Objective:
To develop an international core outcome set (COS), a minimal collection of outcomes that should be measured and reported in all future clinical trials evaluating treatments of acute simple appendicitis in children.
Summary of Background Data:
A previous systematic review identified 115 outcomes in 60 trials and systematic reviews evaluating treatments for children with appendicitis, suggesting the need for a COS.
Methods:
The development process consisted of 4 phases: (1) an updated systematic review identifying all previously reported outcomes, (2) a 2-stage international Delphi study in which parents with their children and surgeons rated these outcomes for inclusion in the COS, (3) focus groups with young people to identify missing outcomes, and (4) international expert meetings to ratify the final COS.
Results:
The systematic review identified 129 outcomes which were mapped to 43 unique outcome terms for the Delphi survey. The first-round included 137 parents (8 countries) and 245 surgeons (10 countries), the second-round response rates were 61% and 85% respectively, with 10 outcomes emerging with consensus. After 2 young peoples' focus groups, 2 additional outcomes were added to the final COS (12): mortality, bowel obstruction, intraabdominal abscess, recurrent appendicitis, complicated appendicitis, return to baseline health, readmission, reoperation, unplanned appendectomy, adverse events related to treatment, major and minor complications.
Conclusion:
An evidence-informed COS based on international consensus, including patients and parents has been developed. This COS is recommended for all future studies evaluating treatment ofsimple appendicitis in children, to reduce heterogeneity between studies and facilitate data synthesis and evidence-based decision-making.
Keywords: appendicitis, appendicitis research, core outcome set, nonoperative treatment, simple appendicitis
Appendicitis, the most common acute surgical condition in children, affects approximately 1 in every 12 people in the world, with the highest incidence between ages 10 and 19 years. 1,2 Although appendicitis may be complicated by rupture, most cases are uncomplicated, or “simple.” In the last decade, there has been interest in alternative minimally invasive approaches and non-operative treatment of simple appendicitis. 3
Randomized clinical trials are needed to inform treatment strategies in clinical practice. However, applicability of trial results depends on the outcomes being measured and reported. To inform clinical decisionmaking, these outcomes need to be relevant to both clinicians and patients. 4 Inconsistent selection and reporting of outcomes limits the ability to adequately interpret and compare clinical trial results and subsequent meta-analysis. 5,6 Between 1973 and 2013, 51 randomized controlled trials, and 9 systematic reviews evaluating treatments of appendicitis in children reported a total of 115 unique outcomes. 7 This demonstrates the need for standardized outcomes that are relevant to both patients and clinicians. These outcomes should be measured and reported, as a minimum, in all clinical trials in specific areas of health or health care. 8 Core outcome sets (COSs) provide an evidence-based approach to standardize outcome selection and allow for unified measuring and reporting which in turn facilitates data synthesis. 9,10
Although a COS for simple appendicitis in children has recently been developed for the UK, 11 the differences between countries in treatment practices, resources, and cultural aspects, means there remains a need for a COS with an international scope. The aim of this study was to develop such an international COS to be measured and reported in all future clinical trials investigating any type of treatment for acute simple appendicitis in children, including surgical treatment and non-operative treatment.
Methods
Study Registration, Methods, and Protocol
This study was registered with the COMET initiative (registration number: 1119) on February 11, 2018. 12 Development consisted of 4 phases: (1) compilation of a list of outcomes for the Delphi study, by an update of the 2015 systematic review, 7 identifying all previously reported outcomes, (2) a 2-round international Delphi study to identify a set of core outcomes for which there is consensus among parents (including, where appropriate, their child) and surgeons, (3) focus groups with young people to identify missing outcomes (4) international expert meetings with parents, surgeons, and researchers to ratify the final COS. A previously published protocol describes the study design, rationale, and methods in more detail, 13 including a completed COS-STAD (Core Outcome Set-STAndards for Development) recommendations check-list. 14 An updated protocol including the focus groups methodology has been published on the Open Science Framework. 15
International Steering Committee
An international steering committee was established by contacting research groups involved in trials or trials that had been completed after 2014 on the treatment of simple acute appendicitis in children listed on ClinicalTrials.gov. Ten of the 13 identified countries agreed to participate. In addition to a lead investigator from each participating center, the steering committee consisted of the authors, and a parent/patient representative of the Dutch Foundation Children and Hospital, see Supplement S1, http://links.lww.com/SLA/C830. The steering committee agreed on the final protocol and provided input throughout the project. Within the steering committee, a smaller study management group (Knaapen M, Hall NJ, Van der Lee JH, Butcher NJ, Offringa M, Bakx R, Gorter RR) met regularly in videoconference meetings.
