Abstract
Timely receipt of colorectal cancer screening can reduce morbidity and mortality. This is the first known study to adopt Andersen's model of health services use to identify factors associated with colorectal cancer screening among U.S. adults. The data from National Health Interview Survey from 2019 was utilized to conduct the analyses. Multivariable logistic regression was used to separately analyze data from 7,503 age-eligible women and 6,486 age-eligible men. We found similar colorectal cancer screening levels among men (57.7%) and women (57.6%). Factors associated with higher screening odds in women were older age, married/cohabitating with a partner, Black race, >bachelor's degree, having a usual source of care, and personal cancer history. Factors associated with lower odds for women were American Indian/Alaska Native race, living in the United States for ≤10 years, ≤138% federal poverty level (FPL), uninsured or having Medicare, and in fair/poor health. For men, factors associated with higher screening odds were older age, homosexuality, married/cohabitating with a partner, Black race, >high school/general educational development education, having military insurance, having a usual source of care, and personal cancer history. Factors associated with lower odds for men were being a foreign-born U.S. resident, living in the South or Midwest, ≤138% FPL, and being uninsured or having other insurance. Despite lower screening rates in the past, Black adults show a significantly higher likelihood of colorectal cancer screening than White adults; yet, screening disparities remain in certain other groups. Colorectal cancer screening efforts should continue to target groups with lower screening rates to eliminate screening disparities.
Significance:
Timely receipt of colorectal cancer screening can reduce morbidity and mortality. Identification of populations and domains of factors associated with colorectal cancer screening receipt among men and women can help future interventions to alleviate impeding factors and target screening promotion efforts in populations not adherent with screening guidelines.
Introduction
Cancer screenings help identify cancers early and mitigate cancer-associated morbidity and mortality and the growing financial burden of cancer treatment (1). Colorectal cancer is the second most common cancer in men and women in the United States (2). Screening for colorectal cancer can detect precancerous polyps and cancerous lesions in early stages which can be treated effectively (1). As of 2019, the U.S. Preventive Services Task Force (USPSTF) recommended adults aged 50 to 75 years should screen for colorectal cancer using one of a variety of screening options. Research has shown a 60% reduction in mortality and a 73% increase in the 5-year survival rate for adults adherent with colorectal cancer screening recommendations (3). However, more than 60% of colorectal cancer cases are diagnosed when they have either spread locally or metastasized to distant organs due to delayed detection (2).
The Healthy People 2030 goal for colorectal cancer screening among age-eligible adults is 74.4% (4). However, the 2018 National Health Interview Survey (NHIS) revealed only 66.9% of age-eligible adults were screened, with rates similar for men (67.4%) and women (66.5%; ref. 5). Research has identified factors associated with not having colorectal cancer screenings, including lack of awareness, screening test knowledge, social support, access to health care services, and insurance coverage; negative attitudes and beliefs; lower education and income; and language barriers for foreign-born residents (6, 7). However, only a few colorectal cancer screening studies have used nationally-representative data to examine colorectal cancer screening (5, 8, 9), and even fewer studies have rarely identified determinants of screening uptake for men and women separately. No studies were identified that applied the comprehensive Andersen's model of health services (Andersen's model) use using a national representative sample. This study was undertaken to fill these gaps in the literature and to contribute to scientific knowledge of colorectal cancer screening by examining variations in screening receipt using a nationally representative dataset, the NHIS (10).
The Andersen's model categorizes factors affecting service use into three domains: “predisposing,” “enabling,” and “need” factors (11). Predisposing factors are sociodemographic characteristics (e.g., age, sex, race/ethnicity, education level, country of birth) related to service use. Enabling factors are resources that facilitate service use if they are available (e.g., health insurance, transportation). Need factors are real and perceived health conditions that may warrant service use (e.g., medical history, health status; refs. 11, 12). Identification of individual and domains of factors associated with colorectal cancer screening among men and women can inform interventions to target those who are not adherent with screening guidelines.
Materials and Methods
Data Source
The study used data from the 2019 NHIS. The NHIS is conducted annually by the National Center for Health Statistics to monitor the health of the U.S. population on a broad range of health topics by surveying a representative random sample of the U.S. civilian noninstitutionalized population. Data are collected through personally interviewing one adult from each household randomly selected to answer detailed questions about their demographic information and health. More information about the NHIS can be found elsewhere (13).
Study Population
There were 15,989 respondents to the 2019 NHIS who were age-eligible (50–75 years) for USPSTF colorectal cancer screening. As the focus of the study was on routine colorectal cancer screening, respondents who reported colorectal cancer screening for reasons other than a routine examination were excluded from the study population (n = 1,876), resulting in a final analytic sample of 13,989 (women = 7,503, men = 6,486).
