Abstract
Background:
White matter hyperintensities (WMH) are identified on T2-weighted magnetic resonance images of the human brain as areas of enhanced brightness; WMH are a major risk factor of stroke, dementia, and death. Currently, there are no large-scale studies testing associations between WMH and circulating metabolites.
Methods:
We studied up to 9,290 individuals (50.7% females, average age 61 years) from 15 populations of 8 community-based cohorts. WMH volume was quantified from T2-weighted or fluid-attenuated inversion-recovery images or as hypointensities on T1-weighted images. Circulating metabolomic measures were assessed with mass spectrometry and nuclear magnetic resonance spectroscopy. Associations between WMH and metabolomic measures were tested by fitting linear regression models in the pooled sample, and in sex-stratified and statin treatment-stratified subsamples. Our basic models were adjusted for age, sex, age*sex, and technical covariates, and our fully adjusted models were additionally adjusted for statin treatment, hypertension, type 2 diabetes, smoking, body mass index, and estimated glomerular filtration rate. Population-specific results were meta-analyzed using the fixed-effect inverse variance-weighted method. Associations with false discovery rate (FDR)-adjusted p-values (pFDR)<0.05 were considered significant.
Results:
In the meta-analysis of results from the basic models, we identified 30 metabolomic measures associated with WMH (pFDR<0.05), 7 of which remained significant in the fully adjusted models. The most significant association was with higher level of hydroxyphenylpyruvate in males (pFDR.full.adj=1.40×10−7) and in both the pooled sample (pFDR.full.adj=1.66×10−4) and statin-nontreated (pFDR.full.adj=1.65×10−6) subsample. In males, HPP explained 3–14% of variance in WMH. In males and the pooled sample, WMH were also associated with lower levels of lysophosphatidylcholines and hydroxysphingomyelins, and a larger diameter of low-density lipoprotein particles, likely arising from higher triglyceride-to-total-lipids and lower cholesteryl ester-to-total-lipids ratios within these particles. In females, the only significant association was with higher level of glucuronate (pFDR=0.047).
Conclusions:
Circulating metabolomic measures, including multiple lipid measures (e.g., lysophosphatidylcholines, hydroxysphingomyelins, low-density lipoprotein size and composition) and non-lipid metabolites (e.g., hydroxyphenylpyruvate, glucuronate) associate with WMH in a general population of middle-aged and older adults. Some metabolomic measures show marked sex specificities and explain sizable proportion of WMH variance.
Keywords: brain, glucuronic acid, hydroxyphenylpyruvate, lipid ratios, lipidomics, lipids, lysophosphatidylcholines, metabolomics, sphingomyelins, white matter
INTRODUCTION
White matter hyperintensities (WMH) are among the most commonly encountered signal alterations on brain magnetic resonance imaging (MRI) images – they are areas of high intensity in the periventricular and deep cerebral white matter on T2-weighted (T2W) or T2 fluid-attenuated inversion recovery (FLAIR) images. The exact neuropathological mechanisms are not fully understood, but the proposed fundamental features of WMH are loss of myelin and axons, and mild gliosis (reviewed in1,2). These features might arise due to chronic ischemia and dysfunction of the blood-brain barrier (BBB) related to cerebral small vessel disease2.
Evidence from a recent meta-analysis of prospective cohort studies indicates that WMH are of major clinical significance3 – data obtained in 14,529 participants show that higher volume of WMH is associated with higher risk (hazard ratio, HR) of incident stroke (HR=2.45 [1.93–3.12], 95% confidence interval in square brackets), intracerebral hemorrhage (HR=3.17 [1.54–6.52]), ischemic stroke (HR=2.39 [1.65–3.74]), dementia (HR=1.84 [1.40–2.43]), Alzheimer’s disease (HR=1.50 [1.22–1.84]), and all-cause mortality (HR=2.00 [1.69–2.36])3. WMH are common in the general population: the prevalence is ~20% at the age of 60 years and >90% at the age of 80 years and higher4. Hypertension, type 2 diabetes, and smoking are the key cardiometabolic disease risk factors for WMH5–7.
We and others have shown that aberrations in the blood metabolome are associated with brain health: circulating levels of multiple lipid measures are associated with Alzheimer’s disease8,9 and cognitive functioning10, as well as structural properties of the brain, such as T1-weighted (T1W) signal intensity of white matter11,12 and thickness of the cerebral cortex13. The metabolomic associations of WMH, however, are incompletely understood and, to date, only two small studies have been published14,15. Here we used metabolomics technologies to provide a comprehensive characterization of variations in circulating metabolomic measures as a function of WMH volume assessed in general population. We further explored whether these associations are independent of the key risk factors of WMH, including hypertension, type 2 diabetes, and smoking.
METHODS
The authors declare that the summary-level results are available within the article and its online-only Data Supplement. The individual-level data analyzed in this study are available by application to the respective cohort committees.
Study populations
This is a large collaborative work incorporating data from 8 population-based cohort studies: Age, Gene/Environment Susceptibility-Reykjavik Study16, Framingham Heart Study17,18, Insight 46 (a sub-study of the British 1946 birth cohort)19, Lothian Birth Cohort 193620,21, Rotterdam Study22–24, Saguenay Youth Study25, Study of Health in Pomerania26,27, and the Southall And Brent Revisited study28. Study-specific descriptions are given in the Supplemental Methods. After excluding individuals with dementia, stroke, multiple sclerosis, brain surgery, or gross morphological abnormalities of the brain (e.g., cysts, brain tumors), and individuals with poor quality of MRI scans, we analyzed up to 9,290 individuals of mostly European ancestries with brain MRI and blood metabolomic data. All cohort studies were approved by local ethics committees, and all participants have provided their written informed consent (Supplemental Methods).
Phenotype quantifications
Brain MRI and WMH assessment
The brain MRI was carried out in each cohort study separately, and the details are provided in Table S1. In the present analyses, the total volume of WMH (or hypointensities on T1W images) was considered as a continuous variable.
Metabolomic quantifications
We used nuclear magnetic resonance (NMR)-based techniques (Nightingale Health Ltd, Helsinki, Finland; National Phenome Centre, London, UK; Bruker Biospin, Rheinstetten, Germany) and mass spectrometry (MS)-based technologies (Metabolon, Morrisville, North Carolina, USA; Biocrates Life Sciences AG, Innsbruck, Austria; Broad Institute, Cambridge, Massachusetts, USA) that are commonly employed in epidemiological research and are described elsewhere29–33 (Table S1). Using these aforementioned platforms, it was possible to measure a total of 2,217 different metabolomic measures; of these, 1,174 metabolomic measures were quantified in 2 or more of the study populations. We did not include unknown metabolites in our study. Metabolomic quantifications were completed using serum or plasma samples, typically extracted from blood samples drawn after overnight fasting (Table S1). Blood samples were drawn before or at the time of MRI in all cohorts except for the Rotterdam Study, in which MRI scans have been conducted at multiple time-points and metabolomic data that were from closest in time to the MRI scans were analyzed (Table S2).
Statistical analyses
Linear regression models
The cross-sectional associations between log-transformed WMH and circulating metabolomic measures were studied using linear regression. Prior to model fitting, the log-transformed WMH and metabolomic measures were scaled to standard deviation (SD) units, which enables the comparison and meta-analysis of data in different units and varying numerical ranges that originate from the multiple quantification methods used (Table S1). We fitted the regression models in five analytical samples (i.e., pooled sample and sex- or statin treatment-stratified subsamples) using a simple covariate structure (basic models) and a more complete covariate structure (fully adjusted models) to test if the associations are independent of key risk factors. The study models were as follows:
-
Pooled sample
basic models: logWMH ~ metabolic measure + age + sex + age*sex + time (if applicable) + fasting duration (if applicable) + intracranial volume or brain size + cohort-specific covariates
fully adjusted models: as above + statin treatment + hypertension + type 2 diabetes + BMI + eGFR + current smoking status
-
Sex-stratified subsamples
basic models: logWMH ~ metabolic measure + age + time (if applicable) + fasting duration (if applicable) + intracranial volume or brain size + cohort-specific covariates
fully adjusted models: as above + statin treatment + hypertension + type 2 diabetes + BMI + eGFR + current smoking status
-
Statin treatment-stratified subsamples
basic models: logWMH ~ metabolic measure + age + sex + age*sex + time (if applicable) + fasting duration (if applicable) + intracranial volume or brain size + cohort-specific covariates
fully adjusted models: as above + hypertension + type 2 diabetes + BMI + eGFR + current smoking status
Here, ‘time’ indicates the time in years between blood sampling and brain MRI, and ‘fasting duration’ denotes the time in hours between the last meal and blood sampling. Moreover, to investigate possible differences in the associations between the analytical subsamples, we fitted models to test for ‘metabolomic measure*sex’ and ‘metabolomic measure*statin use’ interactions; this was done in both basic and fully adjusted models. In the Southall And Brent Revisited study where multiple major ethnicities were present, all models were fitted separately in each ethnic group.
Meta-analyses and multiple testing correction
The association results for metabolomic measures that were present in two or more cohorts (N=1,173) were meta-analyzed. We used inverse variance-weighted fixed-effect meta-analysis to combine the effect estimates and standard errors from each cohort. In case a cohort reported multiple association results for the same metabolic measure (i.e., the metabolic measure was quantified using more than one metabolomic platform within the same cohort), the result that was obtained using a larger number of individuals was included in the meta-analysis.
