Objectives:
People living with chronic pain may use wearable health technology (WHT) in conjunction with an expert-directed pain management program for up to 1 year. WHT use may be associated with improvements in key patient outcomes.
Methods:
A 12-month study of WHT use among people with chronic pain was conducted, consisting of iPhone and Apple Watch applications to measure movement, sleep, and self-reported pain. Clinical outcomes among 105 patients enrolled in a multidisciplinary pain program that included WHT use were compared with 146 patients in the same program but without WHT, and to 161 patients receiving medical pain management without WHT.
Results:
Participants used the WHT on average 143.0 (SD: 117.6) out of 365 days. Mixed-effects models revealed participants who used WHT had decreases in depression scores (−7.83, P<0.01) and prescribed morphine milligram equivalents (−21.55, P=0.04) over 1 year. Control groups also showed decreases in depression scores (−5.08, P=0.01; −5.68, P<0.01) and morphine milligram equivalents (−18.67, P=0.01; −10.99, ns). The estimated slope of change among the WHT was not statistically different than control groups.
Discussion:
Patients who used WHT as part of their pain management program demonstrated a willingness to do so for extended periods of time despite living with chronic pain and other comorbidities. Data trends suggest that WHT use may positively impact depression and prescribed medication. Additional research is warranted to investigate the potential of WHT to improve the negative consequences of chronic pain.
Key Words: pain management, multidisciplinary pain program, pain app, opioids, depression
Chronic pain is a persistent and progressively worsening physical condition that may result in changes to the nervous system over time.1 Greater than 30% of adults in the United States are burdened with chronic pain,2 and 36.7% of them experience daily pain symptoms.2,3 Beyond physical functioning, chronic pain may also impair mental functioning, diminish patients’ quality of life, and cause a loss in productivity.4–6 In addition, numerous epidemiological studies have reported a significant association between chronic pain and depression, and the estimated prevalence of depression among chronic pain patients ranges from 11% to 35%.7–10
At Geisinger (Danville, PA), patients who have complex chronic pain with biopsychosocial sequelae and failed conventional outpatient management are treated by a medical pain management (MPM) team of health care professionals (HCPs). Each visit with the MPM team provides comprehensive and multidisciplinary pain management ranging from counseling to aggressive medication adjustments as appropriate. These include, but are not limited to, referrals to interventional pain management, acupuncture, physical therapy, aquatic therapy, occupational therapy, psychology/psychiatry, social and counseling services, addiction counseling, and drug and alcohol treatment. Because chronic pain is influenced by a variety of biological and psychological factors,11 both the treatment and management of chronic pain often require a multidisciplinary approach. Interdisciplinary and multimodal chronic pain management programs have been observed to produce the greatest impact on patients with the most severe and persistent pain.12–14 The Department of Pain Medicine at Geisinger designed and developed the multidisciplinary pain program (MPP) as a comprehensive outpatient program designed to improve function and quality of life for individuals living with chronic pain. In addition to the treatment outlined as part of the MPM, patients in the MPP also receive a 3‐day multidisciplinary educational class with up to 1 year of follow-up visits and communications with the MPP team. The 3-day classes include sessions on the biopsychosocial model of care, acute and chronic pain, preventing misuse of prescription pain medication, medication therapy disease management, and goal setting. MPP patients are also introduced to behavioral medicine and life-skill topics including yoga-based relaxation, stress management, nutrition, sleep hygiene, art and horticulture therapy, and mindfulness. Participation in the educational sessions is a requirement for enrollment in the MPP, thus all MPP patients take all classes. People who miss a session are given the opportunity to attend the next available class.
Because of the subjective nature of pain, all health care professionals treating patients with chronic pain depend on patients’ perceptions of their pain and their ability to recall and communicate their pain experience.15 The introduction of recall bias from the time delay between the pain episode and patient self-reporting may lead to a vague and distorted memory of the pain experience.15–17 The clinicians’ ability to effectively manage patients and optimize long-term treatment regimens is limited by a lack of timely access to more objective data about symptoms. Chronic pain interventions enhanced with wearable health technology (WHT), such as activity monitors or smartphone apps designed to capture self-reported pain episodes, have the potential to minimize the time delay between pain episodes and reporting. Pain apps have already been developed with acceptable psychometric properties compared with traditional standardized questionnaires and relatively high levels of user satisfaction.18–20 WHT has been shown to be effective in improving mobility among patients with disabilities21 and may have a positive influence on chronic pain intensity, management, and quality of life.20–29 Published WHT studies focusing on chronic musculoskeletal pain have a time horizon ranging from 1 to 12 weeks.24,25,29–33 To the best of our knowledge to date, there have not been data published demonstrating that patients would use WHT for longer periods or on the long-term effects of WHT on health outcomes.
In this study, we wanted to determine whether patients who were willing to use WHT in conjunction with an expert-directed pain management program would do so for an extended period of up to 1 year. Furthermore, we wanted to assess whether the addition of WHT to an MPP program resulted in measurable improvements in patient outcomes, including depression, opioid use, pain severity, and disability.
METHODS
Study Design
A prospective, nonrandomized, nonblinded observational study with WHT users and 2 control arms were conducted to assess the effect of the addition of WHT and provider dashboards on patient outcomes. Patients who started Geisinger’s MPP educational seminar between September 2017 and June 2019 were eligible to participate in the WHT+MPP study arm. The MPP treated between 5 and 20 new patients each month, making it challenging to randomly assign enough patients to arms of a study within a practical time frame. HCPs at Geisinger wanted to offer WHT to every patient who was willing to incorporate it into their pain management treatment plan. Thus, the decision was made to enroll all who met the inclusion criteria into the WHT+MPP study arm while using prospective and historical cohorts of MPP and MPM patients for comparison. The study was approved by Geisinger’s Institutional Review Board, and informed consent was obtained from each participant who agreed to use WHT.
All patients were enrolled in Geisinger’s pain management programs and were screened for eligibility using electronic health records (EHRs). These records are utilized by clinicians in both the inpatient and outpatient settings with integrated electronic scheduling, clinical lab, and radiology systems. Clinical data were collected throughout the study during patient-provider visits at multiple Geisinger sites. The WHT program recorded data on patients’ self-reported pain intensity scores and utilization of pain treatment (pain medications and alternative pain treatments), as well as passively collected data on function (sleep and physical activity). For each cohort, the earliest date of program initiation was defined as the index date.
