Abstract
Background and aims
Disruption of the developing microbiota by Caesarean birth or early exposure to antibiotics may impact long-term health outcomes, which can potentially be prevented by nutritional supplements. This systematic review aimed to summarise the evidence regarding the effects of prebiotics, probiotics and synbiotics on the intestinal microbiota composition of term infants born by Caesarean section or exposed to antibiotics in the first week of life.
Methods
A systematic search was performed from inception to August 2022 in Medline and Embase. Two researchers independently performed title and abstract screening (n = 12,230), full-text screening (n = 46) and critical appraisal. We included randomised controlled trials which included term-born infants who were born following Caesarean section or who were exposed to postpartum antibiotics in the first week of life, pre-, pro- or synbiotics were administered <6 weeks after birth and outcome(s) consisted of microbiota analyses.
Results
Twelve randomised controlled trials investigating Caesarean born infants and one randomised controlled trial including infants exposed to antibiotics were included. Group sizes varied from 11 to 230 with 1193 infants in total. Probiotic (n = 7) or synbiotic (n = 3) supplementation significantly increased the abundance of the supplemented bacterial species (of the Bifidobacterium and Lactobacillus genus), and there was a decrease in Enterobacteriaceae, especially <4 weeks of age. At phylum level, Actinobacteria (two studies), Proteobacteria (one study) and Firmicutes (one study) increased after probiotic supplementation. In three studies on prebiotics, two studies reported a significant increase in Bifidobacteria and one study found a significant increase in Enterobacteriaceae.
Discussion
Prebiotic, probiotic and synbiotic supplements seem to restore dysbiosis after Caesarean section towards a microbial signature of vaginally born infants by increasing the abundance of beneficial bacteria. However, given the variety in study products and study procedures, it is yet too early to advocate specific products in clinical settings.
Introduction
The human gastrointestinal microbiota is a collection of all microorganisms (bacteria, viruses, fungi, protozoa) residing in the gastrointestinal tract. Together, these microorganisms affect processes such as metabolism [1,2] and inflammatory and immunological responses[2], and also influence the integrity and structure of the gastrointestinal tract [2]. The normal gastrointestinal microbiota develops rapidly after birth and is highly dynamic until it shifts towards an adult-like composition around the age of three years [3]. This development is driven by exposure to microbes from maternal, environmental, and dietary sources [4] and can be disrupted by many factors, especially when they occur early in this developmental process.
Caesarean section is one of the most important causes of disrupted microbiota development due to reduced vertical mother-infant transmission of beneficial intestinal bacteria (specifically of the Lactobacillus and Bifidobacterium genus). It has been suggested that the prenatal antibiotic exposure during a Caesarean section also affects the infant’s microbiota development, but a recent randomised controlled trial (RCT) reported that prenatal exposure to antibiotics during caesarean section does not further disrupt the microbiota colonisation [5]. During and after a Caesarean section, the infant becomes predominantly colonised with bacteria from the hospital environment (e.g. Staphylococcus, Corynebacterium and Propionibacterium species) [1,2,6,7]. Dysbiosis after Caesarean section can persist for as long as seven years and is associated with a higher risk of obesity, atopy, and type 1 diabetes mellitus [6].
In addition to Caesarean deliveries, exposure to antibiotics in early life has been associated with dysbiosis [8]. Early life antibiotics have been shown to decrease the abundance of Bifidobacteria [9] and Bacteroidetes [10] and increase the amount of Clostridia [8] and Enterobacteriaceae [9]. Antibiotics are the most frequently prescribed drugs in neonates with 8% of all European infants exposed to antibiotics in the first week of life [11]. The effect of antibiotic exposure, specifically in the first week of life, has been associated with an altered gut microbiota[9,12] a higher risk of wheezing [13], infantile colic [13], gastrointestinal disorders [14], impaired growth [12,15], allergies [16], and asthma [17].
These short- and long-term health effects linked to early dysbiosis through Caesarean delivery and neonatal antibiotic exposure illustrate the need for interventions aimed at restoration of this dysbiosis, and consequently prevention of related health consequences. Supplementation with prebiotics, probiotics or synbiotics has been described as a promising intervention to reduce some of the risks associated with early microbiota disruption. Probiotics are live microorganisms such as Bifidobacteria and Lactobacilli [7], while prebiotics are nutrients that promote growth and activity of bacteria that already exist in the gut [18]. Synbiotics are a combination of pre- and probiotics [18].
The aim of this systematic review is to identify all studies investigating the effects of a pre-, pro- or synbiotic supplement on the gut microbiota of term-born infants born by Caesarean section or exposed to antibiotics in the first week of life.
Methods
Literature search
OVID Medline and Embase were systematically searched from inception to August 10, 2022. The search strategy was constructed in collaboration with a medical librarian (JD) and was composed of the following components:
([c section] OR ([antibiotic treatment] AND [first week of life] OR [first week antibiotics])) AND
[pre- pro- synbiotics]
OR
[dietary supplements] AND [microbiome]
OR
[dietary supplements brands]
In order to reduce recall bias and enhance search results precision VOS-viewer was used to identify terms for NOTing out irrelevant records from databases searched [19]. No other filters or limits were used. The full search term including the specific keywords and combinations of search components can be found in the S1 Table.
Eligibility criteria
The following inclusion criteria were applied, all criteria had to be met for inclusion:
study participants were term-born infants who were born following Caesarean section or exposed to antibiotics in the first week of life (born vaginally or following Caesarean section),
administration of pre-, pro- or synbiotic dietary supplements was started within six weeks after birth,
reported outcome(s) consisted of microbiota analyses,
study design was a randomised controlled trial.
Exclusion criteria were:
studies including infants with major congenital malformations,
studies written in a language other than English,
animal studies,
for the Caesarean-analyses: studies which included both vaginally and Caesarean-delivered infants but performed no subgroup analyses for only the Caesarean-delivered infants
Data collection
All records found in the search were exported into Rayyan after deduplication [20]. Two researchers (NC and KK) independently performed title and abstract screening, as well as full-text screening. Titles and abstracts were screened by determining whether the article could meet the in- and exclusion criteria stated above. After consensus on the included articles, relevant data was extracted by NC in consultation with the other co-authors. Reference lists of the included articles were hand-searched to look for additional relevant studies.
All significant outcomes provided in the main text or supplemental information were summarised in a table, and non-significant results from studies investigating the same outcomes were reported in separate bar charts.
If both “per protocol” and “(modified) intention to treat” analyses were available, only the results from the “(modified) intention to treat” analysis were included in the table.
Critical appraisal
To assess the risk of bias in the included articles, the Cochrane risk-of-bias tool for randomised controlled trials (RoB 2) [21] was used. The RoB 2 assesses the risk of bias of studies in five domains: bias arising from the randomisation process, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results. Risk of bias was independently assessed by two researchers (NC and KK) and any discrepancies were discussed until a consensus was reached. The guidance document of the RoB 2 was used to determine whether articles had a high, some or a low risk of bias. If a study included both vaginally and Caesarean-delivered infants and performed a subgroup analysis on the Caesarean-delivered infants, only the methods used for the relevant subgroup analyses were assessed.
The review and protocol were not registered. This systematic review was conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses [22].
