Abstract
Interleukin-8 (IL-8) production in vivo was monitored in four study groups: normal blood donors, patients with pulmonary tuberculosis (TB), patients with human immunodeficiency virus type 1 (HIV-1) infection, and dually infected (HIV/TB) patients. We show that whereas there was evidence of detectable levels of cell-associated IL-8 (mRNA and protein) in peripheral cells of healthy individuals, this was largely lost in the disease states studied. Coupled with this finding was significantly increased circulating levels of IL-8 in HIV-1-infected individuals with or without concomitant pulmonary TB (P < 0.001). On the other hand, the capacity of peripheral mononuclear cells to produce IL-8 spontaneously ex vivo was enhanced in HIV-1 and TB patients (P < 0.05) and many of the HIV/TB group, but their corresponding capacities to respond to various stimuli, in particular phytohemagglutinin, were significantly diminished compared to those of normal donors (P < 0.05). Circulating levels of IL-8 in a group of HIV/TB patients were significantly positively correlated with the percentage of polymorphonuclear leukocytes (PMN) in the peripheral circulation (r = 0.65; P = 0.01), the proportions of IL-8 receptor A (IL-8RA)-expressing (r = 0.86; P < 0.01) and IL-8RB-expressing (r = 0.77; P < 0.01) PMN, and the capacity of PMN to migrate in response to IL-8 as chemoattractant (r = 0.68; P < 0.01). IL-8RB fluorescence intensity, however, was negatively correlated with plasma IL-8 levels (r = −0.73; P < 0.01). Our results suggest that altered regulation of IL-8 in HIV-1 may have important implications for antimicrobial defenses and for normal immune processes.
The development of tuberculosis (TB) occurs as an early complication of human immunodeficiency virus (HIV) infection in 50 to 67% of HIV-infected individuals (7), the result of either reactivation of a latent mycobacterial infection (32), a reinfection with Mycobacterium tuberculosis (35), or the rapid progression of a recently acquired infection (6). Studies have shown that patients with active TB who are coinfected with HIV have a lower survival rate than patients without HIV infection (27, 36). The proposal that M. tuberculosis accelerates the progression of HIV type 1 (HIV-1) infection is supported by both clinical studies (21, 41, 43) and in vitro studies, which have demonstrated that phagocytosis of tubercle bacilli by HIV-1-infected monocytic cells induces viral expression (34, 47). Furthermore, the induction of cytokines such as tumor necrosis factor alpha by mycobacterial products may promote HIV-1 expression in latently infected cells (9). In addition, dual infection with both organisms poses an increased risk of these individuals acquiring new opportunistic infections compared to those infected with HIV-1 alone (43).
The chemokine family of cytokines, known to mediate a variety of inflammatory processes such as activation and directed migration of neutrophils, monocytes, and lymphocytes (25, 28), constitutes two subfamilies that share a highly conserved structural motif of four cysteine residues. In the first subfamily, the first two cysteine residues are adjacent (CC), whereas in the second subfamily, the first cysteine pair is separated by an intervening amino acid (CXC). Interleukin-8 (IL-8), a member of the CXC chemokine subfamily, has been described mainly as a neutrophil chemotactic factor which is produced by stimulated human blood mononuclear leukocytes (46), T lymphocytes, endothelial cells, keratinocytes, and fibroblasts (19), polymorphonuclear neutrophils (2), and the most prominent source, monocytes/macrophages (3, 46). When infected in vitro with HIV-1, monocytes/macrophages give rise to increased IL-8 production (8, 40). Similarly, IL-8 release from macrophages is enhanced after exposure to M. tuberculosis and its cell wall components (48) and following phagocytosis of M. tuberculosis by human monocytic cell lines (11).
Elevated levels of IL-8 have been found in the serum (22) and plasma (39) samples of HIV-1-infected patients. Increased production of IL-8 has also been found in the bronchoalveolar lavage fluid from HIV-1-infected individuals (5, 20) and from patients infected with M. tuberculosis (47). Circulating levels of IL-8 in plasma of TB patients have been found to be increased with time as a result of anti-TB therapy (13), and high admission plasma levels of IL-8 are of clinical prognostic significance in TB (12).
IL-8, by binding to either of its two specific receptors, CXCR-1 (IL-8 receptor A [IL-8RA]) (15) or CXCR-2 (IL-8RB) (26), induces a variety of cell-type-specific responses (19), including the induction of neutrophil respiratory burst, lysosomal enzyme release, enhanced killing of various microorganisms, including M. tuberculosis, and chemotaxis of neutrophils, T cells, and basophils. Specific neutrophil functions mediated by IL-8 have been assigned to each IL-8R (16). We have recently shown that both IL-8RA and IL-8RB are down-regulated on polymorphonuclear leukocytes (PMN) in the presence of HIV-1 infection or pulmonary TB and in coinfection (24) and that this diminished expression is associated with impaired migration of these cells in response to IL-8. The expression of either IL-8R on lymphocytes was also reduced in HIV-1 infection. Considering that a number of IL-8-dependent functions of PMN and lymphocytes are therefore likely to be altered in vivo, the question of how the major ligand for these receptors is modulated in HIV-1 infection and pulmonary TB becomes important. This study was therefore undertaken to address aspects relating to IL-8 production that may have an impact on its role as an antimicrobial chemokine in the presence of HIV-1 infection, pulmonary TB, and coinfection. We first questioned if IL-8 production was differentially modulated in the different disease states and sought to identify the IL-8 producer cell type in vivo. Included were evaluations of peripheral circulating and cell-associated IL-8 levels, the capacity of mononuclear cells to produce IL-8 spontaneously or when stimulated in vitro, and the production of IL-8 mRNA in specific mononuclear cell fractions. PMN, although formerly thought to have limited protein-synthesizing capacity, when induced have the ability to produce a number of cytokines, including IL-8 (1). The possible contribution of PMN to IL-8 production in either HIV-1 or M. tuberculosis infection, and coinfection was also addressed. More specifically, we attempted to correlate increased levels of peripheral IL-8 that are associated with HIV-1 infection with altered immune activity as measured by PMN migration in response to IL-8.
MATERIALS AND METHODS
Subjects.
Serum samples were collected from four subject groups that included 40 healthy blood donors (ND group), individuals diagnosed with pulmonary TB (TB group) patients seropositive for HIV-1 (HIV group), and HIV-1-seropositive patients with pulmonary TB (HIV/TB group) and were immediately processed and stored. There were approximately equivalent numbers of male and female subjects, all aged between 25 and 40 years, in each group. Apart from age, sex, and disease diagnosis, no other information was available for these individuals. A further four subject groups consisting of 16 of each of ND, TB, HIV, and HIV/TB individuals were recruited for further evaluations. All TB and HIV/TB patients were receiving standard four-drug (rifampin, isoniazid, pyrazinamide, and ethambutol) anti-TB therapy for the first 2 months and then therapy excluding ethambutol for the remaining period. The mean durations of anti-TB treatment did not differ significantly between the TB and HIV/TB groups (P > 0.05) and were 25.5 and 27 weeks, respectively. Blood was collected by venipuncture into EDTA Vacutainers (Becton Dickinson, San Jose, Calif.). The blood was processed immediately upon collection, and plasma was stored at −70°C until testing. The immunological characteristics of the recruited individuals within each group are shown in Table 1. For mononuclear cell fractionation studies, blood was drawn separately from another four individuals belonging to each of the four groups. Their CD4 and CD8 cell counts are shown in Fig. 5. A further 14 HIV/TB patients with characteristics similar to those described in Table 1 were recruited for comparative determinations on each sample of plasma IL-8 levels, PMN capacity to migrate in response to IL-8, and expression of each of the two IL-8 receptors, A and B, on whole-blood PMN. This study was approved by the University of the Witwatersrand Ethical Committee; patients were recruited after informed consent was obtained, and confidentiality of all records was ensured.