Many metabolic measures are highly correlated and, thus, the number of independent tests is lower than the number of metabolomic measures tested. To correct for multiple testing, we estimated false discovery rate (FDR)-adjusted p-values using a method developed by Benjamini and Hochberg34. Here, all original p-values from the meta-analyses (including all analytical samples and both basic and fully adjusted models, and all metabolomic measures analyzed, including all lipid ratios) were included in the numeric vector of p-values used for estimating FDR-adjusted p-values (pFDR); all associations with pFDR<0.05 were considered significant. Testing for ‘metabolomic measure*sex’ and ‘metabolomic measure*statin treatment’ interactions were considered exploratory and no correction for multiple comparisons was applied.
All statistical analyses were conducted using R35.
RESULTS
Characteristics of the study populations are given in Table 1, Table S2 and Figures S1–S2. In the meta-analysis, we identified 416 metabolomic measures showing nominally significant associations with WMH in at least one of the study models. Out of these, 30 (basic models) and 7 (fully adjusted models) associations remained significant after correction for multiple testing (pFDR<0.05). An overview of the associations between circulating metabolomic measures and WMH in all basic and fully adjusted models is given in Figure 1. The relative importance metrics for the fully adjusted model of the most significant metabolite, hydroxyphenylpyruvate, are given in Table S3. All meta-analyzed results from the basic models and fully adjusted models are tabulated in Tables S4 and S5, respectively, and the results for ‘metabolomic measure’-by-sex and ‘metabolomic measure’-by-’statin treatment’ interactions are given in Tables S6 and S7. Cohort-specific associations results are given in Tables S8–S22. Figure S3 illustrates cohort-specific results of the metabolomic measures showing FDR-significant association with WMH. Figure S4 shows a comparison of the meta-analyzed results reported here versus the meta-analyzed results obtained in participants of European ancestries only. We found the cohort-specific results to be highly similar across the cohorts, with very little heterogeneity observed (Figure S3, Tables S4 and S5).
Table 1. Sample characteristics.
Values are mean ± standard deviation of the pooled data of 15 populations from 8 cohort studies. The population-specific characteristics are given in Table S2.
| Characteristic | Combined | Females | Males | Not on statin | On statin |
|---|---|---|---|---|---|
|
| |||||
| Number of individuals | 9,290 | 4,711 | 4,579 | 7,565 | 1,633 |
| Males (%) | 49.3 | 0 | 100 | 47.2 | 58.7 |
| Smokers (%) | 13.6 | 14.0 | 13.3 | 14.5 | 12.4 |
| On statin (%) | 17.7 | 14.4 | 21.0 | 0 | 100 |
| Hypertension (%) | 47.1 | 44.9 | 49.3 | 41.3 | 74.4 |
| Type 2 diabetes (%) | 7.0 | 6.1 | 7.9 | 4.0 | 20.7 |
| Age (years) | 61.0 ± 7.3 | 60.7 ± 7.4 | 61.4 ± 7.2 | 59.6 ± 7.5 | 67.9 ± 5.6 |
| BMI (kg/m2) | 27.3 ± 4.4 | 27.1 ± 4.7 | 27.5 ± 4.0 | 27.1 ± 4.4 | 28.2 ± 4.1 |
| logWMH | 5.35 ± 0.95 | 5.17 ± 0.93 | 5.35 ± 0.95 | 5.05 ± 0.91 | 6.78 ± 1.07 |
| Total-TG (mmol/L) * | 1.38 ± 0.65 | 1.37 ± 0.62 | 1.39 ± 0.65 | 1.36 ± 0.63 | 1.45 ± 0.69 |
| Total-C (mmol/L) * | 4.95 ± 1.00 | 5.10 ± 0.97 | 4.81 ± 0.97 | 5.18 ± 0.93 | 4.17 ± 0.89 |
| LDL-C (mmol/L) ** | 2.39 ± 0.67 | 2.49 ± 0.67 | 2.31 ± 0.66 | 2.57 ± 0.64 | 1.74 ± 0.54 |
| HDL-C (mmol/L) ** | 1.51 ± 0.36 | 1.58 ± 0.34 | 1.43 ± 0.31 | 1.54 ± 0.63 | 1.39 ± 0.33 |
Pooled data of 9 populations for which total-TG and total-C were available.
Pooled data of 10 populations for which LDL-C and HDL-C were available.
BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; and WMH, white matter hyperintensities.
Figure 1. Metabolomic associations of white matter hyperintensities.
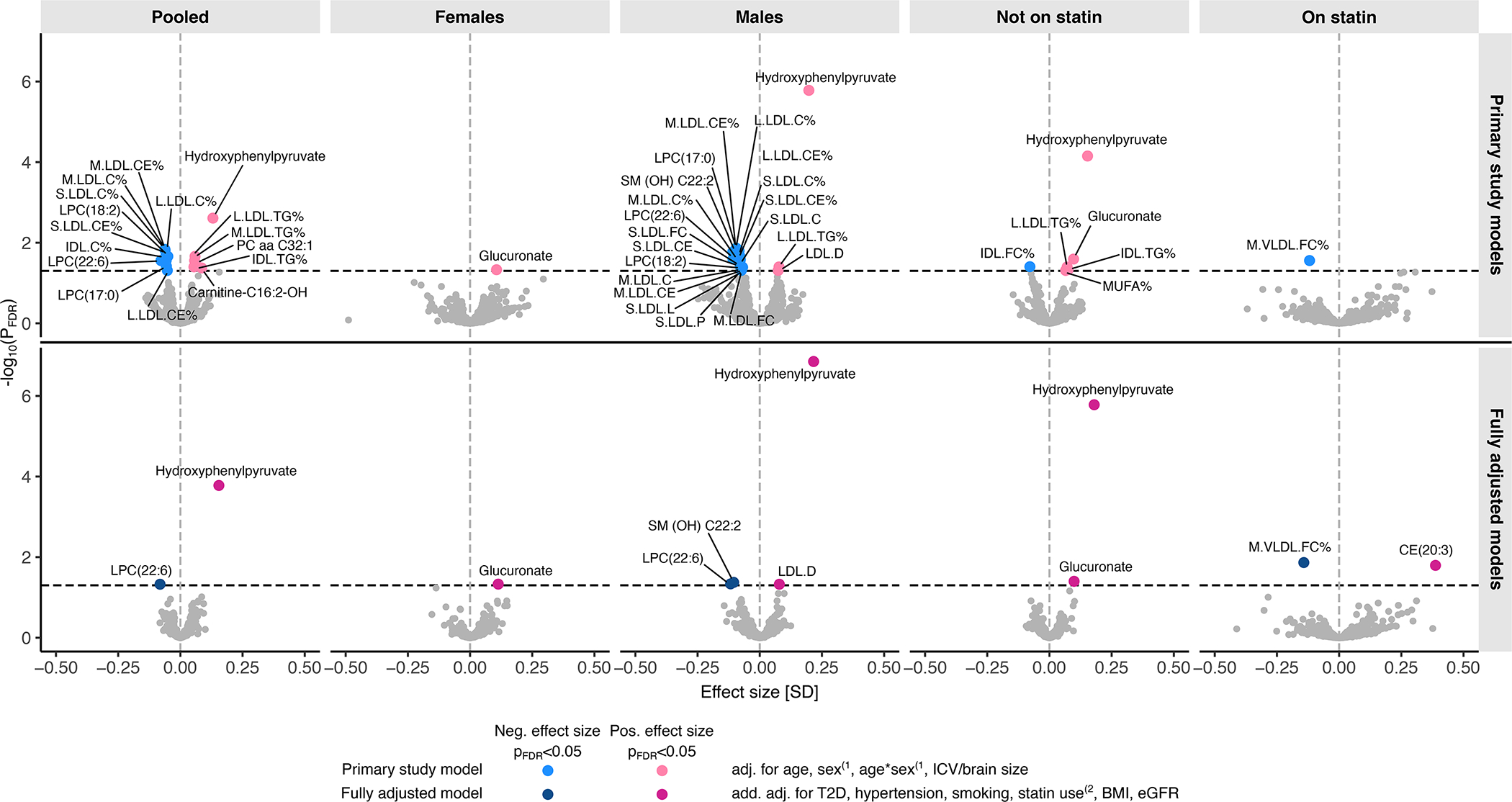
The plot shows the effect sizes (x-axis) and statistical significance (y-axis) of the metabolomic associations of WMH as obtained in the meta-analysis of up to 9,290 individuals from 15 populations of mostly European ancestry. Metabolomic associations of log-transformed WMH were determined by fitting linear regression models separately in a pooled sample, and in sex or statin treatment-stratified subsamples (columns). Population-specific effect sizes and standard errors were combined with inverse variance-weighted fixed effect meta-analysis. The metabolomic measures showing a significant (pFDR<0.05) association with WMH are labelled. Primary study models (top row): The associations were adjusted for age and intracranial volume or brain size, and also for sex and age-by-sex interaction in the pooled sample and in statin treatment-stratified subsamples(1. Fully adjusted models (bottom row): The associations were additionally adjusted for type 2 diabetes (T2D), hypertension, current smoking status, body mass index (BMI), estimated glomerular filtration rate (eGFR), and hypertension, and for statin treatment in the pooled sample and in sex-stratified subsamples(2. Where relevant, all models were also adjusted for fasting duration, time between blood sampling and brain MRI, and cohort study-specific covariates. C indicates cholesterol; CE, cholesteryl ester; FC, free cholesterol; FDR, false discovery rate; ICV, intracranial volume; IDL, intermediate-density lipoprotein; L, large; LDL, low-density lipoprotein; LPC, lysophosphatidylcholines; M, medium; MUFA, monounsaturated fatty acid; S, small; SM-OH, hydroxylated sphingomyelin; TG, triglycerides; and VLDL, very-low-density lipoprotein.