Program Description
Wearable Health Technology (WHT + MPP)
Patients who consented were provided with the WHT equipment, which consisted of an iPhone and an Apple Watch, and a 30-minute training session on how to use the technology with the option of additional training during office visits. Patients were instructed to wear the WHT for at least 20 hours each day, including wearing the watch at night and keeping the phone close by while sleeping. They were instructed to remove the watch while bathing or recharging the device. Devices were equipped with 3 applications (apps): “Activity” app (Apple Inc.) and “Pillow” app (Neybox Digital Ltd.), freely and publicly available, and the “Pain Watch” app, which was developed specifically for use in this study by the Steele Institute for Health Innovation at Geisinger. The “Pain Watch” app was designed to remind and guide patients to document self-reported pain intensity (scale 0=no pain to 10=immobilizing) on a daily basis at approximately the same time each day as chosen by the patient. Patients were also asked to document any experiences of breakthrough pain, including location and description of pain, and any use of alternative therapies such as stretching, mindfulness, and hot or cold therapy. The “Pain Watch” app also helped patients to monitor their pain trends daily, weekly, and monthly. Self-reported pain experiences, along with the data synced from the “Activity” and “Pillow” apps, were collected in real-time by a central data processing server housed at Geisinger and incorporated into the patients’ EHR. Pillow was designed to track and analyze sleeping pattern. At the completion of the study, participants were informed that they would be able to keep the watch and phone and were asked about their overall experience using the WHT. Participants reported being satisfied with the WHT and no participant reported any user issues that prevented them from participating in the study.
HCP Dashboard
The patient-specific data gathered from the WHT were available to physicians, physician assistants, and nurse practitioners within the MPP via a customizable dashboard. These HCPs were trained on dashboard use by the software development team. Patients were informed that HCPs would have access to their WHT data, and HCPs reviewed this data with patients before each office visit during the study.
Study Participants and Data Collection
Patients who agreed to use WHT were consented and enrolled in the WHT+MPP cohort. Enrollment was strictly voluntary. Patients who declined to use WHT remained in the MPP and were also included in the MPP cohort as a control group. Patients were followed for 12 months from the day of enrollment. Additional patients receiving MPM but not enrolled in the MPP who met the inclusion and exclusion criteria were assigned to the MPM cohort as a prospective control group receiving the pain care, as noted by the Institute of Medicine Committee on Advancing Pain Research, Care, and Education.34 Outcome data were collected during regularly scheduled office visits and recorded using Geisinger’s EHR system. The data were pulled from the EHR system at the completion of the study for analysis.
Study inclusion criteria for MPM and MPP cohorts were (1) age 18 to 75 years at enrollment; and (2) completion of the 3-day MPP training (MPP cohort) or at least 2 encounters within the MPM department (MPM cohort) between September 2017 and June 2019. Inclusion criteria for the WHT+MPP cohort were (1) age 18 years or above at enrollment, (2) persistent pain for at least 6 months, (3) completion of the 3-day MPP training between September 2017 and June 2019, (4) ability to understand and complete the informed consent form before the initiation of any study procedures, (5) English-speaking, and (6) willingness and sufficient motor skills to utilize WHT.
Patient exclusion criteria for all the 3 cohorts were (1) currently or previously enrolled in the other cohorts before the index date (the first date of each program); (2) diagnosed with cancer, acquired immunodeficiency syndrome, end-stage liver disease, and/or end-stage renal disease at any time; (3) hospitalization with a length of stay >30 days during the 12 months before study index date; (4) known to have been in a nursing home or under hospice care during the 12 months before the index date; (5) patients who actively opted-out of any research; (6) had no assessment using pain scales within 3 months of baseline pain-related visit; (7) had a terminated Medication Use Agreement on their active EHR problem list, indicating substance abuse/dependence, or completed treatment for substance abuse/dependence before enrollment in MPP.
Measures and Outcomes
This study outcome measures included the Patient Health Questionnaire-9 (PHQ-9), numeric rating scale (NRS), Oswestry Disability Index (ODI) Neck, ODI Back, and morphine milligram equivalents (MME). The PHQ-9, NRS, and ODI measures were included because they assess clinical outcomes (depression, pain, functionality) that are often at the center of patients’ and HCPs’ pain management goals within the MPP and MPM. Furthermore, these measures are used in pain management research, are relatively easy to administer during regularly scheduled office visits, and are routinely used within the MPP.
The PHQ is a self-administered diagnostic instrument for common mental disorders. The PHQ-9 is the 9-item depression module taken from the full PHQ, which measures the severity of depression and response to treatment.35 The scale ranges from 0 to 27, indicating the following levels of depression severity: none (0 to 4), mild (5 to 9), moderate (10 to 14), moderately severe (15 to 19), and severe (20 to 27). The NRS is self-reported by the patient on a scale of 0 to 10, with the following scale labels: no pain (0), mild pain (2), moderate pain (4), severe pain (6), very severe pain (8), and worst pain possible (10). The ODI is a self-administered questionnaire assessing symptoms and severity of back or neck pain on a scale from 0 to 100. This measurement evaluates the loss of function in activities of daily living.36 Two ODI questionnaires were used in this study, 1 for neck pain and 1 for back pain. Interpretation of ODI scoring is as follows: minimal disability (0 to 20), moderate disability (21 to 40), severe disability (41 to 60), and crippled (61 to 80). Scores over 80 suggest that the patient may be bedbound or exaggerating their symptoms and careful evaluation is recommended.
Opioid use as part of pain management should always be carefully considered. Geisinger provides HCPs with guidelines on opioid use and programs aimed at reducing use. Preliminary analyses revealed the number of patients (and percentage of cohort) with at least 1 opioid prescription during the study period in the MPM, MPP, and WHT+MPP cohorts was 120 (74.5%), 101 (69.2%), and 54 (51.4%), respectively, and no patients had documented misuse. MME was included as an outcome so that this study could track opioid use over time, determine whether WHT could be a tool to help patients with the goal to reduce opioid use, and potentially add to the national dialogue on opioid use.
The MME for each patient per month was calculated by multiplying the units prescribed in each month, the strength of the medication per unit, and an MME conversion factor specific to each drug compound.37 If there was more than 1 oral opioid prescription on the same day, then the total MME was calculated by summation. Patients without any oral opioid prescriptions in a month were assumed to have an MME of zero.
Statistical Analyses
Baseline characteristics of patients in the 3 cohorts (MPM, MPP, and WHT+MPP) during the 12-month period before study enrollment (index date) were summarized using descriptive statistics (eg, means and percentages). The cohorts were compared using analysis of variance for continuous variables or χ2 tests or Fisher exact tests for categorical variables, where appropriate.