Results
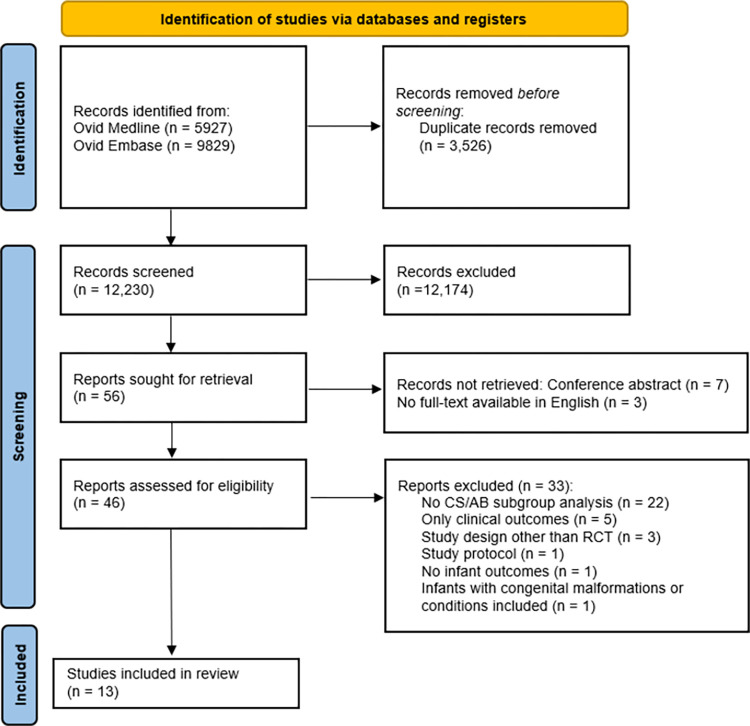
Of the 15,756 records, 12,230 remained after deduplication. After title and abstract screening, 56 articles were deemed suitable for full-text screening. Finally, 13 articles were included for analysis (Fig 1). Hand-searching the reference lists of these articles did not result in any more inclusions.
Fig 1. Flowchart showing article selection.
Adapted from the PRISMA 2020 flow diagram [22].
Study characteristics
In total, 13 articles were included, based on 12 randomised controlled trials (Fig 1); Lay et al [23] published results of a subgroup analysis based on the RCT by Chua et al. [24].
The 12 microbiota studies investigated the effect of prebiotics [23–26] (n = 3), synbiotics [23,24,27,28] (n = 3) and probiotics [29–35] (n = 7) (Table 1). The interventions were started between birth and the first three weeks of life, and treatment duration varied between five days after birth and six months of age. All studies investigated the effect of these interventions on infants born via Caesarean section, except for one study which included infants who received antibiotic treatment within three days after birth [35].
Table 1. General characteristics of the included randomised controlled trials.
| First author | Country | Study period (year published) | # Participants1 | AB or CS SG? |
Feeding method | Intervention | Control | Start of intervention | Duration intervention | Outcomes (relevant subgroup) | Follow-up | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | T | ||||||||||||
| Chua [24] | Singapore & Thailand | 2011–2013 (2017, 2021) | 52 + 51 | 50 | 153 | CS | Mixed (FF + BF) | Prebiotic (scGOS/lcFOS) or synbiotic (scGOS/ICFOS and Bifidobacterium breve M-16V) | Control formula | 1–3 D | 16 W | Total faecal Bifidobacterium, Bifidobacterium species abundance, other members of the gut microbiota, pH, sc fatty acids, lactate | 3, 5 D 2, 4, 8, 12, 16, 22 W |
Infants born via CS were also exposed to intrapartum AB prophylaxis. Lay et al.: some results were based on a SG |
| Lay [23] | 39 + 44 | 44 | 127 | |||||||||||
| Berger [25] | Italy & Belgium | 2012–2015 (2020, 2017) | 19 | 24 | 43 | CS SG | Exclusive FF | Prebiotics: 2 HMOs (2’-fucosyllactose and lacto-N-neotetraose) | Control formula | 0–14 D | 6 M | Stool microbiota diversity | 3, 12 M | - |
| Korpela [29] | Finland | 2000–2003 (2018, 2018, 2009, 2017) | 35 | 44 | 79 | CS SG | Exclusive BF, mixed feeding or FF | Probiotic: Lactobacillus rhamnosus LC705, Bifidobacterium breve Bb99, Propionibacterium freudenreichii spp., shermanii JS | Placebo (micro-crystalline cellulose) | 36 W gestation + from birth | 6 M | Microbiota composition | 3 M | Infants had to be at risk for atopic disease (at least one parent with asthma, allergic rhinitis or eczema) and this intervention was initiated prenatally (36 W gestation) |
| Baglatzi [30] | Greece | 2009–2011 (2016) | 84 | 80 | 164 | CS | Exclusive or mixed FF | Probiotic: regular dose of Bifidobacterium lactis | Low dose of B. lactis | Birth | 6 M | Detection of B. lactis | 12 M | No control group that was fed formula without pre-, pro-, or synbiotics |
| Cooper [27] | South Africa | 2008–2013 (2016) | 92 | 101 | 193 | CS SG | Exclusive FF | Synbiotic: BMOs (containing GOS and MOS such as 3’- and 6’ sialyllactose) + Bifidobacterium lactis CNCM-I-3446 | Control formula | Birth (≤3 D) | 6 M | Faecal (bifido)bacteria, anthropometrics, faecal pH, lean mass, fat mass and bone mineral content, digestive tolerance, immune parameters, HIV infection status, frequency of morbidity episodes | 1 Y | All included infants had HIV+ mothers and all mothers and infants received antiretroviral medication. Infants who tested positive for HIV were excluded |
| Estorninos [26] | Philippines | 2016–2018 (2022) | 115 | 115 | 230 | CS SG | Exclusive FF | Prebiotic: bovine MOS (GOS and sialylated-oligosaccharides) | Control formula | 3 W | 6 M | Phylogenetic distance/microbiota composition, Bifidobacteria abundance | 4 M | At the 2.5 month time point, only a subgroup of 75 infants for each group provided a faecal sample |
| Frese [31] | USA | 2015–2016 (2017) | 11 | 9 | 20 | CS SG | Any | Probiotic: Bifidobacterium infantis EVC001 | None | 7 D | 27 D | Microbiota composition, relative abundances of the most abundant taxonomic groups | 60 D | Significantly more mothers in the control group were primiparous |
| Garcia Rodenas [32] | Greece | 2010–2011 (2016) | 11 | 10 | 21 | CS SG | Exclusive FF | Probiotic: Lactobacillus reuteri DSM 17938 | Control formula | <72 H | 6 M | Relative abundance of OTUs, weighted UniFrac distances, relative abundance of Bifidobacterium | 4 M | - |
| Hurkala [33] | Poland | 2014–2017 (2020) | 71 | 77 | 148 | CS | Exclusive FF | Probiotic: Bifidobacterium breve PB04 and Lactobacillus rhamnosus KL53A | None | <1 H | Until discharge (5 or 6 D) | Abundance of lactobacilli in faeces, populations of Bifidobacterium in faeces, populations of potentially pathogenic bacteria | 1 M | Significantly more missing stool samples from the control group (29 compared to 13 in the intervention group) |
| Roggero [34] | Italy | 2015–2016 (2020) | 16 | 16 | 32 | CS SG | Exclusive FF | Probiotic: Lactobacillus paracasei CBA L74 | Control formula | <7 D | 3 M | sIgA production, antimicrobial peptides, microbiota diversity, metabolome, abundance of bacterial genera | 90 D | Infants may have been breastfed for a few days before enrolment |
| Yang [28] | China | 2018 (2021) | 7 + 7 | 9 | 23 | CS | BF | Synbiotic: high and low dose of Bifidobacterium lactis Bi-07 and Lactobacillus rhamnosus HN001 + GOS | No probiotic | Birth | 28 D | Diversity of gut microbiota, gut microbiota composition, COGs | 28 D | - |
| Zhong [35] | China | 2017–2018 (2021) | 25 + 13 | 17 | 55 | 1 week AB | Any | Probiotic: Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis during or after AB treatment | None | Beginning or end of AB treatment (AB treatment started <3 D after birth) | 42 D | Gut microbiota, relative abundance of OTUs | 42 D | Children were not necessarily born via CS, but received AB in the first week of life |
1 # participants in a subgroup, if applicable.