Systematic Review for Delphi Survey
To develop a list of outcomes for the Delphi survey, we updated an earlier published systematic review 7 to identify any new unique outcomes measured in trials evaluating treatments of simple appendicitis in children. Additional information on the search strategy/study selection and data extraction can be found in online Supplement S2, http://links.lww.com/SLA/C831.
Participants Delphi Study
Two stakeholder groups were invited to complete a 2-round online Delphi survey: parents and surgeons. The parent group comprised of parents of children and young people (5–18 years) treated for acute simple appendicitis either with initial non-operative treatment or with surgery in the preceding 24 months. Parents were asked to discuss the answers they provided with their child whilst filling out the Delphi survey. Parents were included from 11 centers in 8 countries: USA (Nationwide Children’s Hospital, Ohio; Children’s Mercy Hospital, Kansas City); Sweden (Karolinska University Hospital, Stockholm); Australia (Children’s Hospitals Network, Sydney); Singapore (KK Women’s and Children’s Hospital, Singapore); Canada (British Columbia Children’s Hospital, Vancouver; The Hospital for Sick Children, Toronto; Montreal Children’s Hospital, Montreal); The Netherlands (Amsterdam University Medical centers, Amsterdam); Finland (Helsinki Children’s Hospital, Helsinki); Malaysia (Universiti Kebangsaan Malaysia, Kuala Lumpur).
The surgeon stakeholder group included general and/or pedi-atric surgeons who treat children in the specified age group. Surgeons were included from 10 countries: USA, Sweden, Australia, Singapore, Canada, The Netherlands, Finland, Malaysia, UK, and France. They were invited through their respective national pediatric surgical associations or directly by the local lead investigators.
All participating centers obtained ethical board approval as appropriate. Participants received an electronic invitation to register for the online Delphi study through DelphiManager, 16 a web-based Delphi survey system. All participants provided digital informed consent.
Delphi Study
The list of outcome terms from the systematic review was formatted into questions, accompanied by a plain language summary for each outcome, see Online Supplement S3, http://link-s.lww.com/SLA/C832. The survey was piloted by a group of laypersons (n = 10) to assure clarity. The English survey was translated into Dutch, French, Finnish, and Swedish by translators familiar with medical terminology. After deliberation with the local investigators, the English questionnaire was used in Malaysia and Singapore.
Participants were asked to score how important each outcome was to determine the effectiveness of a treatment for simple appendicitis using a 1 to 9 Likert scale. Each round had to be completed within 8 to 10 weeks. During that time, non-responders received a reminder email every 2 weeks. Participants who completed Round 1 were invited to participate in Round 2. It consisted of outcomes that did not reach consensus in the first round and additional outcomes suggested by participants. In Round 2 participants could see their individual score from round 1 combined with a histogram showing the scoring distribution for both stakeholder groups. Participants were then asked to re-score the remaining outcomes in the same manner as Round 1.
In the absence of a formal guideline but in accordance with common practice, 8 consensus was defined as follows:
“Consensus-in:”
-
–
>70% of participants in both stakeholder groups scoring the outcome as 7–9 and <15% in both stakeholder groups scoring the outcome as 1–3.
-
–
>90% of participants within 1 stakeholder group scoring the outcome as 7–9. Allowing inclusion of outcomes considered to be very important by only 1 stakeholder group.
“Consensus-out:”
– >70% of participants in both stakeholder groups scoring the outcomes as 1–3 and <15% of participants in both stakeholder groups scoring the outcome as 7–9.
A country-weighted analysis was performed for each outcome to check for skewing as a result of divergent opinions from a single country with a larger group of respondents.
Focus Groups - Outcome Prioritization With Young People
Sixteen young people (12–18 years) treated for acute simple appendicitis in the Netherlands in the preceding 24 months were invited to participate by phone. Approval was provided by the Medical Research Ethics Committee of the Amsterdam UMC, location AMC, who waived the need for complete ethical review (W18_074 # 19.555). All participants and their parents gave written informed consent.
Two meetings were held to identify any outcomes important from the young peoples’ perspective. During the meeting, participants were asked what outcomes they consider important in determining the effectiveness of a treatment for simple appendicitis using a participation board game “All Voices Count” (Teela, Haverman et al publication pending). Subsequently, participants prioritized the mentioned outcomes together with an adjusted list of outcomes from the Delphi survey considered relevant to young people, see Online Supplement S4 for the excluded Delphi outcomes, http://link-s.lww.com/SLA/C833.
Expert Meetings
In April 2020 2 separate videoconference meetings were held to accommodate different international time zones. Due to the COVID-19 pandemic, the initially planned face-to-face meeting was impossible. 14 All local lead investigators, parents, and surgeons from the Delphi study received an electronic invitation for the expert meeting. Both expert panels were asked to ratify the methodology and comment on the final set of outcomes.