To estimate 12-month longitudinal change in the 5 continuous clinical outcomes (PHQ-9, NRS, ODI Back, ODI Neck, and MME), 5 analytic subsets of patients were created that met the following criteria: patients were required to have a baseline measurement during the 6-month period before the index date; the patients were required to have at least 2 measurements during the study period; and the patients’ baseline measurements must have been above the following clinically relevant thresholds: PHQ-9 >4 (at least mild depression), NRS >1 (at least mild pain), ODI Back and ODI Neck >20 (at least moderate disability), and MME >0 (at least some prescription opioid use). In addition, to be included in the MME analysis, patients must have had 12 months of EHR activity after the index date so that an MME value of zero could correctly be assigned to patients without a recorded opioid prescription. This set of requirements were applied to ensure that conclusions from the longitudinal analyses would be drawn from relevant and suitable patients with adequate data and a potential need for improvement in clinical outcomes.
Descriptive analysis summarizing the effect size of mean change per year and linear mixed-effects models incorporating a random intercept and random coefficient for time were fitted in this subset of patients. The linear mixed-effects models estimated the changes in outcome over time for MPM, MPP, and WHT+MPP groups for each clinical outcome. Scores were used as the dependent outcome variables, with the group, time, and an interaction between group and time as independent variables. Age, sex, and body mass index (BMI) were also included in the model to adjust for characteristics that may be associated with chronic pain. Both random intercept and random slope were included in the model to account for correlations among multiple measurements per patient and variation among patients over time. An unstructured covariance between intercept and slope was used modeling every parameter in the covariance matrix. Point estimates of the slope per treatment group and the differences in the slopes comparing MPM and MPP groups versus the WHT+MPP group (reference) with 95% CIs and corresponding P-values were estimated with covariates held at fixed values: either means (for age and BMI) or a fixed 0.5 for sex. P-values of <0.05 were defined as statistically significant for all analyses, and statistical analyses were performed using SAS software version 9.4 (SAS Institute).
RESULTS
Patient Demographics and Clinical Characteristics
For the WHT+MPP study cohort, 285 patients were screened, and 113 patients consented to be enrolled. The primary reasons given for not enrolling were a lack of interest in participating in a study and a lack of interest in using WHT as part of the treatment plan. Out of those 113 enrollees, 8 patients did not use WHT at all and were thereafter excluded from analyses. A search of EHRs identified 161 MPM patients and 146 MPP patients who met all inclusion and exclusion criteria. Therefore, the final study consisted of 412 patients, with 39% (n=161) in the MPM, 35% (n=146) in the MPP, and 26% (n=105) receiving WHT+MPP.
Baseline demographics of the patients in all the 3 cohorts are shown in Table 1. Overall, the mean patient age was 49.0 years (SD: 12.4), and MPM patients were significantly younger in comparison to MPP and WHT+MPP patients (means of 47.1 vs. 50.4 and 50.1, respectively). The majority of patients were female (68.9% overall) and non-Hispanic White (96.4% overall). The most common insurance coverage was through the system-affiliated Geisinger Health Plan (52.2%).
TABLE 1.
Patient Demographics
| Patient Demographics | All | MPM | MPP | WHT+MPP | P |
|---|---|---|---|---|---|
| No. patients, n | 412 | 161 | 146 | 105 | — |
| Age (y), n (%) | |||||
| Mean (SD) | 49.0 (12.4) | 47.1 (12.5) | 50.4 (11.8) | 50.1 (12.8) | 0.036 |
| 18-29 | 26 (6.3) | 14 (8.7) | 5 (3.4) | 7 (6.7) | 0.048 |
| 30-44 | 117 (28.4) | 49 (30.4) | 42 (28.8) | 26 (24.8) | — |
| 45-54 | 124 (30.1) | 55 (34.2) | 36 (24.7) | 33 (31.4) | — |
| 55-64 | 100 (24.3) | 26 (16.2) | 48 (32.9) | 26 (24.8) | — |
| 65+ | 45 (10.9) | 17 (10.6) | 15 (10.3) | 13 (12.4) | — |
| Sex, n (%) | |||||
| Female | 284 (68.9) | 110 (68.3) | 93 (63.7) | 81 (77.1) | 0.074 |
| Race, n (%) | |||||
| White | 392 (95.2) | 151 (93.8) | 139 (95.2) | 102 (97.1) | 0.570 |
| African American | 17 (4.1) | 9 (5.6) | 5 (3.4) | 3 (2.9) | — |
| Asian | 1 (0.2) | 0 (0.0) | 1 (0.7) | 0 (0.0) | — |
| Native American | 1 (0.2) | 0 (0.0) | 1 (0.7) | 0 (0.0) | — |
| Unknown | 1 (0.2) | 1 (0.6) | 0 (0.0) | 0 (0.0) | — |
| Ethnicity, n (%) | |||||
| Non-Hispanic or Latino | 397 (96.4) | 153 (95.0) | 142 (97.3) | 102 (97.1) | 0.514 |
| Hispanic or Latino | 15 (3.6) | 8 (5.0) | 4 (2.7) | 3 (2.9) | — |
| Insurance type, n (%) | |||||
| Geisinger Health Plan | 215 (52.2) | 89 (55.3) | 69 (47.3) | 57 (54.3) | 0.344 |
| Public (Medicaid/Medicare) | 102 (24.8) | 38 (23.6) | 45 (30.8) | 19 (18.1) | — |
| Commercial/other | 95 (23.1) | 34 (21.1) | 32 (21.9) | 29 (27.6) | — |
MPM indicates medical pain management; MPP, multidisciplinary pain program; WHT, wearable health technology.
The baseline clinical characteristics are shown in Table 2. A majority of patients in all the 3 cohorts were overweight (BMI≥25 kg/m2) and the overall mean BMI was 32.9 kg/m2 (SD: 8.5). The proportions of nonsmokers and nondrinkers of alcohol were 71.4% and 60.9%, respectively. There were significantly more nonsmokers in the WHT+MPP cohort compared with MPM and MPP cohorts (87.6% vs. 60.9% and 71.2%, respectively; P<0.001). Overall, anxiety and depression were among the most common comorbidities (35.4% and 29.1%, respectively) and there were significantly more patients with depression in the WHT+MPP cohort than in MPM and MPP cohorts (36.2% vs. 21.1% and 32.9%; P=0.014). Chronic obstructive pulmonary disease (COPD) and diabetes were common among patients as well (29.1% and 26.5%, respectively).
TABLE 2.
