Abstract
Zonula occludens toxin (Zot) is produced by toxigenic strains of Vibrio cholerae and has the ability to reversibly alter intestinal epithelial tight junctions, allowing the passage of macromolecules through the mucosal barrier. In the present study, we investigated whether Zot could be exploited to deliver soluble antigens through the nasal mucosa for the induction of antigen-specific systemic and mucosal immune responses. Intranasal immunization of mice with ovalbumin (Ova) and recombinant Zot, either fused to the maltose-binding protein (MBP-Zot) or with a hexahistidine tag (His-Zot), induced anti-Ova serum immunoglobulin G (IgG) titers that were approximately 40-fold higher than those induced by immunization with antigen alone. Interestingly, Zot also stimulated high anti-Ova IgA titers in serum, as well as in vaginal and intestinal secretions. A comparison with Escherichia coli heat-labile enterotoxin (LT) revealed that the adjuvant activity of Zot was only sevenfold lower than that of LT. Moreover, Zot and LT induced similar patterns of Ova-specific IgG subclasses. The subtypes IgG1, IgG2a, and IgG2b were all stimulated, with a predominance of IgG1 and IgG2b. In conclusion, our results highlight Zot as a novel potent mucosal adjuvant of microbial origin.
The development of mucosal vaccines could be very useful for protection against pathogens that infect the host through mucosal surfaces. Mucosal vaccination offers several advantages over systemic vaccination (21, 27). It is a very efficient way to stimulate mucosal immunoglobulin A (IgA), which can prevent microbial adhesion and invasion of the host tissues. Moreover, mucosal delivery of antigens can induce secretion of antigen-specific IgA antibodies in mucosal districts distant from the site of immunization. Finally, in addition to secretory IgA, mucosal immunization elicits high IgG and IgA responses at the systemic level (21). These properties, together with the noninvasive nature of mucosal antigen delivery, make mucosal vaccination a potentially successful and flexible means to prevent both infection and disease. However, mucosal administration of soluble proteins does not generally induce an immune response and requires the use of specific mucosal adjuvants (27). It is interesting that two bacterial enterotoxins, Escherichia coli heat-labile enterotoxin (LT) and the related cholera toxin (CT) produced by Vibrio cholerae, are the most powerful mucosal adjuvants known so far (26).
Zonula occludens toxin (Zot) is a single polypeptide chain of 44.8 kDa encoded by the filamentous bacteriophage CTXΦ, present in toxigenic strains of V. cholerae (1, 7, 30). Zot has the ability to increase the permeability of the small intestine by affecting the structure of epithelial tight junctions (8, 9), thus allowing the passage of macromolecules by the paracellular route. This effect has been clearly demonstrated in vivo and in vitro for rabbit and rat intestinal epithelium (10, 11) and is dependent upon the binding of Zot to specific intestinal receptors (11, 12). The mechanism of action of Zot involves a rearrangement of the epithelial cell cytoskeleton due to protein kinase Cα-dependent F-actin polymerization, which results in opening of tight junctions (9). Zot does not cause tissue damage, and its effect on intestinal permeability is time and dose dependent and fully reversible (8, 9). Because of these properties, Zot can be exploited for the mucosal delivery of drugs and macromolecules that normally do not cross the epithelial barrier, with the aim of developing therapeutic and preventive strategies. Indeed, we have previously shown that intestinal perfusion of diabetic rats with insulin and Zot lowers serum glucose levels and increases the survival time of diabetic rats (10).
The present study was undertaken to investigate whether Zot could be exploited for mucosal vaccination. In particular, we sought to determine whether Zot would act on the murine nasal mucosa to promote antigen-specific systemic and mucosal immune responses to an intranasally codelivered protein antigen.
MATERIALS AND METHODS
Mice.
Female BALB/c mice used throughout the study were 6 to 8 weeks of age and were obtained from Charles River (Calco, Italy).
