1.53 ± 0.42 | 19.13 ± 1.46 | 19.98 ± 1.58 | 18.48 ± 0.8 | 9.48 ± 0.96 |
| pYV− | 1.73 ± 0.42 | 14.19 ± 2.17 | 11.24 ± 1.25 | 5.91 ± 1.04 | 3.46 ± 0.63 |
| pYV+ | 1.50 ± 0.85 | 12.04 ± 2.23 | 1.89 ± 1.26b | 1.44 ± 0.34b | 0.94 ± 0.17b |
| pBC7 YadA− Yops+ | 1.34 ± 0.59 | 11.94 ± 1.76 | 2.63 ± 1.63b | NDc | ND |
| pSW2276 YadA+ Yops− | 2.42 ± 0.47 | 13.39 ± 1.46 | 12.48 ± 0.85 | ND | ND |
| pGC1152 YadA+ YopH− | 2.49 ± 1.19 | 20.07 ± 6.03 | 3.61 ± 1.84b | ND | ND |
Granulocytes were preincubated with medium or a Y. enterocolitica strain (ratio of 10 bacteria to 1 granulocyte) for 30 min. After removal of nonadherent bacteria, O2− production by granulocytes during stimulation was assessed by measuring the reduction of the level of ferricytochrome c. Stimulation with pYV− or pYV+ Y. enterocolitica was performed at a ratio of 50 bacteria to 1 granulocyte. Results are expressed in nanomoles per 106 cells per 30 min (means ± standard errors of results from at least three independent experiments).
Significantly different (P < 0.05) from values found for granulocytes preincubated with pVY− Y. enterocolitica in response to the same stimulus, according to Student’s paired two-tailed t test.
ND, not done.
FIG. 2.
Binding of fluorescein N-formyl hexapeptides to their receptors on granulocytes preincubated with various strains of preopsonized Y. enterocolitica. Granulocytes were preincubated with medium only, pVY− or pYV+ Y. enterocolitica, or Y. enterocolitica W22703(pGC1152) YadA+ YopH− for 30 min. After removal of nonadherent bacteria, the pellet was incubated with N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys-FITC at 4°C and analyzed by flow cytometry (gray bars). The specificity of the binding of this fluorescein hexapeptide to granulocytes was determined by measuring the residual binding of N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys-FITC after preincubation of the granulocytes with 10 μM f-MLP for 5 min at 4°C (black bars). Values for controls are represented by the open bars. Results are expressed as the means of the values determined for fluorescence intensity. Data are representative of results from two independent experiments. A.U., arbitrary units.
Role of YadA and Yops in inhibition of f-MLP-receptor dependent O2− production by human granulocytes.
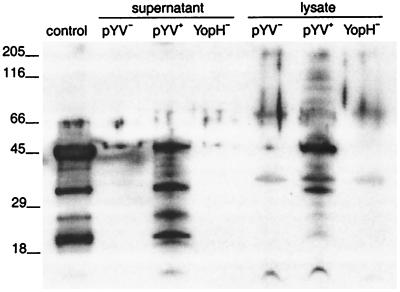
To investigate which plasmid-borne factors are involved in the inhibition of f-MLP-receptor-dependent O2− production by granulocytes, the O2− production by granulocytes preincubated with various mutant strains of Y. enterocolitica and stimulated with f-MLP was determined. Preincubation of granulocytes with Y. enterocolitica 22703(pSW2276) YadA+ Yops− did not inhibit O2− production, whereas preopsonized Y. enterocolitica W22703(pBC7) YadA− Yops+ inhibited O2− production during stimulation with f-MLP to the same extent as Y. enterocolitica preincubated with pYV+ (Table 2). Immunofluorescence microscopy confirmed that YadA was not expressed by the pYV− strain and by Y. enterocolitica W22703(pBC7) but that it was expressed by all other strains (data not shown). Preincubation with Y. enterocolitica W22703(pGC1152) YadA+ YopH− also inhibited the O2− production almost completely during stimulation with f-MLP. Control experiments showed that YopH was not detectable in culture supernatant or bacterial lysate of the pYV− or YopH− strain but that it was detectable in culture supernatant and bacterial lysate of pYV+ Y. enterocolitica (Fig. 3). Preincubation of granulocytes with various mutant Y. enterocolitica strains did not inhibit O2− production during stimulation with PMA. Together, these results indicate that the inhibition of f-MLP-receptor-mediated O2− production does not involve YadA or YopH but that it involves other Yop-mediated mechanisms.
