Abstract
Antibodies against the class 4 outer membrane protein (OMP) from Neisseria meningitidis have been purified from sera from vaccinees immunized with the Norwegian meningococcal group B outer membrane vesicle vaccine. The human sera and purified antibodies reacted strongly with the class 4 OMP in immunoblots, whereas experiments with whole bacteria showed only weak reactions, indicating that the antibodies mainly reacted with parts of the class 4 molecule that were not exposed. The purified human anti-class 4 OMP antibodies and the monoclonal antibodies (MAbs) were neither bactericidal nor opsonic against live meningococci. Three new MAbs against the class 4 OMP were generated and compared with other, previously described MAbs. Three linear epitopes in different regions of the class 4 OMP were identified by the reaction of MAbs with synthetic peptides. The MAbs showed no blocking effect on bactericidal activity of MAbs against other OMPs. However, one of the eight purified human anti-class 4 OMP antibody preparations, selected from immunoblot reactions among sera from 27 vaccinees, inhibited at high concentrations the bactericidal effect of a MAb against the class 1 OMP. However, these antibodies were not vaccine induced, as they were present also before vaccination. Therefore, this study gave no evidence that vaccination with a meningococcal outer membrane vesicle vaccine containing the class 4 OMP induces blocking antibodies. Our data indicated that the structure of class 4 OMP does not correspond to standard β-barrel structures of integral OMPs and that no substantial portion of the OmpA-like C-terminal region of this protein is located at the surface of the outer membrane.
The major outer membrane proteins (OMPs) of Neisseria meningitidis have been designated class 1 (PorA) through class 5 (Opa) (50). The class 1 and 2/3 proteins are porins; they show antigenic variation and have been used to define serosubtypes and serotypes, respectively (13). The class 4 OMP, also called reduction modifiable protein (Rmp), due to its shift in mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after reduction, is closely related to protein III (PIII) of Neisseria gonorrhoeae (7, 25, 31, 48). The class 4 and PIII OMPs are constitutively expressed, antigenically invariable, and closely associated with the porin molecules (31, 35). Both proteins have been extensively studied, and the genes have been cloned and sequenced. There is 96% homology between the DNA sequences of the PIII and class 4 OMP genes studied (18, 25). According to its amino acid sequence, the molecular mass of the mature class 4 protein is about 24 kDa. However, the class 4 molecule contains two disulfide loops and migrates in SDS-PAGE gels at about 32 kDa under reducing conditions. No free C-terminal amino acid could be released by carboxypeptidase digestion of PIII, suggesting that the carboxy terminus is blocked or unavailable for cleavage (7). By SDS-PAGE, variations in migration are observed between class 4 OMPs from different strains (4, 56a). The amino acid sequence of class 4 OMP is homologous to that of the C-terminal part of OmpA from Escherichia coli and to that of OprF from Pseudomonas aeruginosa (7, 47, 60).
The function of the Rmp, both in the pathogenesis and in the physiology of the organism, remains unknown. The related OmpA and OprF OMPs probably have a structural role in maintaining the integrity of the outer membrane, and a pore-forming activity has been shown previously for both these proteins (46); however, no porin activity has been shown for the PIII or class 4 OMPs. The rmp gene is found exclusively in chromosomal DNA of pathogenic neisseriae, indicating that this protein contributes to the virulence of N. gonorrhoeae and N. meningitidis (58). A possible function of the PIII protein for optimal invasion of gonococci into human cervical cells has been reported previously (40). Some murine monoclonal antibodies (MAbs) against PIII and class 4 OMP have been reported to block the serum bactericidal activity (SBA) of other antibodies against gonococci and meningococci (23, 34, 41, 52–54). Furthermore, for some volunteers who had previously suffered a gonococcal infection and were vaccinated with a gonococcal protein I vaccine, with less than 10% PIII, a fall in SBA was observed after vaccination. This fall in bactericidal activity was associated with the development of anti-PIII antibodies (5, 19), and the presence of such antibodies was shown to increase susceptibility to gonococcal infections (37). The blocking action was ascribed to anti-PIII antibodies which competed for binding with other antibody complexes on the gonococcal surface and resulted in the deposition of C5b-9 in a nonbactericidal form, preventing killing of the bacterium (23). These observations led to warnings against Rmp as a component in gonococcal and meningococcal vaccines (17, 34). Since class 4 OMP is present in the Norwegian group B outer membrane vesicle (OMV) vaccine (6, 14), we wanted to study if any induction of blocking antibodies after vaccination with this vaccine occurred. In addition, the functional activities and epitope specificities of different murine MAbs and human anti-class 4 OMP antibodies isolated from volunteers immunized with this vaccine were studied, and putative topological models for the class 4 OMP are discussed.
(This study was presented in part at the 10th International Pathogenic Neisseria Conference, Baltimore, Md., 8 to 13 September 1996.)
MATERIALS AND METHODS
Bacterial strains.
Meningococcal strain 44/76-SL (B:15:P1.7,16:L3,7,9) was used to produce the Norwegian group B OMV vaccine (14). Strain 24/88 is another systemic B:15:P1.7,16:L3,7,9 meningococcal isolate from Norway. Strains 44/76 Rmp− and 24/88 Rmp− are their class 4 OMP-deficient descendants, prepared by genetic manipulation of strains 44/76 and 24/88, respectively, as described previously (25).
OMV preparations from meningococcal strains 44/76-SL and 44/76 Rmp− were obtained after sodium deoxycholate extraction of cells grown in modified Frantz’s medium, as described previously (14).
MAbs.
Three new MAbs against class 4 OMP (155,B-4 [immunoglobulin M (IgM); 173,G-1 [IgG1]; and 185,H-8 [IgG2a]), were generated at the National Institute of Public Health (NIPH), Oslo, Norway (Table 1). The MAbs were obtained by subcutaneous immunization of 7-week-old BALB/c mice with 50 μg of OMVs from strain 44/76 in 0.1 ml of saline mixed with 0.1 ml of Freund’s complete adjuvant, followed by a booster injection 2 weeks later with the same mixture. The fusions with NSO myeloma cells were made by standard methods within the next 2 to 11 months. The mice were given 50 μg of OMP in saline 4 days prior to fusion. Hybridoma culture supernatants were screened by enzyme-linked immunosorbent assay (ELISA) against OMVs from 44/76 and 44/76 Rmp−. Supernatants from positive clones were further analyzed by immunoblotting. Hybridoma cells were cloned by limiting dilution and injected into Pristane-primed mice to obtain ascites. Isotyping of MAbs in hybridoma culture medium was performed in ELISA with OMVs as coating antigen, with a kit from Zymed Laboratories, Inc., San Francisco, Calif. In addition, two MAbs against class 4 OMP, generated at Fundaçao Oswaldo Cruz, Rio de Janeiro, Brazil (AE3) (9), and at Max-Planck-Institut für Molekulare Genetik, Berlin, Germany (V414) (2), respectively, were tested. Also, six MAbs against PIII (SM50 to SM55), generated at the University of Southampton Medical School, Southampton, United Kingdom, were used (52). As effector bactericidal antibodies in blocking experiments and as positive controls in immunoelectron microscopy (IEM), flow cytometry, and phagocytic assays, MAb 151,F-9 (IgG2b), directed against the P1.16 epitope, and MAb 207,B-4 (IgG2a), directed against the P1.7 epitope, were used (44). As a negative control, MAb 144,H-3, directed against a conserved epitope on bacterial L7/L12 ribosomal protein, was used. These MAbs were generated and characterized at the NIPH as described previously (27).
TABLE 1.
