Abstract
Purpose:
Children’s Oncology Group study ACNS1123 tested the efficacy of reduced dose and field of radiation therapy (RT) for patients with localized nongerminomatous germ cell tumors (NGGCT) who achieved a complete (CR) or partial response (PR) to chemotherapy. Here, we evaluate the quality of RT and patterns of failure for patients eligible for reduced RT in this phase 2 trial.
Methods and Materials:
Patients with localized NGGCT with CR/PR after induction chemotherapy received reduced RT to 30.6 Gy whole ventricular irradiation and 54 Gy tumor-bed total dose. An atlas was provided to assist with complex RT volumes. Early interventional review was performed for the initial RT plan. Complete RT plans for all patients and images of relapsed patients were centrally reviewed at completion of therapy.
Results:
Between May 2012 and September 2016, 107 eligible patients were enrolled and 66 achieved a CR/PR after induction chemotherapy (± second-look surgery) and were eligible for reduced RT. Median follow-up was 4.4 years. Median age was 11.0 years (3.7–21.6), and 75% were male. Progression-free survival and overall survival at 4 years were 87.9% ± 4.0% and 92.4% ± 3.3% for 66 evaluable patients, respectively. Eight patients relapsed: 6 with isolated spinal relapse and 2 with disease in the brain and spine. After central review, 62 (94%) patients had RT targets contoured and dose delivered per protocol. None of the patients with deviations (n = 4) have progressed.
Conclusions:
Patterns of failure suggest the spine is at risk for recurrence for patients with localized NGGCT who receive reduced RT after a CR/PR to induction chemotherapy. Although survival data are encouraging, the pattern of failure has influenced the next prospective trial design. RT compliance was excellent despite complexity of radiation volumes, suggesting that providing visual guidance in the form of an online atlas contributes to higher quality RT plans.
Introduction
Nongerminomatous germ cell tumors (NGGCT) demonstrate a more aggressive histology with increased risk of failure both locoregionally and in the spine compared with pure germinoma.1 They are less treatment sensitive, and the 5-year survival with radiation alone is poor, ranging from 20% to 45%.2 The addition of chemotherapy to radiation therapy (RT) has resulted in markedly improved disease outcomes, and combined modality treatment is now the standard of care.3–6 The The Children’s Oncology group (COG) ACNS0122 trial (NCT00047320) trial assessed the use of 6 cycles of induction chemotherapy with carboplatin/ etoposide alternating with ifosfamide/etoposide before 36 Gy craniospinal irradiation (CSI) and 54 Gy to the primary tumor/tumor bed with or without second-look surgery.3 Among 102 patients enrolled, 69% achieved a complete response (CR) or partial response (PR) to induction chemotherapy. The 5-year event-free survival and overall survival (OS) of all patients enrolled was 84% ± 4% and 93% ± 3%, respectively, which is the best published outcome to date.3 Seventy-nine patients (76%) had localized tumors, including bifocal lesions. Analyses revealed a 3-year event-free survival of 92% for patients with localized disease who achieved a CR or PR to induction chemotherapy or with documentation of absence of malignant elements at second-look surgery, with the majority of failures being local.3
The COG ACNS1123 stratum 1 (NCT01602666) was developed for patients with localized NGGCT who responded well to induction chemotherapy and were most appropriate for consideration of reduced-intensity RT. Patients on COG ACNS1123 received the same induction chemotherapy regimen used on COG ACNS0122, and if they achieved a CR or PR on imaging with normalization of tumor markers, they were eligible for reduced RT, defined as 30.6 Gy whole ventricular irradiation (WVI) and 54 Gy tumor-bed dose, compared with 36 Gy CSI plus 54 Gy tumor-bed dose on ACNS0122. The primary objective was to evaluate the effect of reduced RT on progression free survival (PFS), as has been previously reported.7 Overall, 66 of 107 (61.7%) patients with nonmetastatic NGGCT achieved a CR/PR and proceeded to reduced RT, resulting in a 4-year PFS and OS of 87.9% and 92.4%, respectively. There were no identifiable prognostic markers for those patients more likely to have a CR/PR, and there was no difference in PFS based on tumor location or levels of serum or cerebrospinal fluid (CSF) β-human chorionic gonadotropin (HCGβ) or α-fetoprotein (AFP) elevation.