Measures
Outcome
Being up-to-date with colorectal cancer screening was defined as receiving a recommended screening test at specified intervals as per the USPSTF guidelines (see Table 1). The tests recommended are: High-sensitivity guaiac fecal occult blood test (gFOBT), fecal immunochemical test (FIT), stool DNA test with FIT (sDNA-FIT), CT/virtual colonography, flexible sigmoidoscopy, flexible sigmoidoscopy with FIT, and colonoscopy. The NHIS questionnaire asked respondents to indicate whether they had received any of those tests, the time since they last received the test, and whether the screening was part of a routine examination. Respondents were coded as having had a recommended colorectal cancer screening if they had had one of those recommended screening tests within the recommended frequency for the test as part of a routine examination. Respondents were coded as not having a recommended colorectal cancer screening if they had not had one of the tests or had had one test but outside the USPSTF recommended frequency. Response options “Refused,” “Not ascertained,” and “Don't know” were coded as missing.
TABLE 1.
USPSTF Colorectal Cancer Screening Guidelines
| Age range | Screening tests | Screening frequency |
|---|---|---|
| Adults between the ages of 50–75 years | High-sensitivity guaiac fecal occult blood test (gFOBT) | Every year |
| Fecal immunochemical test (FIT) | ||
| Stool DNA test with FIT (sDNA-FIT) | Every 1–3 year(s) | |
| CT colonography | Every 5 years | |
| Flexible sigmoidoscopy | ||
| Flexible sigmoidoscopy with FIT | Flexible sigmoidoscopy every 10 years plus FIT every year | |
| Colonoscopy | Every 10 years |
NOTE: Source: U.S. Preventive Service Task Force, colorectal cancer screening recommendations (2016).
Factors representing the Andersen's model three domains were selected for the analysis based on previous research (14, 15). The operational definitions of the variables are shown in Supplementary Table S1.
Predisposing Factors
Age, sexual orientation, race/ethnicity, education, marital status, nativity, urban-rural residence classification, and region of residence.
Enabling Factors
Employment status in past 12 months, income level, health insurance coverage, problems paying medical bills in past 12 months, worry about paying medical bills if sick/in an accident, usual source of medical care, and number of children in household.
Needs Factors
Perceived health status, personal history of cancer, and body mass index (BMI) categories.
Statistical Analysis
We computed weighted percentages and weighted 95% confidence intervals (CI) for categorical variables and weighted means and SEs for continuous variables to describe age-eligible adults by sex who were up-to-date with USPSTF colorectal cancer screening.
In separate multivariable logistic regression models for men and women, we estimated the association between colorectal cancer screening and the selected predisposing, enabling, and need factors. The magnitude and direction of the associations were captured by odds ratios (ORs), and the uncertainty around the estimates were captured by 95% CIs.
Multicollinearity among independent variables was assessed by computing the variance inflation factor. Statistical significance was determined at an a priori α = 0.05. The complex design of the survey was accounted for with sampling adult weights and other design variables. All descriptive and regression analyses were conducted with STATA/SE 16 (16).
Data Availability
The data analyzed in this study were obtained from the National Center for Health Statistics NHIS at https://www.cdc.gov/nchs/nhis/2019nhis.htm.
Results
Colorectal Cancer Screening
Within the study population, 57.6% of women and 57.7% of men received routine colorectal cancer screening as recommended by USPSTF guidelines.
Characteristics of Age-Eligible Women and Men Who Received a Colorectal Cancer Screening
Results of the analyses to profile women and men up-to-date with colorectal cancer screening based on the predisposing, enabling, and need factors are described below and also in Supplementary Table S1.
Women
Predisposing Factors
The mean age of women up-to-date with colorectal cancer screening was 62.9 years (SE:0.11). More than half (58.4%) of straight women and 51.7% of homosexual women received colorectal cancer screening. Receipt of screening was lowest among American Indian/Alaska Native (AI/AN) women (36.2%), followed by Hispanic (46.5%), Asian (51.7%), other race/ethnicity (55.7%), White (59.8%), and Black (60.2%) women. Women with less than high school/general educational development education (HS/GED) had the lowest colorectal cancer screening (46.8%) versus women with more education. More than half of (59.7%) women born in the United States were up-to-date with colorectal cancer screening versus 24.3% of foreign-born women living in the United States for ≤10 years. Women living in the Midwest had the highest percentage of colorectal cancer screening (60.3%). Women living in the South had the lowest percentage of colorectal cancer screening (54.9%). The distribution of colorectal cancer screening in large central, large fringe, and medium-small metropolitan areas was 58.0%, 57.5%, and 58.7%, respectively.
Enabling Factors
Over half of employed women (54.5%) received colorectal cancer screening vs. 61.6% of unemployed women. Women who had problems paying medical bills (50.4%) had lower screening uptake than women without problems paying medical bills (59.2%). Only 47.0% of women who were very worried about medical bills received the screening versus 63.0% of women who were not at all worried about it. Less than half (44.6%) of less affluent women (incomes ≤138% FPL) received colorectal cancer screening vs. 64.5% of women with incomes >400% FPL. Uninsured women had the lowest percentage of receiving colorectal cancer screening (28.2%). For the other health insurances, the percentages were: Medicaid 46.6%; military insurance, 56.5%; Medicare, 61.2%; and private insurance, 60.2%. Twenty-seven percent of women without a usual source of medical care received colorectal cancer screening versus 60.3% with usual source of care in a doctor's office and 43.6% with usual source of care in other medical facilities. The mean number of children in the household was 0.17 (SE:0.01) for women up-to-date with colorectal cancer screening.