The final COS was categorized according to the 4 core areas of the OMERACT 2.0 filter 17 and the Dodd et al outcome taxonomy to maximize data harmonization. 18 This study is reported in accordance with the COS-STAR (COS-STAndards for Reporting) statement 19 and GRIPP2 (Guidance for Reporting on Involvement of Patients and Public) reporting checklist. 20 The completed checklists can be found in the Online Supplements S5, http://links.lww.com/SLA/C834 and S6, http://links.lww.com/SLA/C835.
Results
Systematic Review for Delphi Survey
The updated systematic review included 10 additional studies, published between January 2014 and November 2017, see Online Supplement S7 for the PRIMSA flowchart, http://links.lww.com/SLA/C836. It yielded 77 unique outcomes which were grouped to general and unique outcome terms. Fifty four of the 77 outcomes could be grouped to the original 38 outcome terms from the systematic review by Hall et al. 7 For 23 of the 77 outcomes, 8 new outcome terms were formulated (Fig. 1). The final 43 outcome terms were mapped to the 4 core areas (death, life impact, resource use, pathophysiological manifestations), with adverse events of treatment labelled separately. 17
Figure 1.
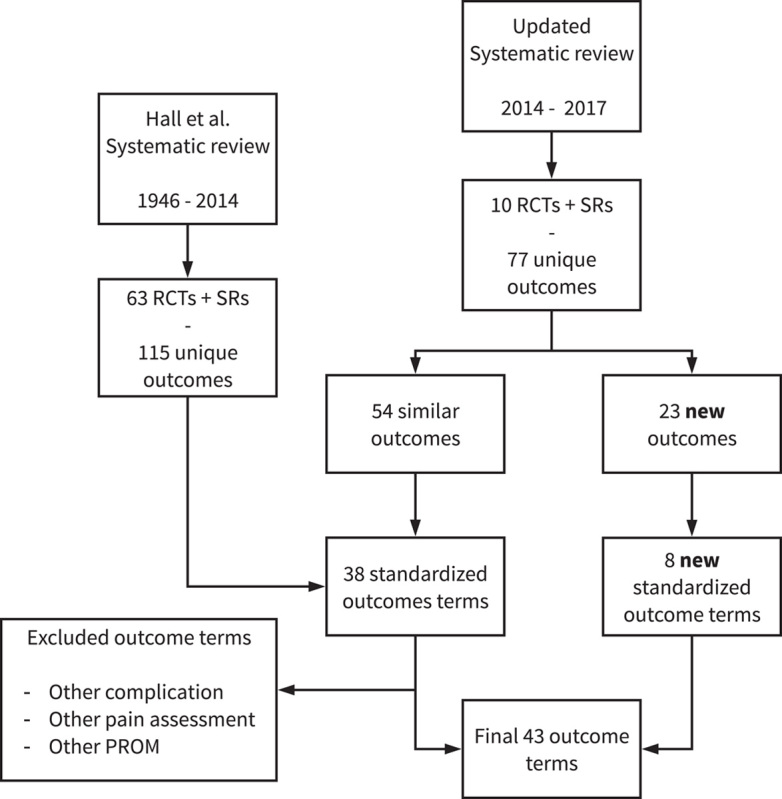
Schematic depiction of outcome term identification from SRs. RCTs indicates randomized controlled trials; SRs, systematic reviews.
Delphi Study
A total of 566 parents and 546 surgeons received an invitation to participate in the Delphi study. Round was 1 completed by 148 parents from 8 countries and 245 surgeons from 10 countries. During survey registration, 74 (50%) parents indicated they would complete the survey together with their child. Participants’ demographics are provided in Tables 1 and 2.
Table 1.
Participant Demographics of Parents Completing the First Delphi Round
| Demographic | Total (n = 148) |
|---|---|
| Male, n (%) | 34 (23) |
| Age parent in years, mean (SD) | 43 (7) |
| Geographic location, n (%) | |
| North America | 34 (23) |
| Europe | 46 (31) |
| Asia | 23 (30) |
| Australia | 23 (16) |
| Education level n (%) | |
| Primary School | 1 (1) |
| High School | 33 (22) |
| College | 23 (16) |
| Bachelor | 53 (36) |
| Master | 24 (16) |
| Other | 14 (10) |
| Age child in years, mean (SD) | 11 (4) |
| Time since diagnosis of child with appendicitis, n (%) | |
| 0–6 mo | 50 (34) |
| 7–12 mo | 53 (36) |
| 13–24 mo | 35 (24) |
| 25–48 mo | 8 (5) |
| Longer than 49 mo | 2 (1) |
| Experience with NOT, n (%) | 27 (18) |
| Child involved in appendicitis research, n (%) | 35 (24) |
| Child suffered complications of appendicitis treatment, n (%) | 23 (16) |
NOT indicates non-operative treatment; SD, standard deviation.