I Intervention.
C Control.
T Total.
CS Caesarean section.
SG subgroup.
BF breastfeeding.
FF formula feeding.
HMOs human milk oligosaccharides.
(sc) GOS: (short chain) galactooligosaccharides.
(lc) FOS: (long chain) fructooligosaccharides.
Spp. Several species.
BMOs bovine milk oligosaccharides.
MOS: Milk oligosaccharides.
D days.
M months.
W weeks.
H hours.
Y year.
AB antibiotic.
LRTI lower respiratory tract infection.
URTI upper respiratory tract infection.
OTU operational taxonomic unit.
sIgA secretory Immunoglobulin A.
COG: Clusters of orthologous groups of proteins.
Critical appraisal
The assessment of the risk of bias of the included studies is provided in Table 2. Of the 12 studies, 10 were determined to have a high risk of bias, mainly due to issues in adhering to the intervention. Most studies did not address the extent to which participants adhered to the intervention, and if they did, the appropriate analyses necessary to estimate the effect of the non-adherence to the intervention were not applied.
Table 2. Risk of bias of the included studies.
| First author | Domains of the Cochrane risk-of-bias tool for randomised controlled trials (RoB-2) | |||||
|---|---|---|---|---|---|---|
| Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Total | |
| Chua [24] | ||||||
| Lay [23] | ||||||
| Berger [25] | ||||||
| Estorninos [26] | ||||||
| Korpela [29] | ||||||
| Baglatzi [30] | ||||||
| Cooper [27] | ||||||
| Frese [31] | ||||||
| Garcia Rodenas [32] | ||||||
| Hurkala [33] | ||||||
| Roggero [34] | ||||||
| Yang [28] | ||||||
| Zhong [35] | ||||||
Green: Low risk of bias, yellow: Some risk of bias, red: High risk of bias.
Domain 1: Risk of bias arising from the randomisation process.
Domain 2: Risk of bias due to deviations from the intended interventions (effect of adhering to intervention).
Domain 3: Missing outcome data.
Domain 4: Risk of bias in measurement of the outcome.
Domain 5: Risk of bias in selection of the reported result.
The effect of pre-, pro- and synbiotics after antibiotics in the first week of life
The effects of the interventions were divided in three time clusters: 0–1 weeks, 1–4 weeks, or >4 weeks. The only study during or after antibiotic treatment in the first week of life investigated the effect of a probiotic supplement containing Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus faecalis [35]. At the phylum level they found a significant increase in abundance of Actinobacteria and Proteobacteria at 0–1 weeks and >4 weeks and an increase in Actinobacteria at 1–4 weeks. At the genus level, they reported a significant increase in the relative abundance of Bifidobacterium at 0–1 weeks and >4 weeks (Table 3).
Table 3. Effects of pre-, pro- or synbiotic interventions on microbiota composition.
| First author | Intervention | # Participants1 | Analysis techniques | Time points | Outcomes: microbiota composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-/pro-/synbiotic | Start | Duration | I | C | T | Diversity + Compositional differences | Phylum level | Family level | Genus level | Species level | Other | |||
| 0–1 Week | ||||||||||||||
| Cooper [27] | Synbiotic: BMOs (containing GOS and MOS such as 3’- and 6’ sialyllactose) + B. lactis CNCM-I-3446 | Birth (≤3 D) | 6 M | 92 | 101 | 193 | PCR, FISH | 3 D | n.s. | |||||
| Chua [24] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 | 45 | 84 | 16S rRNA sequencing + FISH + qPCR | 3/5 D | % Bifidobacteria: ↑ | |||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 1–3 D | 16 W | 45 | 45 | 90 | 3/5 D | % Enterobacteriaceae: ↓ | Estimated mean of total Bifidobacterium gene count: ↑ % Bifidobacteria: ↑ Bifidobacteria count: ↑ |
B. breve M-16V [intervention] detected in infant: ↑ | Acetate: ↑ pH: ↓ |
||||
| Lay [23] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 | 44 | 83 | Shotgun 16S rRNA sequencing of the V3-V6 region, shotgun metagenomics, metatranscriptomics and metabolomics |
3/5 D | n.s. | |||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 1–3 D | 16 W | 44 | 44 | 88 | 3 D | Compositional difference | Relative abundance of strict anaerobes**: ↑ Relative abundance of facultative anaerobes/aerobes***: ↓ Bifidobacteriaceae: ↑ |
Bifidobacterium: ↑ |
Abundance of B. breve [intervention]: ↑ |
||||
| 5 D | Compositional difference | Relative abundance of strict anaerobes**: ↑ Relative abundance of facultative anaerobes/aerobes***: ↓ Bifidobacteriaceae: ↑ |
Bifidobacterium: ↑ Haemophilus: ↓ |
Abundance of B. breve [intervention]: ↑ |
||||||||||
| Yang [28] | High dose of synbiotic: B. lactis Bi-07 and L. rhamnosus HN001 + GOS | Birth | 28 D | 7 | 9 | 16 | 16S rRNA gene sequencing of the V3-V4 region + PCR | 3 D | Relative abundance of Bifidobacterium: ↑ Relative abundance of Lactobacillus: ↑ |
|||||
| 6 | 8 | 14 | 7 D | Relative abundance of Lactobacillus: ↑ | ||||||||||
| Low dose of synbiotic: B. lactis Bi-07 and L. rhamnosus HN001 + GOS | 7 | 9 | 16 | 3 D | Relative abundance of Bifidobacterium: ↑ Relative abundance of Lactobacillus: ↑ |
|||||||||
| 5 | 8 | 13 | 7 D | Relative abundance of Lactobacillus: ↑ | ||||||||||
| Hurkala [33] | Probiotic: B. breve PB04 and L. rhamnosus KL53A | <1 H | 5 or 6 D | 71 | 77 | 148 | PCR | 5/6 D | Abundance of Lactobacilli: ↑ Abundance of Bifidobacterium: ↑ |
L. rhamnosus [intervention]: ↑ B. breve [intervention]: ↑ |
||||
| Zhong [35] | Probiotic: B. longum, L. acidophilus and E. faecalis during AB treatment | <3 D | 42 D | 25 | 17 | 42 | 16S rRNA gene sequencing of the V3-V4 region + PCR | 1 W | Relative abundance Actinobacteria: ↑ Relative Abundance Proteobacteria: ↑ |
Relative abundance of Bifidobacterium: ↑ | ||||
| Probiotic: B. longum, L. acidophilus and E. faecalis after AB treatment | 7 D | 42 D | 13 | 17 | 30 | 1 W |
n.s. |
|||||||
| 1–4 Weeks | ||||||||||||||
| Cooper [27] | Synbiotic: BMOs (containing GOS and MOS such as 3’- and 6’ sialyllactose) + B. lactis CNCM-I-3446 | Birth (≤3 D) | 6 M | 92 | 101 | 193 | PCR, FISH | 10 D | Faecal Bifidobacterium counts: ↑ Faecal detection rate of Bifidobacteria: ↑ Faecal detection rate of Bacteroides: ↑ |