FIG. 3.
Analysis by immunoblotting of YopH expression by various Y. enterocolitica strains. Proteins isolated from culture supernatants and lysates of equal numbers of bacteria of pYV− Y. enterocolitica, pYV+ Y. enterocolitica, or Y. enterocolitica W22703(pGC1152) YadA+ YopH− were dissolved in SDS sample buffer, subjected to polyacrylamide gel electrophoresis, and transferred to nitrocellulose. YopH was detected by enhanced chemiluminescence immunodetection. Lines indicate positions of molecular mass markers (in kilodaltons). The major band of 45 kDa detected in the culture supernatant and lysate of pYV+ Y. enterocolitica corresponds to YopH. YopH was not detected in the culture supernatant and lysate of pYV− Y. enterocolitica or Y. enterocolitica W22703(pGC1152) YadA+ YopH−. Recombinant YopH was used as a positive control. Data are representative of results from two independent experiments.
Tyrosine-specific protein phosphorylation in human granulocytes during stimulation with f-MLP.
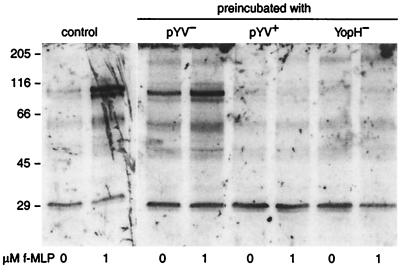
To investigate the effect of Y. enterocolitica on tyrosine kinase activity in response to f-MLP, granulocytes were preincubated with various strains of preopsonized Y. enterocolitica and the pattern of tyrosine-phosphorylated proteins in granulocyte lysates during stimulation with f-MLP was determined. When granulocytes were preincubated in medium without Y. enterocolitica, tyrosine phosphorylation of proteins with apparent molecular masses of 112, 60, and 50 kDa occurred during f-MLP stimulation (Fig. 4). When granulocytes were preincubated with pYV− Y. enterocolitica, phosphorylation of proteins with similar apparent molecular masses occurred without f-MLP stimulation and increased upon stimulation (Fig. 4). When granulocytes were preincubated with the pYV+ or the YopH− strain, tyrosine phosphorylation of these proteins did not occur without or during stimulation with f-MLP (Fig. 4), even when stimulation with f-MLP was prolonged to 5 min (data not shown).
FIG. 4.
Protein tyrosine phosphorylation of granulocytes preincubated with various strains of preopsonized Y. enterocolitica and stimulated with f-MLP. Granulocytes were preincubated with medium only (control), pYV− or pYV+ Y. enterocolitica or Y. enterocolitica W22703(pGC1152) YadA+ YopH− for 90 min. After removal of nonadherent bacteria, the pellet was stimulated with 1 μM f-MLP. After Western blotting, tyrosine-phosphorylated proteins were detected by enhanced chemiluminescence immunodetection. Data are representative of results from three independent experiments. Lines indicate positions of molecular mass markers (in kilodaltons).
An additional phosphotyrosine-containing protein with an apparent molecular mass of 200 kDa was observed in lysates of granulocytes preincubated with the pYV− or the YopH− strain but not in lysates of control granulocytes or granulocytes preincubated with the pYV+ strain. Phosphorylation of this protein did not increase upon stimulation with f-MLP.
These results indicate that the inhibition of f-MLP receptor-induced tyrosine phosphorylation of granulocyte proteins does not involve YopH but that it does involve other Yop-mediated mechanisms.