Table 2.
Participant Demographics of Surgeons Completing First Delphi Round
| Demographic | Total (n = 245) |
|---|---|
| Male, n (%) | 144 (59) |
| Age in years, mean (SD) | 45 (10) |
| Geographic location, n (%) | |
| North America | 50 (20) |
| Europe | 111 (45) |
| Asia | 66 (27) |
| Australia | 18 (7) |
| Type of surgeon, n (%) | |
| Pediatric surgeon | 198 (81) |
| General surgeon | 47 (19) |
| Place of work, n (%) | |
| Academic/university hospital | 159 (65) |
| Teaching hospital | 66 (27) |
| Non-teaching hospital | 20 (8) |
| Years’ work experience as surgeon, n (%) | |
| Resident | 17(7) |
| 0–5 yr | 51 (21) |
| 5–10 yr | 47 (19) |
| 10–20 yr | 67 (27) |
| Longer than 20 yr | 63 (26) |
| Children treated for appendicitis past year, n (%) | |
| 0–5 patients | 24 (10) |
| 6–10 patients | 30 (12) |
| 11–20 patients | 47 (19) |
| 21–30 patients | 46 (19) |
| 31–40 patients | 29 (12) |
| 41–50 patients | 28 (11) |
| More than 51 patients | 41 (17) |
| Experience with NOT, n (%) | 196 (80) |
| Involved in appendicitis research, n (%) | 162 (66) |
After Round 1, 7 of the 43 outcome terms met the threshold for “consensus-in” in both stakeholder groups (Table 3). None of the outcome terms met the “consensus-out” threshold. Country-weighted analysis showed no difference in consensus results. An overview ofall scores per outcome, per country, is provided in Online Supplement S8, http://links.lww.com/SLA/C837. Two new outcome terms were identified from the outcomes suggested by parents and surgeons; “mental health problems,” and “intensive care admission.”
Table 3.
Scoring for Core Outcome Set of Outcome Terms in the Delphi Survey as Critical
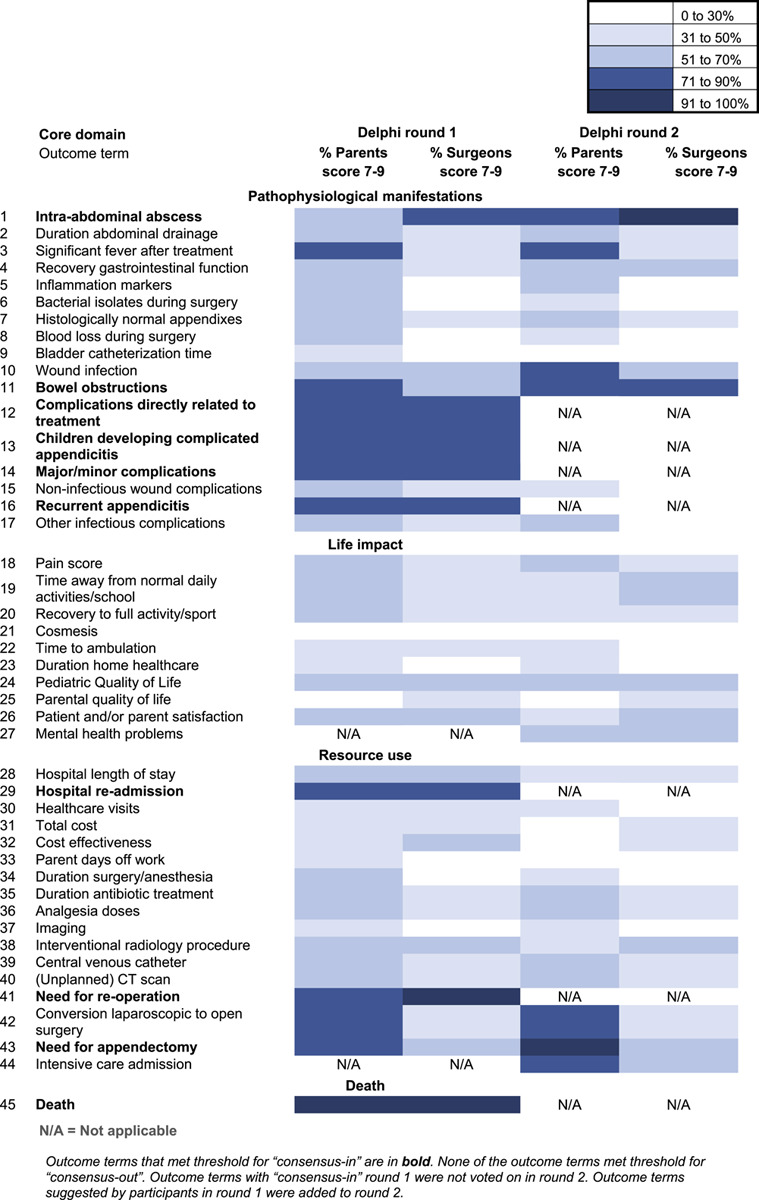
Round 2 consisted of 38 outcome terms, including the 36 outcome terms that had not yet reached consensus and the 2 newly suggested outcome terms. Round 2 was completed by 91 of 148 parents (61%), and 209 of 245 surgeons (85%) that completed Round 1. Three additional outcome terms met the threshold for “consensus-in” (Table 3). The outcome term “Need for appendectomy” only met the “consensus-in” threshold (>90%) in the parent stakeholder group. None of the outcome terms met the “consensus-out” threshold. Country-weighted analysis showed no difference in consensus results between countries. After 2 rounds consensus was reached on 10 of 45 (22%) outcome terms evaluated in the Delphi survey.