Antigens and adjuvants.
Ovalbumin (Ova) was purchased from Sigma (St. Louis, Mo.). Zot fused with the maltose-binding protein (MBP-Zot) was obtained and purified according to a previously described method (10). Briefly, the zot gene was fused in frame with the MBP gene, using vector pMal-c2 (11). The fusion product was expressed in E. coli, and the MBP-Zot fusion protein was purified by affinity chromatography with an amylose column (MBP-fusion purification system; New England Biolabs, Beverly, Mass.). A recombinant form of Zot that corresponds to the whole Zot sequence tagged at the N terminus with a hexahistidine tail (His-Zot) (29) was employed. The biological activities of MBP-Zot and His-Zot were tested in vitro by using Ussing chambers or in vivo in the rabbit ileal loop assay (10). In some experiments, MBP-Zot and His-Zot were heat inactivated for 30 min at 100°C. LT was kindly provided by M. Pizza (Chiron S.p.A., Siena, Italy).
Immunization schedules and sample collection.
Groups of five mice were intranasally immunized five times at weekly intervals (days 0, 7, 14, 21, and 28) with 5 μg of Ova in the absence or presence of mucosal adjuvants. Mice received either 10 μg of MBP-Zot or 5 μg of His-Zot as a mucosal adjuvant. Identical immunization schedules were employed for experiments with heat-inactivated MBP-Zot and His-Zot. In one experiment, groups of five mice were intranasally immunized twice (days 0 and 14) with 5 μg of Ova alone or together with either 1 μg of MBP-Zot or 1 μg of LT. For intranasal immunization, mice were lightly anesthetized by intraperitoneal injection of ketamine and xylazine, and a final volume of 15 to 20 μl (i.e., 7.5 to 10 μl per nostril) of a solution containing antigen with or without adjuvant diluted in sterile phosphate-buffered saline (PBS) was administered. Serum samples, vaginal washes, and fecal pellets were generally collected every week, 24 h before each immunization and 1 week after the last immunization, unless otherwise stated. Vaginal washes were obtained by flushing the vaginal cavity with 150 μl of sterile PBS. After centrifugation, supernatants were stored at −20°C until assayed. The extracts from fecal pellets were prepared as described elsewhere (20). Briefly, 0.1 g of pellet was mixed with 1 ml of PBS containing 0.1% NaN3 and vortexed for 10 min. After centrifugation, supernatants were collected and stored at −20°C until assayed.
Analysis of antibody isotypes and IgG subclasses.
Anti-Ova antibodies were titrated in individual serum and mucosal samples by using enzyme-linked immunosorbent assay methods (4). Microplates (Microtest III; Becton Dickinson, Oxnard, Calif.) were coated with a 100-μl solution of Ova (45 μg/ml) in PBS and incubated overnight at 4°C. Plates were then washed three times with PBS containing 0.05% Tween 20 (Sigma) and blocked for 2 h with 200 μl of PBS containing 1% bovine serum albumin (BSA), and serial dilutions of serum or mucosal samples were added to duplicate wells. IgG and IgA titers were determined by addition of γ- or α-chain-specific biotin-conjugated goat anti-mouse antibodies (Sigma) diluted 1:1,000 in PBS containing 0.1% BSA and 0.025% Tween 20. To determine the patterns of IgG subclasses, γ1-, γ2a-, γ2b-, or γ3-chain-specific biotin-conjugated rat anti-mouse monoclonal antibodies (PharMingen, San Diego, Calif.) were used as previously described (20). After incubation and washing steps, a 100-μl aliquot of horseradish peroxidase-conjugated streptavidin (Dako, Glostrup, Denmark), diluted 1:2,000 in PBS containing 0.1% BSA and 0.025% Tween 20, was added, and color was developed with 3,3′,5,5′-tetramethylbenzidine substrate (Kirkegaard and Perry, Gaithersburg, Md.). The color reaction was terminated after 5 to 10 min with 50 μl of 0.2 M H2SO4, and the absorbance at 450 nm was determined with an enzyme-linked immunosorbent assay plate reader. Antibody titers are expressed as the reciprocal of the sample dilution corresponding to an optical density of 0.3 units (for IgG) or 0.2 units (for IgA and IgG subclasses) above those of controls.