Non-lipid measures
The most robust association – in terms of both effect size and p-value – from across all studied metabolites was observed between WMH and higher circulating concentration of an amino acid derivative hydroxyphenylpyruvate (HPP; Figure 2): this association was most significant in the male subsample (betabasic=0.20, pFDR.basic=1.65×10−6; betafull.adj=0.22, pFDR.full.adj=1.40×10−7), but it was also significant in the pooled sample (betabasic=0.13, pFDR.basic=0.002; betafull.adj=0.15, pFDR.full.adj=1.66×10−4) and in statin-nontreated subsample (betabasic=0.15, pFDR.basic=7.04×10−5; betafull.adj=0.18, pFDR.full.adj=1.65×10−6). The HPP-by-sex interaction reached nominal significance in both the basic and fully adjusted models (psexINT.basic=0.0094, psexINT.full.adj=0.0077), suggesting that the positive effect size is larger in males than females.
Figure 2. White matter hyperintensities associations with circulating levels of selected nonlipid metabolites.
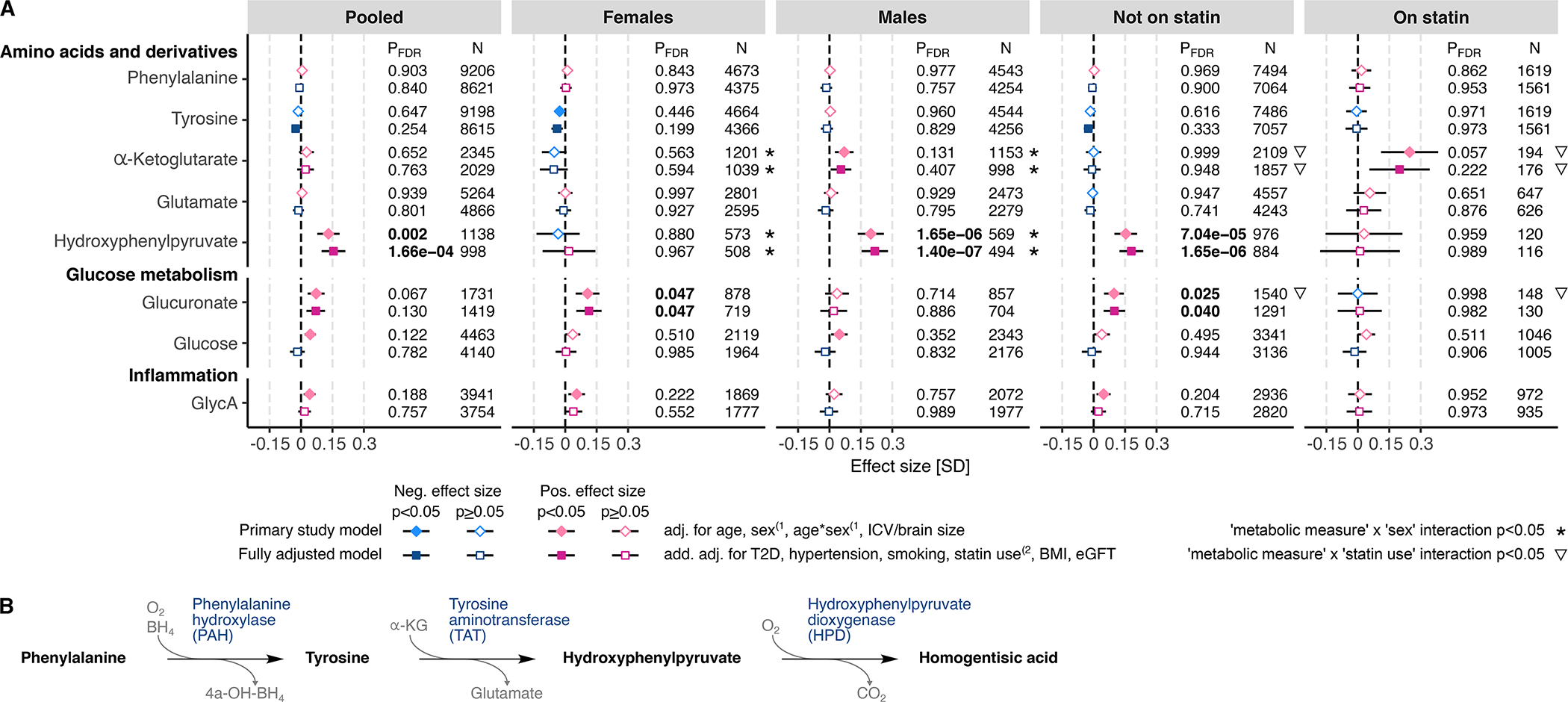
A) The associations between WMH and circulating metabolomic measures were determined using linear regression. The primary study models (diamonds) were adjusted for age and intracranial volume or brain size, and, in the pooled sample and in statin treatment-stratified subsamples also for sex and age-by-sex interaction(1. The fully adjusted models (squares) were additionally adjusted for type 2 diabetes (T2D), hypertension, current smoking status, body mass index (BMI), and estimated glomerular filtration rate (eGFR), and, in the pooled sample and in sex-stratified subsamples, also for statin treatment(2. Where relevant, all models were adjusted for fasting duration, time between blood sampling and brain MRI, and possible cohort study-specific covariates. Blue color indicates negative effect size and red color indicates positive effect size. Error bars indicate 95% confidence intervals. P-values below the threshold for multiple testing correction (pFDR<0.05) are indicated with bold font. B) Catabolic pathway of phenylalanine and tyrosine into hydroxyphenylpyruvate and homogentisic acid by phenylalanine hydroxylase (PAH), tyrosine aminotransferase (TAT) and hydroxyphenylpyruvate dioxygenase (HPD). FDR indicates false discovery rate; and GlycA, glycoprotein acetylation.
In females, the only significant association, after correction for multiple testing, was observed between WMH and higher circulating concentration of glucuronate (betabasic=0.11, pFDR.basic=0.047; betafull.adj=0.11, pFDR.full.adj=0.047; Figure 2); this association was significant also in statin-nontreated subsample (betabasic=0.10, pFDR.basic=0.025; betafull.adj=0.10, pFDR.full.adj=0.040). The glucuronate-by-sex interaction did not reach statistical significance (psexINT.basic=0.053, psexINT.full.adj=0.129).
Lipid measures
WMH volume was associated with lower circulating concentrations of lysophosphatidylcholines (LPCs) and hydroxylated sphingomyelins (SM-OHs) (Figure 3). Among the studied LPCs, the strongest association was seen with LPC(22:6), which was significant in the pooled sample (betabasic=−0.078, pFDR.basic=0.028; betafull.adj=−0.082, pFDR.full.adj=0.047) and in the male subsample (betabasic=−0.11, pFDR.basic=0.025; betafull.adj=−0.12, pFDR.full.adj=0.046). Among the studied SM-OHs, the strongest association was observed with SM (OH) C22:2, and this association was significant in the male subsample only (betabasic=−0.11, pFDR.basic=0.017; betafull.adj=−0.10, pFDR.full.adj=0.042). Typically, the interactions between LPC measures and sex or statin treatment did not reach statistical significance (Tables S5 and S6); the exceptions were nominally significant interaction with sex for LPC(17:0) in the basic and fully adjusted models (psexINT.basic=0.027, psexINT.fully.adj=0.045, respectively), and with statin treatment for LPC(20:4) in the fully adjusted model (pstatinINT.fully.adj=0.015) (Figure 3). The SM-OH species-by-sex interactions were nominally significant in both basic and fully adjusted models for SM (OH) C14:1 (psexINT.basic=0.018, psexINT.fully.adj=0.033), SM (OH) C16:1 (psexINT.basic=0.027, psexINT.fully.adj=0.044), and SM (OH) C22:2 (psexINT.basic=0.0061, psexINT.fully.adj=0.013). Also, SM (OH) C14:1-by-statin treatment interaction was nominally significant in the fully adjusted model (pstatinINT.fully.adj=0.047).
Figure 3. WMH associations with circulating levels of lysophosphatidylcholines (LPC) and hydroxylated sphingomyelins (SM (OH)).