MAbs to class 4 OMP studied
| MAb | Isotype | Epitope | Reference(s) |
|---|---|---|---|
| SM50 | IgG2a | I | 52–54 |
| SM51 | IgG1 | I | 52–54 |
| SM52 | IgG1 | I | 52–54 |
| SM54 | IgG2b | —a | 52–54 |
| SM55 | IgG2a | —a | 52–54 |
| AE3 | IgG2b | II | 2 |
| V414 | IgG2b | II | 9 |
| 155,B-4 | IgM | III | This study |
| 185,H-8 | IgG2a | III | This study |
| 173,G-1 | IgG1 | III | This study |
—, no linear epitopes were identified with the 14-mer peptides shown in Table 2.
Sera and human antibodies.
Pre- and postvaccination sera (6 weeks after the second dose) from all 27 adult vaccinees from the phase II-9 vaccine trial in Norway (43) were tested on immunoblots. Postvaccination sera from eight of the vaccinees, all showing binding to class 4 OMP in immunoblots, were selected for purification of anti-class 4 OMP specific antibodies. In addition, acute (1 to 3 days) and convalescent (12 to 79 days) sera from three patients (K-147, K-187, and K-188) with systemic group B meningococcal disease, who showed antibodies to class 4 OMP on blots, were studied for reactions with peptides. Pre- and postvaccination sera from 32 other vaccinees immunized twice with the Norwegian group B OMV vaccine were studied for SBA against strains 44/76 and 24/88 and their class 4 OMP-deficient variants (Rmp−). These were a subgroup of vaccinees from the phase II-3 vaccine trial (20).
Specific antibodies to class 4 OMP were purified by absorption with OMVs from strain 44/76 Rmp−. Briefly, after isolation of the Ig fraction from the serum by protein G-Sepharose chromatography (Pharmacia, Uppsala, Sweden), purified Igs were incubated overnight at 4°C with OMV from strain 44/76 Rmp− at an Ig/OMV protein concentration ratio of 1:2 (wt/wt). After 2 h of ultracentrifugation (150,000 × g), the supernatant was precipitated with 50% ammonium sulfate and the pellet was resuspended and dialyzed against phosphate-buffered saline (PBS). The protein concentration was determined as described by Lowry et al. (30).
Immunoblotting assay.
SDS-PAGE was performed in 12% polyacrylamide gels (29). The proteins were electroblotted onto 0.45-μm-pore-size nitrocellulose filters (Bio-Rad Laboratories, Hercules, Calif.) for 1 h at room temperature, in a solution containing 0.025 M Tris-HCl, 0.15 M glycine, and 20% methanol (pH 8.3). Binding of IgG antibodies was detected as previously described (55, 56).
ELISA.
Maxisorp plates (Nunc, Roskilde, Denmark) were coated with 100 μl (per well) of either 5 μg of OMVs from strain 44/76 per ml or whole-cell preparations in 0.1 M Tris-HCl, pH 8.5, with 0.02% sodium azide, as described previously (43). Plates were washed four times in PBS with 0.05% Tween 20 before serum dilutions in 0.1% bovine serum albumin (BSA) in PBS were added. After incubation for 3 h at 37°C and washing, bound antibodies were detected by incubation for 1 h at 37°C with either alkaline-phosphatase anti-mouse IgG conjugate (Sigma, St. Louis, Mo.) for MAbs or alkaline-phosphatase conjugate of swine anti-human IgG (Orion Diagnostica, Helsinki, Finland) for purified anti-class 4 OMP human Ig. The substrate p-nitrophenyl phosphate (1 mg/ml) in 0.5 M diethanolamine buffer, pH 9.8, was used to develop the antigen-antibody reaction, and absorbance was read after 20 to 45 min at 405 nm.
Colony blotting.
Colony blots with anti-class 4 OMP MAbs were performed as described previously (33). Briefly, bacteria were grown overnight on brain heart infusion (BHI) agar at 37°C in a candle jar. From these, a fresh inoculum was prepared by growing bacteria on BHI agar plates for 4 h at 37°C in a candle jar. A suspension, corresponding to the inoculum in SBA, containing approximately 100 CFU was spread onto BHI agar plates and incubated overnight at 37°C in a candle jar. The following day, the colonies were blotted directly onto nitrocellulose membranes. The nitrocellulose membranes were placed in blocking buffer containing 3% BSA in PBS and incubated with slow shaking for 30 min. The filters were incubated with the appropriate antibodies, washed, and incubated for 1 h with anti-mouse Ig-peroxidase conjugate (Sigma). Ig binding was detected as described previously (55).
IEM.
Electron microscopy of freshly killed, heat-inactivated (56°C for 30 min), and boiled bacteria of meningococcal strains 44/76 and 44/76 Rmp− was carried out by an on-grid immunogold labelling technique, as previously described (3). Briefly, bacterial suspensions adhering to carbon film grids were incubated on droplets of the anti-class 4 OMP MAbs and with the anti-class 1 OMP MAb as a positive control. After several washings, the specimens were incubated with the secondary antibody, goat anti-mouse IgG conjugated to colloidal gold particles (GAM IgG-10 nm; BioCell Research Laboratories, Cardiff, United Kingdom), washed in buffer, and negatively stained with 0.5% phosphotungstic acid (pH 7.0). Negative controls were carried out with buffer replacing the antibodies and with strain 44/76 Rmp−. The buffer used throughout the experiments was ammonium acetate, pH 7.2, containing 0.5% BSA. Before incubation with the primary MAbs, 1% BSA was used to block nonspecific binding. The specimens were examined in a JEOL 1010 electron microscope operated at 100 keV.
Flow cytometry.
Expression of the class 4 OMP was analyzed on viable and ethanol-killed meningococci by flow cytometry. Bacteria were grown overnight on BHI agar plates at 37°C in 5% CO2, transferred to a second plate of BHI agar, and allowed to grow for 4 h under the same conditions to reach log phase. Bacteria were then harvested, washed, and resuspended in Hanks balanced salt solution (Gibco Laboratories, Chicago, Ill.) supplemented with 0.2% BSA (HBSS-BSA). Alternatively, the bacteria were killed with 70% ethanol for 16 h and stored at room temperature. Approximately 106 cells were mixed with antibodies diluted in HBSS-BSA and incubated for 1 h at room temperature. After a wash with the same buffer, fluorescein isothiocyanate isomer I-labelled sheep anti-mouse Ig (produced in our laboratory) was added, and incubation was continued for 1 h. After washing, antigen-antibody binding was measured by flow cytometry as mean fluorescence intensity with a Coulter EPICS XL flow cytometer, with four-decade logarithmic amplification.
Phagocytic assay.
Phagocytosis was assayed as respiratory bursts by flow cytometry as previously described (1). Briefly, human peripheral blood polymorphonuclear leukocytes (effector cells, 5 × 106/ml) were washed with HBSS-BSA. N. meningitidis 44/76-SL (target cells, 109/ml) was washed in HBSS-BSA and tested either as viable or as ethanol-killed cells (see above) that had been stored at −85°C. Target cells (5 μl) were mixed with a titration series of antibodies diluted in HBSS (50 μl) in U-bottomed microtiter plates and incubated for 30 min at 37°C. Thereafter, 5 μl of human serum from an individual without opsonic or bactericidal activity was added as a complement source and incubated under agitation in a microtiter plate shaker at 37°C for 12 min. Effector cells were subsequently added, and the mixture was incubated under agitation for an additional 12 min at 37°C. The effector cells were mixed with dihydrorhodamine 123 (Molecular Probes, Eugene, Oreg.) (10 μg/ml) just before they were added to the opsonized bacteria. Phagocytosis was stopped by placing the microtiter plates on ice until flow cytometry analysis was performed within 2 h.
Bactericidal and blocking assays.