RESULTS
Adjuvant effect of Zot on antigen-specific serum IgG responses.
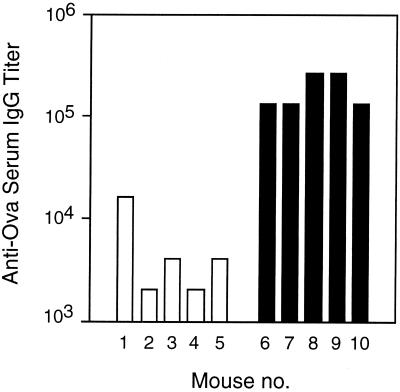
To investigate whether intranasally administered Zot, together with a soluble protein antigen, would act as an adjuvant for induction of systemic antigen-specific antibody responses, we performed a first set of experiments with MBP-Zot (11). Groups of BALB/c mice were intranasally immunized with 5 μg of Ova in the presence or absence of 10 μg of MBP-Zot. When Ova-specific serum IgG responses were measured following five immunizations, we observed that the titers in the group of mice that received Ova with MBP-Zot were consistently higher than those of mice immunized with Ova alone (Fig. 1). Although one mouse of the control group showed a high titer of anti-Ova IgG, this was still at least 10 times lower than titers observed in the presence of MBP-Zot.
FIG. 1.
Serum IgG responses to Ova in mice intranasally immunized with Ova alone (□) or together with 10 μg of MBP-Zot (■). Groups of five mice were immunized five times at weekly intervals, and serum antibody titers were measured 1 week after the last immunization.
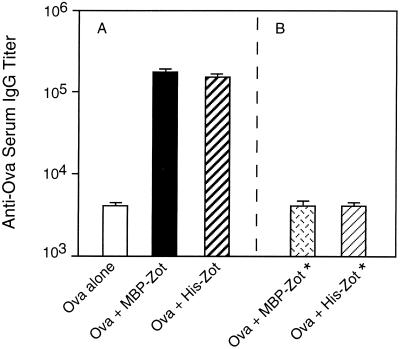
To rule out a possible contribution of MBP on the adjuvanticity of MBP-Zot, we employed His-Zot, which is devoid of MBP (29). To directly compare the adjuvant activities of the two Zot preparations, we intranasally immunized mice with Ova in the absence or presence of MBP-Zot or His-Zot. Indeed, since Zot accounts for approximately 50% of the molecular weight of MBP-Zot, we administered to mice a dose of either 10 μg of MBP-Zot or 5 μg of His-Zot and measured Ova-specific serum IgG responses following five immunizations. It was interesting that His-Zot exhibited an adjuvant activity that was comparable to that of MBP-Zot (Fig. 2A). In fact, sera from mice immunized with Ova and either His-Zot or MBP-Zot displayed IgG titers that were 37 and 42 times higher, respectively, than those of mice immunized with Ova alone. Therefore, we excluded any contribution of MBP to the adjuvanticity of MBP-Zot. To further exclude the possibility that other contaminants (e.g., lipopolysaccharide) could account for the immunomodulatory effect of recombinant MBP-Zot or His-Zot, we included in the same experiment two groups of mice that were immunized with heat-inactivated MBP-Zot and His-Zot. The analysis of Ova-specific IgG responses in these groups of mice showed that neither of the two inactivated forms of Zot retained adjuvant activity (Fig. 2B).
FIG. 2.