Baseline Clinical Characteristics
| Clinical Characteristics | All | MPM | MPP | WHT+MPP | P |
|---|---|---|---|---|---|
| No. members, n | 412 | 161 | 146 | 105 | — |
| Body mass index (kg/m2), n (%) | |||||
| Mean (SD) | 32.9 (8.5) | 33.1 (9.3) | 32.5 (8.2) | 33.4 (7.7) | 0.683 |
| Normal or underweight (BMI <25) | 66 (16.0) | 22 (13.7) | 31 (21.2) | 13 (12.4) | 0.183 |
| Overweight (BMI 25-29.9) | 94 (23.0) | 45 (28.0) | 27 (18.6) | 22 (21.4) | — |
| Obese (BMI 30-34.9) | 108 (26.4) | 41 (25.5) | 41 (28.3) | 26 (25.2) | — |
| Severely obese (BMI ≥35) | 141 (34.5) | 53 (32.9) | 46 (31.7) | 42 (40.8) | — |
| Smoking status, n (%)* | |||||
| No | 294 (71.4) | 98 (60.9) | 104 (71.2) | 92 (87.6) | <0.001 |
| Yes | 115 (27.9) | 63 (39.1) | 41 (28.1) | 11 (10.5) | — |
| Alcohol use, n (%)* | |||||
| No | 251 (60.9) | 98 (60.9) | 89 (61.0) | 64 (61.0) | 0.731 |
| Yes | 151 (36.7) | 57 (35.4) | 55 (37.7) | 39 (37.1) | — |
| Comorbidities, n (%) | |||||
| Anxiety | 146 (35.4) | 54 (33.5) | 52 (35.6) | 40 (38.1) | 0.748 |
| Depression | 120 (29.1) | 34 (21.1) | 48 (32.9) | 38 (36.2) | 0.014 |
| Diabetes with chronic complication | 76 (18.5) | 37 (23.0) | 23 (15.8) | 16 (15.2) | 0.163 |
| Diabetes without chronic complication | 33 (8.0) | 11 (6.8) | 11 (7.5) | 11 (10.5) | 0.545 |
| COPD | 120 (29.1) | 52 (32.3) | 36 (24.7) | 32 (30.5) | 0.318 |
| Heart disease | 19 (4.6) | 9 (5.6) | 5 (3.4) | 5 (4.8) | 0.663 |
There were patients with unknown smoking status or alcohol use.
BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; MPM, medical pain management; MPP, multidisciplinary pain program; WHT, wearable health technology.
Use of WHT
Length of use was defined as the time from the day of the first WHT use to the last day of WHT use up to 365 days, and WHT engagement days were defined as those on which the user entered data on the WHT apps. Within the WHT+MPP cohort (n=105), there was a total of 15,013 engagement days during the 12-month follow-up period, representing 63.2% of all measured days and a mean of 143.0 days of WHT engagement (SD: 117.6) per patient. Sixty-eight (64.8%) patients used the WHT for at least 6 months and 26 (24.8%) patients for the entire 1-year observation period. Figure 1 shows the distribution of active WHT use for all WHT patients by the length of use.
FIGURE 1.

WHT engagement versus number of days enrolled for patients in the WHT+multidisciplinary pain program cohort (n=105). The enrollment period is the time from the first date to the last date when the “Pain Watch,” “Activity,” or “Pillow” app was used. App activity level was measured by the percentage of days with any app use during the enrollment period. WHT indicates wearable health technology.
Clinical Outcomes
The number and percent of patients in each cohort who met the criteria for inclusion in the mixed-effect analyses for each clinical outcome are presented in Table 3. The percent of patients in the MPM group who met the criteria for the PHQ-9, ODI Back, and ODI Neck analyses (19.3%, 22.4%, and 16.8%, respectively) were lower than in the MPP (51.4%, 54.8%, and 39.7%) and WHT+MPP (31.4%, 51.4%, and 33.3%) groups. The percentage of patients in the WHT+MPP group who met the criteria for the MME analysis (37.1%) was lower than in the MPM (52.2%) and MPP (50.7%) groups. The percent of patients in the MPM, MPP, and WHT+MPP groups who met the criteria for the NRS analyses (86.3%, 92.5%, and 75.2%, respectively) were relatively high compared with the other outcome measures.
TABLE 3.
Sample Attrition for Subset of Patients Included in the Mixed Model Analyses
| No. Patients N (% of Original) | ||||
|---|---|---|---|---|
| Clinical Outcomes | Attrition Step | MPM (Original N=161), n (%) | MPP (Original N=146), n (%) | WHT+MPP (Original N=105), n (%) |
| PHQ-9 | Baseline measurement within 6-mo before the index date | 62 (38.5) | 105 (71.9) | 69 (65.7) |
| At least 2 measurements during the study period | 62 (38.5) | 105 (71.9) | 69 (65.7) | |
| Baseline measurement above clinical threshold | 31 (19.3) | 75 (51.4) | 33 (31.4) | |
| NRS | Baseline measurement within 6-mo before the index date | 148 (91.9) | 146 (100) | 89 (84.8) |
| At least 2 measurements during the study period | 148 (91.9) | 146 (100) | 89 (84.8) | |
| Baseline measurement above clinical threshold | 139 (86.3) | 135 (92.5) | 79 (75.2) | |
| ODI back | Baseline measurement within 6-m before the index date | 37 (23) | 81 (55.5) | 58 (55.2) |
| At least 2 measurements during the study period | 37 (23) | 81 (55.5) | 58 (55.2) | |
| Baseline measurement above clinical threshold | 36 (22.4) | 80 (54.8) | 54 (51.4) | |
| ODI neck | Baseline measurement within 6-mo before the index date | 27 (16.8) | 59 (40.4) | 35 (33.3) |
| At least 2 measurements during the study period | 27 (16.8) | 59 (40.4) | 35 (33.3) | |
| Baseline measurement above clinical threshold | 27 (16.8) | 58 (39.7) | 35 (33.3) | |
| MME | 12 mo EHR activity after the index date | 154 (95.7) | 144 (98.6) | 79 (75.2) |
| Baseline measurement within 6-mo before the index date | 154 (95.7) | 144 (98.6) | 79 (75.2) | |
| At least 2 measurements during the study period | 154 (95.7) | 144 (98.6) | 79 (75.2) | |
| Baseline measurement above clinical threshold | 84 (52.2) | 74 (50.7) | 39 (37.1) | |
MME indicates morphine milligram equivalent; MPM, medical pain management; MPP, multidisciplinary pain program; NRS, numeric rating scale; ODI, Oswestry Disability Index; PHQ-9, Patient Health Questionnaire-9; WHT, wearable health technology.