Need Factors
Among women in excellent health, 61.5% had received colorectal cancer screening versus 50.5% of women in fair/poor health. Colorectal cancer screening levels were nearly the same for women who were overweight or obese (57.5% and 57.9%). Among women diagnosed with cancer, 66.2% were up-to-date with colorectal cancer screening versus 56.2% without a history of cancer.
Men
Predisposing Factors
The mean age of men up-to-date with colorectal cancer screening was 62.6 (SE:0.13). More than half (58.3%) of straight men and 71.8% of homosexual men received colorectal cancer screening. Six in ten (61.7%) men who were married/cohabitating received colorectal cancer screening vs. 49.1% of unmarried men. Less than half of Hispanic men (41.8%) were up-to-date with colorectal cancer screening, followed by Asian (47.0%), AI/AN (47.3%), other race/ethnicity (51.8%), Black (58.3%), and White (61.3%) men. Men with <HS/GED were least up-to-date with colorectal cancer screening (41.1%). Six in 10 men (61.4%) who were born in the United States were up-to-date with colorectal cancer screening vs. 46.6% of foreign-born men who had resided in the United States >10 years and 19.2% of foreign-born men living in the United States for ≤10 years. About 63.6% of men living in the Northeast, 56.7% living in the Midwest, and 56.7% living in the West were up-to-date with colorectal cancer screening. Men living in the South had the lowest screening uptake (55.6%).
The distribution of colorectal cancer screening in large central, large fringe, and medium-small metropolitan areas was 54.2%, 62.4%, and 59.4%, respectively.
Enabling Factors
More than half of employed men and unemployed men (55.2% and 62.7%) received colorectal cancer screening. About 48.3% of men with problems paying medical bills received the screening versus 59.3% of those without such financial problems paying medical bills. Among men with incomes ≤138% FPL, 40.1% received colorectal cancer screening versus 66.9% of men with incomes >400% FPL. Uninsured men were least up-to-date with colorectal cancer screening (21.2%), followed by men with Medicaid (41.8%), other insurance (60.1%), private insurance (61.4%), Medicare (63.0%), and military insurance (67.7%). Only 18.3% of men without a usual source of care received colorectal cancer screening versus 62.1% with a usual source of care at a doctor's office and 54.5% who had other places as a usual source of care. The mean number of children in the household for men who received colorectal cancer screening (57.7%) was 0.19 (SE:0.01).
Need Factors
The highest proportion of colorectal cancer screening was reported among men in excellent and very good overall health (61.8% and 60.9%, respectively) versus 52.5% of men in fair/poor overall health and 55.8% of men in good health. Colorectal cancer screenings among overweight and obese men were similar (58.7% and 58.3%, respectively). Among men diagnosed with cancer, 71.4% received colorectal cancer screening versus 55.5% who were not diagnosed with cancer.
Adjusted Associations Between Predisposing, Enabling, and Need Factors and Colorectal Cancer Screening Among Age-Eligible Women and Men
Adjusted associations between predisposing, enabling, and need factors and colorectal cancer screening from sex-specific multivariable logistic regression analyses are reported below, in Table 2, and in Figures 1 and 2.
TABLE 2.
Characteristics associated with colorectal cancer screening among women and men
| Women | Men | |||
|---|---|---|---|---|
| Variables | OR (95% CI)a | OR (95% CI)a | ||
| Predisposing factors | ||||
| Age | 1.06b (1.05–1.08) | 1.07b (1.06–1.09) | ||
| Sexual orientation | ||||
| Homosexual | Ref | — | Ref | — |