COG ACNS1123 used a prospective RT review. Past studies suggest that quality of RT can affect both PFS and OS,8,9 and the severity of RT deviation is also important.10 Here, we evaluate quality of RT for NGGCT and the patterns of failure in patients eligible for reduced dose and field RT in this prospective phase 2 trial.
Methods and Materials
Treatment schema
Patients with localized NGGCT received alternating cycles (6 total) of carboplatin/etoposide and ifosfamide/etoposide followed by response evaluation. Those who became tumor marker negative and achieved radiologic CR or PR were eligible for reduced RT to 30.6 Gy WVI and 54 Gy tumor-bed total dose (details previously published).7 CR was defined as a complete disappearance of disease on imaging, allowing for minimal residual disease or enhancement of not more than 0.5 cm in the suprasellar region or not more than 1 cm in the pineal region. PR was defined as greater than 0.5-cm residual in the suprasellar region or greater than 1 cm in the pineal region and at least a 65% decrease in the sum of the products of the 3 perpendicular diameters of the localized target lesion. The CR and PR definitions mandated normalization of serum and CSF AFP and HCGβ levels.
Radiation review
As part of the COG standard regulations, all proton and photon beam facilities participating in this trial had to undergo compliance testing. Detailed contouring instructions were available as part of the protocol, along with a whole ventricle atlas available on the COG and imaging and radiation oncology core (IROC) websites. Post chemotherapy magnetic resonance imaging (MRI) T2 sequences demonstrate the CSF well, and the use of T2 MRI fusion to define this volume was strongly encouraged. The gross tumor volume for the involved field primary site boost was defined as the tumor at the time of diagnosis (prechemotherapy and presurgery) accounting for shifts in tissue for pushing tumors but not for infiltrating tumors. The involved field boost clinical target volume (CTV) was a 5-mm anatomically constrained expansion on the gross tumor volume. The final whole ventricle CTV was combined with the involved field boost CTV to ensure full dose to the involved field boost. This combined CTV was particularly important when the boost CTV could extend beyond the whole ventricle CTV.
Supportive data submission was also requested and included: operative reports for each surgical procedure, preand postchemotherapy MRI scans used in defining the initial CTV and the local boost volume, pre- and postoperative cranial neuroimaging for each surgical procedure including second surgery after induction chemotherapy, and postchemotherapy (pre-RT) neuroimaging when no second surgery was performed.
IROC was established to facilitate quality assurance for RT and superior outcomes, promoting confidence in study results. Within 3 days of the start of RT, the RT treatment plan, including computed tomography, structures, dose, and plan files for the WVI, was submitted for on-treatment review. Boost data were submitted before the start of the boost, but preferably at the same time. At this time, IROC-affiliated radiation oncologists made suggestions to ensure compliance with protocol-specified fields and radiation doses. Within 1 week of the completion of RT, the RT record (treatment chart), including prescription and daily and cumulative doses to all required areas and organs at risk, was submitted, including any revised documentation or modifications that occurred after the initial, rapid review. These records were reviewed by radiation oncologists on the study committee to identify dose or volume deviations. Minor and major deviations were defined for target volumes, CTV and planning target volume margins, prescription dose coverage, dose uniformity, normal tissue dose volume constraints, and timing of RT delivery (Table 1). If the recommended doses to the organs at risk were exceeded, an explanation was included for review by IROC and the radiation oncology reviewers.
Table 1.