Table 4 describes the unadjusted mean values for each outcome and cohort during the 6-month period before the index date and during the 12-month follow-up period, the effect size of mean change per year, and the number of observations in the follow-up period. Mean PHQ-9 scores for MPM, MPP, and WHT+MPP patients before the index date were 14.63, 15.19, and 14.07, respectively, and 11.31, 12.89, and 11.78 during the follow-up period. Mean NRS scores before the index were 6.48, 6.50, and 5.48, respectively, and 6.15, 6.10, and 5.22 during the follow-up period. Mean ODI Back scores before the index were 51.20, 55.05, and 47.67, respectively, and 46.28, 53.99, and 45.46 during the follow-up period. Mean ODI Neck scores were 53.10, 59.23, and 50.07, respectively, and 51.40, 59.59, and 51.03 during the follow-up period. Mean MMEs before the index date were 43.42, 56.83, and 51.38, respectively, and 43.69, 54.46, and 38.36 during the follow-up period. Most of the Cohen d statistics could be interpreted as representing small (d=0.20) or medium (d=0.50) effect sizes. Decreases in PHQ-9 and NRS tended to be relatively larger than the other effect sizes observed, ranging from −0.37 to −0.63 for PHQ-9 scores and from −0.42 to −0.57 for NRS.
TABLE 4.
Clinical Measures During Baseline, During the 12-month Follow-up Period, Effect Size of Change From Baseline to Outcomes, and Number of Outcome Measurements During the 12-month Follow-up Period, by Cohort
| Clinical Outcomes | Cohort | No. Patients | Baseline Measurement Mean (SD) | Outcomes During the 12-mo Follow-up Mean (SD) | Effect Size of Change from Baseline to Outcomes (Cohen d) | No. outcome Measurements During the 12-mo Follow-up Period Mean (SD) |
|---|---|---|---|---|---|---|
| PHQ-9 | MPM | 31 | 14.63 (4.90) | 11.31 (5.73) | −0.63 | 3.3 (2.8) |
| MPP | 75 | 15.19 (6.34) | 12.89 (6.25) | −0.37 | 3.7 (2.9) | |
| WHT+MPP | 33 | 14.07 (5.65) | 11.78 (6.18) | −0.38 | 4.1 (3.0) | |
| NRS | MPM | 139 | 6.48 (1.70) | 6.15 (1.54) | −0.48 | 8.4 (6.3) |
| MPP | 135 | 6.50 (1.57) | 6.10 (1.36) | −0.57 | 9.2 (5.4) | |
| WHT+MPP | 79 | 5.48 (1.50) | 5.22 (1.53) | −0.42 | 9.0 (7.7) | |
| ODI back | MPM | 36 | 51.20 (11.78) | 46.28 (16.74) | −0.43 | 2.1 (1.5) |
| MPP | 80 | 55.05 (12.43) | 53.99 (12.99) | −0.11 | 2.9 (1.6) | |
| WHT+MPP | 54 | 47.67 (13.54) | 45.46 (14.38) | −0.23 | 3.1 (2.0) | |
| ODI Neck | MPM | 27 | 53.10 (13.70) | 51.40 (17.27) | −0.16 | 1.7 (1.0) |
| MPP | 58 | 59.23 (13.96) | 59.59 (13.79) | 0.04 | 2.8 (1.7) | |
| WHT+MPP | 35 | 50.07 (14.51) | 51.03 (14.50) | 0.11 | 2.8 (1.9) | |
| MME | MPM | 84 | 43.42 (68.62) | 43.69 (51.39) | 0.01 | 12.0 (0.0) |
| MPP | 74 | 56.83 (76.30) | 54.46 (66.51) | −0.04 | 12.0 (0.0) | |
| WHT+MPP | 39 | 51.38 (44.84) | 38.36 (34.88) | −0.31 | 12.0 (0.0) |
MME indicates morphine milligram equivalent; MPM, medical pain management; MPP, multidisciplinary pain program; NRS, numeric rating scale; ODI, Oswestry Disability Index; PHQ-9, Patient Health Questionnaire-9; WHT, wearable health technology.
The changes in clinical outcomes obtained from the mixed-effect models over a 12-month period are shown in Table 5. The changes in PHQ-9, NRS, and MME in each cohort over time are depicted in Figures 2, 3, and 4 respectively. In all the 3 groups (MPM, MPP, and WHT+MPP), PHQ-9 scores showed a statistically significant decrease over time, −5.08, −5.68, and −7.38 per year, respectively (Table 5 and Fig. 2). NRS scores in the MPM showed a significant 1-year decrease (−0.85, P <.01). NRS scores decreased in the MPP and WHT+MPP cohorts but did not achieve statistical significance (Table 5 and Fig. 3). ODI Back and ODI Neck scores did not demonstrate significant change over time, except for the ODI Back scores in the MPM group, which did have a significant 1-year decrease (−11.68, P <.01). Finally, MME showed a significant decrease among the MPM (−18.67, P=0.01) and the WHT+MPP (−21.55, P=0.04). The MPP group declined in MME, but this decline was not statistically significant (Table 5, Fig. 4). None of the estimates of the differences in the slopes comparing the MPM and MPP groups to the WHT+MPP group reached statistical significance.
TABLE 5.
Change in Clinical Outcomes Over Time Using Linear Mixed Models
| Clinical Outcomes | Cohort | No. Patients | Difference in Yearly Change (95% CI) | P |
|---|---|---|---|---|
| PHQ-9 | MPM | 31 | −5.08 (−9.15, −1.01) | 0.01 |
| MPP | 75 | −5.68 (−8.26, −3.11) | <0.01 | |
| WHT+MPP | 33 | −7.83 (−11.77, −3.90) | <0.01 | |
| NRS | MPM | 139 | −0.85 (−1.21, −0.50) | <0.01 |
| MPP | 135 | −0.24 (−0.58, 0.09) | 0.16 | |
| WHT+MPP | 79 | −0.40 (−0.93, 0.14) | 0.14 | |
| ODI Back | MPM | 36 | −11.68 (−19.69, −3.68) | <0.01 |
| MPP | 80 | 0.08 (−4.36, 4.53) | 0.97 | |
| WHT+MPP | 54 | −2.79 (−8.43, 2.85) | 0.33 | |
| ODI neck | MPM | 27 | −4.75 (−15.16, 5.65) | 0.37 |
| MPP | 58 | −0.36 (−5.62, 4.90) | 0.89 | |
| WHT+MPP | 35 | −0.15 (−7.19, 6.90) | 0.97 | |
| MME | MPM | 84 | −18.67 (−32.92, −4.42) | 0.01 |
| MPP | 74 | −10.99 (−26.17, 4.19) | 0.16 | |
| WHT+MPP | 39 | −21.55 (−42.46, −0.64) | 0.04 |
MME indicates morphine milligram equivalent; MPM, medical pain management; MPP, multidisciplinary pain program; NRS, numeric rating scale; ODI, Oswestry Disability Index; PHQ-9, Patient Health Questionnaire-9; WHT, wearable health technology.