Effect of Y. enterocolitica on cytoplasmic F-actin content during stimulation with f-MLP.
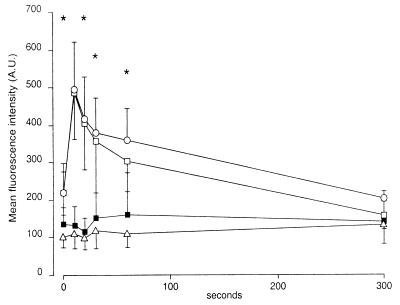
f-MLP induced a rapid and transient increase in the cytoplasmic F-actin contents of granulocytes preincubated with medium without bacteria or preincubated with pYV− Y. enterocolitica (Fig. 5). Preincubation of granulocytes with pYV+ Y. enterocolitica or the YopH− strain resulted in an initially low cellular F-actin content, which did not increase upon stimulation with f-MLP.
FIG. 5.
Time course of f-MLP-induced changes in cellular F-actin contents in human granulocytes. After preincubation of granulocytes with preopsonized Y. enterocolitica and removal of nonadherent bacteria, the pellet was stimulated with 100 nM f-MLP. After permeabilization, granulocytes were stained with fluorescein phalloidin. Fluorescence intensity was measured by flow cytometry. Results are expressed as means (± standard errors) of values determined for fluorescence intensity. ∗, values for pYV+ Y. enterocolitica and Y. enterocolitica W22703(pGC1152) YadA+ YopH− that were significantly different from values for pYV− Y. enterocolitica (P < 0.05, paired Student’s t test). Normal controls (□) and granulocytes preincubated with pYV− Y. enterocolitica (○), pYV+ Y. enterocolitica (■), or Y. enterocolitica W22703(pGC1152) YadA+ YopH− (▵) were tested. A.U., arbitrary units.
DISCUSSION
The main conclusion of this study is that preopsonized plasmid-bearing (pYV+) Y. enterocolitica inhibits O2− production by human granulocytes in response to different receptor-mediated stimuli, including opsonized Y. enterocolitica and f-MLP, through a plasmid-borne mechanism. The inhibition of f-MLP-induced O2− production by granulocytes is dependent on Yops other than YopH and not on the expression of YadA.
Granulocytes incubated with pYV+ Y. enterocolitica, preopsonized with antibody and complement, produced less O2− than cells incubated with the preopsonized pYV− strain. This inhibitory effect of pYV+ Y. enterocolitica was also observed after removal of nonadherent bacteria followed by stimulation with the preopsonized pYV− strain or f-MLP. Since pYV+ and pYV− Y. enterocolitica differ only in the presence of pYV, it is most likely that plasmid-borne factors are involved in the inhibition of O2− production. A similar inhibition, mediated by pYV, has been observed in the luminol-enhanced chemiluminescence response by granulocytes upon incubation with complement-opsonized Y. enterocolitica (15, 34, 42, 49) or complement-opsonized zymosan (42). Together, these results indicate that pYV+ Y. enterocolitica is able to inhibit receptor-mediated O2− production by granulocytes triggered through different classes of receptors, namely, Fcγ receptors, complement receptors (β2 integrin), and f-MLP receptors.
The inhibition of f-MLP-induced O2− production by granulocytes depends on the secretion of Yops and not on the expression of YadA. Studies using the chemiluminescence response by human granulocytes incubated with complement-opsonized Y. enterocolitica and stimulated with complement-opsonized zymosan confirmed the inhibitory role mediated by Yops (42) but found that YadA was also involved (34, 42). This inhibition, mediated by YadA, is probably related to the use of normal human serum for opsonization of Y. enterocolitica. YadA confers resistance to the bactericidal activity of human serum (4) and interferes with complement opsonization (16, 49). Both properties of YadA may affect the chemiluminescence response of granulocytes.