Definitions of radiation protocol deviations
| Minor deviation | Major deviation | |
|---|---|---|
|
| ||
| RT volume | Margins for CTV or PTV less than specified or field(s) excessively large | GTV does not encompass MR-visible residual tumor |
| Prescription dose | Differs from protocol specification by >5% to <10% | Differs from protocol specification by >10% |
| Dose uniformity | <95% of PTV receives prescription dose or >10% of PTV receives >110% of prescription dose | 90% isodose covers <100% of CTV |
| RT timing | RT started 6–9 wk after last chemotherapy or RT course prolonged 7–14 d | RT started >9 wk after last chemotherapy or RT course prolonged >14 d |
Abbreviations: CTV = clinical target volume; GTV = gross tumor volume; MR = magnetic resonance; PTV = planning target volume; RT = radiation therapy.
Pattern of failure review
Failure was initially determined by the treating institution. After patients were identified with progression, diagnostic brain and spine MRI scans with and without contrast at time of progression were collected. These images were reviewed and patterns of failure were documented. The radiation dose data were then coregistered to the images of the progression MRIs and the areas of progression were contoured. The dose to the areas of progression was evaluated and documented.
Statistical methods
The primary objective of the ACNS1123 trial was to determine, as measured by the 3-year PFS rate, whether dose and volume of irradiation can be safely reduced to 30.6 Gy WVI plus 23.4 Gy primary site boost instead of 36 Gy CSI plus primary site boost in a subgroup of children and young adults (aged 3 to ≤21 years) with localized NGGCT. Eligibility included those who met MRI and tumor marker criteria (CSF and serum) for confirmed CR or PR after induction chemotherapy. We also included patients who had less than a PR after induction chemotherapy with negative tumor markers; underwent second-look surgery and were found to have only mature teratoma, residual scar, or fibrosis; and fit the definition of CR/PR after second-look surgery.
Kaplan-Meier estimates were used to estimate PFS, which was calculated from the date the patient began study participation to the date of disease progression, date of death, or date of last follow-up, if the patient was censored. Log-log transformation method was used to calculate confidence intervals for PFS. For this manuscript, time to progression was calculated from RT start date to date of first progression or relapse confirmed on imaging or by tumor markers. Pattern of failure was initially determined by the treating institution. Pattern of failure results and quality of RT for NGGCT are detailed as descriptive results.
Results
Radiation oncology review
The early RT review was performed for 49 of the 70 patients who had initially been designated as achieving a CR/PR. Forty-four (89.8%) had no modification required, 3 (6.1%) had a single modification required and the institution completed the modification, 1 (2.0%) required multiple modifications, which were completed, and 1 (2.0%) had no modification suggested and final review found a major deviation. This last discrepancy between passing initial review and final review finding major deviation is likely due to the fact that only the initial phase of the plan was required for the early interventional review. This patient ended up having an incorrectly contoured boost volume/second phase. The modifications suggested at time of early interventional review included 1 regarding normal tissue dose and 3 regarding the boost volumes. These suggestions were appropriately completed by the treating radiation oncologists. Twenty-one RT plans did not have an interventional review performed because data were not submitted on time. Of these 21 RT plans, 19 (90.5%) ended up being per protocol on final review, 1 (4.8%) resulted in major deviation of incorrect RT dose, and 1 (4.8%) resulted in a minor deviation.
Of note, 4 of 70 patients were treated with reduced RT but should not have been because they did not meet protocol criteria for PR/CR after induction chemotherapy by radiology and by marker assessment. Therefore, central review described here includes a total of 66 patients. After central review, 62 of 66 (94%) patients had RT targets and normal structures contoured and dose delivered per protocol; there were 2 major and 2 minor deviations. One patient was appropriately enrolled on protocol stratum 1 due to HCGβ>100; however, RT treatment doses used were for stratum 2, resulting in a major deviation. The second major deviation included a patient with both suprasellar and pineal disease. The pineal disease had a CR to induction chemotherapy. Only the suprasellar disease was included in the boost volume, but both areas should have been included. The 2 minor deviations were both due to the fact that 95% of the prescription dose covered less than 100% of the CTV; these were done in an effort to meet the normal tissue constraints of the optic apparatus. Importantly, none of the patients with deviations have progressed thus far. Therefore, these few deviations do not explain the patterns of failures in this trial, which are described in the following sections.