FIGURE 2.

Comparison of PHQ-9 over 12 months by the slope using linear models. MPM indicates medical pain management; MPP, multidisciplinary pain program; PHQ-9, Patient Health Questionnaire-9; WHT, wearable health technology.
FIGURE 3.
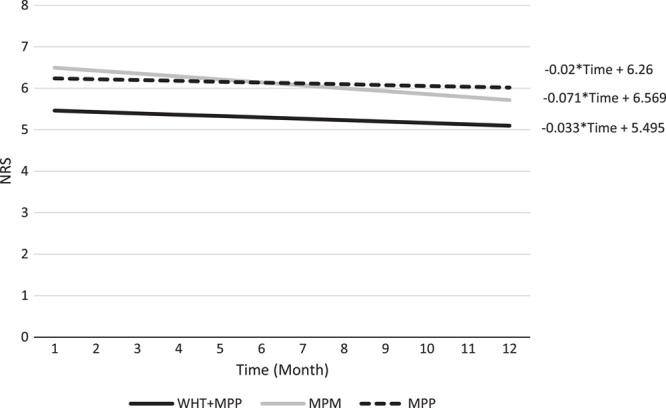
Comparison of NRS over 12 months by the slope using linear models. MPM indicates medical pain management; MPP, multidisciplinary pain program; NRS, numeric rating scale; WHT, wearable health technology.
FIGURE 4.
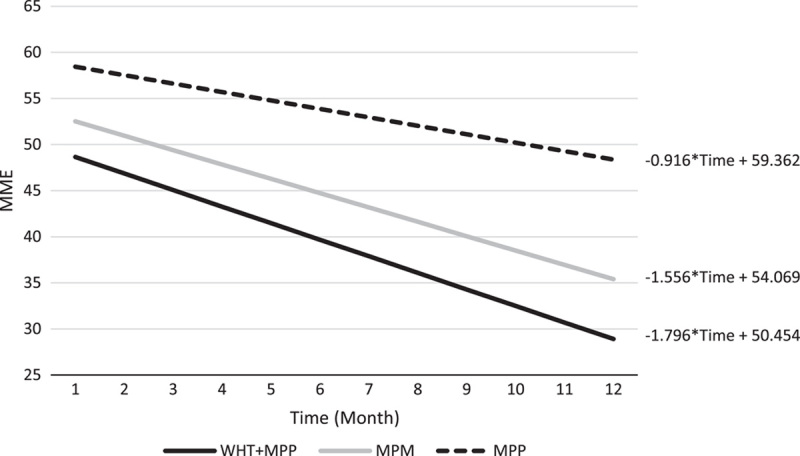
Comparison of MME over 12 months by the slope using linear models. MME indicates morphine milligram equivalent; MPM, medical pain management; MPP, multidisciplinary pain program; NRS, numeric rating scale; WHT, wearable health technology.
DISCUSSION
Chronic pain treatments enhanced by technology are increasingly popular. Though numerous studies have investigated the short-term effects (1 to 12 wk) of a smartphone app or WHT use on chronic pain, this prospective, nonrandomized study with comparison cohorts most likely represents the first investigation of long-term use of WHT.20–23,26–28 Of the 105 patients provided with WHT in the current study, 68 used the WHT for at least 6 months and 26 for the entire year of the study. These results suggest that patients will engage with WHT for extended periods of time when integrated into a pain management program supervised by HCPs. The results of this study also showed that both expert-directed MPM and an expert-directed MPP demonstrated improved severity of depression and reduction in opioid prescribing among chronic pain patients.
At baseline, depression was a common comorbidity; this was expected since previous research has found that patients experiencing chronic pain are 4 times more likely to have depression than patients who do not report pain.38 In this study, 29.1% of the chronic pain patients at baseline presented with depression compared with only 4.7% of all US adults in a 2019 national survey conducted by the Centers for Disease Control and Prevention.39 Patients in all the 3 groups (MPM, MPP, and WHT+MPP) showed a 1-year decrease in depression scores ranging from 5.08 to 7.83 points on the 27-point PHQ-9 scale.
Prescribing patterns of opioids for chronic pain patients are of great interest to clinicians, as balancing pain management with potential risks posed by long-term use of opioids is complex and challenging. Guidelines appropriately recommend caution and vigilance when prescribing opioids for chronic pain, for example, using the lowest effective dose for the shortest duration based on individual treatment goals and only when the benefits outweigh the potential risks.40 Opioid prescribing, as measured by the daily milligrams of morphine equivalents prescribed, decreased over time in the MPM and WHT+MPP cohorts (the MPP cohort also showed a decreasing trend, but this estimate was not statistically significant). Self-reported pain scores significantly decreased in the MPM group. These scores declined in the MPM and WHT+MPM cohorts but were not significant. Outcomes related to functionality were also mixed. The MPM group showed significant improvements in the ODI Back scores, whereas the other cohorts showed little or no improvements in ODI Back or ODI Neck scores.
The addition of WHT to the pain management program at Geisinger did not produce statistically significant improvements in clinical outcomes during the 12-month observational period. The lack of significant results could be a function of relatively low power, differences in attrition rates among the 3 study groups across the various longitudinal outcome measures, inconsistencies in the frequency of WHT use, and the specific functionality of the WHT developed for this study. The sample size in particular was a function of the number of patients in the MPP program, which enrolled 285 patients during the study time frame. Approximately 37% of MPP patients who were offered WHT used it. Although it is hard to interpret this number in isolation, it is worth noting that patients who choose to use WHT in this study (WHT+MPP) were of similar age, BMI, and at similarly elevated risks for a variety of comorbidities including depression, anxiety, diabetes, COPD, and heart disease as patients who did not (ie, the MPP cohort). Thus, we were encouraged that over one third of patients living with chronic pain chose to engage with this new technology given their pain and comorbidities. Although there is no guarantee that a larger sample size would have produced statistical significance, these results were nonetheless encouraging, and future studies should consider drawing WHT users from a larger population of people living with chronic pain.
Different rates of attrition were observed among the 3 study groups when the analytic subset of patients was created for the longitudinal mixed-effects analyses. Attrition was higher among the MPM group than the MPP group for the PHQ-9, ODI Back, and ODI Neck measures. In this observational study, outcome measures were assessed only during regular office visits. The varying rates of attrition suggest that MPM patients attended fewer office visits and were assessed less frequently compared with MPP patients during the 12-month study period. Future research should ensure that follow-up assessments are consistently administered across all groups. Increasing the number of assessments could have provided more power to detect differences among the groups.