TABLE 1.
Immunological status and plasma IL-8 levels of study groups
| Determination | Value for indicated study group (n = 16/group)
|
|||
|---|---|---|---|---|
| ND | TB | HIV | HIV/TB | |
| Count (mean ± SEM) | ||||
| Leukocytes (103/μl) | 5.4 ± 0.2 | 7.16 ± 0.74 | 5.53 ± 0.57 | 6.12 ± 0.43 |
| Monocytes (103/μl) | 0.32 ± 0.02 | 1.12 ± 0.25 | 0.48 ± 0.06 | 0.56 ± 0.08 |
| Granulocytes (103/μl) | 2.8 ± 0.2 (50.6 ± 2.0a) | 3.18 ± 0.34 (44.8 ± 2.4) | 3.03 ± 0.46 (51.9 ± 2.9) | 2.96 ± 0.38 (48.6 ± 4.9) |
| CD4 cells/μl | 977 ± 46 (44.8 ± 1.7) | 1,181 ± 128 (43.1 ± 1.9) | 452 ± 61 (21.6 ± 2.3) | 301 ± 115 (12.2 ± 2.7) |
| CD8 cells/μl | 580 ± 36 (27.8 ± 1.7) | 869 ± 113 (30.3 ± 2.0) | 1,079 ± 117 (53.1 ± 3.4) | 1,556 ± 256 (57.1 ± 3.9) |
| CD4/CD8 ratio (mean ± SEM) | 1.68 ± 0.2 | 1.36 ± 0.14 | 0.42 ± 0.08 | 0.19 ± 0.06 |
| Plasma IL-8 (pg/ml)b | <5.0 (<5.0–19.05) | <5.0 (<5.0–34.67) | 12.94 (<5.0–407.38) | 12.62 (<5.0–63.09) |
Percentage of cells.
Median, with range in parentheses.
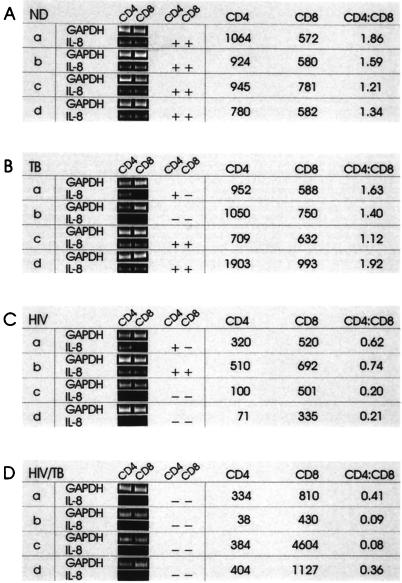
FIG. 5.
IL-8 mRNA expression in CD4 and CD8 mononuclear cell subsets of ND (A), TB (B), HIV (C), and HIV/TB (D) individuals. Mononuclear cells from four individuals (a to d) within each group (their immunological characteristics shown at the right) were fractionated into total PBMC, CD14, CD4, CD8, CD19, and a remaining cell population. mRNA was isolated, and RT-PCR performed with GAPDH- and IL-8-specific primers as described in Materials and Methods. Results are shown for the cell fractions that were consistently positive for IL-8 mRNA in the ND group, namely, CD4 and CD8 cells. Durations of anti-TB treatment: TB-a to TB-d, 8, 15, 26, and 2 weeks, respectively; HIV/TB-a to HIV/TB-d, 7, 10, 13, and 8 weeks, respectively.
IL-8 ELISA.
Plasma and serum IL-8 levels were determined by using the Biotrak human IL-8 enzyme-linked immunosorbent assay (ELISA) system (Amersham, Buckinghamshire, England) according to the manufacturer’s instructions. The minimum detectable dose of IL-8 is 0.5 pg/well. For determination of IL-8 levels in cell lysates, the control IL-8 titration was carried out with lysing solution (1% Triton-X100 in phosphate-buffered saline [PBS]) as the diluent. There was no significant difference in the IL-8 titration curves determined in the presence of the supplied serum control diluent, cell culture growth medium, or lysing solution.
HIV-1 quantitation.
HIV-1 plasma levels were quantitated from 200 μl of patient plasma by using the Amplicor HIV-1 Monitor test (Roche Diagnostic Systems, Nutley, N.J.) according to the manufacturer’s instructions. Amplification reactions were carried out using a Perkin-Elmer GeneAmp PCR System 2400 (Perkin-Elmer, Norwalk, Conn.).
Isolation of peripheral blood leukocytes.
Peripheral blood mononuclear cells (PBMC) were isolated by routine methods from whole blood by centrifugation on Histopaque-Ficoll (Sigma Chemical Co., Oxford, England). PMN were isolated by layering the Ficoll-granulocyte-erythrocyte (RBC) interface onto a second Ficoll gradient, centrifuging it at 1,000 × g for 30 minutes, and again removing the same interface. RBC were lysed in a solution of 0.15 M NH4Cl, 10 mM KHCO3, and 1 mM sodium EDTA (pH 7.0).
Isolation and preparation of PBMC, PMN, and RBC lysates.
Purified PBMC and PMN lysates were prepared by resuspending 106 cells in 100 μl of 1% Triton X-100–PBS lysis buffer and incubated on ice for 40 min. RBC were isolated by washing the RBC pellet four times with PBS, centrifuging at 180 × g for 10 min each time. RBC were adjusted to the original hematocrit for confirmation of the removal of leukocytes (<200/μl) and platelets (<1,000/μl), using a Coulter Counter (Coulter Corp., Hialeah, Fla.). The RBC concentration was then adjusted to 5 × 106 cells/100 μl of lysis buffer, and the cells were incubated as for PBMC and PMN lysates.
In vitro stimulation of primary cell cultures.
PBMC and PMN were adjusted to a concentration of 106 cells/ml in RPMI 1640 medium supplemented with 10 mM HEPES, 100 μg of penicillin per ml, 100 μg of streptomycin per ml, and 10% (vol/vol) fetal calf serum. PBMC were either untreated or stimulated with phytohemagglutinin (PHA; Sigma) purified protein derivative (PPD), or lipopolysaccharide (LPS; Sigma) at final concentrations of 5, 50, and 1 μg/ml, respectively. One hundred-microliter aliquots (105 cells) of each treatment were then placed into four separate wells of a 96-well microtiter plate and incubated at 37°C. After 2 days, the culture supernatants were harvested and replicates were pooled. Levels of IL-8 in each of the supernatants were determined in duplicate, using the Biotrak IL-8 ELISA system (Amersham). PMN cultures were either untreated or stimulated with PPD as described above.