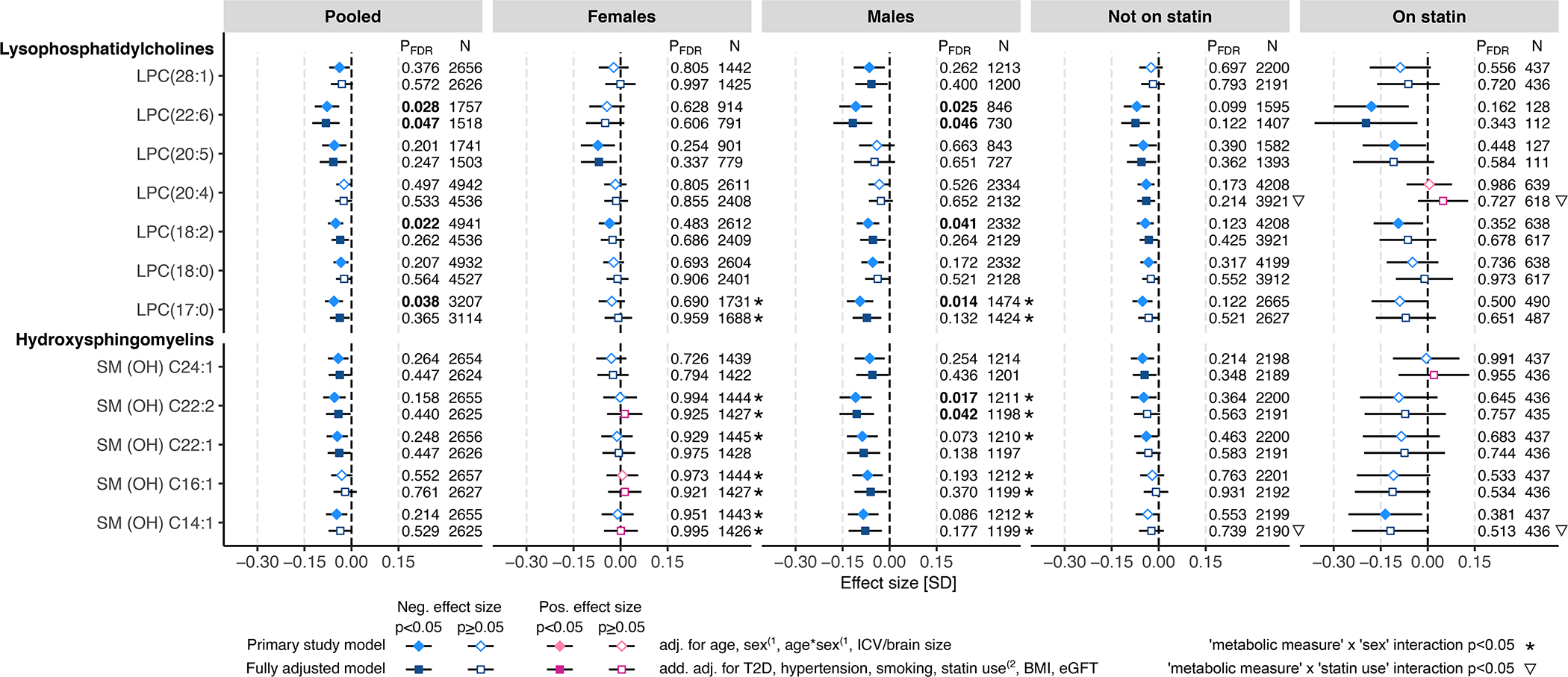
The associations between WMH and circulating metabolomic measures were determined using linear regression. The primary study models (diamonds) were adjusted for age and intracranial volume or brain size, and, in the pooled sample and in statin treatment-stratified subsamples also for sex and age-by-sex interaction(1. The fully adjusted models (squares) were additionally adjusted for type 2 diabetes (T2D), hypertension, current smoking status, body mass index (BMI), and estimated glomerular filtration rate (eGFR), and, in the pooled sample and in sex-stratified subsamples, also for statin treatment(2. Where relevant, all models were adjusted for fasting duration, time between blood sampling and brain MRI, and possible cohort study-specific covariates. Blue color indicates negative effect size and red color indicates positive effect size. Error bars indicate 95% confidence intervals. P-values below the threshold for multiple testing correction (pFDR<0.05) are indicated with bold font. FDR indicates false discovery rate; LPC, lysophosphatidylcholine; and SM (OH), hydroxylated sphingomyelin
A sizable proportion of the studied metabolomic measures were circulating concentrations of lipoproteins and lipoprotein lipids (approximately 24% of the meta-analyzed measures). Out of the 30 metabolomic measures showing FDR-significant associations with WMH in the basic models, 12 were with measures of the lipid composition of the intermediate density and low-density lipoprotein (IDL, LDL) particles (lipid composition calculated as a ratio of a lipid concentration against total lipids concentration within individual lipoprotein subfractions). Specifically, higher WMH volume was associated with higher TG-to-total-lipids ratio and lower cholesteryl ester (CE)-to-total-lipids ratio in the pooled sample and male subsample (Figure 4). The effect sizes were attenuated in the fully adjusted models (Figure 4). Concomitantly, higher WMH volume was associated with larger LDL-particle diameter in the male subsample, and this association remained FDR-significant in the fully adjusted model (betabasic=0.074, pFDR.basic=0.049; betafull.adj=0.078, pFDR.full.adj=0.047). We observed a nominally significant LDL diameter-by-sex interaction (psexINT.basic=0.0093, psexINT.full.adj=0.036). Higher WMH volume was also associated with lower free cholesterol-to-total-lipids-ratio within medium-sized very-low-density lipoprotein (VLDL) subfraction in the statin-treated subsample (Figure 1), but no other significant association with either VLDL or high-density lipoprotein (HDL) subfraction measures was seen (Tables S3 and S4).
Figure 4. WMH associations with LDL particle size and lipid composition measures in IDL and LDL subfractions.
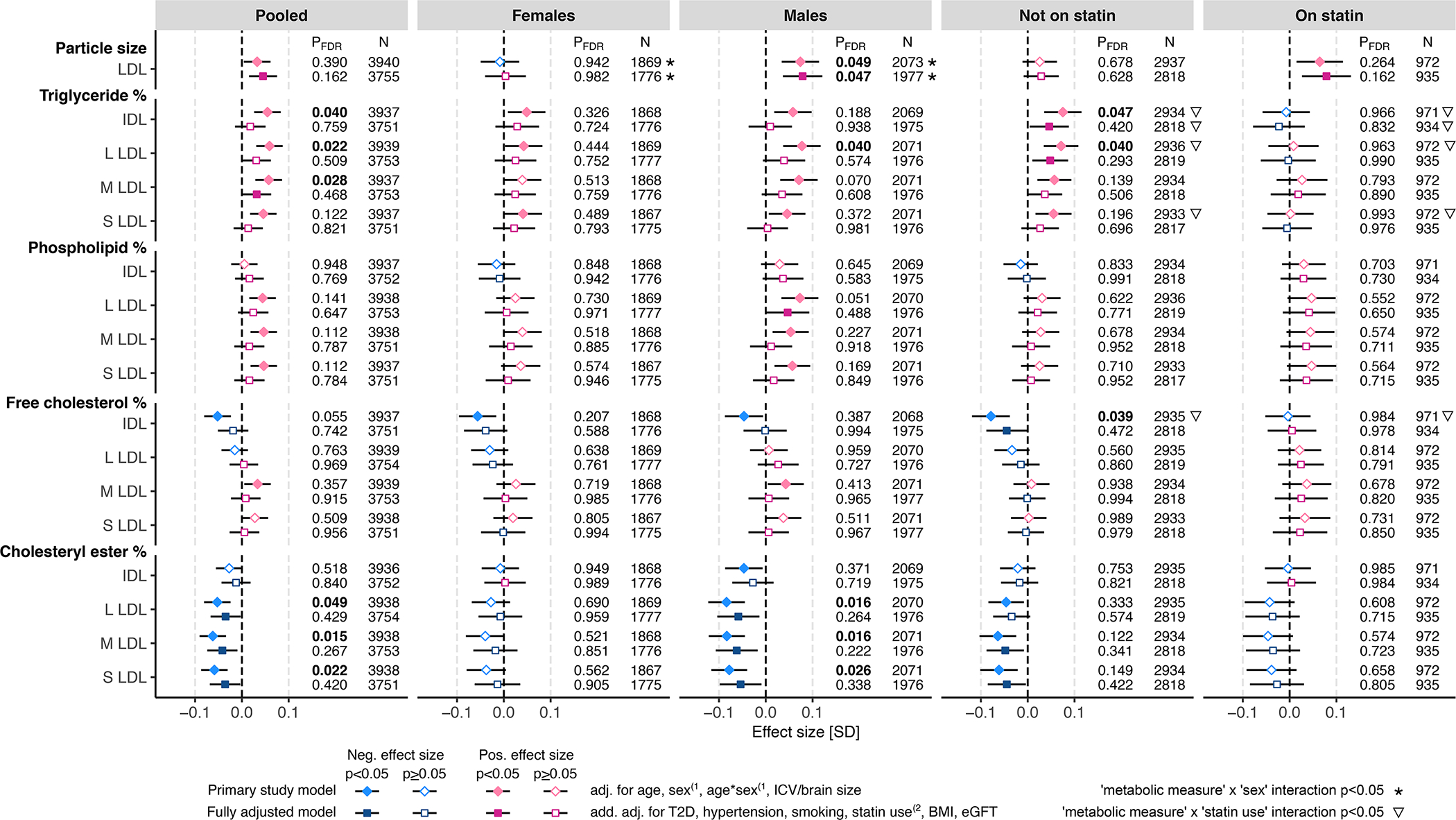
The associations between WMH and circulating metabolic measures were determined using linear regression. The primary study models (diamonds) were adjusted for age and intracranial volume or brain size, and, in the pooled sample and in statin treatment-stratified subsamples also for sex and age-by-sex interaction(1. The fully adjusted models (squares) were additionally adjusted for type 2 diabetes (T2D), hypertension, current smoking status, body mass index (BMI), and estimated glomerular filtration rate (eGFR), and, in the pooled sample and in sex-stratified subsamples, also for statin treatment(2. Where relevant, all models were adjusted for fasting duration, time between blood sampling and brain MRI, and possible cohort study-specific covariates. Blue color indicates negative effect size and red color indicates positive effect size. Error bars indicate 95% confidence intervals. P-values below the threshold for multiple testing correction (pFDR<0.05) are indicated with bold font. FDR indicates false discovery rate; IDL, intermediate-density lipoprotein; L, large; LDL, low-density lipoprotein; M, medium; and S, small.