Participants in the WHT+MPP group were instructed to use the WHT on a daily basis and encouraged by HCPs to do so. Given the observational nature of the study, we expected that participants would not choose to or be able to achieve this goal. Study results revealed that on average patients used the WHT about 63% of days. Although this number is difficult to interpret in isolation, we were encouraged by this level of engagement. Future studies could consider additional ways to promote regular WHT use. With larger sample sizes, statistical analyses could examine the possibility of a relationship between frequency of WHT use and the clinical outcomes.
To the best of authors’ knowledge, only a small number of studies have investigated the effects of a smartphone app or WHT on pain intensities for patients with chronic musculoskeletal pain,21–25 and these studies varied in WHT functionality. Some studies showed significant improvement in pain,21,24,25 whereas the rest reported no significant changes.20,23 The WHT in this study, developed in response to HCP feedback and patient usability testing, consisted of motivational elements such as daily reminders, educational information about nonopioid therapies such as mindfulness, movement monitoring, an electronic diary of pain experiences, passive measurements of sleep, user-feedback of trends in self-reported pain and activity, and a dashboard of results available to HCPs. Nonetheless, rapid advances in technology will make the WHT developed for this study (in 2017) obsolete. Newer devices should make reporting of subjective outcomes more convenient for users, and the measurement of physiological responses (such as sleep, heart rate, respiration, and weight management) more accurate. Much research is needed to identify the specific combination of WHT functionalities, if any, that can reliably impact clinically meaningful changes in patient outcomes.41 These new devices will no doubt create an enormous amount of user-generated data. Even the WHT used in this study had the ability for patients to record average daily pain, as well as breakthrough pain, pain intensity, pain location on the body, and pain descriptors such as stabbing, throbbing, freezing, and shooting. Advances in artificial intelligence and machine learning will further allow for the identification of relationships between WHT data and impactful clinical decision-making.41
Study limitations should be considered when interpreting the results of this study and in the design of future studies. First, enrollment into the cohorts was not randomized, and patients chose whether or not they were willing to incorporate WHT into their pain management program. Therefore, the usual caveats with respect to causal inference in a nonrandomized setting apply.42 In particular, it is reasonable to assume that only patients who were interested or motivated to use WHT agreed to take part in this study. If WHT was randomly assigned to all MPP members, we would expect to see lower rates of WHT use (ie, greater rates of refusals, shorter length of use, and fewer active use days). We chose not to randomize enrollment because we wanted to offer this technology to all interested patients. Thus, results should only be generalized to people who are willing to use WHT. Future studies, perhaps with larger populations of potential participants, should consider some form of randomization to reduce these self-selection biases. Second, patients in the WHT cohort were aware that they were participating in a study of new technology and thus could be subject to Hawthorne effects related to self-consciousness.43 In research on other multimodal interventions, authors warn about the risk of performance bias on the overinterpretation of outcomes for patients assessed within controlled parameters that do not reflect general real-world practice (eg, attention-deficit/hyperactivity disorder and cognitive behavioral therapy).44,45 Third, patients who participated in this study were from a single health system and were predominantly overweight White females with preexisting conditions such as depression, anxiety, COPD, and uncomplicated diabetes. Care should be taken when generalizing to other populations. Fourth, all study outcomes except for MME were based on self-reported data from patients and therefore subject to individual variance and recall or reporting bias. Despite these limitations, to the best of our knowledge, this study represents the longest-term investigation of WHT in chronic pain management. Its results warrant further investigation into the long-term effectiveness of WHT in reducing the devastating impact of chronic pain.
CONCLUSIONS
Chronic pain management is a complex condition that can have multidimensional negative effects on quality of life. Patients who were open to using WHT as part of an expert-directed MPM program demonstrated that they would do so for extended periods of time. Trends in the data suggest that the WHT may positively impact depression and MME use. Continued research using WHTs of increased sophistication is warranted to investigate the potential of WHT to improve the negative consequences of chronic pain.
ACKNOWLEDGMENTS
The authors thank the following people for assistance in developing the wearable health technology and data collection: At Geisinger, Danville, PA; Kelly Baylor, Research Coordinator, Dept of Pain Medicine, Jeff Border, Sr Designer, Institute for Advanced Application (IAA), Matt Hackenberg, Director of Innovation Implementation, IAA, and Amanda Milo, Product Manager, IAA. We thank the following people for insights on study design: At Purdue Pharma L.P., Stamford, CT; Stacy Baldridge, Exec Director, Public Health Initiatives, Nancy Crudele, Exec Director, Medical Affairs, Jennifer Erensen, Exec Director, Medical Affairs, Danielle Lewis Levy*, Exec Director, Corporate Comms & Corporate Social Responsibility, Tracy Mayne*, Exec Director, Medical Affair, Jaromir Mikl, Director, Medical Affairs, Rita Mulvihill, Sr. Medical Science Liaison, and Brian Sullivan, Director, IT. We thank the following people for analytic support: At Genesis Research, Hoboken, NJ; Yingjie Ding, Director, RWE Solutions, Emma East, Senior Manager, Evidence Strategy, Ji Haeng Heo, Associate Director, RWE Analytics, Serena Hsu, Principal Scientist, Biostatistics, Steven Wang, Director, RWE Analytics, Xin Zhao, Principal Scientist, Biostatistics.
Footnotes
This study was sponsored by Purdue Pharma L.P., Stamford, CT. T.A. is an employee of Purdue Pharma L.P. The remaining authors declare no conflict of interest.
Contributor Information
John J. Han, Email: jhan@geisinger.edu.
Jove H. Graham, Email: jhgraham1@geisinger.edu.
Dawn I. Snyder, Email: disnyder@geisinger.edu.
Thomas Alfieri, Email: thomas.alfieri@pharma.com.
REFERENCES
- 1. National Institutes of Health. Pain: hope through research. 2020. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Pain-Hope-Through-Research. Accessed October 18, 2022.