We found that insertional inactivation of the yopH gene by transposon mutagenesis abrogated the inhibition of phagocytosis of this antibody- and complement-opsonized Y. enterocolitica strain by human granulocytes (data not shown) but not of f-MLP-induced O2− production by granulocytes or tyrosine phosphorylation of granulocyte proteins. However, others have reported that YopH is involved in the inhibition of the chemiluminescence response of granulocytes stimulated by complement-opsonized zymosan (42) and of murine macrophages stimulated by IgG2a-opsonized Yersinia bacteria (9) but not those stimulated by unopsonized zymosan (29).
Recently, it was demonstrated that YopH dephosphorylates focal adhesion kinase (FAK) and p130Cas, which leads to disruption of peripheral focal complexes and inhibits β1-integrin-mediated uptake of Y. pseudotuberculosis by HeLa cells (36). FAK and p130Cas are also phosphorylated upon stimulation of Fc receptors in platelets or β2 integrin receptors in lymphocytes (27, 37). It can be hypothesized that YopH-induced dephosphorylation of FAK or p130Cas may be involved in the inhibition of the complement receptor or the Fc receptor-induced chemiluminescence response of granulocytes to Y. enterocolitica. The absence of such an inhibitory role of YopH in f-MLP-induced O2− production by granulocytes may be explained by the fact that specific targets for YopH are not present in the f-MLP receptor-linked signal transduction pathways (22) and that other Yop-mediated mechanisms are involved.
The Yop-mediated inhibition of O2− production by granulocytes occurs early in the signal transduction pathways of the NADPH oxidase, i.e., before activation of protein kinase C, since PMA-stimulated O2− production by granulocytes was not inhibited by pYV+ Y. enterocolitica. Signal transduction pathways activated by the G protein-linked f-MLP receptor involve phosphorylation of regulatory proteins, including the Ras/Raf/microtubule-associated protein kinase (MAPK) pathway (1, 54). Inhibitors of the MAPK pathway inhibit f-MLP-induced O2− production by granulocytes (1, 54). Recently, it has been demonstrated that YopP is involved in the deactivation of MAPKs, resulting in the suppression of tumor necrosis factor alpha release by mouse monocyte/macrophage cell lines upon stimulation with lipopolysaccharide (41) or Y. enterocolitica (11). Possibly, YopP is also involved in the inhibition of f-MLP-induced O2− production by granulocytes by blocking the MAPK pathway.
An alternative hypothesis explaining the observed inhibition of O2− production, of tyrosine phosphorylation of granulocyte proteins, and of F-actin formation in granulocytes in response to f-MLP is that a Yop protein of Y. enterocolitica uncouples the receptor-G protein interaction by phosphorylating serine and/or threonine residues in the cytoplasmic domain of the f-MLP receptor (3, 24, 48). A possible candidate is YopO (or YpkA), an 84-kDa virulence protein, which is translocated to the inner surfaces of the plasma membranes in HeLa cells (28) and which has homology to eukaryotic Ser/Thr protein kinases (25). The determination of which Yop is involved in the inhibition of f-MLP-induced O2− production by granulocytes and at which level of the signal transduction pathway of the f-MLP receptor this inhibition occurs requires further study.
Products of plasmid-borne Yop genes play central roles in the inhibition of phagocytosis of Y. enterocolitica by human granulocytes (42, 50). In addition, the outer membrane protein YadA, whose gene is plasmid borne, contributes to the reduced susceptibility of Y. enterocolitica to complement (4) and to antimicrobial polypeptides present in the granules of granulocytes (51). The Yop-mediated ability to inhibit O2− production by granulocytes triggered by different classes of receptors is another mechanism that enables virulent Y. enterocolitica to obstruct antimicrobial functions of granulocytes and to survive within the host.