Serum IgG responses to Ova in mice intranasally immunized with Ova and two different forms of recombinant Zot. (A) IgG titers in mice receiving Ova alone or together with 10 μg of MBP-Zot or with 5 μg of His-Zot. (B) IgG titers in mice receiving Ova with either 10 μg of heat-inactivated MBP-Zot (MBP-Zot*) or 5 μg of heat-inactivated His-Zot (His-Zot*). Mice were immunized as described in the legend to Fig. 1. Titers of anti-Ova IgG are expressed as geometric means of individual titers + standard errors.
Taken together, these data clearly show that Zot acts as an adjuvant for the induction of serum IgG responses to an intranasally codelivered protein antigen.
Adjuvant effect of Zot on serum and mucosal IgA responses.
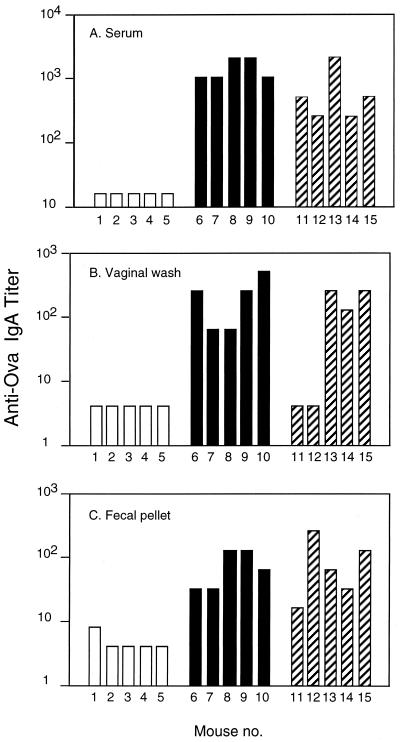
The observation that intranasal administration of Zot and Ova resulted in high levels of antigen-specific serum IgG prompted us to investigate whether serum IgA responses were also induced. Figure 3A shows that the sera of mice receiving MBP-Zot or His-Zot exhibited high IgA titers compared to sera of control mice, in which IgA responses were undetectable. We then investigated whether IgA responses were also induced at the mucosal level by measuring Ova-specific IgA titers in vaginal washes and fecal pellets from the same mice (Fig. 3B and C). It was interesting that MBP-Zot induced consistently high titers of IgA in vaginal washes from all mice, whereas only three of five mice immunized with His-Zot exhibited vaginal IgA titers that were nevertheless comparable to those induced by MBP-Zot (Fig. 3B). The lack of response by two mice in the His-Zot group possibly reflected hormone influences on the immune response (4). Indeed, high titers of IgA were found in serum (Fig. 3A) and in fecal samples (Fig. 3C) from the same two mice immunized with His-Zot. Finally, the fecal pellets from all mice immunized with either MBP-Zot or His-Zot exhibited high titers of anti-Ova IgA (Fig. 3C).
FIG. 3.
IgA responses to Ova measured in serum, vaginal washes, and fecal pellets. Mice were intranasally immunized with Ova alone (□) or together with either 10 μg of MBP-Zot (■) or 5 μg of His-Zot (▨) by following the protocol described in the legend to Fig. 1. Sera and mucosal samples were collected 1 week after the last immunization. Serum IgA titers below 16 and mucosal IgA titers below 4 were considered undetectable.
Altogether these data show that intranasally delivered MBP-Zot and His-Zot both act as mucosal adjuvants for the induction of antigen-specific IgA in serum and in mucosal secretions.
Comparison of Zot and LT adjuvant activities.