- 2. Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. [DOI] [PubMed] [Google Scholar]
- 3. Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams LM, Turk DC. Psychosocial Factors and Central Sensitivity Syndromes Curr Rheumatol Rev. 2015;11:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawai K, Kawai AT, Wollan P, et al. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Family Practice. 2017;34:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. [DOI] [PubMed] [Google Scholar]
- 7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity. Arch Intern Med. 2003;163:2433–2445. [DOI] [PubMed] [Google Scholar]
- 8. Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk. J Pain. 2009;10:619–627. [DOI] [PubMed] [Google Scholar]
- 9. Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107:54–60. [DOI] [PubMed] [Google Scholar]
- 10. Munce SEP, Stewart DE. Gender differences in depression and chronic pain conditions in a national epidemiologic survey. Psychosomatics. 2007;48:394–399. [DOI] [PubMed] [Google Scholar]
- 11. Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- 12. Worley SL. New directions in the treatment of chronic pain national pain strategy will guide. Prev Manag and Res. 2016;41:107–114. [PMC free article] [PubMed] [Google Scholar]
- 13. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55–64. [DOI] [PubMed] [Google Scholar]
- 14. Ringqvist Å, Dragioti E, Björk M, et al. Moderate and stable pain reductions as a result of interdisciplinary pain rehabilitation—A cohort study from the Swedish Quality Registry for Pain Rehabilitation (SQRP). J Clin Med. 2019;8:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erskine A, Morley S, Pearce S. Memory for pain: a review. Pain. 1990;41:255–265. [DOI] [PubMed] [Google Scholar]
- 17. Karimi Z, Pilenko A, Held SM, et al. Recall bias in patients with chronic low back pain: individual pain response patterns are more important than pain itself! Int J Behav Med. 2016;23:12–20. [DOI] [PubMed] [Google Scholar]
- 18. Jamison RN, Jurcik DC, Edwards RR, et al. A pilot comparison of a smartphone app with or without 2-way messaging among chronic pain patients: who benefits from a pain app. Clin J Pain. 2017;33:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suso-Ribera C, Castilla D, Zaragozá I, et al. Validity, reliability, feasibility, and usefulness of pain monitor. Clin J Pain. 2018;34:900–908. [DOI] [PubMed] [Google Scholar]
- 20. Hauser-Ulrich S, Künzli H, Meier-Peterhans D, et al. A smartphone-based health care chatbot to promote self-management of chronic pain (SELMA): pilot randomized controlled trial. JMIR mHealth uHealth. 2020;8:e15806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skrepnik N, Spitzer A, Altman R, et al. Assessing the impact of a novel smartphone application compared with standard follow-up on mobility of patients with knee osteoarthritis following treatment with Hylan G-F 20: a randomized controlled trial. JMIR mHealth uHealth. 2017;5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson SC. No exchange, same pain, no gain: risk–reward of wearable healthcare disclosure of health personally identifiable information for enhanced pain treatment. Health Informatics J. 2019;25:1675–1691. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Wei Q, Ge Y, et al. Smartphone-based remote self-management of chronic low back pain: a preliminary study. J Healthc Eng. 2019;2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin D, Xu Y, Geng D, et al. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2010;89:10–15. [DOI] [PubMed] [Google Scholar]
- 25. Lewis GK, Langer MD, Henderson CR, et al. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound Med Biol. 2013;39:1429–1439. [DOI] [PubMed] [Google Scholar]
- 26. Kristjánsdóttir ÓB, Fors EA, Eide E, et al. A smartphone-based intervention with diaries and therapist-feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain: randomized controlled trial. J Med Internet Res. 2013;15:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Palacios A, Herrero R, Belmonte MA, et al. Ecological momentary assessment for chronic pain in fibromyalgia using a smartphone: a randomized crossover study. Eur J Pain. 2014;18:862–872. [DOI] [PubMed] [Google Scholar]
- 28. Forbes G, Newton S, Cantalapiedra Calvete C, et al. MEMPHIS: a smartphone app using psychological approaches for women with chronic pelvic pain presenting to gynaecology clinics: a randomised feasibility trial. BMJ Open. 2020;10:e030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillory J, Chang P, Henderson CR, et al. Piloting a text message-based social support intervention for patients with chronic pain: establishing feasibility and preliminary efficacy. Clin J Pain. 2015;31:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perraudin CGM, Illiano VP, Calvo F, et al. Observational study of a wearable sensor and smartphone application supporting unsupervised exercises to assess pain and stiffness. Digit Biomark. 2018;2:106–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gordon R, Bloxham S. Influence of the Fitbit Charge HR on physical activity, aerobic fitness and disability in non-specific back pain participants. J Sports Med Phys Fitness. 2017;57:1669–1675. [DOI] [PubMed] [Google Scholar]
- 32. Ferguson SA, Berner RS, Bridger MA, et al. Patient and practitioner experience with clinical lumbar motion monitor wearable technology. Health Technol. 2019;9:289–295. [Google Scholar]
- 33. Inoue M, Orita S, Inage K, et al. Comparison of the activity level of the upper limbs and trunk in patients with low back pain evaluated using a wearable accelerometer: a validation study. Spine Surg Relat Res. 2019;3:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. IOM (Institute of Medicine). Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press. 2011. [PubMed] [Google Scholar]
- 35. Beard C, Hsu KJ, Rifkin LS, et al. Validation of the PHQ-9 in a psychiatric sample. J Affect Disord. 2016;193:267–273. [DOI] [PubMed] [Google Scholar]
- 36. Wittink H, Turk DC, Carr DB, et al. Comparison of the redundancy, reliability, and responsiveness to change among SF-36, Oswestry Disability Index, and Multidimensional Pain Inventory. Clin J Pain. 2004;20:133–142. [DOI] [PubMed] [Google Scholar]
- 37. The Centers for Medicare & Medicaid Services. Opioid morphine equivalent conversion factors.; 2015. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovcontra/downloads/opioid-morphine-eq-conversion-factors-march-2015.pdf. Accessed May 17, 2021. [PubMed]
- 38. Kleiber D, Jain T, Kleiber B, et al. Depression and pain: implications for symptomatic presentation and pharmacological treatments. Psychiatry (Edgmont). 2005;2:12–18. [PMC free article] [PubMed] [Google Scholar]
- 39. Clarke TC, Schiller JS, Boersma P. Early release of selected estimates based on data from the 2019 national health interview survey. 2019. Available at: https://www.cdc.gov/nchs/data/nhis/earlyrelease/EarlyRelease202009-508.pdf. Accessed April 28, 2021.
- 40. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leroux A, Rzasa-Lynn R, Crainiceanu C, et al. Wearable devices: current status and opportunities in pain assessment and management. Digit Biomark. 2021;5:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glass TA, Goodman SN, Hernán MA, et al. Causal inference in public health. Annu Rev Public Health. 2013;34:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McCarney R, Warner J, Iliffe S, et al. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Institute for Clinical and Economic Review. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers & Long-Term Effectiveness in All Ages Supplementary Data and Analyses to the Comparative Effectiveness Review of the Agency for Healthcare Research and Quality Final Report. 2012. Available at: www.icer-review.org. Accessed October 18, 2022. [PubMed]
- 45. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther. 2005;43:831–842. [DOI] [PubMed] [Google Scholar]


