Meet the First Author, see p 869
The long-term impacts of COVID-19 infection have emerged as a major global health challenge. Survivors present an elevated risk for cerebrovascular and ischemic heart disease, among other thromboembolic disorders. ICU-admitted survivors exhibited increased risks and 12-month burden of incident cardiovascular diseases over 300 per 1000 individuals.1 Increased platelet activation and hyperreactivity are landmarks of the COVID-19 hypercoagulable state and are implicated in the severity and mortality.2–4 However, little is known about these patients’ hypercoagulable mechanism or whether increased platelet activation persists after hospital discharge. Here we investigate platelet activation, hyperreactivity, and circulating procoagulant tissue factor (TF)-bearing extracellular vesicles in COVID-19 pneumonia survivors up to 6 months after symptom onset.
Between October 2020 and November 2021, blood samples of 46 COVID-19 pneumonia hospitalization survivors (age 51.5 years; [interquartile range, IQR: 40–57.5] 58.6% male) were collected 77.5 (IQR: 59–114) days after symptom onset during a follow-up appointment. COVID-19 survivors had a median length of hospitalization of 8 days (IQR: 3.75–14), with 36 (78.3%) of them having moderate pneumonia, 10 (21.7%) severe pneumonia, and 5 (10.8%) of them requiring invasive mechanical ventilation. All patients reported at least 1 persisting symptom after COVID-19 recovery, with 73% of them presenting respiratory symptoms. A total of 29 age, sex, race/ethnicity, and comorbidity-matched voluntary healthy donors (age 46 years; [IQR: 34.5–55] 41% male) who tested negative for SARS-CoV-2 weekly from April 2020 until sample collection date were also included in this study. Cancer diagnosis and the recent or current use of antiplatelet medications were exclusion factors. None of the subjects experienced post-discharge thrombotic events to this date.
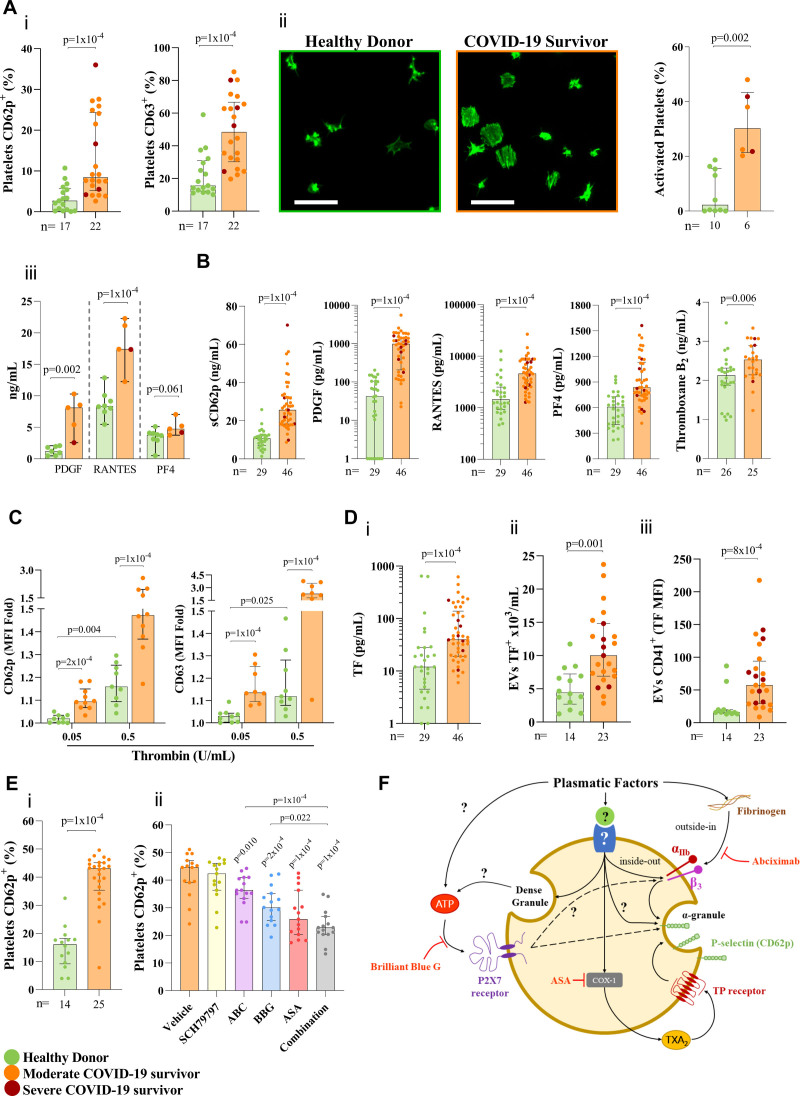
Platelets were isolated and labeled for flow cytometry as previously described.2 COVID-19 survivors had a higher percentage of platelets presenting markers of α (CD62p) and dense granule (CD63) degranulation (Figure Ai), increased spread when allowed to adhere to fibrinogen-coated surface (Figure Aii), increased granule products secretion (Figure Aiii), and elevated platelet granule-derived and newly synthetized products in the plasma (Figure B), indicating increased platelet activation, a feature markedly found in hospitalized COVID-19 patients.2,3 Platelets from survivors were also responsive to subthreshold thrombin stimulation (0.05 U/mL) and hyperreactive to 0.5 U/mL, which activates platelets of healthy donors in a lower extent (Figure C). Circulation of hyperreactive platelets primed to activation were observed in hospitalized COVID-19 patients,3 and may also contribute to the hypercoagulable state of COVID-19 survivors.