| Straight/heterosexual | 1.38 | 0.89–2.14 | 0.61c | 0.38–0.98 |
| Marital status | ||||
| Unmarried | Ref | — | Ref | — |
| Married/cohabiting with a partner | 1.41b | 1.23–1.62 | 1.40b | 1.21–1.62 |
| Race/Ethnicity | ||||
| White | Ref | — | Ref | — |
| Black | 1.51b | 1.22–1.86 | 1.37c | 1.08–1.74 |
| Asian | 0.82 | 0.56–1.20 | 0.90 | 0.61–1.32 |
| AI/AN | 0.48c | 0.24–0.95 | 0.82 | 0.42–1.59 |
| Hispanic | 0.94 | 0.72–1.22 | 1.07 | 0.59–1.93 |
| Other race/ethnicity | 1.17 | 0.74–1.87 | 0.98 | 0.75–1.30 |
| Educational attainment | ||||
| <HS/GED | Ref | — | Ref | — |
| HS/GED | 1.00 | 0.77–1.29 | 1.35c | 1.02–1.78 |
| Some college/no degree | 1.12 | 0.86–1.46 | 1.50c | 1.12–2.00 |
| Associate-Bachelor's | 1.23 | 0.94–1.61 | 1.53d | 1.16–2.02 |
| >Bachelor's | 1.57d | 1.15–2.14 | 1.70d | 1.21–2.38 |
| Nativity | ||||
| U.S. citizen by birth | Ref | — | Ref | — |
| Living in the United States for ≤10 years | 0.44c | 0.21–0.93 | 0.25d | 0.10–0.64 |
| Living in the United States for >10 years | 0.99 | 0.78–1.27 | 0.79c | 0.63–1.00 |
| Region of residence | ||||
| Northeast | Ref | — | Ref | — |
| Midwest | 1.00 | 0.82–1.22 | 0.73d | 0.59–0.90 |
| South | 0.90 | 0.75–1.07 | 0.77d | 0.64–0.92 |
| West | 1.05 | 0.86–1.29 | 0.86 | 0.70–1.11 |
| Area of residence | ||||
| Large central metropolitan | Ref | — | Ref | — |
| Large fringe metropolitan | 0.89 | 0.75–1.07 | 1.17 | 0.96–1.42 |
| Medium-small metropolitan | 1.02 | 0.86–1.20 | 1.09 | 0.92–1.29 |
| Non-metropolitan | 0.84 | 0.69–1.03 | 0.88 | 0.70–1.11 |
| Enabling factors | ||||
| Employment status | ||||
| Unemployed | Ref | — | Ref | — |
| Employed | 0.90 | 0.77–1.04 | 0.96 | 0.80–1.16 |
| Problems paying medical bills | ||||
| No problems paying medical bills | Ref | — | Ref | — |
| Problems paying medical bills | 0.96 | 0.78–1.18 | 0.88 | 0.69–1.12 |
| Worry about paying medical bills | ||||
| Not at all worried | Ref | — | Ref | — |
| Somewhat worried | 0.92 | 0.79–1.07 | 0.91 | 0.78–1.06 |
| Very worried | 0.90 | 0.74–1.10 | 1.01 | 0.79–1.28 |
| Federal poverty level | ||||
| >400% FPL | Ref | — | Ref | — |
| ≤138% FPL | 0.71c | 0.55–0.91 | 0.59b | 0.45–0.77 |
| >138%–250% FPL | 0.95 | 0.78–1.15 | 0.71d | 0.56–0.89 |
| >250%–400% FPL | 0.89 | 0.75–1.05 | 0.79c | 0.66–0.95 |
| Insurance coverage | ||||
| Private insurance | Ref | — | Ref | — |
| Uninsured | 0.43b | 0.33–0.58 | 0.45b | 0.33–0.61 |
| Medicaid | 0.98 | 0.70–1.37 | 0.88 | 0.59–1.32 |
| Military insurance | 0.82 | 0.50–1.34 | 1.74c | 1.14–2.65 |
| Medicare | 0.79c | 0.64–0.96 | 0.94 | 0.74–1.19 |
| Other insurance | 0.87 | 0.72–1.07 | 0.76c | 0.60–0.96 |
| Usual source of care | ||||
| No usual source of care | Ref | — | Ref | — |
| Usual source of care–Doctor's office | 2.71b | 1.89–3.88 | 4.67b | 3.45–6.32 |
| Usual source of care–Other medical facility | 1.77d | 1.16–2.71 | 3.48b | 2.44–4.96 |
| Children in household | 0.91 | 0.80–1.03 | 0.91 | 0.81–1.03 |
| Need factors | ||||
| Health status | ||||
| Excellent health | Ref | — | Ref | — |
| Very good health | 0.98 | 0.82–1.17 | 0.88 | 0.73–1.07 |
| Good health | 0.87 | 0.72–1.06 | 0.87 | 0.71–1.06 |
| Fair/Poor health | 0.74d | 0.60–0.92 | 0.82 | 0.64–1.05 |
| BMI category | ||||
| Healthy weight | Ref | — | Ref | — |
| Underweight | 0.97 | 0.63–1.50 | 1.05 | 0.38–2.95 |
| Overweight | 0.99 | 0.84–1.15 | 1.05 | 0.89–1.24 |
| Obese | 1.10 | 0.94–1.29 | 1.08 | 0.89–1.30 |
| History of any cancer | ||||
| No personal history of cancer | Ref | — | Ref | — |
| Personal history of cancer | 1.36d | 1.14–1.62 | 1.30c | 1.05–1.60 |
NOTE: Definition of NHIS and data source can be found at https://www.cdc.gov/nchs/nhis/about_nhis.htm.
Abbreviation: Ref, reference category.
aPercentage and 95% CI values are weighted.
b P < 0.001.
c P < 0.05.
d P <0.01.