Bactericidal tests were performed in microtiter plates as described previously (20). Briefly, bacteria were grown overnight on BHI agar at 37°C in a candle jar and then inoculated onto a second plate of BHI agar and allowed to grow for 4 h under the same conditions. Twofold dilutions of sera or MAbs were tested with an inoculum of 80 to 100 CFU per well, in the presence of 25% human plasma, devoid of bactericidal activity in itself, as a complement source. Complement was added after the inoculum, followed by 30 min of incubation at 37°C in an ordinary atmosphere. Then agar was added, and the plates were incubated overnight in a candle jar at 37°C. Titers were expressed as the reciprocal of the final serum dilution giving at least a 50% reduction of CFU.
Possible blocking of the bactericidal effect of other antibodies was studied by preincubating the bacteria for 15 min at 37°C in an ordinary atmosphere with dilutions of anti-class 4 OMP antibodies prior to exposure to the bactericidal anti-class 1 OMP MAb and human complement. A dilution of the anti-class 1 OMP MAb giving about 95% killing of strain 44/76 was chosen as optimum in all experiments. After incubation for 30 min at 37°C, the CFU were counted as described above. The effect of simultaneous incubation of anti-class 4 OMP antibodies and the bactericidal P1.16 MAb was also examined. For this, a dilution series of anti-class 4 OMP antibodies was mixed with the bactericidal MAb before exposure to bacteria and complement. The starting dilution of purified Igs against class 4 OMP in both assays was about 200 μg of total Ig per ml. The possible blocking effect of human Ig against class 4 protein on the bactericidal activity of sera from two vaccinees (1480 and 1428) was also studied. Postvaccination sera and purified anti-class 4 OMP human Ig were mixed 1:4 (wt/wt) and serially diluted prior to incubation with bacteria and human complement, as described above.
Epitope mapping.
Solid-phase peptide synthesis was carried out with a commercially available kit (Cambridge Research Biochemicals, Northwich, Cheshire, United Kingdom) based on the methods of Geysen et al. (15). According to the predicted amino acid sequence of class 4 OMP (25), peptides (14-mers) spanning the entire molecule were synthesized on polyethylene pins with adjacent peptides overlapping by seven residues (Table 2). Pins were screened by ELISA for reactivity with MAbs or human anti-class 4 OMP purified Ig, as described by Delvig et al. (11). Results are expressed as means from two independent experiments with two different parallel sets of pins.
TABLE 2.
Amino acid sequences of 14-mer peptides overlapping by seven residues, spanning the entire class 4 OMPa
| Pin no. | Sequence | Pin no. | Sequence | |
|---|---|---|---|---|
| 1 | AGEASVQGYTVSGQ | 16 | QTNIQSVRVEGHTD | |
| 2 | GYTVSGQSNEIVRN | 17 | RVEGHTDFMGSDKY | |
| 3 | SNEIVRNNYGECWK | 18 | FMGSDKYNQALSER | |
| 4 | NYGECWKNAYFDKA | 19 | NQALSERRAYVVAN | |
| 5 | NAYFDKASQGRVEC | 20 | RAYVVANNLVSNGV | |
| 6 | SQGRVECGDAVAAP | 21 | NLVSNGVPVSRISA | |
| 7 | GDAVAAPEPEPEPE | 22 | PVSRISAVGLGESQ | |
| 8 | EPEPEPEPAPVVVV | 23 | VGLGESQAQMTQVC | |
| 9 | PAPVVVVEQAPQYV | 24 | AQMTQVCEAEVAKL | |
| 10 | EQAPQYVDETISLS | 25 | EAEVAKLGAKVSKA | |
| 11 | DETISLSAKTLFGF | 26 | GAKVSKAKKREALI | |
| 12 | AKTLFGFDKDSLRA | 27 | KKREALIACIEPDR | |
| 13 | DKDSLRAEAQDNLK | 28 | ACIEPDRRVDVKIR | |
| 14 | EAQDNLKVLAQRLG | 29 | VDVKIRSIVTRQVV | |
| 15 | VLAQRLGQTNIQSV | 30 | VTRQVVPAHNHHQH |
The peptides were synthesized on polyethylene pins.
Structure and molecular modelling.
For the prediction of the topology of the class 4 OMP, the same criteria were used as applied previously (49). Hydrophilic peaks in the hydropathy profile, which are supposed to correspond to the cell surface-exposed parts, were identified according to the method of Kyte and Doolittle (28). Beta-turns, which are supposed to be present in surface- and periplasmically exposed parts of the protein, were identified by the criteria of Paul and Rosenbusch (36) as adapted for β-sheet proteins. Potential membrane-spanning β-strands were identified by the following criteria: they should be (a) approximately 10 residues long, (b) able to form an amphipathic β-strand (one side of the strand should entirely consist of hydrophobic residues: F, W, Y, M, V, I, M, A, and G; incidentally, a few P, T, or S residues are tolerated, whereas on the other side of the strand both hydrophilic and hydrophobic residues can occur), (c) devoid of turn predictions, (d) not coinciding with a hydrophilic maximum, and (e) preferentially flanked by aromatic residues at the hydrophobic side of the strands.
RESULTS
ELISA and immunoblotting experiments.
Studies with murine MAbs and purified human Igs against class 4 OMP showed that both categories of antibodies reacted well in immunoblots with OMVs from strain 44/76, recognizing only the class 4 OMP, whereas no reactions were observed with OMVs from strain 44/76 Rmp−. Reactions in immunoblots with sera and purified Igs from the eight vaccinees are shown in Fig. 1. When purified human Igs against class 4 OMP were used in ELISA experiments with OMVs or whole cells from strain 44/76, only weak reactions were detected, whereas murine anti-class 4 OMP MAbs reacted well both in OMV ELISA and on immunoblots (results not shown).
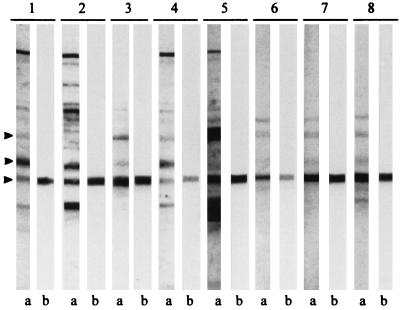
FIG. 1.
Immunoblot with sera from eight selected vaccinees immunized with the Norwegian meningococcal OMV vaccine and their corresponding purified anti-class 4 OMP human Igs against strain 44/76. Lanes a, postvaccination serum; lanes b, purified human anti-class 4 OMP Ig from the same serum. Lanes 1 to 8 show sera from vaccinees 1347, 1429, 1436, 1479, 1480, 1508, 1607, and 1610, respectively. Arrows indicate positions of class 1, class 3, and class 4 OMPs (top to bottom, respectively).
Colony blotting.
Recognition of class 4 OMP in meningococci was tested by colony blotting with the class 4 OMP-specific MAbs. Colony blotting experiments were performed with strains 44/76 and 24/88 and their respective class 4 OMP-deficient variants. Strong binding of all the anti-class 4 OMP MAbs was observed for both strain 44/76 and strain 24/88, while no significant reaction was detected with the Rmp− mutants. Both the anti-P1.16 class 1 OMP and the anti-L7/L12 ribosomal protein MAbs bound strongly to both strains in colony blotting assays, whereas the anti-P1.15 MAb did not (results not shown).
IEM.