Figure.
Persistent platelet activation in COVID-19 survivors. A, Platelet activation. (i) Percentage of platelets expressing CD62p and CD63 assessed by flow cytometry. (ii) Quantification of platelets spreading (stained with Alexa-488-phalloidin) over a fibrinogen coating and images that better represent the median of the results. Bars: 20 µm. Quantifications were performed in images of 3 different fields with at least 60 platelets each, taken by an investigator blinded to experimental groups. (iii) Spontaneous ex-vivo release of α-granule products by platelets incubated for 30 min at 37°C. B, Quantification of platelet-derived factors in the plasma. sCD62p, PDGF, RANTES, PF4, and Thromboxane B2 assessed through ELISA. C, Platelet hyperreactivity in COVID-19 survivors. MFI-fold increase of CD62p and CD63 labeling after stimulation with thrombin (0.05 or 0.5 U/mL). D, Procoagulant extracellular vesicles (EVs) in COVID-19 survivors. (i) Concentration of plasmatic TF. (ii) Concentration of circulating TF-bearing EVs. (iii) MFI of TF labeling in platelet-derived TF-bearing EVs. E, Ex-vivo platelet activation mechanisms. (i) Percentage of healthy donors’ platelets expressing CD62p after 1 h incubation with COVID-19 survivors’ or heterologous healthy donors’ plasma. (ii) Percentage of healthy donors’ platelets expressing CD62p after 30 min of pretreatment with SCH79797 (5 µM), Abciximab (ABC-50 µg/mL), Brilliant Blue G (BBG-5 µM), acetylsalicylic acid (ASA-100 µM), or their combination followed by incubation with COVID-19 survivors’ plasma. F, Schematic representation of platelet activation mechanisms in COVID-19 survivors. Inside-out signaling primes integrins αIIb and β3 for interaction with fibrinogen resulting in α-granule release. ATP released from dense granules in an autocrine/paracrine manner induces α-granule release through P2X7R purinergic signaling. Thromboxane A2 synthesis though COX-1 promotes autocrine activation of TP receptor, leading to α-granule release. Values are represented as the median with interquartile range. Normality was assessed using the Shapiro-Wilk test. Comparisons between 2 groups were performed using the Student t test and the Mann–Whitney U test for parametric and nonparametric distributions, respectively. One-way ANOVA followed by Tukey post hoc test was used to compare differences among 3 groups following a parametric distribution. All statistical analyses were performed using GraphPad Prism version 9.4.1. TF indicates tissue factor.
In addition to elevated d-dimer (521.5 [IQR: 325.8–800.8] versus 257 [IQR: 190–392 ng/mL]; P=0.001), as seen in other COVID-19 survivors’ cohorts,5 the survivors also presented higher d-dimer/albumin ratio (122 [IQR: 74.1–215.3] versus 68 [IQR: 53–102]; P=0.020) and elevated circulating TF levels (Figure Di). High levels of TF bearing extracellular vesicles (EVs) associate with the occurrence of thromboembolic events in hospitalized COVID-19 patients.4 To investigate EVs in COVID-19 survivors, EVs were isolated through differential centrifugation and labeled for flow cytometry. The plasma of the survivors exhibits elevated concentrations of total EVs (1.698; [IQR: 1.432–2.321] versus 1.096, [IQR: 0.729–0.344] EVs×105/mL; P=0.001) and TF-bearing EVs (Figure Dii). Moreover, the expression of TF, assessed through the mean fluorescence intensity was elevated in platelet-derived EVs in survivors (Figure Diii), supporting a role for platelets in the development of the persistent hypercoagulable state in COVID-19 survivors.