Focus Groups - Outcome Prioritization With Young People
Of the 16 Dutch young people invited, 8 agreed to participate in the focus groups. They were aged 12-16 years, with 6/8 (75%) being male. They had been treated for acute simple appendicitis in the past 1 to 20 months, with 3 treated non-operatively with antibiotics and 5 had undergone appendectomy. Young people mentioned 5 outcomes not included in the Delphi survey. Both groups prioritized these 5 outcomes, together with the adjusted list of 22 outcome terms from the Delphi study. Both groups found 6 of the 10 “consensus in” outcomes from the Delphi study to be important, see Table 4 for a summary of the prioritization. In addition, the young people found 2 outcomes important that had not reached “consensus-in” in the Delphi study; “pediatric quality of life”, and “time away from normal daily activities/school.” The young people found none of the 5 outcomes that were newly mentioned in the focus group to be very important.
Table 4.
Outcome Prioritization Results of the Focus Groups With Young People
| Outcome term | Group 1 (n = 4) | Group 2 (n = 4) | Delphi Consensus-in |
|---|---|---|---|
| Very important | |||
| Recurrent appendicitis | Most important | Most important | Yes |
| Major/minor complications | Very important | Most important | Yes |
| Need for appendectomy | Most important | Important | Yes |
| Need for re-operation | Very important | Very important | Yes |
| Hospital readmission | Very important | Very important | Yes |
| Pediatric quality of life | Very important | Important | No |
| Time away from normal daily activities/school | Important | Very important | No |
| Complications directly related to treatment | Very important | Not important | Yes |
| Important | |||
| Recovery to full activity/sport | Important | Important | No |
| Pain score | Important | Important | No |
| Fear* | Important | Important | No |
| Hospital length of stay | Important | Important | No |
| Return to sport/full activity | Important | Important | No |
| Children developing complicated appendicitis | Important | Not important | Yes |
| Bowel obstructions | Important | Not important | Yes |
| Intra-abdominal abscess | Important | Not important | Yes |
New outcome from the focus group with young people not in the part of the Delphi survey.
Expert Meetings
In total, 19 people took part in the 2 expert meetings, excluding the study management group. The group consisted of 11 pediatric surgeons (local lead investigators), 6 pediatric and general surgeons, and 2 parents. All attendees participated in both rounds of the Delphi survey. Both meetings ratified the study’s methodology and results. All experts agreed to add the outcomes found to be important by young people in the focus groups to the final COS: “return to school/normal activity” and, “quality of life.” After a discussion, it was decided that these 2 outcomes reflect an overlapping general quality of life concept, consistent with the comments made by young people during the focus groups. Therefore, “return to school/normal activity” and, “quality of life” were combined in the outcome “time to return to baseline health status.”
Final Core Outcome Set
The 4 phases of this COS development process resulted in a final set containing 12 outcomes in 5 core areas, 18 see Table 5. The outcome “death” was further specified as “disease or treatment related mortality” because all-cause mortality did not seem relevant in pediatric simple appendicitis. The outcome “bowel obstruction” signifies obstruction as a result of mechanical obstruction, not functional or paralytic. “Reoperation” means requiring an operation for the second time; not including radiological drainage. The outcome “minor/major complications” was divided into 2 outcomes: “major complications,” and “minor complications,” major referring to a complication that occurred as a result of appendicitis or its treatment, requiring invasive treatment. Minor complications refers to complications that resolve with limited or no treatment.
Table 5.