DISCUSSION
In this study, we investigated associations between WMH volume and circulating metabolomic measures in up to 9,290 individuals. As discussed in the following text, several aspects of our metabolomic findings support a possible vessel-related pathophysiology of WMH, and some suggest the involvement of myelin disruption and neuron injury. Further, a number of the observed associations showed sex specificities indicating that distinct metabolomic features accompany WMH in males and females.
In the present study, the most robust association was the positive association between WMH volume and HPP in males (pFDR.full.adj=1.40×10−7); the association was also significant in the pooled sample (pFDR.full.adj=1.66×10−4) and statin-nontreated individuals (pFDR.full.adj=1.65×10−6). HPP is a potentially toxic compound derived from the catabolism of phenylalanine and tyrosine36 (Figure 2). Higher circulating levels of phenylalanine and tyrosine have been associated with higher risk of cardiovascular disease (CVD) in prior studies37,38, but, to our knowledge, HPP was not examined in those studies. We did not see strong associations with phenylalanine or tyrosine and, thus, the association between WMH and HPP likely arises downstream from phenylalanine hydroxylase (PAH) (Figure 2). The enzymatic alterations possibly contributing to higher level of HPP could be a higher activity of tyrosine aminotransferase (TAT) or lower activity of hydroxyphenylpyruvate dioxygenase (HPD). In tyrosine breakdown, TAT converts tyrosine and α-ketoglutarate to HPP and glutamate39 and, thus, higher TAT activity could contribute to lower α-ketoglutarate and, at the same time, to higher glutamate. In males and statin-treated participants, however, we found only a nominal association between WMH and circulating α-ketoglutarate that was not in the anticipated direction and no association with glutamate (Figure 2). Therefore, in the view of these results, lower HPD activity appears as the most likely mechanism driving the association between WMH and circulating HPP. HPD requires oxygen to convert HPP to homogentisic acid (Figure 2) and, consistent with this requirement, hypoxia promotes accumulation of HPP40. The deep and periventricular white matter, which is the predilection site for WMH, is characterized by sparse vasculature consisting of long, narrow end arteries/arterioles that are vulnerable to oxygen desaturation41. Thus, higher circulating HPP in association with higher WMH may be an indicator of ischemic hypoxia promoting WMH. As indicated above, the association of HPP with WMH volume was present in the pooled sample, in males, and in the statin-nontreated subsample, but not in females. In males, HPP explained 3.3% and 14.3% of variance in WMH in Insight46 and the 3rd Generation of Framingham Heart Study (FHS-GEN3), respectively, the two cohorts providing results for HPP in the fully adjusted model (Table S3). Consistent with previous research demonstrating that vascular risk factors, including hypertension, explain only up to 2% of variance in WMH volume7,42, we found the respective proportions of variance explained by hypertension, diabetes, and smoking to be 0.1%, 2.9%, and 0.03% in males from Insight46 and 1.1%, 1.0% and 0.1% in males from FHS-GEN3 (Table S3). Taken together, our results suggest that circulating HPP may be a strong biomarker of WMH in males, but its potential in clinical use requires further research.
Glucuronate was the only metabolite demonstrating a robust association with WMH in females (pFDR.full.adj=0.047); the association was also significant in statin-nontreated participants (pFDR.full.adj=0.040). Glucuronate is derived from glucose, and, in humans, it is involved in the elimination of toxic substances by making them more water-soluble in a process called glucuronidation36. In addition, hormones can be glucuronidated to enable easier transport36. A key enzyme of glucuronidation is UDP-glucuronosyltransferase, which is highly expressed in endothelial cells of the blood-brain barrier (BBB) and associated astrocytes, where the enzyme contributes to the protection of the brain from systemic toxic substances (reviewed by Ouzzine et al.43). Also, endothelial cells use glucuronate to synthesize glycosaminoglycans, which are constituents of the glycocalyx layer that tightens the endothelial barrier and limits vascular permeability44. The observed WMH association with glucuronate may involve altered glucose metabolism in endothelial cells, which might compromise the molecular mechanisms enabling the protective functions of the BBB. Of note, the association between WMH and glucuronate remained significant after adjusting for type 2 diabetes in the fully adjusted model. In our study, WMH association with (mostly fasting) glucose level did not reach statistical significance, which is in line with some45 but not all46 previous reports.
In addition to the above-discussed non-lipid measures, we found that WMH were associated with several circulating lipids – most notably with LPCs and SM-OHs. These associations were almost exclusively negative and reached statistical significance predominantly in males and, in some cases, also in the pooled sample. LPCs are phospholipids generated by partial hydrolysis of phosphatidylcholines, which are the building blocks of cell membranes, such as those of blood cells and endothelial cells. In circulation, LPCs modulate inflammation and oxidative stress47,48, and they may alter the integrity of endothelial membranes, including the BBB49,50. Lower circulating levels of LPCs, such as LPC(18:2), have been associated with higher CVD risk51 and adverse cognitive outcomes52. Consistent with these previous reports, we found that lower LPC(18:2) is associated with higher WMH volume, but similar to most other tested LPCs, the association reached only nominal significance in the fully adjusted model. The only LPC that remained significantly associated with WMH in the fully adjusted model was LPC(22:6) (males: pFDR.full.adj=0.046; pooled sample: pFDR.full.adj=0.047). Docosahexaenoic acid (DHA, 22:6n-3) is one of the two predominant fatty acids in the human brain and a structural component of neuronal cell membranes53,54. DHA plays a crucial role in neuronal survival, neurogenesis, and synaptic function53. Evidence obtained in mice suggests that oral administration of LPC-DHA, but not free DHA, increases DHA content of the brain and improves spatial learning and memory55. Thus, the associations of circulating LPCs with WMH observed here suggest the BBB and neuron injury-related pathobiologies of WMH.
Similar to LPCs, the associations between WMH and SM-OHs were negative, with the most significantly associated SM-OH being SM (OH) C22:2 in males (pFDR.full.adj=0.042). SM-OHs are important components of myelin sheaths56, which are lipid-rich cell membranes wrapped around neuronal axons, protecting the axons physically and providing trophic support, as well as increasing the conduction speed of action potentials57. The negative associations between WMH and SM-OHs could point towards disruption of myelin sheaths56, which is one of the key features of WMH1. In line with our findings, previous evidence suggests that low level of serum total SM is associated with cross-sectional memory impairment58.
Regarding the associations with lipoprotein measures, we observed that WMH were associated with larger LDL-particle diameter (pFDR.full.adj=0.047 in males), likely arising from altered LDL-lipid composition, namely relatively higher TG and lower CE content. Putatively, an altered interaction between lipoproteins and enzymes modulating their lipid composition could play a role in these associations. For example, the endothelium-bound lipoprotein lipase (LPL), which catalyzes the hydrolysis of lipoprotein TGs, interacts directly with LDL-receptor related protein59 that is involved in the regulation of the BBB permeability60; as such, LPL displays functions related to both blood metabolome and WMH found in areas with enhanced permeability of the BBB61. Statin treatment may have a small confounding effect on these associations, as statin-treated individuals have the highest WMH volumes together with the lowest cholesterol levels (Table 1). But considering the facts that the effect estimates of these measures were consistent across all analytical subsamples, including statin-nontreated individuals, and, that the effect estimates were only marginally attenuated in the statin use-adjusted models compared with the basic models (Figure 4), statin use does not seem to be a key factor driving the negative associations between WMH and lower CE content in the LDL subfractions.
In the present study, several of the FDR-significant associations in the fully adjusted models showed nominally significant metabolite-by-sex interactions suggesting that the effect sizes vary depending on sex. These associations included the amino acid-derivative HPP (psexINT.full.adj=0.0077), and two lipid measures, namely SM (OH) C22:2 (psexINT.full.adj=0.013) and LDL-particle size (psexINT.full.adj=0.036): for all three, the effect sizes were nominally greater in males than in females. These sex specificities were observed despite similar sample sizes of males and females, and no major sex differences in the distributions of WMH and key traits, such as BMI, cigarette smoking or clinical lipid traits (Table 1). Also, the metabolomic measures did not show major sex differences, except for the circulating levels of SM (OH) C22:2, which tended to be lower in males than in females (Figure S1), as reported previously62. Considering that hydroxysphingomyelins are important for the maintenance of myelination56, the lower levels of this lipid in males than in females could contribute to the nominally greater negative effect size of SM (OH) C22:2 on WMH in males than in females. Regarding HPP, the circulating levels were similar between males and females (Figure S1), and the biological mechanism of the nominally greater positive effect of HPP on WMH in males vs. females remains inconclusive. Nevertheless, it could be that circulating HPP is a marker of ischemic hypoxia that is of a similar magnitude in both males and females (as reflected by similar circulating levels of HPP in the two sexes), but the ischemic hypoxia is of a greater impact in males than in females. This is supported by previous research indicating that, in males compared with females, ischemic hypoxia in the brain induces greater oxidative stress and, thus, possibly greater tissue damage and more extensive WMH63,64. Finally, with respect to the LDL-particle size, this measure also did not show consistent sex differences across the analyzed cohorts (Figure S1), and the biological mechanisms of the nominally greater positive effect size of LDL-particle size on WMH in males than in females are not clear. Previous literature on sex differences in WMH is not extensive65–67. Sex hormones may play a role, as they show direct effects on the endothelium68 and multiple brain cells, including oligodendrocytes producing myelin69 and neurons70. In our study, female hormonal status was not likely a major factor in the observed sex specificities, as female participants were likely menopausal (average age >60 years). We cannot, however, exclude the possibility that the use of hormonal replacement therapy and a greater lifetime exposure to female sex hormones would modulate the associations examined in older age. Overall, our results indicating sex-specific associations between metabolomic measures and WMH add to the growing body of research reporting sex differences in the associations between risk factors and cerebrovascular outcomes71–75.