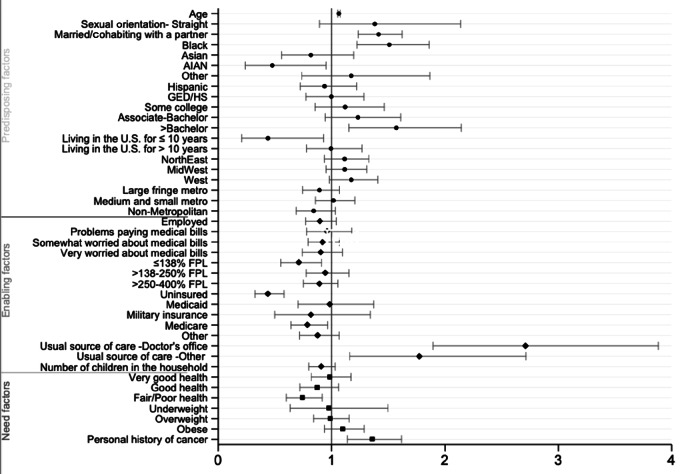
FIGURE 1.
Predicting factors associated with receipt of colorectal cancer screening in women. Multivariable logistic regression results showing ORs with CI values of various predisposing, enabling, and need factors and their associations with colorectal cancer screening uptake in women.
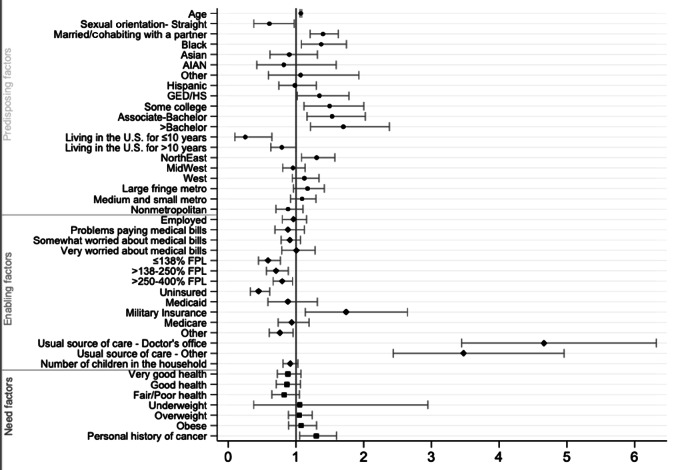
FIGURE 2.
Predicting factors associated with receipt of colorectal cancer screening in men. Multivariable logistic regression results showing ORs with CI values of various predisposing, enabling, and need factors and their associations with colorectal cancer screening uptake in men.
Women
Predisposing Factors
For each year increase in age past the mean age for screening-eligible women, the odds of colorectal cancer screening increased by 1.06 (P < 0.001). The odds of having colorectal cancer screening for married/cohabitating women were 1.41 times the odds for their unmarried counterparts (P < 0.001). The odds of having colorectal cancer screening for Black women were 1.51 times the odds for White women (P < 0.001). In contrast, the odds of having colorectal cancer screening for AI/AN women were 0.48 times the odds for White women (P = 0.036). The odds of having colorectal cancer screening for women with >bachelor's were 1.57 times the odds for women with <HS/GED (P = 0.004). The odds of having colorectal cancer screening for foreign-born women residing in the United States for ≤10 years were 0.44 times the odds for women who were born in the United States (P = 0.032).
Enabling Factors
The odds of having colorectal cancer screening for women at ≤138% FPL were 0.71 times the odds for women at >400% FPL (P = 0.007). The odds of having colorectal cancer screening for uninsured women and those with Medicare insurance were 0.43 (P < 0.001) and 0.79 (P < 0.021) times the odds for women with private insurance. The odds of having colorectal cancer screening for women with a usual source of care in a doctor's office were 2.71 times the odds for women without a usual source of care (P < 0.001) and 1.77 times for women with a usual source of care in other types of medical facilities compared with the odds of those without a usual source of care (P = 0.008).
Need Factors
The odds of having colorectal cancer screening for women with poor/fair health were 0.74 times the odds for women with excellent health (P = 0.006). The odds of having colorectal cancer screening for women with a history of cancer diagnosis were 1.36 times the odds for women without a history of cancer diagnosis (P = 0.001). See Table 2 and Figures 1 and 2.
Men
Predisposing Factors
For each year increase in age among men, the odds of colorectal cancer screening receipt increased by 1.07 (P < 0.001). The odds of having colorectal cancer screening for married/cohabitating men were 1.40 times the odds of unmarred men (P < 0.001). The odds of having colorectal cancer screening for Black men were 1.37 times the odds for White men (P = 0.010). The odds of having colorectal cancer screening for men with a HS/GED, some college education, an associate or bachelor's, and >bachelor's degree were greater (ORs: 1.35, 1.50, 1.53, and 1.70, respectively; all P < 0.05) than the odds for men with <HS/GED. The odds of having colorectal cancer screening for foreign-born men with ≤10 years of residence in the United States and foreign-born men with >10 years of residence in the United States were 0.25 (P = 0.004) and 0.79 (P = 0.047) times the odds for men born in the country. The odds of having colorectal cancer screening for men living in the South and Midwest were 0.77 (P = 0.005) and 0.73 (P = 0.004) times the odds of those living in the Northeast.