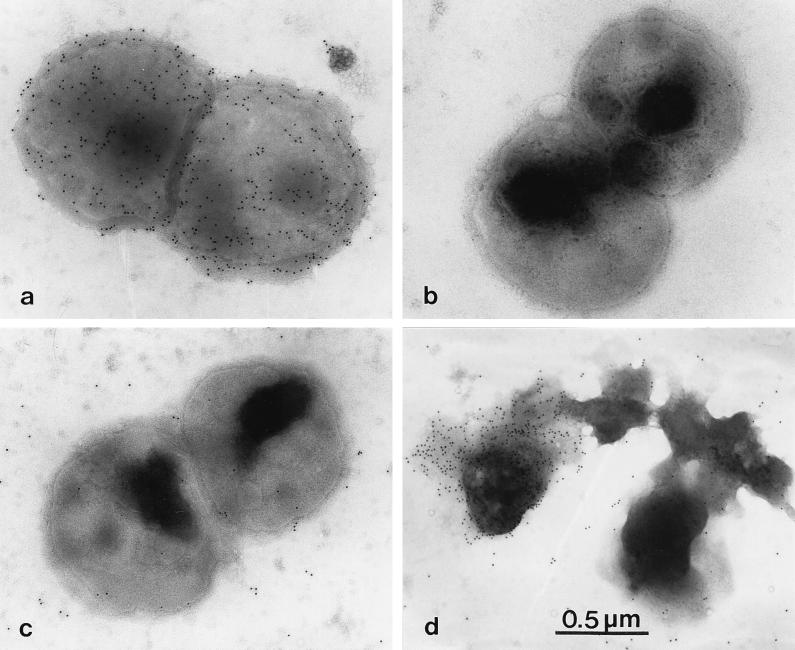
No significant immunogold labelling of freshly killed bacteria (strain 44/76) was observed with any of the anti-class 4 OMP MAbs (Fig. 2c). In contrast, bacteria heated to 100°C showed labelling of cells and released membrane fragments (blebs) with all anti-class 4 OMP MAbs tested (Fig. 2d). With the anti-class 1 OMP MAb, a strong labelling was observed with cells from both strain 44/76 (Fig. 2a) and strain 44/76 Rmp−. No immunogold labelling was observed in any of the negative controls.
FIG. 2.
Electron micrographs of meningococcal strains 44/76 (a, c, and d) and 44/76 Rmp− (b) immunogold labelled with 10-nm-colloidal-gold-labelled goat anti-mouse Ig after incubation with anti-class 1 OMP MAb (151,F-9) (a) or anti-class 4 OMP MAb (185,H-8) (b to d). For panel d, the bacteria had been heated to 100°C before labelling. Magnification, ×40,000.
Flow cytometry.
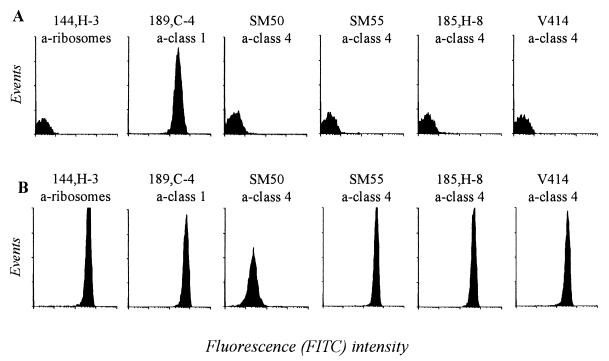
Expression of the class 4 OMP on viable and killed meningococci was measured by flow cytometry with a panel of different MAbs. With viable or freshly killed bacteria (Fig. 3A), no significant binding was observed with any of the MAbs against class 4 OMP, compared to the strong binding obtained with the anti-class 1 OMP MAb. In contrast, all anti-class 4 OMP MAbs, and surprisingly also the anti-ribosomal protein MAb (144,H-3), bound strongly to ethanol-killed bacteria, stored at room temperature for several days (Fig. 3B).
FIG. 3.
Flow cytometry histograms showing binding of different MAbs to viable (A) and ethanol-killed (B) cells of meningococcal strain 44/76. The ethanol-killed bacteria had been stored for 1 month at room temperature. FITC, fluorescein isothiocyanate.
Serum bactericidal and opsonic activity.
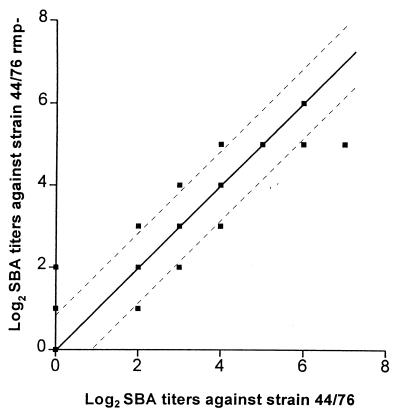
Pre- and postvaccination sera from 32 vaccinees were tested for SBA against strains 44/76 and 24/88 and their respective class 4 OMP-deficient variants. When the titers against these strains were compared, no significant differences in SBA between the parent strain and the Rmp− mutant were observed (Fig. 4).
FIG. 4.
SBA of postvaccination sera from vaccinees immunized with the Norwegian OMV vaccine against strains 44/76 and 44/76 Rmp−. Observations along the diagonal show sera with the same titer against both strains; broken lines indicate ±1 titer step difference. Data for SBA against strains 24/88 and 24/88 Rmp− were similar (not shown).
Neither purified human anti-class 4 OMP Igs nor anti-class 4 OMP MAbs were significantly bactericidal or opsonic against live strain 44/76 in the concentrations tested. However, in some experiments with ethanol-killed bacteria, a weak, positive opsonic activity was observed with anti-class 4 OMP MAbs 155,B-4 and 185,H-8, indicating that the epitope(s) may be variably expressed from batch to batch.
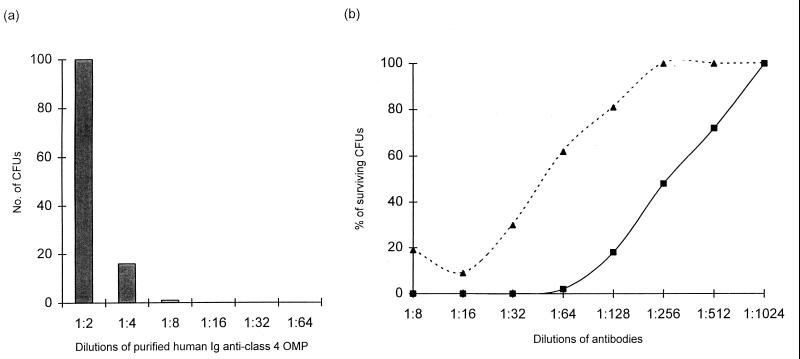
Blocking activity.
To investigate a possible blocking activity of anti-class 4 OMP antibodies, SBA was assayed as described in Materials and Methods. No blocking of SBA was observed with the MAbs. Only purified anti-class 4 OMP Igs from one of the eight vaccinees (1607) showed an inhibition of the bactericidal activity of the anti-P1.16 MAb at the highest concentration of Ig tested (Fig. 5a). The human Igs against class 4 OMP were also tested for the ability to inhibit the bactericidal effect of other postvaccination sera. The two human postvaccination serum samples selected as effector sera had high SBA titers against strain 44/76 and contained antibodies against different meningococcal antigens. Both sera were mixed with a high concentration of purified human Ig against class 4 OMP to study a possible blocking effect. Purified human anti-class 4 OMP Ig from vaccinee 1607 inhibited moderately (about fourfold) the bactericidal effect of these sera (Fig. 5b), whereas no effect was detected with the other seven anti-class 4 OMP Igs tested. Immunoblot analysis of sera from this person, taken throughout the vaccination schedule, showed that this vaccinee had antibodies against class 4 OMP before vaccination and showed no further increased response to the immunizations.
FIG. 5.