Pattern of failure review
After median follow-up of 4.4 years, 8 patients demonstrated progression. The 4-year PFS and OS was 87.88% (±4.02) and 92.42 % (±3.26) for the 66 evaluable patients, respectively. Median time to progression was 3.5 months (range, 1.7–19.1) from initiation of RT. Four (50%) patients had recurrence noted at their first end of treatment evaluation. Importantly, all failures had a distal component, completely out of the RT field (Table 2). Six patients had spine only progression (Fig. 1) and 2 had progression in brain and spine. Of those with intracranial relapse, 1 (patient 2) recurred locally in the suprasellar primary with the recurrence extending into the anterior ventricles; dose to the area of the recurrence was V54 of 53%, V30.6 of 100%, mean 52 Gy, maximum 56 Gy, and minimum 31 Gy (Fig. 2). Of note, this was the only patient with local progression, and this was accompanied by a spinal recurrence. The second patient (patient 6) with intracranial relapse had leptomeningeal relapse and all areas of recurrent disease had received a mean dose of 10 Gy or less (Fig. 3). Of note, 3 of 8 relapsed patients had a CR/continuous complete response, and 2 of these initially presented with bifocal disease. None of the PR relapsed patients had bifocal disease. Of note, there was no relationship between time to RT start and progression.
Table 2.
Patient-specific characteristics for patients with relapse
| Patient ID | Age (y) | Sex | Histology | Initial HCGß | Initial AFP | Tumor location | Postinduction response | Relapse location | Relapse detail |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 12 | M | Mixed GCT | ⬆ | ⬆ | Pineal | PR | Spine | T4–6, T9, L spine |
| 2 | 18 | M | NA | ⬆ | NL | Bifocal | CR | Brain and spine | Local, sacrum |
| 3 | 15 | M | NA | ⬆ | ⬆ | Bifocal | CR | Spine | Thecal sac |
| 4 | 18 | M | Embryonal carcinoma | NL | NL | Suprasellar | PR | Spine | C, T, L spine |
| 5 | 11 | M | Germinoma | ⬆ | ⬆ | Pineal | CCR | Spine | Lumbar mass |
| 6 | 17 | M | Mixed GCT | ⬆ | ⬆ | Pineal | PR | Brain and spine | LMD, C3, T2, T10–12, thecal sac |
| 7 | 6 | M | NA | NL | ⬆ | Ventricle | PR | Spine | T7, T8 |
| 8 | 11 | M | Embryonal carcinoma | ⬆ | ⬆ | Pineal | PR | Spine | C2, L3 through cauda equina |
Abbreviations: ⬆ = elevated; AFP = α-fetoprotein; C = cervical; CCR = continuous complete response; CR = complete response; GCT = germ cell tumor; HCGβ = β-human chorionic gonadotropin; ID = identification; L = lumbar; LMD = leptomeningeal disease; M = male; NA = not available; NL = normal; PR = partial response; T = thoracic.