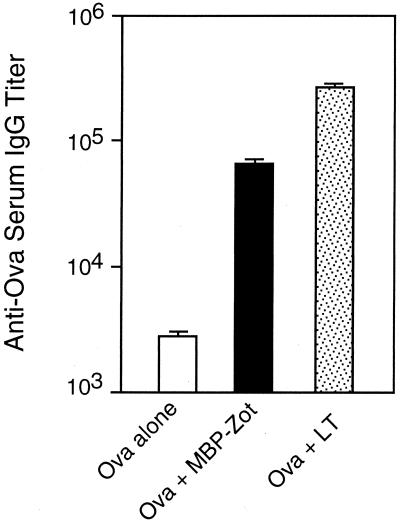
LT is commonly used as a mucosal adjuvant because of its extraordinary ability to induce very high systemic and mucosal immune responses to coadministered antigens (2, 26). Furthermore, its effect is exerted at relatively low dosages and after few immunizations (4, 14). It was thus interesting to relate the degree of Zot adjuvanticity to that of LT. To this end, we lowered the Zot dose and decreased the number of immunizations. Mice were intranasally immunized with 1 μg of either MBP-Zot or LT and 5 μg of Ova on days 0 and 14, and Ova-specific serum IgG responses were measured 2 weeks after the second immunization. We observed that mice immunized with MBP-Zot or LT exhibited IgG titers that were 18- and 124-fold higher, respectively, than those of control mice (Fig. 4). These data show that the adjuvant activity of MBP-Zot is only about seven times lower than that of LT, at least when 1-μg doses of the two proteins are compared, and demonstrate that Zot exerts its adjuvant effect also at low doses and after two immunizations. Interestingly, no antigen-specific IgA was found after two immunizations in the sera of mice immunized with either MBP-Zot or LT (data not shown).
FIG. 4.
Serum IgG responses to Ova in mice intranasally immunized with Ova alone or together with either 1 μg of MBP-Zot or 1 μg of LT. Groups of five mice were immunized twice (on days 0 and 14), and sera were collected 2 weeks after the second immunization. Titers of anti-Ova IgG are expressed as geometric means of individual titers + standard errors.
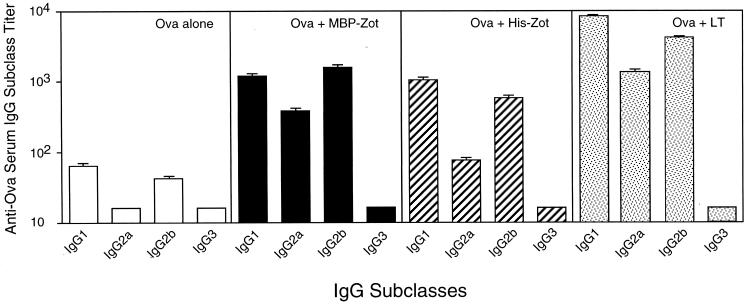
Antigen-specific IgG subclasses following intranasal administration of Zot or LT as a mucosal adjuvant.
It has been shown that mucosal administration of LT together with protein antigens stimulates both Th1- and Th2-type cells, which in turn lead to secretion of antigen-specific IgG1, IgG2a, and IgG2b subclasses (14, 28). We therefore analyzed the pattern of IgG subclasses induced by Zot and determined whether there were any qualitative differences between Zot and LT in this regard. Mice were intranasally immunized five times with Ova alone or in combination with MBP-Zot (10 μg), His-Zot (5 μg), or LT (1 μg). The results in Fig. 5 show that mice immunized with Ova alone exhibited only low Ova-specific IgG1 and IgG2b titers. On the other hand, all the groups that received an adjuvant displayed enhancement of IgG1, IgG2a, and IgG2b responses. In particular, MBP-Zot-immunized mice showed comparable titers of IgG1 and IgG2b and lower levels of IgG2a antibodies, while His-Zot-immunized mice displayed higher levels of IgG1 followed by IgG2b and IgG2a. Similarly, administration of LT resulted in high titers of IgG1 followed by IgG2b and IgG2a. The IgG3 subclass was undetectable in all groups of mice. Overall the results indicate that following intranasal administration of Zot, the pattern of IgG subclasses induced resembles that of LT, which is indicative of stimulation of both Th1- and Th2-type cells (13, 22, 28).
FIG. 5.