Magnetic bead cell separations.
Freshly isolated PBMC were immunomagnetically separated into different cell compartments, using the MACS magnetic cell sorting system (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, 5 × 106 PBMC were resuspended in 80 μl of wash buffer (PBS containing 5 mM EDTA and 0.5% bovine serum albumin) to which 10 μl of the appropriately conjugated MACS magnetic microbead solution was added. After a 15-min incubation at 4°C, 500 μl wash buffer was added to the magnetically labeled cell suspension, which was then pipetted onto a MiniMACS separation column. The suspension was then allowed to run through the column, 500 μl wash buffer was added to wash the column, and the effluent was collected as the negative fraction. After being washed twice with 500 μl of wash buffer, the column was removed from the separation unit, 1 ml of wash buffer was added to the top of the column and flushed through with a plunger, and the effluent was collected as the positive cell fraction. The negative cell fraction was then pelleted and resuspended in 80 μl of wash buffer, and the next volume of antibody-conjugated magnetic microbeads was added to positively select the second cell population in a similar manner. This process was continued, allowing sequentially the positive selection of CD14, CD4, CD8, and then CD19 cells. Total PBMC and the cell fraction remaining after the specific microbead isolations were also collected.
RNA isolation and RT-PCR.
Cytoplasmic RNA was isolated from total PBMC and mononuclear cell fractions by Nonidet P-40 lysis extraction (14). cDNA was synthesized by using 60 pmol of primer p(dT)15 (Boehringer Mannheim, Mannheim, Germany) and 8 U of avian myeloblastosis reverse transcriptase (AMV RT) (Promega, Madison, Wis.) in the presence of 40 U of RNAsin and 500 μM each dATP, dCTP, dGTP, and dTTP, 50 mM Tris-HCl (pH 8.3), 50 mM KCl, 10 mM MgCl2, and 10 mM dithiothreitol in a final reaction volume of 20 μl. Five microliters of cDNA (which corresponded to approximately 104 cells) was amplified by PCR using 25 pmol of IL-8-specific primers or control glyceraldehyde phosphate dehydrogenase (GAPDH) primers (Stratagene, La Jolla, Calif.) and 2.5 U of Taq DNA polymerase (Promega) in a reaction mix containing 50 mM KCl, 10 mM Tris-HCl (pH 9), 0.1% Triton X-100, 850 μM MgCl2, and 50 μM each dATP, dCTP, dGTP, and dTTP. Template was denatured at 98°C for 10 min prior to addition of enzyme, buffer, and MgCl2 at 55°C. PCR for thirty-five cycles of 93°C for 30 s, 55°C for 30 s, and 72°C for 1 min was followed by a 10-min primer extension at 72°C. PCR products (10 μl of 50-μl total) were then analyzed by electrophoresis on 6% polyacrylamide gels and stained with ethidium bromide (1 μg/ml).
Fluorescent labeling of IL-8 receptors on whole-blood PMN and flow cytometry.
Fluorescent staining was performed on each of the samples from the study subjects as described before (24). Briefly, IL-8RA (9H1) and IL-8RB (10H2) antibodies (Genentech, South San Francisco, Calif.) and their appropriate isotype control antibodies (immunoglobulin G1 [IgG1] and IgG2a, respectively; Serotec, Oxford, England) were separately added to 50-μl aliquots of whole blood. After incubation for 20 min at room temperature, samples were washed twice with 3 ml of wash solution and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Dako, Copenhagen, Denmark) was added. After further incubation for 20 min at room temperature, samples were washed, the RBC were lysed with 2 ml of 1× FACS (fluorescence-activated cell sorting) lysing solution (Becton Dickinson), the samples were washed again, and the cells were resuspended in 200 μl of fixative (1.5% [vol/vol]) formaldehyde with 2% [wt/vol] bovine serum albumin). A Becton Dickinson FACSort flow cytometer with a 488-nm argon laser was used for all analyses. Forward and side light scatter characteristics were used in gating the granulocyte population. The data were analyzed with using Cellquest version 1.0 software and expressed as the percentage of cells expressing IL-8RA or IL-8RB and their respective fluorescence intensities or median channel shift (MCS) values (median channel number of the sample stained with IL-8R antibodies minus median channel number of the corresponding isotype antibody control sample).
Isolation of PMN and chemotaxis in response to IL-8.
PMN were isolated from anticoagulated peripheral blood by centrifugation on a double-layered Histopaque-Ficoll (Sigma) gradient, RBC were lysed, and chemotaxis of PMN in response to IL-8 was carried out via a Transwell chemotaxis assay as described before (24). Results are expressed as mean chemotactic index from duplicate determinations: ratio of number of PMN migrating in response to IL-8 divided by the number of randomly migrating PMN in unstimulated controls.
Statistical evaluation.
All statistics were determined with Statgraphics software (STSC, Inc., Rockville, Md.). Differences in peripheral IL-8 levels and cellular IL-8 production between ND, TB, HIV, and HIV/TB groups were compared by using the nonparametric Mann-Whitney U test. Relationships between various parameters were determined by simple correlation (Spearman’s rank coefficient).
RESULTS
IL-8 levels in the peripheral circulation of ND, HIV, TB, and HIV/TB groups.
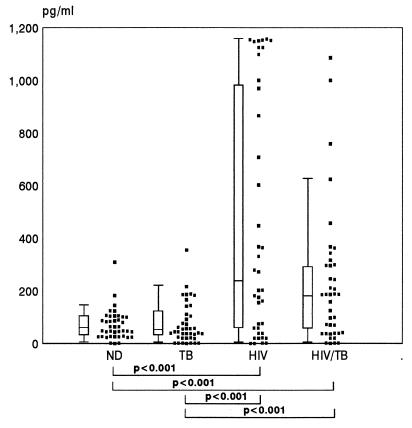
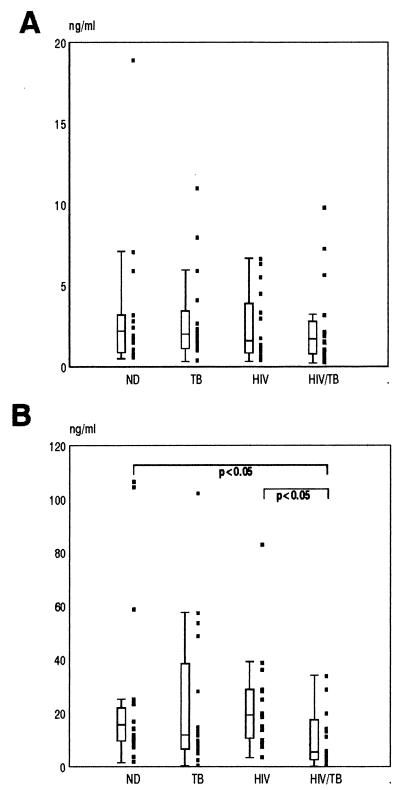
A preliminary screen was first conducted to quantitate IL-8 levels in stored serum samples from 160 individuals, 40 within each of the four study groups described previously. The median concentrations of IL-8 in the serum samples of the HIV and HIV/TB groups were both significantly higher than in the ND and TB groups, which in turn did not differ from each other (Fig. 1). As no additional information other than diagnosis was available for these individuals, we recruited a further 16 individuals in each of the ND, TB, HIV, and HIV/TB groups in order to study in more detail a number of aspects relating to IL-8 production in vivo, including circulating IL-8 plasma levels together with their respective levels of cell-associated IL-8 and cellular capacities to secrete IL-8 ex vivo. The immunological characteristics of these groups of individuals are shown in Table 1. The median viral load determinations in plasma of HIV and HIV/TB groups were 10,064/ml (range, 240 to 284,308/ml) and 34,788 (range, 549 to 308,832/ml), respectively. Both CD4 cell counts and viral load determinations were not significantly different between the HIV and HIV/TB groups (P > 0.05). For this group of individuals only plasma samples were available, and the sensitivity of the ELISA for IL-8 detection has been shown to be less than that found for serum (unpublished observations). However, similar results were obtained as with serum in that the median IL-8 values for the HIV and HIV/TB groups were significantly higher (P < 0.05) than for the ND group, whose median value was below the level of detection of the assay (Table 1). Once again the HIV group showed higher IL-8 levels than the TB group (P < 0.05). No significant correlations were found between levels of plasma IL-8 and copies of HIV-1 RNA per milliliter in either the HIV or HIV/TB group. Similarly, no correlations were found between the IL-8 plasma levels within each group and their corresponding CD4 or CD8 T-cell counts. An interesting correlation found was a negative one between absolute CD8 count and viral load in the HIV/TB group (P < 0.005; r = −0.735). Furthermore, the majority of TB (7 of 11 [63.6%]) and HIV/TB (5 of 7 [71.4%]) patients with undetectable levels of IL-8 were on anti-TB therapy for more than 6 months.