The strengths and limitations of this study should be considered. We analyzed data from multiple population-based cohorts that enables a large sample size, yielding the statistical power required to detect reliably the small effect sizes and to replicate/meta-analyze the results. Although the study sample included individuals from multiple geographical areas and diverse ethnic backgrounds, the vast majority of participants were of European ancestries, and, thus, replication of the findings in other ethnicities would be of high value. The fact that we analyzed metabolomic data quantified with multiple platforms could be considered as both a strength and a limitation: while covering a wide spectrum of circulating lipids and metabolites, some of the metabolic measures are underrepresented and, therefore, we may lack statistical power to detect associations with these measures. Further, due to the multiple testing correction to minimize the risk of false positive associations, some biologically relevant associations may be missed. For instance, many of the associations between WMH and lipid concentrations within IDL and LDL subfractions were negative with non-null-containing 95% confidence intervals; however, only a minority of these associations reached FDR-significance. As lipoprotein metabolism is an interconnected system, it may be more meaningful to look at the big picture instead of interpreting the associations on the level of individual metabolic measures. In most cohorts, WMH were quantified with automated image-analysis techniques. Therefore, we cannot exclude the possibility that small lacunar lesions (also a feature of small vessel disease2,76) were included in the quantified WMH volume. This potential misclassification is expected to be low, however, as lacune prevalence is not high in the general population77 and, when present, lacunes usually occur as singular or in low numbers, and the volume of WMH surrounding small lacunes is low. Manual segmentation of WMH is extremely labor-intensive and, therefore, it is not frequently used in large-scale cohort studies including thousands of participants6, such as the ones included here. In future studies, automatic segmentation of multi-modal MR images may allow one to compare metabolic profiles of different types of tissue abnormalities present in white matter. Additionally, it would be of high value to assess the causal inferences between circulating metabolites and WMH in future studies once suitable genetic instruments are available. Considering the results of our study, it is likely necessary to test the causality in sex-specific analyses. Finally, further insight into the pathophysiology of WMH awaits the results of LACI-2 trial, as this trial is testing two repurposed licenced drugs with effects on endothelium-dependent vasodilation and BBB integrity to prevent progression of cerebral small disease78.
In summary, this is the first large-scale study to determine associations between WMH and circulating metabolomic measures. While no causal associations can be inferred from the present findings, our findings indicate that alterations in circulating metabolism are closely linked with WMH in a general population of middle aged and older adults. The present findings suggest that the metabolomic profiles accompanying WMH in males and females may differ.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
This is the first large-scale study to identify circulating metabolomic measures associated with white matter hyperintensities (WMH) volume, which is the most common brain-imaging marker of small vessel disease and a major risk factor for incident stroke, dementia, and all-cause mortality.
The metabolomic profile of WMH volume appears largely sex specific.
The most significant metabolite, hydroxyphenylpyruvate, explains up to 6% and 14% of variance in WMH volume in the pooled sample and in males, respectively, which is comparatively more than the proportions of variance explained by hypertension (1%), type 2 diabetes (1–3%) or smoking (<0.1%).
What are the clinical implications?
Hydroxyphenylpyruvate is a new potentially useful clinical biomarker explaining more variance in WMH volume than the established WMH risk factors.
The marked sex specificities in the metabolomic associations of WMH volume add to the growing body of research suggesting that sex-specific molecular pathways underlie the associations between risk factors and cerebrovascular outcomes.
Some associated metabolites support vessel-related pathophysiology of WMH, while others suggest the involvement of myelin disruption and neuron injury.
Acknowledgements
SYS: The authors thank the following individuals for their contributions in data acquisition and storage in the SYS: Manon Bernard (database architect, The Hospital for Sick Children) and Hélène Simard (CÉGEP de Jonquière). The authors thank all participants who took part in the Saguenay Youth Study.
Support in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
Insight 46: The authors thank the study members who helped in the design of the study and the LHA and Insight 46 study teams for their contributions in data acquisition, analysis and storage.
We thank the LBC1936 participants and team members who contributed to these studies.
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. All study participants, general practitioners, specialists, researchers, institutions and funders of all other studies from this thesis are appreciatively acknowledged. Metabolomics measurements of Nightingales platform were funded by Biobanking and Biomolecular Resources Research Infrastructure (BBMRI)–NL(184.021.007).
Funding sources
SYS: The Saguenay Youth Study has been funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Foundation for Innovation, and National Institutes for Health (R01AG056726).
Insight 46 is principally funded by grants from Alzheimer’s Research UK, the Medical Research Council Dementias Platform UK and the Wolfson Foundation, and the British Heart Foundation. The National Survey of Health and Development is funded by the Medical Research Council). JS is supported by the University College London/University College London Hospitals National Institute for Health Research Biomedical Research Centre.
The LBC1936 is supported by Age UK (Disconnected Mind project, which supports S.E.H.), the Medical Research Council (MRC) (G0701120, G1001245, MR/M013111/1, MR/R024065/1, which supports S.R.C.), and the University of Edinburgh. MRI brain imaging was supported by MRC grants G0701120, G1001245, MR/M013111/1 and MR/R024065/1. Metabolomics were supported by the Dementia Platform UK, Medical Research Council grant MR/L023784/2, the Medical Research Council & National Institute for Health Research [grant number MC_PC_12025] and infrastructure support was provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
Support in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR001881, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
Conflict of interest disclosures
HJG has received travel grants and speaker honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag as well as research funding from Fresenius Medical Care.
Non-standard Abbreviations and Acronyms:
- BBB
blood-brain barrier
- CE
cholesteryl ester
- FDR
false discovery rate
- HPD
hydroxyphenylpyruvate dioxygenase
- HPP
hydroxyphenylpyruvate
- IDL
intermediate-density lipoprotein
- LDL
low-density lipoprotein
- LPC
lysophosphatidylcholines
- LPL
lipoprotein lipase
- MRI
magnetic resonance imaging
- PAH
phenylalanine hydroxylase
- SM-OH
hydroxylated sphingomyelin
- T1W
T1-weighted
- T2W
T2-weighted
- TAT
tyrosine aminotransferase
- TG
triacylglycero
- WMH
white matter hyperintensities
Footnotes
References