Serum IgG subclass responses to Ova in groups of five mice intranasally immunized with Ova alone or together with either 10 μg of MBP-Zot, 5 μg of His-Zot, or 1 μg of LT. The immunization protocol was the same as described in the legend to Fig. 1. Titers of anti-Ova IgG subclasses are expressed as geometric means of individual titers + standard errors. Serum IgG subclass titers below 16 were considered undetectable.
DISCUSSION
We have shown that Zot, a protein that reversibly affects the structure of intestinal tight junctions, acts as a mucosal adjuvant for antigens delivered through the murine nasal mucosa. We have used two different forms of recombinant Zot, MBP-Zot and His-Zot. When intranasally delivered with Ova to mice, MBP-Zot and His-Zot induced titers of Ova-specific IgG and IgA in the serum and Ova-specific IgA in mucosal secretions that were much higher than those elicited by the antigen alone. Anti-Ova IgA titers were generally more consistent in the mice that received MBP-Zot as an adjuvant. However, the lack of Ova-specific IgA in the vaginal washes from two mice immunized with His-Zot may likely reflect the intrinsic variability of the immune responses in the female reproductive tract (4), rather than a lower efficacy of His-Zot for the induction of vaginal IgA responses. This is supported by two observations: (i) the three responsive mice immunized with His-Zot showed vaginal IgA titers comparable to those of mice receiving MBP-Zot, and (ii) all mice immunized with His-Zot secreted IgA antibodies in serum and intestine. The ability of intranasally delivered Zot to induce antigen-specific IgA antibodies in mucosal districts distant from the immunization site is particularly useful when a local response is required in mucosae that are not easily accessible to direct immunization or in mucosae where it is generally difficult to elicit an immune response, such as that of the female reproductive tract (4, 16).
The comparison between MBP-Zot and LT made with 1 μg of each protein showed that the adjuvanticity of Zot is only sevenfold lower than that of LT, which is considered one of the most powerful mucosal adjuvants. Furthermore, as for LT, the adjuvant effect of Zot on serum IgG was already evident after two immunizations. Thus, Zot is a very potent novel mucosal adjuvant of microbial origin. Indeed, in light of the recent finding that Zot and CT are encoded by phage CTXΦ, present in toxigenic strains of V. cholerae (30), Zot and CT now have to be considered mucosal adjuvants of bacteriophage origin.
The effects of MBP-Zot and His-Zot on tight junctions first had been shown in vitro and in vivo for rat and rabbit intestinal epithelium (10, 11), where Zot interacts with a receptor whose regional distribution varies, being more represented in the jejunum and distal ileum and virtually absent in the colon (11, 12). The results shown here suggest that a receptor for Zot may also be present on the nasal epithelium, where Zot may exert an effect on tight junctions similar to that shown for the intestinal epithelium, resulting in increased permeabilization of the nasal mucosal barrier. Thus, the mucosal adjuvanticity of Zot could be the result of an increased delivery of antigen into the nasal lymphoreticular tissue. In this regard, it has been shown that the increased permeability observed in the intestinal and nasal mucosae exposed to CT contributes to its adjuvant activity (15, 18). However, it is possible that besides acting as a permeabilizing agent, Zot may exert some immunomodulatory effects on immune cells, such as those reported for LT and CT (3, 17, 19, 20, 28). Indeed, similarly to LT, Zot induced a pattern of antigen-specific IgG subclasses (i.e., IgG1, IgG2a, and IgG2b) that is indicative of stimulation of mixed Th1- and Th2-type cells (13, 22, 28). Although we did not study the cytokine profile of antigen-specific T-helper cells induced by Zot, it is tempting to speculate that Zot could influence the T-cell polarization toward Th1- and Th2-type cells. Obviously, further studies are needed to investigate the action of Zot on immune cells, and we are currently addressing these issues.