Blocking effect of anti-class 4 OMP antibodies from vaccinee 1607 on the bactericidal activity of P1.16 MAb and postvaccination sera. (a) Inhibition of the bactericidal activity of anti-class 1 OMP MAb (151,F-9) by purified Ig against class 4 OMP. The concentration of the anti-class 1 OMP MAb (effector antibody) was kept constant at the last dilution, giving 100% killing of strain 44/76, and the anti-class 4 OMP Ig (blocking antibody) was titrated. (b) Inhibition of the bactericidal activity of postvaccination serum from vaccinee 1428 by anti-class 4 OMP Ig from vaccinee 1607. ■, numbers of colonies surviving after incubation with different dilutions of serum (vaccinee 1428) without purified Ig against class 4 OMP; ▴, numbers of colonies surviving after incubation with different dilutions of the 1:4 (vol/vol) mixture of serum from vaccinee 1428 and purified Ig against class 4 OMP from vaccinee 1607. There was no indication of blocking effect with purified anti-class 4 OMP Igs from the other seven vaccinees.
Epitope specificity.
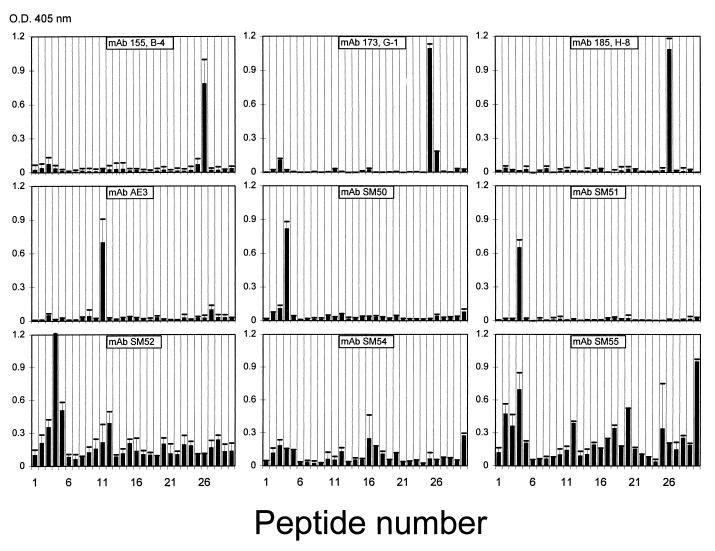
In order to detect linear epitopes on the class 4 OMP, the MAbs and purified human Igs were tested by ELISA with peptides of 14 residues synthesized on polyethylene pins. The amino acid sequences of peptides that the MAbs recognized were as follows: for SM50 and SM51, 21NYGECWKNAYFDKA34 (epitope I); for AE3 and V414, 70DETISLSAKTLFGF83 (epitope II); for 173,G-1, 168EAEVAKLGAKVSKA181; and for 185,H-8 and 155,B-4, 175GAKVSKAKKREALI188 (epitope III). MAbs SM52 to SM55 showed binding to multiple peptides, indicating that they were reacting mainly with conformational epitopes (Fig. 6).
FIG. 6.
ELISA reactivities of nine anti-class 4 OMP MAbs with synthetic 14-mer peptides from class 4 OMP on pins. The reaction of MAb V414 was similar to the reaction of MAb AE3. Amino acid sequences corresponding to peptide numbers are given in Table 2.
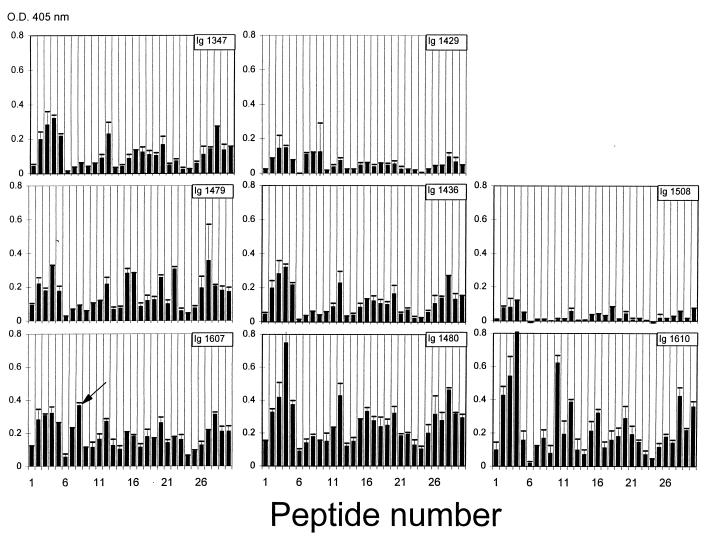
Also, the purified polyclonal anti-class 4 OMP Igs from the eight vaccinees reacted with multiple peptides, all with strong reactions around epitopes I and II (Fig. 7). The Igs from the vaccinee with a blocking effect for SBA (1607) showed their strongest reaction to peptide 8 (Table 2).
FIG. 7.
ELISA reactivities with synthetic 14-mer peptides from class 4 OMP on pins with purified anti-class 4 OMP antibodies (purified Igs) from eight vaccinees immunized with the Norwegian OMV vaccine. Amino acid sequences corresponding to peptide numbers are given in Table 2. O.D., optical density. The arrow in panel Ig 1607, corresponding to peptide no. 8, indicates the most-marked detected difference between this vaccinee and the others.
Pre- and postimmunization sera from three vaccinees with anti-class 4 OMP antibodies detected by immunoblot were also tested with the peptides by ELISA. Varying reaction patterns were observed, with the main positive difference occurring between two vaccinees (1508 and 1610) against peptides 10 and 11 (63EQAPQYVDETISLS76 and 70DETISLSAKTLFGF83), corresponding to epitope II. Similar analyses with acute and convalescent sera from three patients who had suffered systemic meningococcal group B disease showed a varying binding pattern with no clear increase in binding to any one common peptide (data not shown).
DISCUSSION
The class 4 OMP constitutes about 10% of the proteins in the Norwegian group B meningococcal vaccine. As detected on immunoblots, about 10 to 20% of the vaccinees responded to the class 4 OMP after immunization (44). In the present study, sera from eight selected individuals, immunized twice with this vaccine, were used for purification of antibodies to the class 4 OMP. These antibodies were purified by protein G chromatography and absorbed with OMVs from bacteria lacking the class 4 OMP (44/76 Rmp−) to remove antibodies against other meningococcal antigens. The purified human anti-class 4 OMP Igs reacted strongly with the class 4 OMP in immunoblots (Fig. 1), whereas only weak reactions were observed in ELISA with whole cells and OMVs from strain 44/76, indicating that most of these antibodies were directed against parts of the class 4 OMP molecule that were poorly or not surface exposed. In contrast, MAbs against different epitopes of the class 4 OMP reacted strongly in colony blots, whole-cell ELISA, and flow cytometry with ethanol-killed bacteria. However, neither purified human Ig against class 4 OMP nor murine anti-class 4 OMP MAbs were bactericidal against strain 44/76-SL, and no significant reactions with anti-class 4 OMP MAbs were observed in immunofluorescence binding experiments with flow cytometry or in IEM with intact cells, whereas binding of MAbs was observed in IEM to cells heated to 100°C and to outer membrane blebs (Fig. 2). This lack of reaction with intact cells in IEM is in concordance with previously published observations by Poolman et al. (38) with other anti-class 4 OMP MAbs. It is therefore possible that the positive reactions in colony blots and in immunofluorescence with ethanol-fixed cells were with partially lysed cells or membrane fragments where the class 4 OMP is exposed. The positive reactions with MAb 144,H-3 support this possibility strongly, since this MAb is directed against an epitope on ribosomal proteins. The epitopes on the class 4 OMP may also be masked to some extent by other membrane components, e.g., by the lipopolysaccharide (LPS) side chains. Blake et al. (7) have shown that PIII in gonococci is resistant to proteolytic cleavage; however, once extracted from the membrane, it is very sensitive to enzymatic digestion.