Enabling Factors
The odds of having colorectal cancer screening for men at ≤138% FPL, at >138%–250% FPL, and at >250%–400% FPL were lesser (ORs:0.59, 0.71, 0.79, respectively; P < 0.001, <0.01, <0.05, respectively) than the odds for men at >400% FPL. The odds of having colorectal cancer screening for uninsured men and men with other types of health insurance were lesser than the odds for men with private insurance (OR:0.45, P < 0.005; and 0.76, P = 0.020). In contrast, the odds of having colorectal cancer screening for men with military insurance was 1.74 times the odds for men with private insurance (P = 0.010). The odds of having colorectal cancer screening for men who had a usual source of care at a doctor's office were 4.67 times the odds for men without a usual source of care (P < 0.001). For those who had a usual source of care at other medical facilities, their odds of receiving colorectal cancer were 3.48 times the odds of those without a usual source of care (P < 0.001).
Need Factors
The odds of having colorectal cancer screening for men with a history of cancer diagnosis were 1.30 times the odds for men without a history of cancer diagnosis (P = 0.014). See Table 2 and Figures 1 and 2.
Discussion
Using the Andersen's model as a comprehensive conceptual framework, this study showed receipt of guideline concordant colorectal cancer screenings was associated with several predisposing, enabling, and need factors. While some factors were common for both women and men, other factors were unique to each sex.
Interestingly, our analyses reveal Black respondents exhibited significantly higher odds of colorectal cancer screening receipt compared to White respondents. To the best of our knowledge, this is the first study to show significantly higher odds of colorectal cancer screening among Black adults as compared to White adults in a nationally representative sample. Black adults have historically had lower colorectal cancer screening levels than White adults (17), but this disparity has been decreasing in recent years (17, 18). Our findings suggest that targeted efforts toward modifying enabling factors to increase access (19, 20) and insurance coverage for Black adults (21, 22) may have been successful in improving colorectal cancer screening uptake.
Factors associated with significantly higher odds of colorectal cancer screening uptake for both sexes were: age, being married/cohabitating with a partner, educational attainment higher than a bachelor's degree, having a usual source of care, and having a personal history of cancer. These associations are consistent with the past literature (5, 6, 23–27). As the risk of colorectal cancer increases with age, so does colorectal cancer screening knowledge and awareness (5, 23, 24). Prior research suggests that being married/cohabitating with a partner provides social support and promotes preventive health seeking behaviors (25, 28). Higher educational attainment and engagement in preventive health behaviors have a well-established association (5, 6, 24). We also find that having an established source of care and personal history of cancer increases health care visits which can promote cancer screening uptake (6, 26, 27). Research suggests having insurance coverage leads to having a usual source of care (26, 27) which in turn promotes the use of preventive health care services (21, 29, 30). Conversely, factors correlated with significantly lower odds of colorectal cancer screening uptake in both sexes were: being born outside the United States and living in the United States for ≤10 years, poverty ≤138% FPL, and lack of insurance coverage. These factors are consistent with prior literature, as lack of insurance coverage is often associated with lower cancer screening uptake due to access and financial barriers (6, 23). Being born outside the United States and living in the United States for ≤10 years may pose barriers, including limited access to government health care insurance and other resources in addition to linguistic barriers hindering screening uptake (7).
Among women only, lower odds of colorectal cancer screening was associated with being AI/AN, having Medicare insurance, and being in fair/poor health. Our findings are consistent with prior research which has shown women in fair/poor health may not prioritize colorectal cancer screening above other health conditions (31–33). There is limited research with AI/AN regarding cancer screening behavior, and more research is needed to understand the lower odds of colorectal cancer screening among AI/AN. More research is also needed to understand the disparities for those with Medicare insurance, as having insurance coverage generally improves access by eliminating financial barriers.
Among men only, those with educational attainment greater than HS/GED and having military insurance showed a significantly higher screening uptake. Our findings are consistent with prior research which has shown colorectal cancer screenings have been shown to be high among those with military insurance (34). Factors among men associated with lower screening uptake were being straight/heterosexual, born outside the United States and living in the United States for >10 years, living in the Midwest and South, below 138%–400% FPL, and having other insurance. Our finding that straight men had significantly lower odds of colorectal cancer screening compared to gay or bisexual men is consistent with prior studies (35, 36). One of the reasons for lower screening odds among straight/heterosexual men may be men who have sex with men have higher rates of anal cancers as compared to straight men (37) and, therefore, are more likely to prioritize colorectal cancer screening tests. Foreign-born adults living in the United States for ≤10 years and foreign-born men may face restrictions accessing government health care insurance, language barriers, and other access barriers, regardless of the length of stay in the United States (7). Men living in the South and Midwest had lower odds of receiving colorectal cancer screening, and it is not clear why men from these two U.S. regions are less likely to be screened. Colorectal cancer mortality rates are higher in these regions compared to other U.S. regions (38). Additional research is needed to identify regional cultures or other factors that may affect the willingness of men from these regions to follow colorectal cancer screening recommendations.