FIG. 1.
Circulating IL-8 levels determined by ELISA in the serum samples of 40 individuals in each of the ND, TB, HIV, and HIV/TB study groups. Data are presented as individual values (solid squares), medians (horizontal bars), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between the groups are indicated.
Peripheral cell-associated levels of IL-8.
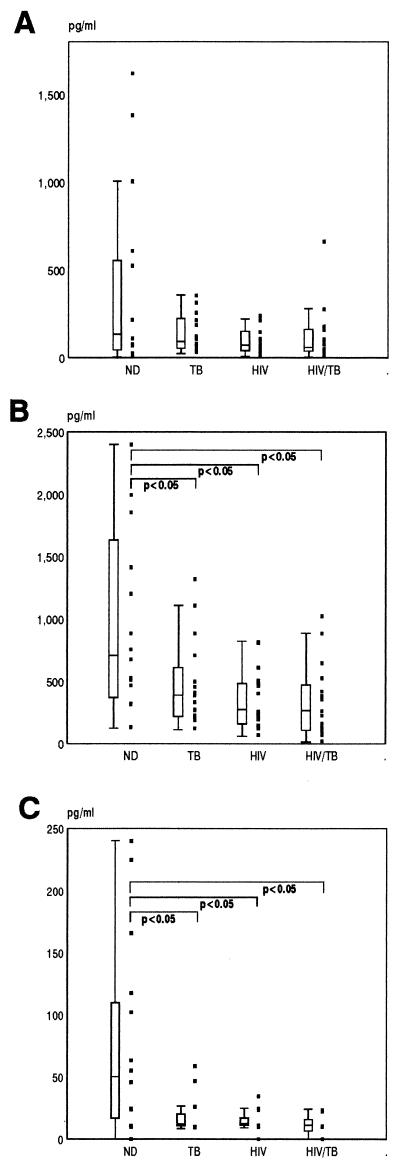
In addition to cell-free detection of IL-8, we thought it important to quantitate levels of IL-8 protein that may be associated with specific cell compartments in vivo. Peripheral cells isolated from whole blood were fractionated by Ficoll density centrifugation into mononuclear cells, PMN, and RBC, these populations were enumerated and lysed, and IL-8 was then quantitated by ELISA (Fig. 2). In general, the highest cell-associated levels were found for PMN in all subject groups (Fig. 2B). Within the ND group there was wide variability with a strong correlation between levels of IL-8 associated with each compartment. Although some ND subjects had higher PBMC-associated levels of IL-8 compared to the other groups, the median was not significantly different (P > 0.05) (Fig. 2A). However, IL-8 levels associated with RBC and PMN in the ND group were significantly higher than those in TB, HIV, and HIV/TB groups (Fig. 2B and C).
FIG. 2.
Peripheral cell-associated levels of IL-8 from the ND, TB, HIV, and HIV/TB study groups. Cells were isolated and lysed as described in Materials and Methods. IL-8 was quantitated by ELISA in cell lysates of PBMC (A), PMN (B), and RBC (C).
Spontaneous and induced secretion of IL-8 by PBMC.
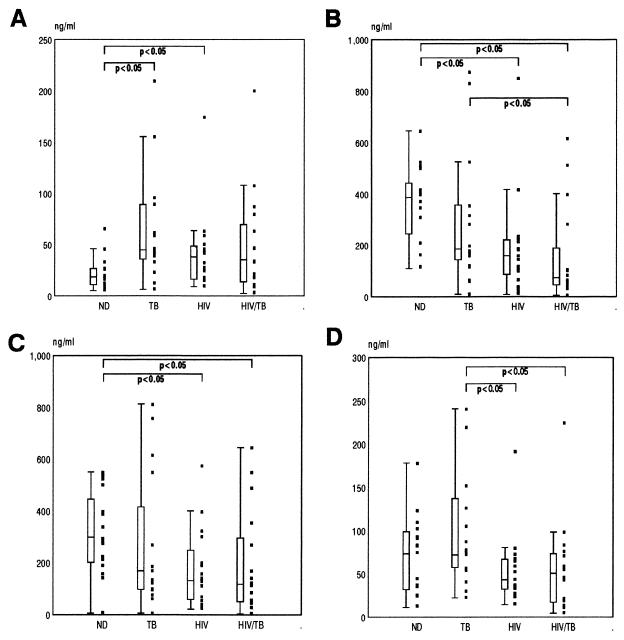
The ability of peripheral mononuclear cells to spontaneously produce IL-8 and their subsequent capacity to produce IL-8 in response to agonists was determined by culturing PBMC either without any stimulus or in the presence of PHA, PPD, or LPS. In general, the levels of IL-8 released spontaneously by PBMC cultures of infected individuals (TB, HIV, and HIV/TB groups) were higher than those released by cultures of individuals of the ND control group, although attaining significance only in the HIV and TB groups (Fig. 3A).
FIG. 3.
Spontaneous and induced production of IL-8 in PBMC ex vivo. PBMC from the 16 individuals in each study group (Table 1) were either cultured without an agonist to determine spontaneous IL-8 release (A) or stimulated with PHA (B), PPD (C), or LPS (D) for 48 h, and then supernatant IL-8 levels were determined by ELISA. Data are presented as individual values (solid squares), medians (horizontal bars), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between the groups are indicated.