Some MAbs against PIII have previously been shown to block the effect of bactericidal MAbs against meningococcal OMP (34), whereas other anti-PIII MAbs have been reported to be bactericidal to gonococci (52, 53). The observed blocking activity led to warnings against including the class 4 OMP as a component in a meningococcal vaccine (17, 34). In contrast to this, none of the MAbs against class 4 OMP or PIII blocked the bactericidal effect of the anti-class 1 OMP MAb in our studies. With purified human antibodies against class 4 OMP, one of eight selected Igs from 27 vaccinees reduced the bactericidal effect of the anti-class 1 OMP MAb and of postvaccination sera, but only at a very high concentration (Fig. 5). The anti-class 4 OMP antibodies from this vaccinee (1607) were present also before vaccination and were not induced by vaccination. The distinct anti-class 4 OMP antibodies of this vaccinee could possibly be the result of previous neisserial infections or cross-reactive antibodies elicited to epitopes on similar molecules, e.g., OmpA from E. coli.
When postvaccination sera from 32 vaccinees were studied for SBA against two meningococcal strains and their respective Rmp− mutants, no marked difference in SBA titers was observed (Fig. 4). There was no negative correlation between anti-class 4 OMP IgG levels in immunoblots and SBA titers, indicating that the presence of anti-class 4 OMP antibodies did not affect the bactericidal activity of other meningococcal antibodies (44). In contrast to the previously reported blocking effects with immunization with gonococcal OMP vaccines containing PIII (5, 19, 23, 37), the present study did not indicate that vaccination with a meningococcal OMV vaccine induced blocking antibodies. Similar results have been reported in other studies (51, 61). Possibly, the exposure of PIII on gonococci is different from that of class 4 OMP on meningococci. There are many differences between the outer surfaces of gonococci and meningococci, such as the capsule found only in meningococci, differences in LPS structure, and meningococci normally having two porins rather than the gonococcal one. Possibly, such factors could influence the exposure of Rmp.
Three new MAbs against class 4 OMP were generated at the NIPH and compared to other anti-class 4 OMP or PIII MAbs, prepared in different laboratories. Our data showed that at least three separate, linear regions of the class 4 OMP were reacting with these MAbs. Their corresponding epitopes, located between amino acids N21 and A34 (epitope I), D70 and F83 (epitope II), and E68 and I188 (epitope III), were detected by ELISA with synthetic 14-mer peptides from class 4 OMP (Fig. 6). Only the two MAbs (SM50 and SM51) elicited against gonococci reacted with epitope I, whereas epitope II was identified by two MAbs made by immunizing mice with a whole-cell bacterial preparation or crude outer membranes from meningococci. Epitope III was identified only by MAbs made by immunization with LPS-depleted OMVs from meningococci. The specificity of MAbs SM50 and SM51 has previously been defined in more detail as the overlapping peptides WKNAYFDK and ECWKNAYFDK, respectively (54). The MAbs SM52 to SM55, which did not react with 10-mer peptides from PIII, reacted with several 14-mer peptides from class 4 OMP, indicating discontinuous epitopes (Fig. 6). MAbs SM52 to SM55 have previously been found to recognize at least two distinct epitopes (52). Purified polyclonal anti-class 4 OMP Igs reacted with multiple peptides, with the strongest reaction occurring around epitopes I, II, and III (Fig. 7). The pre- and postvaccination sera from three vaccinees with anti-class 4 OMP antibodies indicated a response to epitope II (peptides 10 and 11) in two vaccinees (1508 and 1610), whereas sera from one patient showed the strongest increase for peptide 23 (data not shown).
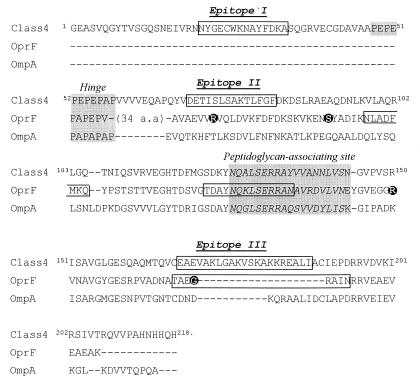
Several questions about the topological model of the class 4 OMP from N. meningitidis have been addressed. Bacterial OMPs do not contain stretches of hydrophobic amino acids long enough to span the outer membrane in an α-helical configuration. Instead, all the OMPs that have been studied in sufficient detail up to now are β-barrels with amphipathic β-strands spanning the membrane (8, 10, 45, 49). By using the criteria described in Materials and Methods, models for the topology of these proteins can be predicted. However, attempts to apply these criteria to predicting the topology of the class 4 OMP were unsuccessful (data not shown). Hence, if class 4 protein is indeed an integral OMP, its structure appears to be different from those of the well-characterized OMPs. This conclusion is consistent with the secondary-structure analysis of two homologues of class 4 OMP, i.e., OmpA of E. coli and OprF of P. aeruginosa, which revealed a high α-helical content in the C termini of these proteins (47). Like OmpA and OprF, class 4 OMP consists of two domains, which are separated by a proline-rich hinge region, PEPEPEPEPAP (Fig. 8). The large C-terminal domain is homologous to the C-terminal domains of OmpA and OprF and is therefore expected to be predominantly α-helical as well. Since, in addition, the N-terminal domain of Rmp is very small (47 residues), it is unlikely that Rmp is a β-barrel protein.
FIG. 8.
Alignment of class 4 OMP, OprF, and OmpA proteins. From OprF and OmpA proteins, only those segments that can be aligned with the class 4 OMP are shown. Epitopes identified in class 4 OMP and in OprF are boxed (21, 39). Exposed parts of OprF, according to epitope insertion experiments (59), are shown as reversed-type amino acids. The hinge region and the putative peptidoglycan-associating region are shaded.
How could Rmp be embedded in the membrane? Two entirely different and mutually exclusive models can be proposed. OmpA is supposed to be embedded in the outer membrane by its N-terminal domain, which forms an eight-stranded β-barrel, with the C-terminal domain completely extending into the periplasm (42). If this model is correct, the C-terminal domain of Rmp would probably be entirely periplasmic as well. The N-terminal domain of Rmp is much shorter than that of OmpA and thus cannot form a β-barrel. At the most, it could contain two membrane-spanning segments, thereby exposing one loop (containing, for example, epitope I) to the surface. Consistent with this hypothesis is that MAb SM52, which strongly reacts with a peptide encompassing epitope I, has been reported to be bactericidal for gonococci (54). However, in our hands, SM52 was not bactericidal for meningococci. Furthermore, since no hydrophobic, or even any amphipathic, stretches of residual segments that are sufficiently long to span the membrane can be distinguished in the N-terminal domain, this domain of the protein might be periplasmic as well. Then, the fractionation of Rmp with the outer membranes might be entirely due to its strong association with the porins (32).