Faecal detection of B. lactis CNCM I-3446 [intervention]: ↑ | Mean faecal pH: ↓ | |||
| 1 M | Faecal Bifidobacterium counts: ↑ Faecal detection rate of Bifidobacteria: ↑ Faecal detection rate of Lactobacillus: ↑ |
Faecal detection of B. lactis CNCM I-3446 [intervention]: ↑ | Mean faecal pH: ↓ | |||||||||||
| Chua [24] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 | 45 | 84 | 16S rRNA sequencing + FISH + qPCR | 2 W | % Enterobacteriaceae: ↑ | |||||
| 4 W | pH: ↓ | |||||||||||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 45 | 45 | 90 | 2 W | % Enterobacteriaceae: ↓ | Estimated mean of total Bifidobacterium gene count: ↑ % Bifidobacteria: ↑ Bifidobacteria count: ↑ |
B. breve M-16V [intervention] detected in infant: ↑ | pH: ↓ | ||||||
| 4 W | % Enterobacteriaceae: ↓ | Estimated mean of total Bifidobacterium gene count: ↑ % Bifidobacteria: ↑ Bifidobacteria count: ↑ |
B. breve M-16V [intervention] detected in infant: ↑ | pH: ↓ | ||||||||||
| Lay [23] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 | 44 | 83 | Shotgun 16S rRNA sequencing of the V3-V6 region, shotgun metagenomics, metatranscriptomics and metabolomics |
2 + 4 W | n.s. | |||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 44 | 44 | 88 | 2 W | Compositional difference | Relative abundance of strict anaerobes**: ↑ Relative abundance of facultative anaerobes/aerobes***: ↓ Bifidobacteriaceae: ↑ |
Bifidobacterium: ↑ | Abundance of B. breve [intervention]: ↑ | ||||||
| 4 W | Clostridiaceae: ↓ | Abundance of B. breve [intervention]: ↑ | ||||||||||||
| Garcia Rodenas [32] | Probiotic: L. reuteri DSM 17938 | <72 H | 6 M | 11 | 10 | 21 | 16S rRNA gene sequencing of the V1- V3 regions + PCR | 2 W | Compositional difference | Relative abundance of Actinobacteria: ↑ Relative abundance of Firmicutes: ↑ |
Relative abundance of Enterobacteriaceae: ↓ Relative abundance of Bifidobacteriaceae: ↑ Relative abundance of Lactobacillaceae: ↑ |
Detectable Bifidobacterium: ↑ Relative abundance of Lactobacillus: ↑ |
Abundance of L. reuteri [intervention]: ↑ | |
|
Zhong [35] |
Probiotic: B. longum, L. acidophilus and E. faecalis during AB treatment | <3 D | 42 D | 25 | 17 | 42 | 16S rRNA gene sequencing of the V3-V4 region + PCR | 2 W | n.s. | |||||
| Probiotic: B. longum, L. acidophilus and E. faecalis after AB treatment | 7 D | 42 D | 13 | 17 | 30 | 2 W | Relative abundance of Actinobacteria: ↑ | |||||||
| Hurkala [33] | Probiotic: B. breve PB04 and L. rhamnosus KL53A | <1 H | 5 or 6 D | 58 | 48 | 106 | PCR | 1 M | Abundance of Lactobacilli: ↑ | |||||
| Yang [28] | High dose of synbiotic: B. lactis Bi-07 and L. rhamnosus HN001 + GOS | Birth | 28 D | 6 | 5 | 11 | 16S rRNA gene sequencing of the V3-V4 region + PCR | 1 M | n.s. | |||||
| Low dose of synbiotic: B. lactis Bi-07 and L. rhamnosus HN001 + GOS | 7 | 5 | 12 | n.s. | ||||||||||
| >4 weeks | ||||||||||||||
| Zhong [35] | Probiotic: B. longum, L. acidophilus and E. faecalis during AB treatment | <3 D | 42 D | 25 | 17 | 42 | 16S rRNA gene sequencing of the V3-V4 region + PCR | 42 D | Relative abundance of Actinobacteria: ↑ Relative abundance of Proteobacteria: ↑ |
Relative abundance of Bifidobacterium: ↑ | ||||
| Probiotic: B. longum, L. acidophilus and E. faecalis after AB treatment | 7 D | 42 D | 13 | 17 | 30 | 42 D | n.s. | |||||||
| Chua [24] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 | 45 | 84 | 16S rRNA sequencing + FISH + qPCR | 12 W | n.s. | |||||
| 16 W | % Enterobacteriaceae: ↑ | |||||||||||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 45 | 45 | 90 | 12 W | % Enterobacteriaceae: ↓ | Bifidobacteria count: ↑ | B. breve M-16V detected in infant: ↑ | |||||||
| 16 W | B. breve M-16V detected in infant: ↑ | |||||||||||||
| Lay [23] | Prebiotic (scGOS/lcFOS) | 1–3 D | 16 W | 39 44 |
44 44 |
83 88 |
Shotgun 16S rRNA sequencing of the V3-V6 region, shotgun metagenomics, metatranscriptomics and metabolomics |
8 W | Staphylococcaceae: ↓ | |||||
| 12, 16, 22 W | n.s. | |||||||||||||
| Synbiotic (scGOS/ICFOS and B. breve M-16V) | 10 | 10 | 20 | 8 W | Shannon diversity: ↑ | B. longum: ↓ | ||||||||
| 12, 16 W | n.s. | |||||||||||||
| 22 W | V. dispar: ↑ | |||||||||||||
| Estorninos [26] | Prebiotic: bovine MOS (GOS and sialylated-oligosaccharides) | 3 W | 6 M | 75 | 75 | 150 | 16S rRNA gene sequencing of the V3-V4 region | 2.5 M | Compositional difference | |||||
| 114 | 112 | 226 | 4 M | Compositional difference | Abundance of Bifidobacterium: ↑ | |||||||||
| Berger [25] | Prebiotics: 2 HMOs (2’-fucosyllactose and lacto-N-neotetraose) | 0–14 D | 6 M | 19 | 24 | 43 | 16S rRNA gene sequencing of the V3-V4 region | 3 M | n.s. | |||||
| Korpela [29] | Probiotic: L. rhamnosus LC705, B. breve Bb99, P. freudenreichii spp., shermanii JS and GOS | 36 W gest. + from birth | 6 M | 35 | 44 | 79 | 16S rRNA gene amplicon sequencing of the V3-V4 region | 3 M |
Bifidobacteriaceae: ↑ Coriobacteriaceae: ↑ Porphyromonadaceae: ↑ Bacteroidaceae: ↑ |
|||||
| Cooper [27] | Synbiotic: BMOs (containing GOS and MOS such as 3’- and 6’ sialyllactose) + B. lactis CNCM-I-3446 | Birth (≤3 D) | 6 M | 92 | 101 | 193 | PCR, FISH | 3 M | Faecal Bifidobacteria counts: ↑ Faecal detection rate of Clostridium/Eubacterieum: ↓ |
Faecal detection of B. lactis CNCM I-3446 [intervention]: ↑ |
Mean faecal pH: ↓ | |||
| Roggero [34] | Probiotic: L. paracasei CBA L74 | <7 D | 3 M | 16 | 16 | 32 | 16s RNA gene sequencing of the V3 region | 3 M | sIgA production: ↑ | |||||
| Baglatzi [30] | Probiotic: regular dose of B. lactis | Birth | 6 M | 84 | 80 | 164 | PCR | 4 M | Positive detection of B. lactis [intervention]: ↑ | |||||
| Garcia Rodenas [32] | Probiotic: L. reuteri DSM 17938 | <72 H | 6 M | 11 | 10 | 21 | 16S rRNA gene sequencing of the V1- V3 regions + PCR | 4 M | Abundance of L. reuteri [intervention]: ↑ | |||||
*: g. bifidobacterium, o. pseudomonadales, f. actinomycetaceae, k. bacteria, g. staphylococcus, g. streptococcus, f. streptococcaceae, f. bifidobacteriaceae, o. enterobacteriales, f. Enterobacteriaceae.