REFERENCES
- 1.Avdi N J, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Mapping pathways for mitogen-activated protein kinase activation. J Biol Chem. 1996;271:33598–33606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 2.Babior B M, Kipnes R S, Curnette J T. Biological defence mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Investig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini M, Boulay F, Badwey J A, Curnutte J T. Activation of neutrophil leukocytes: chemoattractant receptors and respiratory burst. FASEB J. 1993;7:1004–1010. doi: 10.1096/fasebj.7.11.8396540. [DOI] [PubMed] [Google Scholar]
- 4.Balligand G, Laroche Y, Cornelis G. Genetic analysis of virulence plasmid from serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985;48:782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belloc F, Vincendeau P, Freyburger G, Dumain P, Boisseau M R. Flow cytometric study of the activation of polymorphonuclear cells. J Leukocyte Biol. 1990;48:353–358. doi: 10.1002/jlb.48.4.353. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson T, Dahlgren C, Stendahl O, Andersson T. Actin assembly and regulation of neutrophil function: effects of cytochalasin B and tetracaine on chemotactic peptide-induced O2− production and degranulation. J Leukoc Biol. 1991;49:236–244. doi: 10.1002/jlb.49.3.236. [DOI] [PubMed] [Google Scholar]
- 7.Bennett P A, Finan P M, Dixon R J, Kellie S. Tyrosine phosphatase antagonist-induced activation of the neutrophil NADPH oxidase: a possible role for protein kinase C. Immunology. 1995;85:304–310. [PMC free article] [PubMed] [Google Scholar]
- 8.Berkow R L, Dodson R W. Tyrosine-specific protein phosphorylation during activation of human neutrophils. Blood. 1990;75:2445–2452. [PubMed] [Google Scholar]
- 9.Bliska J B, Black D S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect Immun. 1995;63:681–685. doi: 10.1128/iai.63.2.681-685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliska J B, Guan K L, Dixon J E, Falkow S. Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci USA. 1991;88:1187–1191. doi: 10.1073/pnas.88.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. J Clin Lab Investig. 1968;21:77–98. [PubMed] [Google Scholar]
- 14.Chanock S J, El Benna J, Smith R M, Babior B M. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 15.China B, N’Guyen B T, De Bruyere M, Cornelis G R. Role of YadA in resistance of Yersinia enterocolitica to phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1994;62:1275–1281. doi: 10.1128/iai.62.4.1275-1281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.China B, Sory M P, N’Guyen B T, De Bruyere M, Cornelis G R. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect Immun. 1993;61:3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connelly P A, Farrell C A, Merenda J M, Conklyn M J, Showell H S. Tyrosine phosphorylation is an early signaling event common to Fc receptor cross-linking in human neutrophils and rat basophilic leukemia cells (RBL-2H3) Biochem Biophys Res Commun. 1991;177:192–201. doi: 10.1016/0006-291x(91)91967-h. [DOI] [PubMed] [Google Scholar]
- 18.Cornelis G, Sluiters C, Lambert de Rouvroit C, Michiels T. Restriction of DNA of Yersinia enterocolitica detected by recipient ability for a derepressed R factor from Escherichia coli. J Gen Microbiol. 1975;87:285–291. doi: 10.1099/00221287-87-2-285. [DOI] [PubMed] [Google Scholar]
- 19.Cornelis G, Vanootegem J C, Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987;2:367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 20.Elsbach P, Weiss J. Oxygen-independent antimicrobial systems of phagocytes. In: Galin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press Ltd.; 1992. [Google Scholar]
- 21.Fällman M, Andersson K, Håkansson S, Magnusson K E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez R, Suchard S J. Syk activation is required for spreading and H2O2 release in adherent human neutrophils. J Immunol. 1998;160:5154–5162. [PubMed] [Google Scholar]
- 23.Forsberg Å, Bölin I, Norlander L, Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987;2:123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- 24.Franci C, Gosling J, Tsou C L, Coughlin S R, Charo I F. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. Critical role of carboxyl-tail serines/threonines in receptor function. J Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- 25.Galyov E E, Hakansson S, Forsberg A, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 26.Guan K, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 27.Haimovich B, Regan C, DiFazio L, Ginalis E, Ji P, Purohit U, Rowley R B, Bolen J, Greco R. The FcγRII receptor triggers pp125FAK phosphorylation in platelets. J Biol Chem. 1996;271:16332–16337. doi: 10.1074/jbc.271.27.16332. [DOI] [PubMed] [Google Scholar]