Our findings showed there are differences between women and men in the predicting factors that facilitate or constrain colorectal cancer screening. Efforts to improve colorectal cancer screening can be informed by these results, which could be used to guide sex-specific outreach and education interventions. Creating targeted educational materials for women and for men, separately, may generate gains in overall colorectal cancer screenings in the United States. Future research should develop and test colorectal cancer screening information and education based on factors that specifically facilitate or constrain colorectal cancer screenings for women and for men.
Limitations and Strengths
Survey-based studies are susceptible to some inherent limitations. The data were self-reported and may be subject to recall bias and/or response bias. Moreover, the cross-sectional data did not allow us to examine long-term adherence to colorectal cancer screening and did not report the proportion of participants receiving different screening methods. Nevertheless, this study used a large, nationally representative sample to examine colorectal cancer screening among U.S. women and men. This is the first study to apply Andersen's model to examine how predisposing, enabling, and need domains may affect colorectal cancer screening uptake among women and men separately; in doing so, the study provides a more complete examination of the factors that may influence colorectal cancer screenings by simultaneously examining factors from the model's three domains.
Conclusion
Encouragingly, this study showed higher odds of colorectal cancer screening receipt among the Black population in a nationally representative sample. However, the overall colorectal cancer screenings among women and men remain lower than national colorectal cancer screening goals, and disparities among certain populations still exist. Continued monitoring of colorectal cancer screenings may help inform and focus efforts toward alleviating colorectal cancer disparities among the most socially and medically vulnerable populations.
Supplementary Material
Characteristics of Women and Men Who Were up-to-Date with the USPSTF CRC Screening Guidelines
Acknowledgments
This research was supported by University of Arkansas for Medical Sciences Translational Research Institute funding awarded through the National Center for Research Resources and National Center for Advancing Translational Sciences of the NIH (UL1 TR003107). We would like to thank Erin Gloster for reviewing and formatting the manuscript.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Communications Online (https://aacrjournals.org/cancerrescommun/).
Authors’ Disclosures
No disclosures were reported.
Authors’ Contributions
S.K. Shah: Conceptualization, investigation, writing-original draft, project administration, writing-review and editing. M.-R. Narcisse: Software, formal analysis, investigation, methodology, writing-original draft, writing-review and editing. E. Hallgren: Writing-review and editing. H.C. Felix: Validation. P.A. McElfish: Resources, supervision, funding acquisition.
References
- 1. Centers for Disease Control and Prevention. Colorectal (colon) cancer. U.S. Department of Health and Human Services. [accessed 2021. Aug 25]. Available from: https://www.cdc.gov/cancer/colorectal/basic_info/screening/index.htm.
- 2. National Cancer Institute. Cancer stat facts: colorectal cancer. NIH; [accessed 2021. Jul 16]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 3. Maida M, Macaluso FS, Ianiro G, Mangiola F, Sinagra E, Hold G, et al. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther 2017;17:1131–46. [DOI] [PubMed] [Google Scholar]
- 4. Healthy People 2030. Increase the proportion of adults who get screened for colorectal cancer — C‑07. U.S. Department of Health and Human Services [accessed 2021. Dec 7]. Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-adults-who-get-screened-colorectal-cancer-c-07.
- 5. Sabatino SA, Thompson TD, White MC, Shapiro JA, de Moor J, Doria-Rose VP, et al. Cancer screening test receipt – United States, 2018. MMWR Morb Mortal Wkly Rep 2021;70:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah SK, Jones-Carr M, Bimali M, Su LJ, Nakagawa M. An online survey and focus groups for promoting cancer prevention measures. J Cancer Educ 2021; 10.1007/s13187-021-02027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah SK, Nakagawa M, Lieblong BJ. Examining aspects of successful community-based programs promoting cancer screening uptake to reduce cancer health disparity: a systematic review. Prev Med 2020;141:106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu B, Parsons V, Feuer EJ, Pan Q, Town M, Raghunathan TE, et al. Small area estimation of cancer risk factors and screening behaviors in US counties by combining two large national health surveys. Prev Chronic Dis 2019;16:E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sauer AG, Liu B, Siegel RL, Jemal A, Fedewa SA. Comparing cancer screening estimates: behavioral risk factor surveillance system and national health interview survey. Prev Med 2018;106:94–100. [DOI] [PubMed] [Google Scholar]
- 10. National Center for Health Statistics. National Health Interview Survey, 2019. Centers for Disease Control and Prevention. [accessed 2021. Jul 16]. Available from: https://www.cdc.gov/nchs/nhis/2019nhis.htm. [Google Scholar]
- 11. Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav 1995;36:1–10. [PubMed] [Google Scholar]
- 12. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Mem Fund Q Health Soc 2005;83. [PubMed] [Google Scholar]