**: f. Prevotellaceae, f. peptostreptococcaceae, f. ruminococcaceae, o. clostridiales, f. porphyromonadaceae, f. clostridiaceae, f. lachnospiraceae, f. veillonellaceae, f. bacteroidaceae, f. bifidobacteriaceae.
***: o. lactobacillales, o. bacillales, f. pasteurellaceae, f. staphylococcaceae, f. lactobacillaceae, f. enterococcaceae, o. enterobacteriales, f. streptococcaceae, f. Enterobacteriaceae.
1 # participants in a subgroup, if applicable.
I Intervention.
C Control.
T Total.
CS Caesarean section.
SG subgroup.
HMOs human milk oligosaccharides.
(sc) GOS: (short chain) galactooligosaccharides.
(lc) FOS: (long chain) fructooligosaccharides.
BMOs bovine milk oligosaccharides.
MOS: Milk oligosaccharides.
D days.
M months.
W weeks.
H hours.
Y year.
AB antibiotics.
The effect of pre-, pro- and synbiotics after Caesarean section
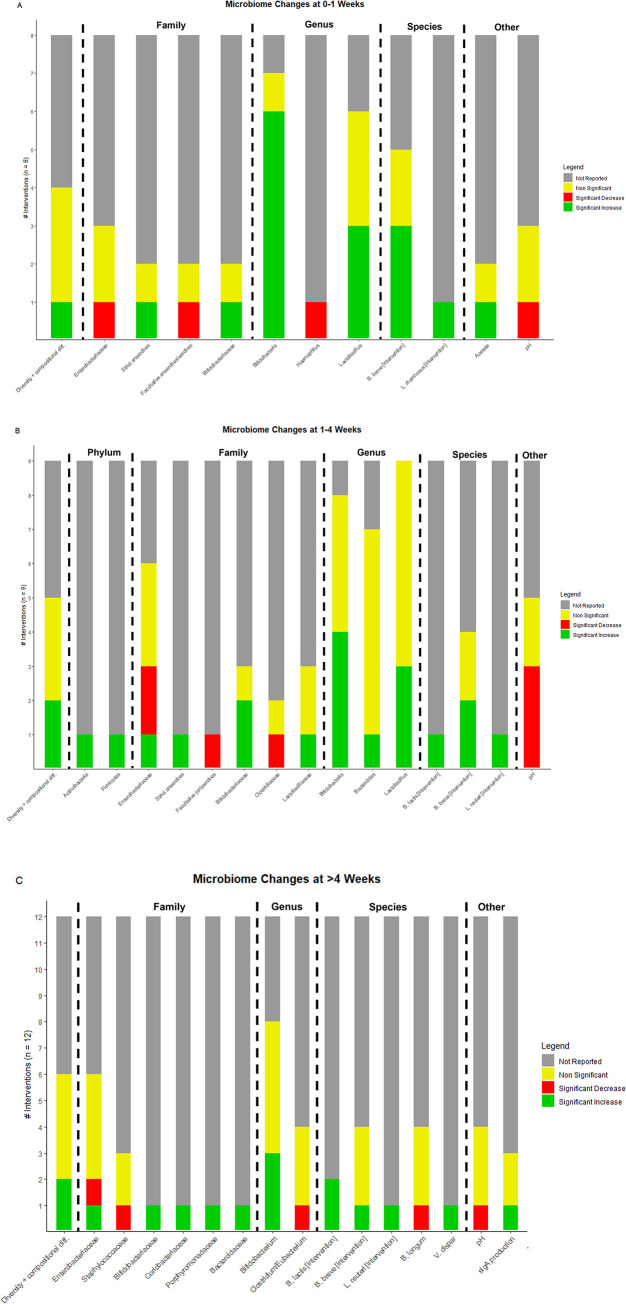
Fig 2 summarises the number of statistically significant and non-significant differences that were found in the three time clusters. The significant microbiota changes in the experimental groups compared to the control groups (described in Table 1) are discussed in further detail below.
Fig 2.
Bar charts showing the number of interventions (several studies used more than one intervention) with a significant effect on the microbiota composition at at least one time point in the clusters of 0–1 weeks (a), 1–4 weeks (b) or >4 weeks (c).
Diversity and compositional differences
One study [23] found compositional differences (using a distance-based redundancy analysis) at 0–1 weeks, 1–4 weeks and >4 weeks in the infants who received a synbiotic compared to those who received a prebiotic or placebo, and an increased diversity at 8 weeks using the Shannon diversity index. Researchers of two other studies [26,32] also measured compositional differences (phylogenetic distance) at 1–4 weeks [32] and >4 weeks [26] and reported a significantly different microbiota composition in infants who received a probiotic [32] or prebiotic [26] compared to a placebo.
Phylum level
Only one study [32] investigated the effect of a probiotic after Caesarean delivery on the phylum level. At 1–4 weeks, they found an increase in both Actinobacteria and Firmicutes in the probiotic group.
Family level
At 0–1 weeks, Chua et al. [24] found a significant decrease in the percentage of Enterobacteriaceae present in the stool of infants who received the synbiotic, but not those who received the prebiotic. Lay et al. [23], who analysed a subgroup of infants from the same study, reported an increase in relative abundance of strict anaerobes, a decrease in relative abundance of facultative anaerobes/aerobes and an increase in Bifidobacteriaceae in the synbiotic group.