PHA and PPD, both potent inducers of IL-8, showed similar patterns of stimulation in PBMC cultures from the different subject groups (Fig. 3B and C). The reduction in the ability of PBMC from TB, HIV, and HIV/TB groups to respond was, however, more evident with PHA. On the other hand, LPS stimulation induced a differential response between the various infected groups of individuals in that those with pulmonary TB produced greater amounts of IL-8 in response to LPS than did HIV-1 seropositive individuals with or without concurrent pulmonary TB (Fig. 3D). Although no direct relationships were noted between duration of anti-TB treatment and either spontaneous or induced IL-8 production in the TB and HIV/TB groups, there was a trend that longer times of treatment did result in a recovery of the capacity to produce IL-8 in response to PHA and PPD. Those patients in either group that had the highest IL-8 levels induced by PHA and PPD were all on anti-TB treatment for >6 months. A number of these, in fact, produced higher levels of IL-8 than stimulated PBMC cultures from the majority of healthy donors.
Spontaneous and induced secretion of IL-8 by PMN cultures.
Levels of IL-8 spontaneously produced in PMN cultures of ND (Fig. 4A) were approximately 100-fold less than that obtained for the equivalent number of unstimulated ND PBMC in culture (Fig. 3A). There were no differences noted between the levels of IL-8 produced spontaneously by PMN cultures between the different study groups (Fig. 4A). However, when PMN cultures were stimulated with PPD, the amount of IL-8 produced was significantly reduced only in the HIV/TB group (Fig. 4B). The TB group showed a trend toward decreased and the HIV group showed a trend toward increased IL-8 induction, although this was not statistically significant (P > 0.05). The duration of anti-TB treatment had no apparent effect on spontaneous or PPD-induced IL-8 production in PMN cultures from TB and HIV/TB patients.
FIG. 4.
Spontaneous and induced production of IL-8 in PMN ex vivo. PMN from the 16 individuals in each study group (Table 1) were either cultured without an agonist to determine spontaneous IL-8 release (A) or stimulated with PPD (B) for 48 h, and then supernatant IL-8 levels were determined by ELISA. Data are presented as individual values (solid squares), medians (horizontal bars), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars). Significant differences between the groups are indicated.
Levels of IL-8, produced spontaneously or induced in PBMC or PMN cultures, showed no correlations with mononuclear cell counts (CD4 and CD8) or PMN cell counts, respectively, or with plasma IL-8 levels (P > 0.05). Furthermore, there was also no significant correlation between (i) levels of IL-8 produced spontaneously or those induced in PBMC or PMN, and (ii) viral load determinations in the HIV and HIV/TB groups (P > 0.05).
Detection of IL-8 mRNA in different mononuclear cell fractions.
As there were no significant differences in cell-associated IL-8 levels detected between PBMC lysates of the individual groups but clear differences seen in production of IL-8 in vitro, we decided to use RT-PCR to determine the presence of IL-8 expression in specific mononuclear cell subsets directly following isolation. PBMC from each of four individuals in the four subject groups were separated into different cell fractions by using antibody-conjugated magnetic beads. Prior to embarking on RT-PCR assays on subject material, we optimized the magnetic bead isolation method to allow for the positive selection of approximately similar numbers of input cells. As the efficiency of positive selection is dependent on the microbead/cell ratio used, this was achieved by adjusting these ratios in such a way as to allow less than optimal selection of the total number of cells within each cell population. This approach ensured that similar cell numbers would be isolated even though specific cell numbers in some compartments differed between the groups, the compromise therefore being on total selection and not on purity of the individual cell fractions. Cell purity was monitored after each positive isolation by flow cytometry using double staining (FITC-phycoerythrin) with CD3-CD4, CD3-CD8, and CD45-CD14 and one-color (FITC) staining with CD19. The affinity of CD4 microbeads for the CD4 molecule on T cells is much greater than for CD4 on monocytes, and therefore CD4-expressing monocytes were found not to be a contaminant of the CD4 cell fraction.
RT-PCR was carried out on mRNA extracted from PBMC, fractionated CD14, CD4, CD8, and CD19 cells, and the cell fractions remaining at the end of the positive selections. GAPDH was used as a control for mRNA integrity, and endpoint titrations of the cDNAs were carried out to ensure that cell input (particularly of CD4 cells in patients with low CD4 cell count) was approximately uniform throughout. The PCR assay as used allows for a semiquantitative analysis of mRNA results; that is, the accumulation of GAPDH and IL-8-specific PCR products did not reach saturation levels within the number of amplification cycles used. IL-8 was detected mainly in the CD4 and CD8 cell fractions (Fig. 5) and in only one CD14 sample (TB-c) and one CD19 cell fraction (ND-d) (data not shown). Only one total PBMC fraction was found positive for IL-8 mRNA (ND-a), which also had detectable IL-8 mRNA in the cell fraction remaining after the positive cell selections. Cells in this latter fraction would include predominantly NK cells and monocytes, plus smaller numbers of each of the other cell fractions positively selected for.
Figure 5 therefore shows results obtained for the CD4 and CD8 cell fractions for individuals within each group as well as their immunological characteristics. All of the four individuals in the ND group were positive for IL-8 mRNA in the CD4 and CD8 cell fractions (Fig. 5A). For the patients with pulmonary TB (Fig. 5B), three of four CD4 cell fractions and two of four CD8 cell fractions were IL-8 mRNA positive. In the HIV group, CD4 cells from two of the four individuals were IL-8 mRNA positive, whereas CD8 cells from only one individual contained IL-8 mRNA (Fig. 5C). In the HIV/TB group, no IL-8 mRNA was detected in any of the cell fractions for any of the individuals (Fig. 5D). The lack of detectable IL-8 mRNA expression within the CD4 cell fraction of most individuals in the HIV and HIV/TB groups was not due to lower cell input resulting from lower absolute CD4 cell counts, as endpoint titrations of input cDNA for GAPDH were found to be similar to those obtained for ND samples (data not shown).
Relationship between IL-8 plasma levels and other immunological parameters.