Platelet activation and hyperactivity are central mechanisms in the progression of the hypercoagulable state found during and after SARS-CoV-2 infection. To explore the mechanisms of platelet activation in COVID-19 survivors, we stimulated healthy donors’ platelets with COVID-19 survivors’ plasma. Incubation with the survivors’ plasma induced a higher percentage of platelets expressing CD62p when compared to stimulation with the plasma from heterologous healthy donors (Figure Ei), indicating that soluble factors in COVID-19 survivors plasma can activate platelets and possibly influence platelet reactivity. To gain insights into the possible pathways involved in platelet activation, we pretreated heathy donors’ platelets with the PAR-1 receptor antagonist SCH79797 (5 µM), the anti-integrin αIIbβ3 monoclonal antibody Abciximab (ABC-50 µg/mL), the ATP-sensitive P2X7 receptor antagonist brilliant blue G (BBG-5 µM), the COX inhibitor acetylsalicylic acid (ASA-100 µM), or a combination of these drugs before incubation with 90% COVID-19 survivors’ or healthy donors’ plasma. Pretreatment with ABC, BBG, ASA, and their combination was capable of partially preventing survivors’ plasma-induced platelet activation (Figure Eii), but not healthy donors’ plasma (not shown). This indicates that mechanisms involving integrin αIIbβ3 inside-out activation through an unknown pathway and subsequent binding to plasma fibrinogen, ATP purinergic signaling and TXA2 generation and autocrine signaling are involved in the amplification of platelet activation by plasmatic mediators (Figure F). Interestingly, thrombin/PAR-1 signaling does not seem to be involved in this priming, and other procoagulant and inflammatory plasmatic mediators should be investigated in future studies.
This study has its limitations, including the reduced sample size and its cross-sectional nature. However, the similar age, gender, and comorbidities profile between subjects and healthy donors increases the reliability of our data.
Together, our findings indicate that platelet activation and hypercoagulable state persists in COVID-19 survivors after hospital discharge, having possible implications for long lasting consequences of COVID-19. Further investigation, with larger cohorts including individuals that experienced post-discharge thrombotic events are required for the development of adequate public health measures to prevent future thromboembolic events in these patients.
Article Information
Acknowledgments
The authors express gratitude to Giulia Caminha and Edson F. Assis for technical assistance, and the multiuser flow cytometry facility and confocal microcopy facility from Rede de Plataformas Tecnológicas FIOCRUZ/RJ. We also thank Vanessa Tiago Narcizo Figueredo and the clinical research support team from the D’Or Institute of Research.
Sources of Funding
This work was supported by grants from Inova Fiocruz, Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosures
The complete and detailed methodology, characterization of the cohort and data are available upon request. This cross-sectional study was conducted in accordance with the Declaration of Helsinki and approved by the National Review Board of Brazil/CONEP (CAAE 5523520.3.0000.5249). Subjects answered a questionnaire and provided written consent before any study-related procedure.
Nonstandard Abbreviations and Acronyms
- ASA
- acetylsalicylic acid
- BBG
- brilliant blue G
- EV
- extracellular vesicles
- IQR
- interquartile range
- TF
- tissue factor
E.D. Hottz and P.T. Bozza contributed equally.
For Sources of Funding and Disclosures, see page 947.
References
- 1.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TM, Kurtz P, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;11:1330–1341. doi: 10.1182/blood.2020007252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;11:1317–1329. doi: 10.1182/blood.2020007214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guervilly C, Bonifay A, Burtey S, Sabatier F, Cauchois R, Abdili E, Arnaud L, Lano G, Pietri L, Robert T, et al. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;3:628–634. doi: 10.1182/bloodadvances.2020003308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, Bergin C, O’Farrelly C, Conlon N, Bourke NM, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;4:1064–1070. doi: 10.1111/jth.15267 [DOI] [PMC free article] [PubMed] [Google Scholar]