At 1–4 weeks, Chua et al. again report a significant decrease in the percentage of Enterobacteriaceae in the synbiotic group, but a significant increase in the prebiotic group [24]. In a subgroup analysis by Lay et al., no significant differences were found in the prebiotic group, but an increase in strict anaerobes, decrease in facultative anaerobes/aerobes and Clostridiaceae and an increase in Bifidobacteriaceae was observed after a synbiotic supplement [23]. In line with their findings, another study[32] also found a significant increase in Bifidobacteriaceae. Additionally, they found a significant increase in Lactobacillaceae. Furthermore, they reported a significant decrease in the percentage of Enterobacteriaceae in their probiotic group, which is similar to Chua et al.’s findings in their synbiotic intervention group.
At >4 weeks, Chua et al. found the same results as at 1–4 weeks: a significant decrease in the percentage of Enterobacteriaceae in the synbiotic group, and a significant increase in the prebiotic group [24]. Moreover, in a subgroup a decrease in Staphylococcaceae was reported [23]. Another study found an increase in Bifidobacteriaceae, Coriobacteriaceae, Porphyromonadaceae and Bacteroidaceae [29].
Genus level
At 0–1 weeks, four articles [23,24,28,33] based on three RCTs reported a significant increase in abundance of the Bifidobacterium genus after administration of a probiotic[33], prebiotic [24], and synbiotic [23,24,28]. Two studies [28,33] also found an increase abundance of the Lactobacillus genus [28,33], and one study [23] reported a decrease of the Haemophilus genus.
At 1–4 weeks, four articles [23,24,27,32] from three RCTs found an increase in the Bifidobacterium genus after administration of a probiotic [27,32] or synbiotic [23,24]. Three [27,32,33] reported an increased (relative) abundance of Lactobacillus and one of these [27] also observed an increased abundance of Bacteroides.
At >4 weeks, three studies [23,26,27] reported an increased Bifidobacterium genus abundance, and one of the two [27] also found a decreased faecal detection rate of Clostridium/Eubacterium.
Species level
At 0–1 weeks, Chua et al. [24] and Lay et al. [23] (based on the same RCT) reported a significant increase of Bifidobacterium breve M-16V detected in the infants who received a synbiotic, which included this Bifidobacterium species.
At 1–4 weeks, three studies [24,27,32] found an increase in the faecal detection of the bacterial species they included in their intervention: Bifidobacterium lactis CNCM I-3446 [27], Bifidobacterium breve [23] and Lactobacillus reuteri [32].
At >4 weeks, three studies again reported an increase in faecal detection of their intervention: Bifidobacterium lactis [27,30] and Lactobacillus reuteri [32]. Another study also found a decreased abundance of Bifidobacterium longum and an increase in Veillonella dispar [23].
Intestinal microenvironment
At 0–1 weeks, one article [24] found a decreased faecal pH and increased acetate after administration of a synbiotic. At 1–4 weeks, the same study [24] and another [27] both reported a decreased faecal pH after administration of a synbiotic [24,27] or prebiotic [24]. At >4 weeks, one of the studies [27] still found a decreased faecal pH in the synbiotic-group [27], and another article [34] observed an increased secretory IgA (sIgA) production in infants who received a probiotic.
Discussion
The aim of this systematic review was to describe the effects of a pre-, pro- or synbiotic supplement on the gut microbiota following Caesarean section or exposure to antibiotics in the first week of life. Only one article investigated the effect of a probiotic on antibiotic-exposed infants; a mixture of three probiotics resulted in an increase in Actinobacteria, Proteobacteria and Bifidobacterium. For the Caesarean-born infants, the key finding was an increase in the supplemented bacterial species (of the Bifidobacterium and Lactobacillus genus) after probiotic or synbiotic supplements, and a decrease in Enterobacteriaceae after synbiotic but an increase after prebiotic supplementation. Furthermore, there were significant increases in Actinobacteria, Proteobacteria and Firmicutes in the probiotic groups compared to the control groups. Moreover, the microbiota composition of the probiotic or synbiotic group was significantly different from the control group in two studies, and bacterial species diversity was increased in one study after administration of a synbiotic.
Prebiotics are less extensively studied, and only few outcome parameters reached statistical significance. However, according to one included study, prebiotics increased the abundance of Enterobacteriaceae, which has been associated with potentially negative health effects such as an increased risk of atopic eczema [36], food allergy [37] and delayed colonisation of beneficial bacterial species [36]. Three articles based on two prebiotic studies reported an increase in Bifidobacteria [23,24,26] and two of the three also found a significantly different microbiota composition [23,26].
Because of the heterogeneity in the interventions in terms of study design and composition of the supplement, it is difficult to compare their efficacy. Generally, probiotics and synbiotics seem more effective in increasing the abundance of beneficial bacteria. Bifidobacteria and Lactobacilli, which were increased in the intervention groups of eight and five studies respectively, are associated with various health effects: both Bifidobacteria and Lactobacilli seem to protect from allergies [38,39] and infantile colic [38] and they are associated with healthy microbiota development [38]. Several species of the Bifidobacterium genus are commonly present in the infant gut, and their function is to digest sugars in human milk, reduce intestinal pH and improve the integrity of the intestinal wall [40]. Delivery via Caesarean section, which was the case in five [23,24,26,28,33] of the six studies investigating the microbiota at the genus level, results in a disrupted vertical transmission of Bifidobacterium [40]. The results in Table 3 indicate that a pro- or synbiotic intervention can alleviate this disruption and shift the neonatal microbiota composition towards that of vaginally born infants. Human milk oligosaccharides present in breast milk can also stimulate colonisation by Bifidobacteria [40]. Interestingly, all five articles that reported a significant increase of Bifidobacterium levels at 0–1 weeks included infants that were (also) breastfed. However, at the time points after this first week, other studies that included infants who were exclusively formula fed also show significant increases in Bifidobacterium levels. Moreover, three studies on pro- and synbiotics also found a significantly different microbiota composition or a more diverse microbiota in the intervention groups. Birth following Caesarean section or exposure to antibiotics in the first week of life reduces bacterial diversity, which makes these infants susceptible to colonisation by bacteria usually found on the mother’s skin such as Staphylococcus, Corynebacterium and Propionibacterium spp., which is associated with an increased risk of gastrointestinal and systemic disorders, including eczema allergies, later in life [41]. The results in Table 3 show that this diversity may (partially) be restored by supplementation with pro- or synbiotics and possibly prebiotics.
To our knowledge, this is the first systematic review evaluating the effects of pre-, pro- and synbiotics specifically in both Caesarean born and antibiotic-exposed infants. While another systematic review about the effects of pre-, pro- and synbiotics on the microbiota of children born via Caesarean section was published recently [42], we identified four additional relevant articles that were not included by Martin-Pelaez et al. Furthermore, some of their included articles did not perform a separate subgroup-analysis for children born via Caesarean section.
It is crucial to analyse these Caesarean born infants separately from vaginally born infants who were not exposed to antibiotics, because the effect of pre-, pro- and synbiotics may differ in infants with a disrupted microbiota from those who were born vaginally. Illustratively, in one of the trials included in our review, the effects of pre-, pro- and synbiotics on the microbiota was only significant in the Caesarean born subgroup [32]. Additionally, Frese et al. [31] did not perform a statistical subgroup analysis for the Caesarean born infants but the differences in the microbiota composition of their cohort seems to be largely driven by Caesarean born infants. Specifically, in their intervention group, they found a significant increase in faecal Bifidobacteriaceae and Bifidobacterium infantis, and a clear decrease in the relative abundances of Enterobacteriaceae, Clostridiaceae, Erysipelotrichaceae, Pasteurellaceae, Micrococcaceae and Lachnospiraceae. These findings suggest that especially infants with a disrupted microbiota might benefit most from an intervention with pre-, pro or synbiotics.