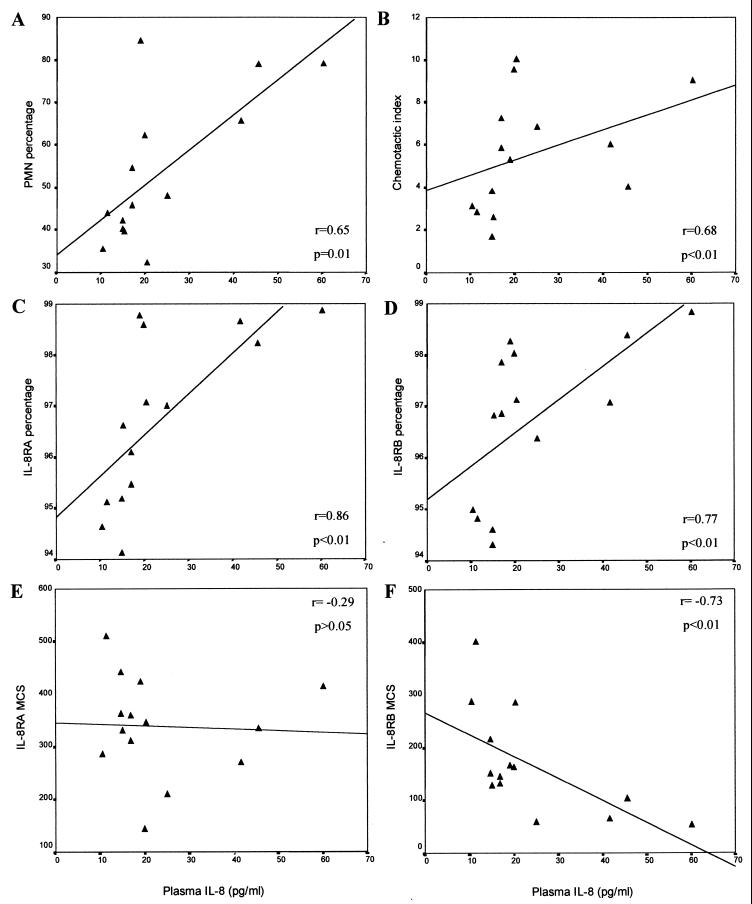
The up-regulation of IL-8 production observed in HIV-1-infected persons is likely to contribute to some form of immune dysregulation. As IL-8-dependent functions of leukocytes present prime candidates for altered regulation by IL-8, we determined the relationships between plasma IL-8 levels and a variety of immune parameters in a group of 14 HIV/TB patients: the proportions of peripheral leukocytes that were PMN, the proportion of PMN that specifically expressed IL-8RA and -B and their corresponding fluorescence intensities or MCS (as a measure of IL-8 receptor density), and the capacity of PMN to migrate in response to IL-8 (Fig. 6). Plasma IL-8 levels were positively correlated with the proportion of PMN in the peripheral circulation (r = 0.65; P = 0.01), the proportion of peripheral PMN that express IL-8RA (r = 0.86; P < 0.01) and IL-8RB (r = 0.77; P < 0.01), and chemotactic index (r = 0.68; P < 0.01). On the other hand, there was a strong negative correlation between IL-8 plasma levels and the intensity of IL-8RB expression (r = −0.73; P < 0.01) on PMN, but no relationship with the intensity of IL-8RA expression on PMN.
FIG. 6.
Relationship between plasma IL-8 concentration and various immune parameters: PMN percentage in peripheral blood (A), chemotactic index (B), proportion of IL-8RA-expressing PMN (C), proportion of IL-8RB-expressing PMN (D), IL-8RA fluorescence intensity (MCS) (E), and IL-8RB fluorescence intensity (MCS) (F).
DISCUSSION
The production of IL-8, an important chemoattractant and cellular activator, if altered is likely to play an important role in the pathogenesis of HIV-1 disease and TB, particularly in the context of secondary microbial infections. Our results confirm the presence of elevated circulating levels of IL-8 in individuals with HIV-1 in our setting, as has been reported by others (22, 39), and further show significantly raised IL-8 levels in HIV-1-infected individuals with concurrent pulmonary TB but not in those patients with pulmonary TB alone. A longitudinal study carried out by Friedland et al. (13) has shown a role for antibiotic therapy in tuberculosis, where both in vivo and LPS-stimulated ex vivo plasma IL-8 concentrations were increased following antibacterial therapy. This finding suggests that both peripheral levels of IL-8 and peripheral cell capacity to produce IL-8 are increased during anti-TB therapy. Our results, however, tend to support an increase in cellular capacity to produce IL-8 with time of anti-TB treatment but not an increase in circulating plasma levels. It is, however, significant that in another study, IL-8 plasma levels upon patient admission were found to be higher in TB patients, with or without HIV-1 infection, who died than in patients who survived (12).
A number of mechanisms may be responsible for altered peripheral IL-8 production in HIV-1-infected individuals. Although HIV and TB patients exhibited similar mononuclear cellular responses in vitro as measured by their abilities to produce IL-8 spontaneously or upon induction, it is possible that different mechanisms underlie cell priming in vivo that leads to the disparate peripheral IL-8 production in the two groups. Increased spontaneous release coupled with a nonresponsiveness to a second stimulus as measured by mononuclear cell cytokine production is well described in HIV-1 infection (10, 45). There is also evidence of priming of PMN in vivo in HIV-1-infected persons, as shown by reports of enhanced phagocytosis of Candida spp. (42) and Escherichia coli (33), reduced FcγRIII (CD16) expression (23), and increased CD11b expression (30). Our results, however, showed that enhanced IL-8 production ex vivo was not one of the consequences of PMN priming in vivo, and therefore PMN seem unlikely producers of the increased peripheral IL-8. Apart from peripheral circulating cells, other cells that could contribute to elevated peripheral levels of IL-8 are noncirculating cells such as endothelial cells, shown to be potent producers of IL-8 in vitro (19). Given the state of immune activation in HIV-1 infection, endothelial cell production of IL-8 could provide for persistently raised levels of this chemokine. Alternatively, levels of IL-8 in the periphery may constitute overflow from other tissue sites of HIV-1-infected individuals such as the lung, where production of cytokines, including IL-8 (20), is known to be substantial.
Another explanation for increased peripheral IL-8 levels could be that chemokine clearance mechanisms are compromised in infected individuals. The presence of IL-8 that we found associated with the PBMC and PMN compartments in healthy individuals may represent either preformed intracellular IL-8 or IL-8 bound to IL-8R on the surface of these cells or both. IL-8 binding to these cell surfaces in ND individuals would not be expected to occur, as any excess IL-8 is thought to be bound either to the promiscuous chemokine receptor, the duffy antigen on RBC (4), or in complexes with free IgG anti-IL-8 antibody (37), thereby removing IL-8 from the circulation and thus preventing peripheral cell activation. The corresponding increased IL-8 levels associated with RBC may therefore represent normal IL-8 turnover in vivo. From our results, the reduced binding of IL-8 to RBC of infected individuals may suggest impaired clearance of circulating IL-8. Increased circulating levels of IL-8, particularly in HIV-1 infection, could be the result of this deficit, and excess IL-8 may then instead bind to IL-8 receptors on leukocytes, giving rise to their activation. This possibility, which is consistent with the immune activation described for both HIV-1 infection and TB, has implications for in vivo regulation of chemokines in general and deserves further evaluation.
Furthermore, our results show that the peripheral mononuclear cell types in vivo that contain IL-8 mRNA are predominantly those identified by the CD4 and CD8 cell surface markers and not, as one might expect, monocytes which are potent producers in vitro (3). A loss of this constitutive IL-8 mRNA expression was noted in some patients with HIV-1 infection or pulmonary TB, with a total abrogation thereof in individuals that were coinfected. The presence or absence of IL-8 mRNA was not related to the extent of immune deficiency as determined by CD4 cell count, as some individuals with low CD4 count had detectable mRNA and others with much higher counts were negative for IL-8 mRNA. The presence of IL-8 mRNA in CD4 and CD8 cells from healthy individuals may form part of a process of normal IL-8 turnover as has been suggested earlier; alternatively, these cells may be poised for a subsequent posttranscriptional stimulus for IL-8 translation and secretion. Our results support the view that the regulation of IL-8 production is cell type dependent. Consistent with this possibility is the finding that T cells upon direct isolation from blood have detectable IL-8 mRNA which ceases to be detected within 24 h upon in vitro culture (39a), whereas freshly isolated monocytes have undetectable expression but rapidly produce IL-8 mRNA upon adherence to plastic in culture (17). This finding suggests that posttranscriptional processes or regulation of secretion of preformed protein may be operational in T cells, whereas monocytes require transcriptional activation for IL-8 production. Whatever the mechanisms of regulation, it is clear that the presence of HIV-1 infection or pulmonary TB, or both, interferes with those mechanisms underlying constitutive IL-8 expression in T cells. It may be significant in this regard that the Tat protein of HIV-1 has the ability to superinduce IL-8 production in T cells, and this could be a factor contributing to altered IL-8 regulation in HIV-1 infection (29).