- 1.Moroni F, Ammirati E, Hainsworth AH, Camici PG. Association of White Matter Hyperintensities and Cardiovascular Disease: The Importance of Microcirculatory Disease. Circ Cardiovasc Imaging. 2020;1–13. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76:81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ. 2010;341:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power MC, Deal JA, Sharrett AR, Jack CR, Knopman D, Mosley TH, Gottesman RF. Smoking and white matter hyperintensity progression. Neurology. 2015;84:841 LP–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sargurupremraj M, Suzuki H, Jian X, Sarnowski C, Evans TE, Bis JC, Eiriksdottir G, Sakaue S, Terzikhan N, Habes M, Zhao W, Armstrong NJ, Hofer E, Yanek LR, Hagenaars SP, Kumar RB, van den Akker EB, McWhirter RE, Trompet S, Mishra A, Saba Y, Satizabal CL, Beaudet G, Petit L, Tsuchida A, Zago L, Schilling S, Sigurdsson S, Gottesman RF, Lewis CE, Aggarwal NT, Lopez OL, Smith JA, Valdés Hernández MC, van der Grond J, Wright MJ, Knol MJ, Dörr M, Thomson RJ, Bordes C, Le Grand Q, Duperron MG, Smith AV, Knopman DS, Schreiner PJ, Evans DA, Rotter JI, Beiser AS, Maniega SM, Beekman M, Trollor J, Stott DJ, Vernooij MW, Wittfeld K, Niessen WJ, Soumaré A, Boerwinkle E, Sidney S, Turner ST, Davies G, Thalamuthu A, Völker U, van Buchem MA, Bryan RN, Dupuis J, Bastin ME, Ames D, Teumer A, Amouyel P, Kwok JB, Bülow R, Deary IJ, Schofield PR, Brodaty H, Jiang J, Tabara Y, Setoh K, Miyamoto S, Yoshida K, Nagata M, Kamatani Y, Matsuda F, Psaty BM, Bennett DA, De Jager PL, Mosley TH, Sachdev PS, Schmidt R, Warren HR, Evangelou E, Trégouët DA, Amouyel P, de Andrade M, Basu S, Berr C, Brody JA, Chasman DI, Dartigues JF, et al. Cerebral small vessel disease genomics and its implications across the lifespan. Nat Commun. 2020;11:6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Allerhand M, Doubal FN, Hernandez MV, Morris Z, Gow AJ, Bastin M, Starr JM, Dennis MS, Deary IJ. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernath MM, Bhattacharyya S, Nho K, Barupal DK, Fiehn O, Baillie R, Risacher SL, Arnold M, Jacobson T, Trojanowski JQ, Shaw LM, Weiner MW, Doraiswamy PM, Kaddurah-Daouk R, Saykin AJ. Serum triglycerides in Alzheimer disease: Relation to neuroimaging and CSF biomarkers. Neurology. 2020;94:e2088–e2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, O’Brien R, Pletnikova O, Troncoso JC, Toledo J, Baillie R, Arnold M, Kastenmueller G, Nho K, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R, Legido-Quigley C, Thambisetty M. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018;15:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, Melo van Lent D, Tynkkynen J, Fischer K, Hernesniemi J, Haller T, Singh-Manoux A, Verhoeven A, Willemsen G, de Leeuw FA, Wagner H, van Dongen J, Hertel J, Budde K, Willems van Dijk K, Weinhold L, Ikram MA, Pietzner M, Perola M, Wagner M, Friedrich N, Slagboom PE, Scheltens P, Yang Q, Gertzen RE, Egert S, Li S, Hankemeier T, van Beijsterveldt CEM, Vasan RS, Maier W, Peeters CFW, Jörgen Grabe H, Ramirez A, Seshadri S, Metspalu A, Kivimäki M, Salomaa V, Demirkan A, Boomsma DI, van der Flier WM, Amin N, van Duijn CM. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimer’s Dement. 2018;14:707–722. [DOI] [PubMed] [Google Scholar]
- 11.Syme C, Pelletier S, Shin J, Abrahamowicz M, Leonard G, Perron M, Richer L, Veillette S, Gaudet D, Pike B, Strug LJ, Wang Y, Xu H, Taylor G, Bennett S, Paus T, Pausova Z. Visceral fat-related systemic inflammation and the adolescent brain: a mediating role of circulating glycerophosphocholines. Int J Obes. 2019;43:1223–1230. [DOI] [PubMed] [Google Scholar]
- 12.Sliz E, Shin J, Syme C, Patel Y, Parker N, Richer L, Gaudet D, Bennett S, Paus T, Pausova Z. A variant near DHCR24 associates with microstructural properties of white matter and peripheral lipid metabolism in adolescents. Mol Psychiatry. 2020; 10.1038/s41380-019-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sliz E, Shin J, Syme C, Black S, Seshadri S, Paus T, Pausova Z. Thickness of the cerebral cortex shows positive association with blood levels of triacylglycerols carrying 18-carbon fatty acids. Commun Biol. 2020;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Leeuw FA, Karamujić-Čomić H, Tijms BM, Peeters CFW, Kester MI, Scheltens P, Ahmad S, Vojinovic D, Adams HHH, Hankemeier T, Bos D, van der Lugt A, Vernooij MW, Ikram MA, Amin N, Barkhof F, Teunissen CE, van Duijn CM, van der Flier WM. Circulating metabolites are associated with brain atrophy and white matter hyperintensities. Alzheimer’s Dement. 2020;205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Misialek JR, Jack CR, Mielke MM, Knopman D, Gottesman R, Mosley T, Alonso A. Plasma metabolites associated with brain MRI measures of neurodegeneration in older adults in the atherosclerosis risk in communities– neurocognitive study (ARIC-NCS). Int J Mol Sci. 2019;20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV., Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, gene/environment susceptibility-reykjavik study: Multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 18.Tsao CW, Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): Overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane CA, Parker TD, Cash DM, Macpherson K, Donnachie E, Murray-Smith H, Barnes A, Barker S, Beasley DG, Bras J, Brown D, Burgos N, Byford M, Jorge Cardoso M, Carvalho A, Collins J, De Vita E, Dickson JC, Epie N, Espak M, Henley SMD, Hoskote C, Hutel M, Klimova J, Malone IB, Markiewicz P, Melbourne A, Modat M, Schrag A, Shah S, Sharma N, Sudre CH, Thomas DL, Wong A, Zhang H, Hardy J, Zetterberg H, Ourselin S, Crutch SJ, Kuh D, Richards M, Fox NC, Schott JM. Study protocol: Insight 46 - a neuroscience sub-study of the MRC National Survey of Health and Development. BMC Neurol. 2017;17:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor AM, Pattie A, Deary IJ. Cohort Profile Update : The Lothian Birth Cohorts of 1921 and 1936. Int J Epidemiol. 2018;47:1042–1042r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Bastin ME, Valde MC, Royle NA, Morris Z, Clayden JD, Sandeman EM, Eadie E, Murray C, Starr JM, Deary IJ. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. 2011;6:547–559. [DOI] [PubMed] [Google Scholar]
- 22.Hofman A, Breteler MMB, Van Duijn CM, Krestin GP, Pols HA, Stricker BHC, Tiemeier H, Uitterlinden AG, Vingerling JR, Witteman JCM. The Rotterdam Study: Objectives and design update. Eur J Epidemiol. 2007;22:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, Klaver CCW, Nijsten TEC, Peeters RP, Stricker BH, Tiemeier H, Uitterlinden AG, Vernooij MW, Hofman A. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, Kieboom BCT, Klaver CCW, de Knegt RJ, Luik AI, Nijsten TEC, Peeters RP, van Rooij FJA, Stricker BH, Uitterlinden AG, Vernooij MW, Voortman T. Objectives, design and main findings until 2020 from the Rotterdam Study. Springer; Netherlands; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, Peron M, Pike GB, Richer L, Séguin JR, Veillette S. Cohort Profile: The Saguenay Youth Study (SYS). Int J Epidemiol. 2017;46:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John U, Hensel E, Lüdemann J, Piek M, Sauer S, Adam C, Born G, Alte D, Greiser E, Haertel U, Hense HW, Haerting J, Willich S, Kessler C. Study of Health in Pomerania (SHIP): A health examination survey in an east German region: Objectives and design. Soz Praventivmed. 2001;46:186–194. [DOI] [PubMed] [Google Scholar]
- 27.Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Jünger M, Mayerle J, Kraft M, Lerch MM, Dörr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Gläser S, Ewert R, Fietze I, Penzel T, Dören M, Rathmann W, Haerting J, Hannemann M, Röpcke J, Schminke U, Jürgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kühn JP, Kühn J, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Völker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W. Cohort profile: The study of health in Pomerania. Int J Epidemiol. 2011;40:294–307. [DOI] [PubMed] [Google Scholar]
- 28.Jones S, Tillin T, Park C, Williams S, Rapala A, Al Saikhan L, Eastwood SV, Richards M, Hughes AD, Chaturvedi N. Cohort profile update: Southall and Brent revisited (SABRE) study: a UK population-based comparison of cardiovascular disease and diabetes in people of European, South Asian and African Caribbean heritage. Int J Epidemiol. 2020;1441–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am J Epidemiol. 2017;186:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dona AC, Jiménez B, Schafer H, Humpfer E, Spraul M, Lewis MR, Pearce JTM, Holmes E, Lindon JC, Nicholson JK. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 2014;86:9887–9894. [DOI] [PubMed] [Google Scholar]
- 31.Römisch-Margl W, Prehn C, Bogumil R, Röhring C, Suhre K, Adamski J. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics. 2012;8:133–142. [Google Scholar]
- 32.Jiménez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, Chetwynd AJ, Cannet C, Fang F, Pearce JTM, Lewis MR, Viant MR, Lindon JC, Spraul M, Schäfer H, Nicholson JK. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by 1H NMR Spectroscopy in a Multilaboratory Trial. Anal Chem. 2018;90:11962–11971. [DOI] [PubMed] [Google Scholar]
- 33.Harris SE, Ritchie SJ, Correia GDS, Jiménez B, Fawns-Ritchie C, Pattie A, Corley J, Maniega SM, Hernández MV, Starr JM, Hill D, Wren P, Bastin ME, Lewis MR, Wardlaw JM, Deary IJ. Plasma lipid and liporotein biomarkers in LBC1936: Do they predict general cognitive ability and brain structure? bioRxiv. Preprint posted online July 17, 2020. doi:10.1101/. [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 35.R Core Team. R: A language and environment for statistical computing. 2021;URL https://www.R-project.org/.