The alternative model takes into account the homology with OprF and the fact that some evidence for the cell surface exposure of several epitopes in Rmp was obtained, for example by colony blotting and flow cytometry with ethanol-killed cells. Multiple observations supporting the cell surface exposure of segments within the C-terminal domain of OprF have been reported. This evidence was obtained by mapping epitopes of MAbs reacting with presumably intact cells (39), by studying the interaction of antibodies raised against synthetic peptides with intact cells (16, 21, 22), and by inserting foreign epitopes into OprF and studying their accessibility (12, 59). One of the C-terminal epitopes identified in OprF (TAEGRAIN) was particularly interesting because epitope III in class 4 OMP mapped in exactly the same position and antibodies to this peptide induced antibodies which were protective in mice upon challenge with P. aeruginosa (16). If all the available data on Rmp and OprF are correct, the C-terminal domains of these proteins should be almost entirely surface exposed. However, such a model would be inconsistent with the presence of a putative peptidoglycan-binding domain in this part of the protein (26). It should be noted that strong evidence for the cell surface exposure of any part of class 4 OMP is still lacking. Of the many different antibodies directed against this protein that were used in this study, not a single one proved bactericidal against meningococci. Furthermore, IEM did not reveal the labelling of intact cells with antibodies directed against Rmp, suggesting that the epitopes are all hidden and therefore possibly localized in the periplasm. However, whereas the intact cells could not be labelled, blebs that are shed from the cells were labelled with MAbs against Rmp (Fig. 2) (38), suggesting that some of these blebs (in part) may have an inside-out topology. The presence of blebs in whole-cell preparations could explain the positive results with MAbs against Rmp. It should be noted that P. aeruginosa also has been reported to shed blebs (24), which could affect the interpretation of the results described for OprF. Furthermore, under all conditions in which a clear reaction was determined with Rmp-specific antibodies, a reaction with the ribosomal protein-specific antibody 144,H-3 was detected as well, indicating a considerable degree of cell lysis. Because of these considerations, we presently favor the former model for the topology of Rmp, in which most of the protein is exposed to the periplasm. Alternatively, the C-terminal part can move through the membrane, but only a portion of the cell exposes this domain at certain stages in the growth phase.
ACKNOWLEDGMENTS
We are grateful to Milan Blake for help in constructing the meningococcal strains 44/76 Rmp− and 24/88 Rmp− and to J. E. Heckels for constructive criticism regarding this work and useful suggestions concerning the manuscript. We also thank M. Achtman, J. E. Heckels, and C. T. Sacchi for kindly supplying MAbs and Gunnhild Rødal, Torunn Marigaard, Anne Klem, and Elisabeth Rønnild for excellent technical assistance.
This work was supported financially by grants from Ninas Minnefond, Norway, and by the WHO Global Program for Vaccines (GPV/V23/181/52).
REFERENCES
- 1.Aase A, Sandlie I, Norderhaug L, Brekke O H, Michaelsen T E. The extended hinge region of IgG3 is not required for high phagocytic capacity mediated by Fca receptors, but the heavy chains must be disulfide bonded. Eur J Immunol. 1993;23:1546–1551. doi: 10.1002/eji.1830230723. [DOI] [PubMed] [Google Scholar]
- 2.Achtman M, Kusecek B, Morelli G, Eickmann K, Wang J, Crowe B, Wall R A, Hassan-King M, Moore P S, Zollinger W D. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J Infect Dis. 1992;165:53–68. doi: 10.1093/infdis/165.1.53. [DOI] [PubMed] [Google Scholar]
- 3.Andersen S R, Kolberg J, Høiby E A, Namork E, Caugant D A, Frøholm L O, Jantzen E, Bjune G. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: possible consequences for vaccine development. Microbiol Pathol. 1997;23:139–155. doi: 10.1006/mpat.1997.0143. [DOI] [PubMed] [Google Scholar]
- 4.Arhin F F, Moreau F, Coulton J W, Mills E L. Subtyping of Neisseria meningitidis strains isolated in Quebec, Canada: correlation between deduced amino acid sequences and serosubtyping techniques. Can J Microbiol. 1997;43:234–238. doi: 10.1139/m97-032. [DOI] [PubMed] [Google Scholar]
- 5.Arminjon F, Cadoz M, Morse S A, Rock J P, Sarafian S K. Abstracts of the Annual Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1987. Bactericidal and opsonic activities of sera from individuals immunized with a gonococcal protein I vaccine, abstr. E-92; p. 118. [Google Scholar]
- 6.Bjune G, Høiby E A, Grønnesby J K, Arnesen O, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 7.Blake M S, Wetzler L M, Gotschlich E C, Rice P A. Protein III: structure, function, and genetics. Clin Microbiol Rev. 1989;2(Suppl.):S60–S63. doi: 10.1128/cmr.2.suppl.s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan S W, Schirmer T, Rummel G, Steiert M, Ghosh R, Paupit R A, Jansonius J N, Rosenbusch J P. Crystal structure explains functional properties of two E. coli porins. Nature. 1992;358:727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- 9.Danelli M G, Batoreu N M, Lacerda M D, Ferreira C R B, Darc Cardoso J, Peralta J M, Frasch C E. Surface antigen analysis of group B Neisseria meningitidis outer membrane by monoclonal antibodies: identification of bactericidal antibodies to class 5 protein. Curr Microbiol. 1995;31:146–151. doi: 10.1007/BF00293545. [DOI] [PubMed] [Google Scholar]
- 10.Dekker N, Merck K, Tommassen J, Verheij H M. In vitro folding of Escherichia coli outer membrane phospholipase A. Eur J Biochem. 1995;232:214–219. doi: 10.1111/j.1432-1033.1995.tb20801.x. [DOI] [PubMed] [Google Scholar]
- 11.Delvig A A, Wedege E, Caugant D A, Dalseg R, Kolberg J, Achtman M, Rosenqvist E. A linear B-cell epitope on the class 3 outer-membrane protein of Neisseria meningitidis recognized after vaccination with the Norwegian group B outer-membrane vesicle vaccine. Microbiology. 1995;141:1593–1600. doi: 10.1099/13500872-141-7-1593. [DOI] [PubMed] [Google Scholar]
- 12.Finnen R L, Martin N L, Siehnel R J, Woodruff W A, Rosok M, Hancock R E W. Analysis of the Pseudomonas aeruginosa major outer membrane protein OprF by use of truncated OprF derivatives and monoclonal antibodies. J Bacteriol. 1992;174:4977–4985. doi: 10.1128/jb.174.15.4977-4985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 14.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm L O, Lindbak A-K, Møgster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–79. [PubMed] [Google Scholar]
- 15.Geysen H M, Rodda S J, Mason T J, Tribbick G, Schoofs P G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 16.Gilleland L B, Gilleland H E., Jr Synthetic peptides representing two protective, linear B-cell epitopes of outer membrane protein F of Pseudomonas aeruginosa elicit whole-cell-reactive antibodies that are functionally pseudomonad specific. Infect Immun. 1995;63:2347–2351. doi: 10.1128/iai.63.6.2347-2351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotschlich E C. The meningococcal serogroup B vaccine protection trials: concluding remarks at the report meeting second day. NIPH Ann. 1991;14:247–250. [Google Scholar]
- 18.Gotschlich E C, Seiff M, Blake M S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. J Exp Med. 1987;165:471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati S, Rice P A, Blake M S, Sarafian S K, Morse S A, Quentin-Millet M J, Arminjon F. Antibody responses in six volunteers immunized with a gonococcal protein I vaccine. In: Achtman M, Kohl P, Marchal C, Morelli G, Seiler A, Thiesen B, editors. Neisseria 1990. Berlin, Germany: Walter de Gruyter; 1990. pp. 229–234. [Google Scholar]
- 20.Høiby E A, Rosenqvist E, Frøholm L O, Bjune G, Feiring B, Nøkleby H, Rønnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann. 1991;14:147–156. [PubMed] [Google Scholar]
- 21.Hughes E E, Gilleland H E., Jr Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against Pseudomonas aeruginosa infection in murine acute pneumonia model. Vaccine. 1995;13:750–753. doi: 10.1016/0264-410x(95)00166-x. [DOI] [PubMed] [Google Scholar]
- 22.Hughes E E, Matthews-Greer J M, Gilleland H E., Jr Analysis by flow cytometry of surface-exposed epitopes of outer membrane protein F of Pseudomonas aeruginosa. Can J Microbiol. 1996;42:859–862. doi: 10.1139/m96-109. [DOI] [PubMed] [Google Scholar]