Altered production of IL-8 in vivo, as evidenced mainly in the presence of HIV-1 infection, may have a number of possible consequences. First, impairment of leukocyte trafficking may occur due to an altered concentration gradient between the periphery and the site of inflammation. As IL-8 is chemotactic for both neutrophils and T lymphocytes, the cellular composition at sites of infection may be altered. Alteration of the migration of HIV-1-infected T cells may have implications for dissemination of virus to other sites of the body. IL-8 is chemotactic for approximately 10% of lymphocytes direct from peripheral blood (18), but this proportion increases when mononuclear cells are activated with anti-CD3 antibody or PPD (44). As these cells are anergic in HIV-1 infection, it seems likely that they in turn would be unresponsive to IL-8 as a second signal for migration. In addition, PMN functions dependent on IL-8 binding to either of its receptors are likely to be altered. Calcium mobilization and chemotaxis in response to IL-8 have been shown to be impaired (24), and nonoxidative killing mechanisms employed by PMN are suggested to be impaired in HIV-1-infected individuals (42). As IL-8 has been shown to regulate the expression of its own receptor (31), persistently elevated levels of IL-8 could result in the down-regulation of IL-8 receptors. Consistent with this view are our recent findings (24) of a significantly diminished expression of both IL-8RA and IL-8RB on peripheral PMN of patients with HIV-1, patients with pulmonary TB, and also those with dual infection. Expression of either receptor on lymphocytes was also markedly decreased in the HIV and HIV/TB groups. This diminished expression occurred irrespective of the patient’s immunological status. In addition, IL-8 shares one of its receptors (IL-8RB) with CXC chemokines GROα, GROβ, GROγ, NAP-2, and ENA-78, further suggesting a possible modulation of their functions by altered IL-8 production. Apart from direct effects of IL-8 on cellular immune function, its role in terms of potentiation or suppression of HIV-1 replication remains to be questioned.
Most notably in this study, we found a significant association between higher circulating levels of IL-8 and a higher proportion of circulating PMN, as well as a higher proportion of PMN expressing IL-8RA and -B, in a group of HIV/TB patients. Higher IL-8 levels were further associated with a correspondingly greater capacity of PMN to migrate in response to IL-8 than what was found for patients in the same group who exhibited low peripheral IL-8 levels. Interestingly, higher IL-8 levels were significantly negatively correlated with the intensity of IL-8RB but not of IL-8RA. We propose that raised peripheral IL-8 levels may be responsible for recruitment of new PMN from the bone marrow as has recently been described (38), hence the increase in the proportions of IL-8R bearing PMN and the subsequent improvement in chemotactic migration of these cells. The proportion of IL-8R-positive PMN is a stronger predictor for reduced chemotactic function in the Transwell assay than is a decrease in receptor density (24). However, the negative relationship between IL-8 levels and IL-8RB fluorescence intensity suggests the down-regulation of these receptors. It seems unlikely that the down-regulating agent in this case would be raised levels of IL-8 itself, as the intensity of fluorescence of IL-8RA, which selectively binds only IL-8 and no other known ligand, shares no relationship with the corresponding circulating IL-8 levels.
In summary, elevated circulating levels of IL-8 in HIV-1-infected persons may therefore have a positive effect in increasing circulating numbers of IL-8R-bearing PMN which quantitatively contribute to maintaining some degree of PMN function, although still significantly less than found in healthy individuals (24). This effect of higher IL-8 levels, on the other hand, is counteracted by the persistent down-regulation of IL-8RB on the surface of peripheral PMN, which in turn is also associated with reduced PMN function (24). These results further add to our previous findings of altered IL-8R expression on peripheral leukocytes and diminished PMN function as a consequence (24), and they suggest that dysregulated production of IL-8 in vivo plays a role in the disease pathogenesis of HIV-1 and M. tuberculosis.
ACKNOWLEDGMENTS
This work was supported by the Poliomyelitis Research Foundation and Medical Research Council of South Africa.
We thank D. Spencer at the HIV outpatient clinic, staff from Rietfontein Hospital, Johannesburg, South Africa, and J. McAnerny and I. Henley from our institute for their cooperation in this study. IL-8R-specific mAbs were kindly supplied by A. Chuntharapai and K. Jin Kim, Department of Bioanalytical Technology, Genentech, Inc.
REFERENCES
- 1.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 2.Cassatella M A, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992;148:3216–3220. [PubMed] [Google Scholar]
- 3.Colditz I, Zwahlen R, Dewald B, Baggiolini M. In vivo inflammatory activity of a neutrophil activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989;134:755–760. [PMC free article] [PubMed] [Google Scholar]
- 4.Darbonne W C, Rice G C, Mohler M A, Apple T, Hébert C A, Valente A J, Baker J B. Red blood cells are a sink for interleukin-8, a leukocyte chemotaxin. J Clin Investig. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denis M, Ghadirian E. Dysregulation of interleukin-8, interleukin-10, and interleukin-12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 1994;10:1619–1627. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- 6.Edlin B R, Tokars J I, Grieco M H, Crawford J T, Williams J, Sordillo E M, Ong K R, Kilburn J O, Dooley S W, Castro K G, Jarvis W R, Holmberg S D. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 7.Ellner J J. Tuberculosis in the time of AIDS. Chest. 1990;98:1051–1052. doi: 10.1378/chest.98.5.1051. [DOI] [PubMed] [Google Scholar]
- 8.Esser R, von Briesen H, Brugger W, Ceska M, Glienke W, Müller S, Rehm A, Rübsamen-Waigmann H, Andreesen R. Secretory repertoire of HIV-infected human monocytes/macrophages. Pathobiology. 1991;59:219–222. doi: 10.1159/000163649. [DOI] [PubMed] [Google Scholar]
- 9.Folks T M, Justement J, Kinter A, Schnittman S, Orenstein J, Poli G, Fauci A S. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J Immunol. 1988;140:1117–1122. [PubMed] [Google Scholar]
- 10.Friberg D, Bryant J, Shannon W, Whiteside T L. In vitro cytokine production by normal human peripheral blood mononuclear cells as a measure of immunocompetence or the state of activation. Clin Diagn Lab Immunol. 1994;1:261–268. doi: 10.1128/cdli.1.3.261-268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedland J S, Remick D G, Shattock R, Griffin G E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992;22:1373–1378. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 12.Friedland J S, Hartley J C, Hartley C G C, Shattock R J, Griffin G E. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–238. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedland J S, Hartley J C, Hartley C G C, Shattock R J, Griffin G E. Cytokine secretion in vivo and ex vivo following chemotherapy of Mycobacterium tuberculosis infection. Trans R Soc Trop Med Hyg. 1996;90:199–203. doi: 10.1016/s0035-9203(96)90141-8. [DOI] [PubMed] [Google Scholar]