Important strengths of this review are the elaborate search strategy developed in collaboration with a medical librarian to include all relevant articles. We also looked for any subgroup analyses of Caesarean-born infants in the full texts, even when the title or abstract did not explicitly state that these were performed. We only included articles that performed analyses on Caesarean-born infants, and not articles that only analysed the total group of participants with vaginally born infants included.
Limitations of this study are that many articles that included a subgroup analysis of Caesarean-born infants reported only a selection of the outcomes for this subgroup. It is unclear whether more analyses were performed and only the significant results were published, which would result in publication bias. Similarly, many articles did not adjust for multiple testing. This is also reflected in the critical appraisal of the articles, which showed that 11 of the 13 included studies had a high risk of bias. Lastly, while some trials mentioned in this review included a reference group with vaginally born and/or exclusively breastfed infants, we chose to focus on analyses between intervention and placebo-controlled groups instead of also comparing the intervention groups to the reference group. For further research, it would be interesting to evaluate whether pre-, pro- and synbiotic interventions could restore the microbiota of infants born through Caesarean section or infants exposed to antibiotics to that of a healthy reference group.
Other recommendations for future research are firstly that, in order to be able to compare the results of different studies, studies should standardise their methods of faecal sample collection, storage, isolation and analyses. Furthermore, microbiota studies should increase their follow-up time to see whether any differences that were found between intervention and control groups persist beyond the duration of the intervention and to search for associations with long-term health outcomes. In addition, since the effect of a pre-, pro- or synbiotic on infants after antibiotic treatment in the first week of life was only investigated by one study [35], more RCTs are necessary in this group of infants. Moreover, to assess the clinical potential of pre-, pro- or synbiotic supplementation, it is crucial that high quality RCTs with predetermined clinical outcomes are conducted. Lastly, only one study [34] explored the effects of their intervention on the metabolome. The metabolome gives an indication of the function of the microbiota, and how the microbiota affects metabolites in urine, faeces and blood serum [43]. While most included studies focused their microbiota analysis on the microbiota composition, the metabolome might reveal important information on the mechanics by which the microbiota influences its host.
Conclusion
Supplementation of pre-, pro- or synbiotics in Caesarean-born infants and infants who received antibiotics early in life mostly increased the phyla, families, genera and species that corresponded to the pro- or synbiotic intervention that was administered, while the effects of a prebiotic generally did not reach statistical significance. Supplementation of these at-risk children to restore the microbiota to a composition more similar to vaginally born infants (i.e. predominant colonisation by Bifidobacteria and Lactobacilli) may alleviate some of the negative consequences of a disrupted microbiota. However, more high-quality research is needed to explicate the clinical effects of such microbiota changes and to determine which pre-, pro- or synbiotic products are most effective.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Korpela K, de Vos WM. Early life colonization of the human gut: microbes matter everywhere. Curr Opin Microbiol. 2018;44:70–8. Epub 2018/08/08. doi: 10.1016/j.mib.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–803. Epub 2015/08/14. doi: 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butel MJ, Waligora-Dupriet AJ, Wydau-Dematteis S. The developing gut microbiota and its consequences for health. J Dev Orig Health Dis. 2018;9(6):590–7. Epub 2018/03/23. doi: 10.1017/S2040174418000119 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy KM, Gerlach MJ, Adam T, Heimesaat MM, Rossi L, Surette MG, et al. Fetal meconium does not have a detectable microbiota before birth. Nat Microbiol. 2021;6(7):865–73. Epub 2021/05/12. doi: 10.1038/s41564-021-00904-0 [DOI] [PubMed] [Google Scholar]
- 5.Dierikx T, Berkhout D, Eck A, Tims S, van Limbergen J, Visser D, et al. Influence of timing of maternal antibiotic administration during caesarean section on infant microbial colonisation: a randomised controlled trial. Gut. 2022;71(9):1803–11. Epub 2021/11/23. doi: 10.1136/gutjnl-2021-324767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagodzinski A, Zielinska E, Laczmanski L, Hirnle L. The early years of life. Are they influenced by our microbiome? Ginekol Pol. 2019;90(4):228–32. Epub 2019/05/07. doi: 10.5603/GP.2019.0041 [DOI] [PubMed] [Google Scholar]
- 7.Munyaka PM, Khafipour E, Ghia JE. External influence of early childhood establishment of gut microbiota and subsequent health implications. Front Pediatr. 2014;2:109. Epub 2014/10/28. doi: 10.3389/fped.2014.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ainonen S, Tejesvi MV, Mahmud MR, Paalanne N, Pokka T, Li W, et al. Antibiotics at birth and later antibiotic courses: effects on gut microbiota. Pediatr Res. 2021. Epub 2021/04/08. doi: 10.1038/s41390-021-01494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Daele E, Kamphorst K, Vlieger AM, Hermes G, Milani C, Ventura M, et al. Effect of antibiotics in the first week of life on faecal microbiota development. Arch Dis Child Fetal Neonatal Ed. 2022. Epub 2022/05/10. doi: 10.1136/archdischild-2021-322861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eck A, Rutten N, Singendonk MMJ, Rijkers GT, Savelkoul PHM, Meijssen CB, et al. Neonatal microbiota development and the effect of early life antibiotics are determined by two distinct settler types. PLoS One. 2020;15(2):e0228133. Epub 2020/02/06. doi: 10.1371/journal.pone.0228133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Herk W, Stocker M, van Rossum AM. Recognising early onset neonatal sepsis: an essential step in appropriate antimicrobial use. J Infect. 2016;72 Suppl:S77–82. Epub 2016/05/26. doi: 10.1016/j.jinf.2016.04.026 [DOI] [PubMed] [Google Scholar]
- 12.Uzan-Yulzari A, Turta O, Belogolovski A, Ziv O, Kunz C, Perschbacher S, et al. Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization. Nat Commun. 2021;12(1):443. Epub 2021/01/28. doi: 10.1038/s41467-020-20495-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oosterloo BC, van Elburg RM, Rutten NB, Bunkers CM, Crijns CE, Meijssen CB, et al. Wheezing and infantile colic are associated with neonatal antibiotic treatment. Pediatr Allergy Immunol. 2018;29(2):151–8. Epub 2018/01/10. doi: 10.1111/pai.12857 [DOI] [PubMed] [Google Scholar]
- 14.Salvatore S, Baldassarre ME, Di Mauro A, Laforgia N, Tafuri S, Bianchi FP, et al. Neonatal Antibiotics and Prematurity Are Associated with an Increased Risk of Functional Gastrointestinal Disorders in the First Year of Life. J Pediatr. 2019;212:44–51. Epub 2019/06/16. doi: 10.1016/j.jpeds.2019.04.061 [DOI] [PubMed] [Google Scholar]