Final Core Outcome Set for Reporting Treatment of Simple Appendicitis in Children
| Core Area/Domain18 | Outcome Domain19 | Outcome |
|---|---|---|
| Death | 1. Mortality/survival | Disease or treatment related mortality |
| Physiological/clinical | 8. Gastrointestinal outcomes | Bowel obstruction |
| 12. Infection and infestation outcomes | Intra-abdominal abscess | |
| Recurrent appendicitis* | ||
| Complicated appendicitis* | ||
| Life impact | 31. Perceived health status | Time to return to baseline health status |
| 35. Hospital | Hospital readmission | |
| Resource use | 36. Need for further intervention | Reoperation |
| Unplanned appendectomy* | ||
| Adverse events | 38. Adverse events/effects | Adverse events as direct result of treatment |
| Major complication | ||
| Minor complication |
Primarily for research involving non-operative treatment of appendicitis.
For further explanations of each outcome’s meaning please see the “final core outcome set” section of the Results. Some suggestions for measurement tools can be found in the Discussion section and online supplement S8, http://links.lww.com/SLA/C837.
Three of the included outcomes are primarily relevant to trials assessing non-operative treatment; “recurrent appendicitis,” “complex appendicitis,” and “unplanned appendectomy.” “Recurrent appendicitis” is defined as a diagnosis of appendicitis that occurs after a patient has recovered from simple appendicitis. “Complex appendicitis” is defined as a diagnosis of complex/complicated appendicitis after simple appendicitis was initially diagnosed and managed. This may be due to disease progression or as the result of error in the initial diagnosis. “Unplanned appendectomy” is defined as the performance of an appendectomy due to a medical indication or at the patients’ or parents' request when the initial treatment plan was non-operative.
Discussion
The development of this set of 12 core outcomes for use in clinical trials of children with acute simple appendicitis aims to reduce the current heterogeneity of outcomes measured in this population. This COS covers the key perspectives of children, parents and physicians on the impact appendicitis and its management, and goes beyond the traditional pathophysiological outcome indicators. It was developed in collaboration with most of the research groups currently undertaking trials on pediatric appendicitis across the world. The use of this international COS in future research should help improve the comparability of clinical trial results and allow for simpler data pooling and meta-analysis, 6,9 maximizing trial utility and reducing research waste. 21 The fact that these outcomes are developed by an international group of stakeholders can also be used as information to be highlighted in the informed consent process of future clinical trials. Moreover, by identifying information that is considered important to stakeholders we can inform the pre-treatment informed consent process in routine clinical practice. Such so called Core Information Sets can be developed with methods similar to COS development, 22,23 and can be conducted alongside a COS development. 24
Most outcomes in this international COS are similar to the recently published UK COS. 11 However, 5 outcomes differ: “complicated appendicitis,” “time to return to baseline health status,” “adverse events as direct result of treatment,” and “major complication” and “minor complication.” The outcome “complicated appendicitis” was only first identified in the updated systematic review, likely reflecting the recent developments in non-operative treatment research. This is because “complicated appendicitis” is often reported as an adverse outcome of antibiotic treatment. The UK COS included “time away from full activity,” and “child’s quality of life.” In the international COS similar outcomes were integrated into “time to return to baseline health status.” “Wound infection,” “wound complication,” “negative appendectomy,” “patient stress/psychological distress,” and “length of hospital stay” were part of our international Delphi survey, but not voted “consensus-in,” as opposed to the UK COS. However, “wound infection,” “wound complication” and “negative appendectomy” may be considered as components of “major and minor complications” which are included in the international COS. Some of the differences between these 2 COSs may be due to interpretation by study teams, and some may be the result of treatment practices, resources and cultural aspects familiar to the respondents. 25 As such it seems appropriate to recommend this international COS be used for trials outside the UK, and specifically for countries with representation on the stakeholder panels.
This study has only established what outcomes should be measured, as the first step in COS development. 8 Additional research is needed to determine how some of these outcomes can be measured best, with which instruments, by whom and at what time point. We recommend that decisions on how to best measure the outcomes from this COS are informed by criteria from COSMIN (COnsensus-based Standards for the selection of health Measurement Instruments) guidelines. 26 Until that time, the use of generally accepted measurement tools and classification systems is recommended particularly where validated for use in children. These suggested classifications and definitions have been listed in online supplement S9, http://links.lww.com/SLA/C838. For example, for the outcomes “major” and “minor complications,” the Clavien-Dindo scoring system is suggested. 27,28 In this system, it is common to consider a score of III to V as a “major complication,” and I to II as a “minor complication.” Regarding “time to return to baseline health status,” it is suggested that children (or their parents, as appropriate) report the time they perceived it took to return to their pre-appendicitis health status (eg, physical, social and school/work). As simple appendicitis is an acute disease with only little and transient impact on quality of life, quality of life questionnaires seems less useful.