- 36.Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018;46:D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, Kangas AJ, Kettunen J, Kaikkonen J, Mikkilä V, Jula A, Kähönen M, Lehtimäki T, Lawlor DA, Gaunt TR, Hughes AD, Sattar N, Illig T, Adamski J, Wang TJ, Perola M, Ripatti S, Vasan RS, Raitakari OT, Gerszten RE, Casas JP, Chaturvedi N, Ala-Korpela M, Salomaa V. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Würtz P, Raiko JR, Magnussen CG, Soininen P, Kangas AJ, Tynkkynen T, Thomson R, Laatikainen R, Savolainen MJ, Laurikka J, Kuukasjärvi P, Tarkka M, Karhunen PJ, Jula A, Viikari JS, Kähönen M, Lehtimäki T, Juonala M, Ala-Korpela M, Raitakari OT. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J. 2012;33:2307–2316. [DOI] [PubMed] [Google Scholar]
- 39.Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, Sidiropoulos K, Cook J, Gillespie M, Haw R, Loney F, May B, Milacic M, Rothfels K, Sevilla C, Shamovsky V, Shorser S, Varusai T, Weiser J, Wu G, Stein L, Hermjakob H, D’Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones DP, Mason HS. Metabolic hypoxia: accumulation of tyrosine metabolites in hepatocytes at low pO2. Biochem Biophys Res Commun. 1978;80:477–83. [DOI] [PubMed] [Google Scholar]
- 41.Sosa SM, Smith KJ. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin Sci. 2017;131:2503–2524. [DOI] [PubMed] [Google Scholar]
- 42.Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, Fawns-Ritchie C, Barbu MC, De Nooij L, Reus LM, Alloza C, Shen X, Neilson E, Alderson HL, Hunter S, Liewald DC, Whalley HC, McIntosh AM, Lawrie SM, Pell JP, Tucker-Drob EM, Wardlaw JM, Gale CR, Deary IJ. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40:2290–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouzzine M, Gulberti S, Ramalanjaona N, Magdalou J, Fournel-Gigleux S. The UDP-glucuronosyltransferases of the blood-brain barrier: Their role in drug metabolism and detoxication. Front Cell Neurosci. 2014;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev. 2018;98:3–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, DeCarli C. Stroke risk profile predicts white matter hyperintensity volume: The Framingham study. Stroke. 2004;35:1857–1861. [DOI] [PubMed] [Google Scholar]
- 46.Scharf EL, Graff-Radford J, Przybelski SA, Lesnick TG, Mielke MM, Knopman DS, Preboske GM, Schwarz CG, Senjem ML, Gunter JL, Machulda M, Kantarci K, Petersen RC, Jack CR, Vemuri P. Cardiometabolic Health and Longitudinal Progression of White Matter Hyperintensity: The Mayo Clinic Study of Aging. Stroke. 2019;50:3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marathe GK, Pandit C, Lakshmikanth CL, Chaithra VH, Jacob SP, D’Souza CJM. To hydrolyze or not to hydrolyze: the dilemma of platelet-activating factor acetylhydrolase. J Lipid Res. 2014;55:1847–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan JJ, Jung JS, Lee JEJ, Lee JEJ, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. [DOI] [PubMed] [Google Scholar]
- 49.Sevastou I, Kaffe E, Mouratis MA, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. Biochim Biophys Acta - Mol Cell Biol Lipids. 2013;1831:42–60. [DOI] [PubMed] [Google Scholar]
- 50.Ousman SS, David S. Rapid Recruitment and Activation of Macrophages In the Adult Mouse Spinal Cord. Glia. 2000;104:92–104. [PubMed] [Google Scholar]
- 51.Ganna A, Salihovic S, Sundström J, Broeckling CD, Hedman ÅK, Magnusson PKE, Pedersen NL, Larsson A, Siegbahn A, Zilmer M, Prenni J, Ärnlöv J, Lind L, Fall T, Ingelsson E. Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease. PLoS Genet. 2014;10:e1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, Macarthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. [DOI] [PubMed] [Google Scholar]
- 54.Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, Lin LE, Ermini L, Post M, Thies F, Bazinet RP. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep. 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zöller I, Meixner M, Hartmann D, Büssow H, Meyer R, Gieselmann V, Eckhardt M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci. 2008;28:9741–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadelmann C, Timmler S, Barrantes-Freer A, Simons M. Myelin in the central nervous system: Structure, function, and pathology. Physiol Rev. 2019;99:1381–1431. [DOI] [PubMed] [Google Scholar]
- 58.Mielke MM, Bandaru VVR, Haughey NJ, Rabins PV., Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2010;31:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beisiegel U, Heeren J. Lipoprotein lipase (EC 3.1.1.34) targeting of lipoproteins to receptors. Proc Nutr Soc. 1997;56:731–737. [DOI] [PubMed] [Google Scholar]
- 60.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. [DOI] [PubMed] [Google Scholar]
- 61.Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, Muñoz-Maniega S, Sakka E, Shuler K, Dennis MS, Thrippleton MJ. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer’s Dement. 2017;13:634–643. [Google Scholar]
- 62.Muilwijk M, Callender N, Goorden S, Vaz FM, van Valkengoed IGM. Sex differences in the association of sphingolipids with age in Dutch and South-Asian Surinamese living in Amsterdam, the Netherlands. Biol Sex Differ. 2021;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Netto CA, Sanches E, Odorcyk FK, Duran-Carabali LE, Weis SN. Sex-dependent consequences of neonatal brain hypoxia-ischemia in the rat. J Neurosci Res. 2017;95:409–421. [DOI] [PubMed] [Google Scholar]
- 64.Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G. Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem. 2016;137:714–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Den Heuvel DMJ, Admiraal-Behloul F, Ten Dam VH, Olofsen H, Bollen ELEM, Murray HM, Blauw GJ, Westendorp RGJ, De Craen AJM, Van Buchem MA. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63:1699–1701. [DOI] [PubMed] [Google Scholar]
- 66.Ammirati E, Moroni F, Magnoni M, Rocca MA, Anzalone N, Cacciaguerra L, Di Terlizzi S, Villa C, Sizzano F, Palini A, Scotti I, Besana F, Spagnolo P, Rimoldi OE, Chiesa R, Falini A, Filippi M, Camici PG. Progression of brain white matter hyperintensities in asymptomatic patients with carotid atherosclerotic plaques and no indication for revascularization. Atherosclerosis. 2019;287:171–178. [DOI] [PubMed] [Google Scholar]
- 67.Jiménez-Sánchez L, Hamilton OK, Backhouse EV, Clancy U, Steward CR, Wardlaw JM. Sex differences in cerebral small vessel disease: a systematic review and meta-analysis. medRxiv. Preprint posted online March 8, 2021. doi:10.1101/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol - Hear Circ Physiol. 2018;315:H1569–H1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cerghet M, Skoff RP, Swamydas M, Bessert D. Sexual dimorphism in the white matter of rodents. J Neurol Sci. 2009;286:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pesaresi M, Soon-Shiong R, French L, Kaplan DR, Miller FD, Paus T Axon diameter and axonal transport: In vivo and in vitro effects of androgens. Neuroimage. 2015;115:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, Kissela BM, Safford MM, Moy CS, McClure LA, Howard G, Cushman M. Sex and Race Differences in the Association of Incident Ischemic Stroke with Risk Factors. JAMA Neurol. 2019;76:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet. 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 73.Madsen TE, Howard G, Kleindorfer DO, Furie KL, Oparil S, Manson JE, Liu S, Howard VJ. Sex Differences in Hypertension and Stroke Risk in the REGARDS Study: A Longitudinal Cohort Study. Hypertension. 2019;74:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O, Khatri P, Flaherty M, De Los Rios La Rosa F, MacKey J, Mistry E, Demel SL, Coleman E, Jasne A, Slavin SJ, Walsh K, Star M, Broderick JP, Kissela BM, Kleindorfer DO. Temporal Trends in Stroke Incidence over Time by Sex and Age in the GCNKSS. Stroke. 2020;1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters SAE, Carcel C, Millett ERC, Woodward M. Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology. 2020;95:e2715–e2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, DeCarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, van Oostenbrugge R, Pantoni L, Speck O, Stephan BCM, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yilmaz P, Ikram MK, Niessen WJ, Ikram MA, Vernooij MW. Practical small vessel disease score relates to stroke, dementia, and death: The Rotterdam study. Stroke. 2018;49:2857–2865. [DOI] [PubMed] [Google Scholar]
- 78.Wardlaw J, Bath PMW, Doubal F, Heye A, Sprigg N, Woodhouse LJ, Blair G, Appleton J, Cvoro V, England T, Hassan A, John Werring D, Montgomery A. Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur Stroke J. 2020;5:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vidal JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, Garcia ME, Van Buchem MA, Harris TB, Gudnason V, Launer LJ. Coronary artery calcium, brain function and structure: The AGES-Reykjavik study. Stroke. 2010;41:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: The lothian birth cohorts of 1921 and 1936. Int J Epidemiol. 2012;41:1576–1584. [DOI] [PubMed] [Google Scholar]
- 82.Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, Campbell H, Whalley LJ, Visscher PM, Porteous DJ, Starr JM. The Lothian Birth Cohort 1936: A study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N, Chaturvedi N, Beauchamp N, Coady E, Collins R, Forouhi N, Gedroyc W, Godsland I, Hattersley A, Hughes A, Majeed F, Mayet J, McKeigue P, Sattar N, Shibata D, Whincup P, Wright A. Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. 2012;41:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Oskarsdottir B, Jonsson PV, Eiriksdottir G, Tamara B, Zijdenbos A, Van Buchem MA, Launer LJ, Gudnason V. Brain tissue volumes in the general population of the elderly The AGES-Reykjavik Study. Neuroimage. 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sudre CH, Cardoso MJ, Willem H, Biessels GJ, Barnes J, Ourselin S. Bayesian Model Selection for Pathological Neuroimaging Data Applied to White Matter Lesion Segmentation. IEEE Trans Med Imaging. 2015;34:2079–2102. [DOI] [PubMed] [Google Scholar]
- 86.de Boer R, Vrooman HA, van der Lijn F, Vernooij MW, Ikram MA, van der Lugt A, Breteler MMB, Niessen WJ. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45:1151–1161. [DOI] [PubMed] [Google Scholar]
- 87.Sudre CH, Smith L, Atkinson D, Chaturvedi N, Ourselin S, Barkhof F, Hughes AD, Jäger HR, Cardoso MJ. Cardiovascular Risk Factors and White Matter Hyperintensities: Difference in Susceptibility in South Asians Compared With Europeans. J Am Heart Assoc. 2018;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Launer LJ, Rosseel Y, Janowitz D, Doshi J, Van Der Auwera S, Von Sarnowski B, Hegenscheid K, Schminke U, Hosten N, Homuth G, Vo H, Hoffmann W, Grabe HJ, Davatzikos C. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. 2016;139:1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