- 15.Kamphorst K, Oosterloo BC, Vlieger AM, Rutten NB, Bunkers CM, Wit EC, et al. Antibiotic Treatment in the First Week of Life Impacts the Growth Trajectory in the First Year of Life in Term Infants. J Pediatr Gastroenterol Nutr. 2019;69(1):131–6. Epub 2019/05/07. doi: 10.1097/MPG.0000000000002360 [DOI] [PubMed] [Google Scholar]
- 16.Kamphorst K, Vlieger AM, Oosterloo BC, Waarlo S, van Elburg RM. Higher risk of allergies at 4–6 years of age after systemic antibiotics in the first week of life. Allergy. 2021. Epub 2021/03/28. doi: 10.1111/all.14829 [DOI] [PubMed] [Google Scholar]
- 17.Stromberg Celind F, Wennergren G, Vasileiadou S, Alm B, Goksor E. Antibiotics in the first week of life were associated with atopic asthma at 12 years of age. Acta Paediatr. 2018;107(10):1798–804. Epub 2018/03/27. doi: 10.1111/apa.14332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moya-Perez A, Luczynski P, Renes IB, Wang S, Borre Y, Anthony Ryan C, et al. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutr Rev. 2017;75(4):225–40. Epub 2017/04/06. doi: 10.1093/nutrit/nuw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. Epub 2010/06/30. doi: 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. Epub 2016/12/07. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. Epub 2019/08/30. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Epub 2021/03/31. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lay C, Chu CW, Purbojati RW, Acerbi E, Drautz-Moses DI, de Sessions PF, et al. A synbiotic intervention modulates meta-omics signatures of gut redox potential and acidity in elective caesarean born infants. BMC Microbiol. 2021;21(1):191. Epub 2021/06/27. doi: 10.1186/s12866-021-02230-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua MC, Ben-Amor K, Lay C, Neo AGE, Chiang WC, Rao R, et al. Effect of Synbiotic on the Gut Microbiota of Cesarean Delivered Infants: A Randomized, Double-blind, Multicenter Study. J Pediatr Gastroenterol Nutr. 2017;65(1):102–6. Epub 2017/06/24. doi: 10.1097/MPG.0000000000001623 [DOI] [PubMed] [Google Scholar]
- 25.Berger B, Porta N, Foata F, Grathwohl D, Delley M, Moine D, et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio. 2020;11(2). Epub 2020/03/19. doi: 10.1128/mBio.03196-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Estorninos E, Lawenko RB, Palestroque E, Sprenger N, Benyacoub J, Kortman GAM, et al. Term infant formula supplemented with milk-derived oligosaccharides shifts the gut microbiota closer to that of human milk-fed infants and improves intestinal immune defense: a randomized controlled trial. Am J Clin Nutr. 2022;115(1):142–53. Epub 2021/10/08. doi: 10.1093/ajcn/nqab336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper P, Bolton KD, Velaphi S, de Groot N, Emady-Azar S, Pecquet S, et al. Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial. Clin Med Insights Pediatr. 2016;10:119–30. Epub 2017/01/18. doi: 10.4137/CMPed.S40134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W, Tian L, Luo J, Yu J. Ongoing Supplementation of Probiotics to Cesarean-Born Neonates during the First Month of Life may Impact the Gut Microbial. American Journal of Perinatology. 2021;38(11):1181–91. Epub 2020/05/24. doi: 10.1055/s-0040-1710559 [DOI] [PubMed] [Google Scholar]
- 29.Korpela K, Salonen A, Vepsalainen O, Suomalainen M, Kolmeder C, Varjosalo M, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6(1):182. Epub 2018/10/18. doi: 10.1186/s40168-018-0567-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baglatzi L, Gavrili S, Stamouli K, Zachaki S, Favre L, Pecquet S, et al. Effect of Infant Formula Containing a Low Dose of the Probiotic Bifidobacterium lactis CNCM I-3446 on Immune and Gut Functions in C-Section Delivered Babies: A Pilot Study. Clin Med Insights Pediatr. 2016;10:11–9. Epub 2016/03/22. doi: 10.4137/CMPed.S33096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, et al. Persistence of Supplemented Bifidobacterium longum subsp. infantis EVC001 in Breastfed Infants. mSphere. 2017;2(6). Epub 2017/12/16. doi: 10.1128/mSphere.00501-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia Rodenas CL, Lepage M, Ngom-Bru C, Fotiou A, Papagaroufalis K, Berger B. Effect of Formula Containing Lactobacillus reuteri DSM 17938 on Fecal Microbiota of Infants Born by Cesarean-Section. J Pediatr Gastroenterol Nutr. 2016;63(6):681–7. Epub 2016/04/02. doi: 10.1097/MPG.0000000000001198 [DOI] [PubMed] [Google Scholar]
- 33.Hurkala J, Lauterbach R, Radziszewska R, Strus M, Heczko P. Effect of a Short-Time Probiotic Supplementation on the Abundance of the Main Constituents of the Gut Microbiota of Term Newborns Delivered by Cesarean Section-A Randomized, Prospective, Controlled Clinical Trial. Nutrients. 2020;12(10). Epub 2020/10/18. doi: 10.3390/nu12103128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roggero P, Liotto N, Pozzi C, Braga D, Troisi J, Menis C, et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat Commun. 2020;11(1):2703. Epub 2020/06/03. doi: 10.1038/s41467-020-16582-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong H, Wang XG, Wang J, Chen YJ, Qin HL, Yang R. Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure: an open-label single-center randomized parallel controlled study. World J Pediatr. 2021;17(4):385–93. Epub 2021/08/01. doi: 10.1007/s12519-021-00443-y [DOI] [PubMed] [Google Scholar]
- 36.Ta LDH, Chan JCY, Yap GC, Purbojati RW, Drautz-Moses DI, Koh YM, et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes. 2020;12(1):1–22. Epub 2020/10/08. doi: 10.1080/19490976.2020.1801964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka M, Korenori Y, Washio M, Kobayashi T, Momoda R, Kiyohara C, et al. Signatures in the gut microbiota of Japanese infants who developed food allergies in early childhood. FEMS Microbiol Ecol. 2017;93(8). Epub 2017/09/15. doi: 10.1093/femsec/fix099 [DOI] [PubMed] [Google Scholar]
- 38.Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015;53:233–45. Epub 2015/02/02. doi: 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J Allergy Clin Immunol. 2019;143(2):467–85. Epub 2019/01/03. doi: 10.1016/j.jaci.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 40.Dalby MJ, Hall LJ. Recent advances in understanding the neonatal microbiome. F1000Res. 2020;9. Epub 2020/06/11. doi: 10.12688/f1000research.22355.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Monaco MH, Donovan SM. Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonatal Med. 2016;21(6):380–7. Epub 2016/05/02. doi: 10.1016/j.siny.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 42.Martin-Pelaez S, Cano-Ibanez N, Pinto-Gallardo M, Amezcua-Prieto C. The Impact of Probiotics, Prebiotics, and Synbiotics during Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients. 2022;14(2). Epub 2022/01/22. doi: 10.3390/nu14020341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brink LR, Mercer KE, Piccolo BD, Chintapalli SV, Elolimy A, Bowlin AK, et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am J Clin Nutr. 2020;111(6):1190–202. Epub 2020/04/25. doi: 10.1093/ajcn/nqaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]