- 13. National Center for Health Statistics. National health interview survey, 2019 survey description. Centers for Disease Control and Prevention; [accessed 2021. Sep 23]. Available from: https://www.cdc.gov/nchs/nhis/2019nhis.htm. [Google Scholar]
- 14. Babitsch B, Gohl D, von Lengerke T. Re-revisiting andersen's behavioral model of health services use: a systematic review of studies from 1998–2011. Psychosoc Med 2012;9:Doc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin SW, Yun Lee H, Lee J. Analyzing factors enabling colorectal cancer screening adherence in Korean Americans using the Andersen's Behavioral Model of Health Services Utilization. J Psychosoc Oncol 2019;37:729–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. STATACorp. Stata Statistical Software: Release 16. StataCorp LLC; 2019. [Google Scholar]
- 17. Rutter CM, Knudsen AB, Lin JS, Bouskill KE. Black and white differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev 2021;30:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao SR, Breen N, Graubard BI. Trends in black-white disparities in breast and colorectal cancer screening rates in a changing screening environment: the peters-belson approach using United States national health interview surveys 2000–2010. Med Care 2016;54:133–9. [DOI] [PubMed] [Google Scholar]
- 19. Kwaan MR, Jones-Webb R. Colorectal cancer screening in black men: recommendations for best practices. Am J Prev Med 2018;55:S95–S102. [DOI] [PubMed] [Google Scholar]
- 20. Waghray A, Jain A, Waghray N. Colorectal cancer screening in African Americans: practice patterns in the United States. Are we doing enough? Gastroenterol Rep (Oxf) 2016;4:136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huguet N, Angier H, Rdesinski R, Hoopes M, Marino M, Holderness H, et al. Cervical and colorectal cancer screening prevalence before and after affordable care act medicaid expansion. Prev Med 2019;124:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among black americans: recent trends and key challenges. U.S. department of health and human services; 2022. [2022 Apr 14]. Available from: https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-black-americans.
- 23. Shah SK, Demmings B, Bimali M, Hadden K, Nakagawa M. Assessing the feasibility of an online module for promoting cancer prevention measures. Cancer Control 2021;28:10732748211037908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White A, Thompson TD, White MC, Sabatino SA, de Moor J, D-R PV, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanske J, Meyer CP, Sammon JD, Choueiri TK, Menon M, Lipsitz SR, et al. The influence of marital status on the use of breast, cervical, and colorectal cancer screening. Prev Med 2016;89:140–5. [DOI] [PubMed] [Google Scholar]
- 26. Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis 2018;15:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao G, Okoro CA, Li J, Town M. Health insurance status and clinical cancer screenings among U.S. adults. Am J Prev Med 2018;54:e11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coughlin SS. Social determinants of colorectal cancer risk, stage, and survival: a systematic review. Int J Colorectal Dis 2020;35:985–95. [DOI] [PubMed] [Google Scholar]
- 29. Hatch B, Hoopes M, Darney BG, Marino M, Rose Templeton A, Schmidt T, et al. Impacts of the affordable care act on receipt of women's preventive services in community health centers in medicaid expansion and nonexpansion states. Womens Health Issues 2021;31:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blewett LA, Johnson PJ, Lee B, Scal PB. When a usual source of care and usual provider matter: adult prevention and screening services. J Gen Intern Med 2008;23:1354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hategekimana C, Karamouzian M. Self-perceived mental health status and uptake of fecal occult blood test for colorectal cancer screening in Canada: a cross-sectional study. Int J Health Policy Manag 2016;5:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miles A, Rainbow S, von Wagner C. Cancer fatalism and poor self-rated health mediate the association between socioeconomic status and uptake of colorectal cancer screening in England. Cancer Epidemiol Biomarkers Prev 2011;20:2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sultan S, Conway J, Edelman D, Dudley T, Provenzale D. Colorectal cancer screening in young patients with poor health and severe comorbidity. Arch Intern Med 2006;166:2209–14. [DOI] [PubMed] [Google Scholar]
- 34. de Moor JS, Cohen RA, Shapiro JA, Nadel MR, Sabatino SA, Robin Yabroff K, et al. Colorectal cancer screening in the United States: trends from 2008 to 2015 and variation by health insurance coverage. Prev Med 2018;112:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heslin KC, Gore JL, King WD, Fox SA. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care 2008;46:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kilgore S. Cancer screening practices among sexual orientation groups. Health Sciences Research Commons; 2018. [accessed 2021 Dec 7]. Available from: https://hsrc.himmelfarb.gwu.edu/son_dnp/11. [Google Scholar]
- 37. Poon MKL, Wong JPH, Li ATW, Manuba M, Bisignano A, Owino M, et al. HIV-positive MSM's knowledge of HPV and anal cancer self-sampling: a scoping review. Curr Oncol 2018;25:e83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Cancer Society. Colorectal cancer facts & figures 2020–2022. American Cancer Society; [accessed 2021. Dec 7]. Available from: https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of Women and Men Who Were up-to-Date with the USPSTF CRC Screening Guidelines
Data Availability Statement
The data analyzed in this study were obtained from the National Center for Health Statistics NHIS at https://www.cdc.gov/nchs/nhis/2019nhis.htm.