Strengths and Limitations
Including the views of parents and children and using the Delphi methodology are strengths of this study. Outcomes considered important by patients and their parents are essential to a meaningful and complete COS. 29 The Delphi approach is an established method for reaching consensus in a large group of experts, including patients, without the need for face-to-face. 10,30 This Delphi survey was designed to be completed by parents with input from their children; half of parents involved in the Delphi study declared to have done so, although it is unknown to what extent parents complied with that instruction. To ensure no large discrepancies existed between the opinions of parents who participated together with their child and of young persons without their parents, we developed a focus group with prioritization methodology to involve young people directly. There was good agreement between the focus groups and the Delphi study. However, as the focus groups were only done with a small group of Dutch children, its results should be interpreted with caution. It was not feasible to organize prioritization meetings in all participating countries due to a lack of experienced interviewers and resources. However, more research is needed on the optimal methodology for including children in COS research. 31
Respondents from 10 different countries participated in this international COS. Involving patients from different countries is not done in all COS developments. A study in 2017 found that only 23% of COS projects included patients from more than 4 countries. 25 However, as can be seen in the Delphi consensus results per outcome, per country, in Online Supplement S8, http://links.lww.com/SLA/C837, there is quite some inter-country variability, especially amongst parents. For instance, the outcome “time away from normal daily activities/school” is only voted as critical by 23% of Finnish parents compared to 100% of Malaysian parents. These results emphasize the importance of including representatives from multiple countries to make a COS internationally applicable. With the exception of Malaysia, all included countries were high-income countries, which could limit the generalizability of our results to low and middle-income countries. Ideally a COS development would include countries from all continents, with a variety of resources and cultural backgrounds.
The protocol intended to achieve consensus on more than 80% of all outcome terms in the Delphi study, 14 if this was achieved after 2 rounds, a third round was not warranted. However, after 2 rounds consensus was only reached on 10 of 45 (22%) outcomes, it was decided in the expert meetings not to perform a third round, as this would most likely not result in the intended 80% consensus. Secondly, a low response rate in the third round could result in attrition bias.
A steering committee with research groups from 10 of the 13 countries involved in simple appendicitis research was assembled for this project. By including future implementers as stakeholders in the development of this COS, we hope to facilitate uptake. Research on COS uptake and implementation is limited. 8,32 This COS will also be sent to relevant journal editors and funding bodies, for instance the National Institutes of Health and UK Medical Research Council. A final step would be to introduce the COS as a requirement for future funding decisions or publication of simple appendicitis trials. Another area of implementation could be uptake and recording of these core outcomes in clinical databases, for example, the American College of Surgeons National Surgical Quality Improvement Program registry, or as a part of quality indicators for governmental oversight authorities or health insurance companies.
In conclusion, this international COS for simple appendicitis, developed using Delphi methods, consists of 12 outcomes that are important to children who suffered from simple appendicitis, their parents, and surgeons from 4 different continents. It is recommended this COS be incorporated in the design of future clinical trials evaluating treatments for acute simple appendicitis in children.
Acknowledgments
The authors acknowledge Hester Rippen as the representative of the Dutch Foundation Children and Hospital for her advice and support in drafting the protocol. We would like to thank all children, young people, parents and health professionals who were willing to dedicate time to participate in the Delphi surveys and expert meetings.
Expert Meeting Participants
In addition to the lead investigators in our steering committee, we would like to thank the following people for being part of our expert panel; Saskia Koning (parent, Netherlands), Darcy Moulin (parent, USA), Ankush Gosain (surgeon, USA) and Henry E. Rice (surgeon, USA).