- 23.Joiner K, Scales R A, Warren K A, Frank M M, Rice P A. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985;76:1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadurugamuwa J L, Beveridge T J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klugman K P, Gotschlich E C, Blake M S. Sequence of the structural gene (rmpM) for the class 4 outer membrane protein of Neisseria meningitidis, homology of the protein to gonococcal protein III and Escherichia coli OmpA, and construction of meningococcal strains that lack class 4 protein. Infect Immun. 1989;57:2066–2071. doi: 10.1128/iai.57.7.2066-2071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koebnik R. Proposal for a peptidoglycan-associating alpha-helical motif in the C-terminal regions of some bacterial cell-surface proteins. Mol Microbiol. 1995;16:1269–1270. doi: 10.1111/j.1365-2958.1995.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolberg J, Høiby E A, Lopez R, Sletten K. Monoclonal antibodies against Streptococcus pneumoniae detect epitopes on eubacterial ribosomal proteins L7/L12 and on streptococcal elongation factor Ts. Microbiology. 1997;143:55–61. doi: 10.1099/00221287-143-1-55. [DOI] [PubMed] [Google Scholar]
- 28.Kyte J, Doolittle R F. A simple method for displaying the hydrophobic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Lytton E J, Blake M S. Isolation and partial characterization of the reduction-modifiable protein of Neisseria gonorrhoeae. J Exp Med. 1986;164:1749–1759. doi: 10.1084/jem.164.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDade R L, Johnston K H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980;141:1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran E E, Brandt B L, Zollinger W D. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect Immun. 1994;62:5290–5295. doi: 10.1128/iai.62.12.5290-5295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munkley A, Tinsley C R, Virji M, Heckels J E. Blocking of bactericidal killing of Neisseria meningitidis by antibodies directed against class 4 outer membrane protein. Microb Pathog. 1991;11:447–452. doi: 10.1016/0882-4010(91)90041-8. [DOI] [PubMed] [Google Scholar]
- 35.Newhall W J, Sawyer W D, Haak R A. Cross-linking analysis of outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980;28:785–790. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul C, Rosenbusch J P. Folding patterns of porins and bacteriorhodopsin. EMBO J. 1985;4:1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plummer F A, Chubb H, Simonsen J N, Bosire M, Slaney L, Maclean I, Ndinya-Achola J O, Waiyaki P, Brunham R C. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest. 1993;91:339–343. doi: 10.1172/JCI116190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poolman J T, Wientjes F B, Hopman C T P, Zanen H C. Influence of the length of lipopolysaccharide molecules on the surface exposure of class 1 and 2 outer membrane proteins of Neisseria meningitidis 2996 (B:2b:P1.2) In: Schoolnik G K, Brooks G F, Falkow S, Frasch C E, Knapp J S, McCutchan J A, Morse S A, editors. The pathogenic neisseriae. Washington, D.C: American Society for Microbiology; 1985. pp. 562–570. [Google Scholar]
- 39.Rawling E G, Martin N L, Hancock R E W. Epitope mapping of the Pseudomonas aeruginosa major outer membrane porin protein OprF. Infect Immun. 1995;63:38–42. doi: 10.1128/iai.63.1.38-42.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rest R F, Chen G C, Gotschlich E C. Neisseria gonorrhoeae mutants lacking outer membrane protein Rmp (PIII) are deficient in invasion of human epithelial cells. In: Zollinger W D, Frasch C E, Deal C D, editors. Tenth International Pathogenic Neisseria Conference, Baltimore, Md., September 1996. 1996. pp. 267–269. [Google Scholar]
- 41.Rice P A, Vayo H E, Tam M R, Blake M S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ried G, Koebnik R, Hindennach I, Mutschler B, Henning U. Membrane topology and assembly of the outer membrane protein OmpA of Escherichia coli K12. Mol Gen Genet. 1994;243:127–135. doi: 10.1007/BF00280309. [DOI] [PubMed] [Google Scholar]
- 43.Rosenqvist E, Wedege E, Høiby E A, Frøholm L O. Serogroup determination of Neisseria meningitidis by whole-cell ELISA, dot-blotting and agglutination. APMIS. 1990;98:501–506. [PubMed] [Google Scholar]
- 44.Rosenqvist E, Høiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schirmer T, Keller T A, Wang Y F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science. 1994;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara E, Nikaido H. Pore-forming activity of OmpA protein from Escherichia coli. J Biol Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- 47.Sugawara E, Steiert M, Rouhani S, Nikaido H. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J Bacteriol. 1996;178:6067–6069. doi: 10.1128/jb.178.20.6067-6069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson J, Mayer L W, Tam M R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982;38:668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tommassen J. Biogenesis and membrane topology of outer membrane proteins in Escherichia coli. In: Op den Kamp J A F, editor. Membrane biogenesis. NATO ASI series. Berlin, Germany: Springer-Verlag; 1988. pp. 352–373. [Google Scholar]
- 50.Tsai C M, Frasch C E, Mocca L F. Five structural classes of major outer membrane proteins in Neisseria meningitidis. J Bacteriol. 1981;146:69–78. doi: 10.1128/jb.146.1.69-78.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Voort E R, van der Ley P, Van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun. 1996;64:2745–2751. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virji M, Zak K, Heckels J E. Outer membrane protein III of Neisseria gonorrhoeae: variations in biological properties of antibodies directed against different epitopes. J Gen Microbiol. 1987;133:3393–3401. doi: 10.1099/00221287-133-12-3393. [DOI] [PubMed] [Google Scholar]
- 53.Virji M, Heckels J E. Non-bactericidal antibodies against Neisseria gonorrhoeae: evaluation of their blocking effect on bactericidal antibodies directed against outer membrane antigens. J Gen Microbiol. 1988;134:2703–2711. doi: 10.1099/00221287-134-10-2703. [DOI] [PubMed] [Google Scholar]
- 54.Virji M, Heckels J E. Location of a blocking epitope on outer-membrane protein III of Neisseria gonorrhoeae by synthetic peptide analysis. J Gen Microbiol. 1989;135:1895–1899. doi: 10.1099/00221287-135-7-1895. [DOI] [PubMed] [Google Scholar]
- 55.Wedege E, Frøholm L O. Human antibody response to a group B serotype 2a meningococcal vaccine determined by immunoblotting. Infect Immun. 1986;51:571–578. doi: 10.1128/iai.51.2.571-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wedege E, Michaelsen T E. Human immunoglobulin G subclass immune response to outer membrane antigens in meningococcal group B vaccine. J Clin Microbiol. 1987;25:1349–1353. doi: 10.1128/jcm.25.8.1349-1353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56a.Wedege, E. Unpublished data.
- 57.Weiss M S, Abele U, Weckesser J, Welte W, Schiltz E, Schultz G E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991;254:1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- 58.Wolff K, Stern A. Identification and characterization of specific sequences encoding pathogenicity associated proteins in the genome of commensal Neisseria species. FEMS Microbiol Lett. 1995;125:255–264. doi: 10.1111/j.1574-6968.1995.tb07366.x. [DOI] [PubMed] [Google Scholar]
- 59.Wong R S Y, Wirtz R A, Hancock R E W. Pseudomonas aeruginosa outer membrane protein OprF as an expression vector for foreign epitopes: the effects of positioning and length on the antigenicity of the epitope. Gene. 1995;158:55–60. doi: 10.1016/0378-1119(95)00155-y. [DOI] [PubMed] [Google Scholar]
- 60.Woodruff W A, Hancock R E W. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989;171:3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zollinger W, Kuschener R A, Brandt B, Moran E E, Shoemaker D, Blake M, Mays J. Phase I study of two meningococcal outer membrane protein vaccines prepared from a class 4 outer membrane protein negative mutant and its isogenic parent. In: Evans J S, Jost S E, Maiden M C J, Feavers I M, editors. Proceedings of the Ninth International Pathogenic Neisseria Conference, Winchester, England, September 1994. 1994. p. 449. [Google Scholar]