It is interesting that the most effective mucosal adjuvants studied so far are toxins mainly produced by enterobacteria or by other bacteria that infect mucosal surfaces. These include LT, CT, and pertussis toxin (24), and Zot can now be considered a novel enterotoxin with mucosal adjuvant activity. Interestingly, for most of these toxins the enzymatic activity is responsible for toxicity whereas the adjuvant effect, at least for some of them, is independent from the enzymatic activity (5, 6, 31) and depends on toxin binding to cells (23, 25). It is thus tempting to speculate that from an evolutionary point of view, these toxins developed to perform a main function (that would be dependent on enzymatic activity) and that they acquired adjuvant activity as a “side effect,” probably dependent on their binding to cell membranes. Similarly to the above-mentioned toxins, Zot has a dual function, that is, to participate in CTXΦ phage assembly (30) and to modulate epithelial tight junctions (9). Although an enzymatic activity has not been formally demonstrated for Zot, the two functions of this protein seem to be associated with two different domains (29).
In conclusion, the facts that the effect of Zot on tight junctions is reversible and that Zot does not cause tissue damage at the epithelial level or induce acute systemic side effects upon oral delivery (10), in conjunction with the evidence that it acts as a potent adjuvant when intranasally delivered to mice, make Zot a promising tool for the oral delivery of therapeutic agents (10) and for the development of mucosal vaccines against pathogens of the respiratory, gastrointestinal, and urogenital systems.
REFERENCES
- 1.Baudry B, Fasano A, Ketley J, Kaper J B. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 3.Cong Y, Weaver C T, Elson C O. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–5308. [PubMed] [Google Scholar]
- 4.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenighini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douce G, Fontana M, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fando R, Pérez J L, Rodriguez B L, Campos J, Robert A, García L, Silva A, Benitez J A. Promoter activities in Vibrio cholerae ctxΦ prophage. Infect Immun. 1997;65:1561–1565. doi: 10.1128/iai.65.4.1561-1565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper J B, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum S E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, Uzzau S. Modulation of intestinal tight junctions by zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Investig. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin (ZOT) on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112:839–846. doi: 10.1053/gast.1997.v112.pm9041245. [DOI] [PubMed] [Google Scholar]
- 12.Fasano, A. Unpublished data.
- 13.Finkelman F D, Holmes J, Katona I M, Urban J F J, Beckman M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 14.Giuliani M M, Del Giudice G, Giannelli V, Douce G, Dougan G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gizurarson S, Tamura S, Aizawa C, Kurata T. Stimulation of the transepithelial flux of influenza HA vaccine by cholera toxin B subunit. Vaccine. 1992;10:101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- 16.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J-P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T-K, Fox B S. Cholera toxin B subunit binding to an antigen-presenting cell directly co-stimulates cytokine production from a T cell clone. Int Immunol. 1998;8:1849–1856. doi: 10.1093/intimm/8.12.1849. [DOI] [PubMed] [Google Scholar]
- 18.Lycke N, Karlsson U, Sjolander A, Magnusson K E. The adjuvant action of cholera toxin is associated with an increased intestinal permeability for luminal antigens. Scand J Immunol. 1991;33:691–698. doi: 10.1111/j.1365-3083.1991.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 19.Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol. 1997;148:504–520. doi: 10.1016/s0923-2494(98)80144-2. [DOI] [PubMed] [Google Scholar]
- 20.Marinaro M, Boyaka P N, Finkelman F D, Kiyono H, Jackson R J, Jirillo E, McGhee J R. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. J Exp Med. 1997;185:415–427. doi: 10.1084/jem.185.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–174. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 23.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunit of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J R, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan M, McCarthy L, Rappuoli R, Mahon B P, Mills K H G. Pertussin toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2, and CD28. Int Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 26.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staats H F, Jackson R J, Marinaro M, Takahashi I, Kiyono H, McGhee J R. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Uzzau, S., and A. Fasano. Unpublished data.
- 30.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]