Supplementary Material
Footnotes
Pediatric appendicitis COS development group: Francois I Luks, PhD - Hasbro Children’s Hospital & Alpert Medical School of Brown University, Providence, USA
Olivier Abbo, MD - Hôpital des Enfants de Toulouse - CHU de Toulouse, France Alexix P Arnaud, MD - Hôpital Sud, University Hospital, Rennes, France Agostino Pierro, PhD - The Hospital for Sick Children & University of Toronto, Toronto, ON, Canada Mark Stasiewicz, MsC - The Hospital for Sick Children & University of Toronto, Toronto, ON, Canada Nadia Safa, MD - Montreal Children’s Hospital, Montreal, QC, Canada Crystal Ng - British Columbia Children’s Hospital, Vancouver, BC, Canada Zarina A Latiff MD - Department of Pediatric Surgery, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia Marjmin Osman MD - Department of Pediatric Surgery, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia. Azrina S K Zaman MD - Department of Pediatric Surgery, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia. This research was supported by the foundation of research and management projects in pediatric surgery (KCHOMP), from the Department of Pediatric Surgery, Amsterdam UMC.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1. Addiss DG, Shaffer N, Fowler BS, et al. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910–925. [DOI] [PubMed] [Google Scholar]
- 2. Anderson JE, Bickler SW, Chang DC, et al. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995-2009. World J Surg. 2012;36:2787–2794. [DOI] [PubMed] [Google Scholar]
- 3. Podda M, Gerardi C, Cillara N, et al. Antibiotic treatment and appendectomy for uncomplicated acute appendicitis in adults and children: a systematic review and meta-analysis. Ann Surg. 2019;270:1028–1040. [DOI] [PubMed] [Google Scholar]
- 4. Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet (London England). 1998;351:47–52. [DOI] [PubMed] [Google Scholar]
- 6. Kirkham JJ, Gargon E, Clarke M, et al. Can a core outcome set improve the quality of systematic reviews?–a survey of the Co-ordinating Editors of Cochrane review groups. Trials. 2013;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall NJ, Kapadia MZ, Eaton S, et al. Outcome reporting in randomised controlled trials and meta-analyses of appendicitis treatments in children: a systematic review. Trials. 2015;16:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williamson PR, Altman DG, Bagley H, et al. The COMET handbook: version 1.0. Trials. 2017;18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials. 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinha IP, Smyth RL, Williamson PR. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLoSMed. 2011;8:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sherratt FC, Allin BSR, Kirkham JJ, et al. Core outcome set for uncomplicated acute appendicitis in children and young people. Br J Surg. 2020;107:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Protocol for the Development of a Global Core Outcome Set for Treatment of Uncomplicated Appendicitis in Children: Core Outcome Measures in Effectiveness Trials Initiative (COMET). Available at: http://www.comet-initia-tive.org/studies/details/1119. Accessed March 5, 2018.
- 13. Kirkham JJ, Davis K, Altman DG, et al. Core outcome set-standards for development: the COS-STAD recommendations. PLOS Med. 2017;14:e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knaapen M Hall NJ, van der Lee JHet al. Establishing a core outcome set for treatment of uncomplicated appendicitis in children: study protocol for an international Delphi survey. BMJ Open. 2019;9:e028861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knaapen M. Open Source Protocol International Core Outcome Set for the Treatment of Simple Appendicitis in Children. Open Science Framework. doi: 10.17605/OSF.IO/ZHY34. [DOI]
- 16. COMET DelphiManager. 2017. Available at: http://www.comet-initiative.org/ delphimanager/. Accessed December 4, 2018.
- 17. Boers M, Kirwan JR, Gossec L, et al. How to choose core outcome measurement sets for clinical trials: OMERACT 11 approves filter 2.0. J Rheumatol. 2014;41:1025–1030. [DOI] [PubMed] [Google Scholar]
- 18. Dodd S, Clarke M, Becker L, et al. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkham JJ, Gorst S, Altman DG, et al. Core outcome Set-standards for reporting: the COS-STAR statement. PLoS Med. 2016;13:e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet (London England). 2009;374:86–89. [DOI] [PubMed] [Google Scholar]
- 22. Main BG, McNair AGK, Huxtable R, et al. Core information sets for informed consent to surgical interventions: baseline information of importance to patients and clinicians. BMC Med Ethics. 2017;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blazeby JM, Macefield R, Blencowe NS, et al. Core information set for oesophageal cancer surgery. Br J Surg. 2015;102:936–943. [DOI] [PubMed] [Google Scholar]
- 24. McNair AGK, Whistance RN, Main B, et al. Development of a core information setforcolorectalcancersurgery: a consensusstudy. BMJOpen. 2019;9:e028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biggane AM, Brading L, Ravaud P, et al. Survey indicated that core outcome set development is increasingly including patients, being conducted internationally and using Delphi surveys. Trials. 2018;19:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prinsen CAC, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” - a practical guideline. Trials. 2016;17:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dindo D, Demartines N, Clavien P.-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 29. Sanderson T, Morris M, Calnan M, et al. What outcomes from pharmacologic treatments are important to people with rheumatoid arthritis? Creating the basis of a patient core set. Arthritis Care Res (Hoboken). 2010;62:640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy MK, Black NA, Lamping DL, et al. Consensus development methods, and their use in clinical guideline development. HealthTechnol Assess.1998;2:i-iv. 1–88. [PubMed] [Google Scholar]
- 31. Sherratt FC, Bagley H, Stones SR, et al. Ensuring young voices are heard in core outcome set development: international workshops with 70 children and young people. Res Involv Engagem. 2020;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Akinremi A, Turnbull AE, Chessare CM, et al. Delphi panelists for a core outcome set project suggested both new and existing dissemination strategies that were feasibly implemented by a research infrastructure project. J Clin Epidemiol. 2019;114:104–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