- 28.Hakansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 29.Hartland E L, Green S P, Phillips W A, Robins-Browne R M. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect Immun. 1994;62:4445–4453. doi: 10.1128/iai.62.10.4445-4453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heesemann J, Gross U, Gruter L. Genetic manipulation of virulence of Yersinia enterocolitica. Contrib Microbiol Immunol. 1987;9:312–316. [PubMed] [Google Scholar]
- 31.Heesemann J, Gross U, Schmidt N, Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986;54:561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson A, Sarndahl E, Andersson T, Bengtsson T, Lundqvist H, Dahlgren C. Chemoattractant-induced NADPH oxidase activity in human monocytes is terminated without any association of receptor-ligand complex to cytoskeleton. Inflammation. 1995;19:179–191. doi: 10.1007/BF01534460. [DOI] [PubMed] [Google Scholar]
- 33.Lian C J, Hwang W S, Pai C H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987;55:1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian C J, Pai C H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Y. enterocolitica. Infect Immun. 1985;49:145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petruzzelli L, Takami M, Herrera R. Adhesion through the interaction of lymphocyte function-associated antigen-1 with intracellular adhesion molecule-1 induces tyrosine phosphorylation of p130Cas and its association with c-CkrII. J Biol Chem. 1996;271:7796–7801. doi: 10.1074/jbc.271.13.7796. [DOI] [PubMed] [Google Scholar]
- 38.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruckdeschel K, Machold J, Roggenkamp A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases: extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 42.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze-Koops H, Burkhardt H, Heesemann J, von der Mark K, Emmrich F. Plasmid-encoded outer membrane protein YadA mediates specific binding of enteropathogenic yersiniae to various types of collagen. Infect Immun. 1992;60:2153–2159. doi: 10.1128/iai.60.6.2153-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sklar L A, Finney D A, Oades Z G, Jesaitis A J, Painter R G, Cochrane C G. The dynamics of ligand-receptor interactions. Real-time analysis of association, dissociation, and internalization of a N-formyl peptide and its receptors on the human neutrophil. J Biol Chem. 1984;259:5661–5669. [PubMed] [Google Scholar]
- 45.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 47.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. . (Review.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tardif M, Mery L, Brouchon L, Boulay F. Agonist-dependent phosphorylation of N-formylpeptide and activation peptide from the fifth component of C (C5a) chemoattractant receptors in differentiated HL60 cells. J Immunol. 1993;150:3534–3545. [PubMed] [Google Scholar]
- 49.Tertti R, Eerola E, Lehtonen O P, Stahlberg T H, Viander M, Toivanen A. Virulence-plasmid is associated with the inhibition of opsonization in Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Exp Immunol. 1987;68:266–274. [PMC free article] [PubMed] [Google Scholar]
- 50.Visser L G, Annema A, van Furth R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect Immun. 1995;63:2570–2575. doi: 10.1128/iai.63.7.2570-2575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visser L G, Hiemstra P S, van den Barselaar M T, Ballieux P A, van Furth R. Role of YadA in resistance to killing of Yersinia enterocolitica by antimicrobial polypeptides of human granulocytes. Infect Immun. 1996;64:1653–1658. doi: 10.1128/iai.64.5.1653-1658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiles M E, Dykens J A, Wright C D. Human neutrophil (PMN) oxygen radical production and the cytoskeleton. Life Sci. 1995;57:1533–1546. doi: 10.1016/0024-3205(95)02114-x. [DOI] [PubMed] [Google Scholar]
- 53.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worthen G S, Avdi N, Buhl A M, Suzuki N, Johnson G L. FMLP activates Ras and Raf in human neutrophils. Potential role in activation of MAP kinase. J Clin Investig. 1994;94:815–823. doi: 10.1172/JCI117401. [DOI] [PMC free article] [PubMed] [Google Scholar]