- 14.Gough N M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Ann Biochem. 1988;173:93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- 15.Holmes W E, Lee J, Kuang W J, Rice G C, Wood W I. Structure and functional expression of a human interleukin 8 receptor. Science. 1991;253:1278–280. [PubMed] [Google Scholar]
- 16.Jones S A, Wolf M, Qin S, Mackay C R, Baggiolini M. Different functions for the interleukin-8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA. 1996;93:6682–686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasahara K, Strieter R M, Chensue S M, Standiford T J, Kunkel S L. Mononuclear cell adherence induces neutrophil chemotactic factor/interleukin-8 gene expression. J Leukoc Biol. 1991;50:287–295. doi: 10.1002/jlb.50.3.287. [DOI] [PubMed] [Google Scholar]
- 18.Larsen C G, Anderson A O, Appella E, Oppenheim J J, Matsushima K. Neutrophil activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 19.Liles W C, Van Hoorhis W C. Nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis. 1995;172:1573–1580. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- 20.Lipschik G Y, Doerfler M E, Kovacs J A, Travis W D, Andrawis V A, Lawrence M G, Dichter J R, Ognibene F P, Shelhamer J H. Leukotriene B4 and interleukin-8 in human immunodeficiency virus-related pulmonary disease. Chest. 1993;104:763–769. doi: 10.1378/chest.104.3.763. [DOI] [PubMed] [Google Scholar]
- 21.Martin D J, Sim J G M, Sole G J, Rymer L, Shalekoff S, Van Niekerk A B N, Becker P, Weilbach C N, Iwanik J, Keddy K, Miller G B, Ozbay B, Ryan A, Viscovic T, Woolf M. CD4+ lymphocyte count in African patients co-infected with HIV and tuberculosis. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:386–390. [PubMed] [Google Scholar]
- 22.Matsumoto T, Miike T, Nelson R P, Trudeau W L, Lockey R F, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meddows-Taylor S, Martin D J, Tiemessen C T. Altered expression of FcγRIII (CD16) on polymorphonuclear neutrophils from individuals with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. Clin Diagn Lab Immunol. 1997;4:789–791. doi: 10.1128/cdli.4.6.789-791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meddows-Taylor S, Martin D J, Tiemessen C T. Reduced expression of interleukin-8 receptors A and B on polymorphonuclear neutrophils from persons with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. J Infect Dis. 1998;177:921–930. doi: 10.1086/515232. [DOI] [PubMed] [Google Scholar]
- 25.Miller M D, Krangel M S. Biology and biochemistry of the chemokines: a novel family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 26.Murphy P M, Tiffany H L. Cloning of complementary DNA encoding on functional human interleukin-8 receptor. Science. 1992;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 27.Nunn P, Brindle R, Carpenter L, Odhiambo J, Wasunna K, Newnham R, Githui W, Gathua S, Omwega M, McAdam K. Cohort study of human immunodeficiency virus infection in patients with tuberculosis in Nairobi, Kenya. Am Rev Respir Dis. 1992;146:849–854. doi: 10.1164/ajrccm/146.4.849. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 29.Ott M, Lovett J L, Mueller L, Verdin E. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-κB factors. J Immunol. 1998;160:2872–2880. [PubMed] [Google Scholar]
- 30.Palmer S, Hamblin A S. Increased CD11/CD18 expression on the peripheral blood leukocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993;93:344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samanta A K, Oppenheim J J, Matsushima K. Interleukin-8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990;265:183–189. [PubMed] [Google Scholar]
- 32.Selwyn P A, Hartel D, Lewis V A, Schoenbaum E E, Vermund S H, Klein R S, Walker A T, Friedland G H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 33.Shalekoff S, Tiemessen C T, Gray C M, Martin D J. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without HIV-1 infection. Clin Diagn Lab Immunol. 1998;5:41–44. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shattock R J, Friedland J S, Griffin G E. Phagocytosis of Mycobacterium tuberculosis modulates human immunodeficiency virus replication in human monocytic cells. J Gen Virol. 1994;75:849–856. doi: 10.1099/0022-1317-75-4-849. [DOI] [PubMed] [Google Scholar]
- 35.Small P M, Shafer R W, Hopewell P C, Singh S P, Murphy M J, Desmond E, Sierra M F, Schoolnik G K. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N Engl J Med. 1993;328:1137–1144. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 36.Stoneburner R, Laroche E, Prevots R, Singh T, Blum S, Terry P, Reatrice S, Adler J. Survival in a cohort of human immunodeficiency virus infected tuberculosis patients in New York City. Arch Intern Med. 1992;152:2033–2037. [PubMed] [Google Scholar]
- 37.Sylvester I, Yoshimura T, Sticherling M, Schröder J, Ceska M, Peichl P, Leonard E J. Neutrophil attractant protein-1-immunoglobulin G immune complexes and free anti-NAP-1 antibody in normal human serum. J Clin Investig. 1992;90:471–481. doi: 10.1172/JCI115883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terashima T, English D, Hogg J C, van Eeden S F. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood. 1998;92:1062–1069. [PubMed] [Google Scholar]
- 39.Thea D M, Porat R, Nagimbi K, Baangi M, St. Louis M E, Kaplan G, Dinarello C A, Keusch G T. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 39a.Tiemessen, C. Unpublished observations.
- 40.Tsai W, Hirose K, Nara P L, Kuang Y, Conley S, Li B, Kung H, Matsushima K. Decrease in cytokine production by HIV-infected macrophages in response to LPS-mediated activation. Lymphokine Cytokine Res. 1991;10:421–429. [PubMed] [Google Scholar]
- 41.Wallis R S, Vjecha M, Amir-Tahmasseb M, Okwera A, Byekwaso F, Nyole S, Kabengera S, Mugerwa R D, Ellner J J. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated β2-microglobulin in HIV-1 associated tuberculosis. J Infect Dis. 1993;167:43–48. doi: 10.1093/infdis/167.1.43. [DOI] [PubMed] [Google Scholar]
- 42.Wenisch C, Parschalk B, Zedwitz-Liebenstein K, Graninger W, Rieger A. Dysregulation of the polymorphonuclear leukocyte-Candida spp. interaction in HIV-positive patients. AIDS. 1996;10:983–987. doi: 10.1097/00002030-199610090-00008. [DOI] [PubMed] [Google Scholar]
- 43.Whalen C, Horsburgh C R, Hom D, Lahart C, Simberkoff M, Ellner J J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson P C, Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 1992;149:2689–2694. [PubMed] [Google Scholar]
- 45.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–1320. [PubMed] [Google Scholar]
- 46.Yoshimura T, Matsushima K, Tanaka S, Robinson E A, Appella E, Oppenheim J J, Leonard E J. Purification of human monocyte derived neutrophil chemotactic factor that shares sequence homology with other host defence cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Nakata K, Weiden M, Rom W N. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Investig. 1995;95:2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Riebman J, Rom W N. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Investig. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]