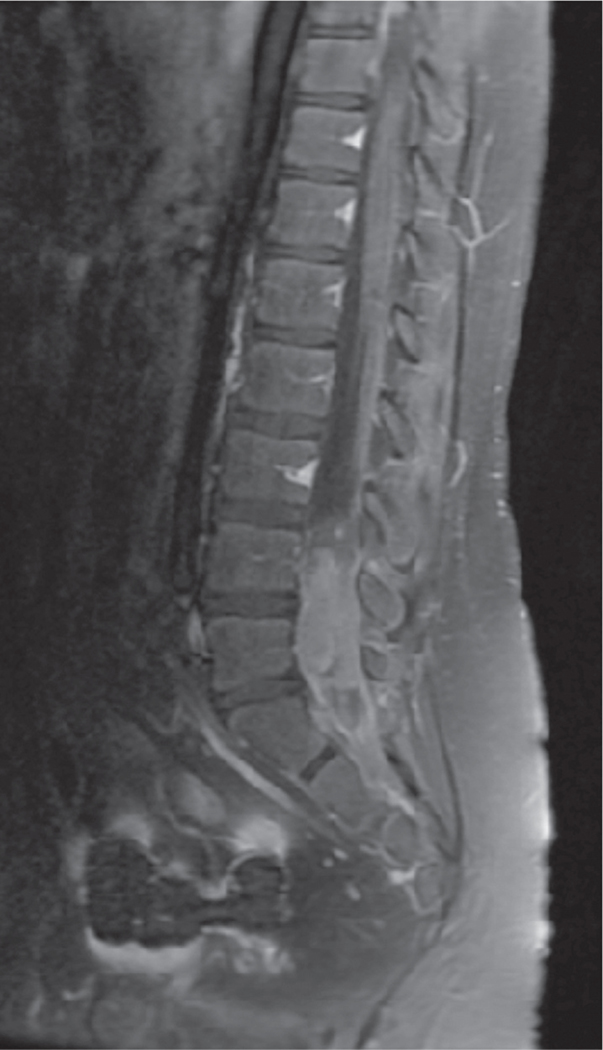
Fig. 1.
Patient 5 with pineal primary tumor location progressed in the spine. Representative sagittal contrast enhanced thoracolumbar spine magnetic resonance imaging (MRI) demonstrating progression with a lumbar mass.
Fig. 2.
Patient 2 with bifocal suprasellar and pineal location primaries with locoregional and distant progression. Axial fluid-attenuated inversion recovery (FLAIR) brain magnetic resonance imaging (MRI) of patient 2 demonstrating (a) intraventricular progression, (b) hypothalamic progression, and (c) prepontine space progression. (d) Sagittal computed tomography (CT) of the head demonstrating area of progression (dark blue contour) with radiation therapy (RT) dose coregistered (green color wash = 30.6 Gy whole ventricular irradiation [WVI], red color wash = 54 Gy primary site boost, red contour line: primary site boost volume)
Fig. 3.
Patient 6 with pineal primary location with multifocal leptomeningeal progression. Axial contrast-enhanced brain magnetic resonance imaging (MRI) demonstrating areas of progression (a) in the right temporal area (light blue contour), midfrontal area (light green contour) with radiation therapy (RT) dose coregistered (mint blue color wash = 18 Gy, purple color wash = 30.6 Gy, yellow color wash = 54 Gy primary site boost), and (b) bilateral cerebellar recurrence = light blue contour. (c) Contrast-enhanced sagittal thoracolumbar spine MRI demonstrating thecal sac leptomeningeal disease.
Discussion
Adherence to RT guidelines in clinical trials is critical in interpreting results appropriately and often can affect outcomes. Major deviations in RT protocol adherence can negatively affect outcomes across different cancer diagnoses.8,9 The Korean prospective trials G051 and G081 evaluated an upfront chemotherapy followed by response-adapted RT approach for central nervous system (CNS) germinoma (n = 91).8 These were reported together, and the RT adherence rate for both dose and volume was 43%; all 4 patients with recurrence in that trial were noncompliant with RT protocol guidelines. On a much larger scale, a prospective phase 3 international head and neck cancer trial evaluated RT with concurrent cisplatin plus tirapazamine for advanced head and neck cancer (Trans-Tasman Radiation Oncology Group 02.02 [TROG 02.02]) and determined that 208 (25%) of 820 RT plans were noncompliant.9 Those with major deficiencies in their treatment plans (n = 87 out of 502) had a significantly worse 2-year OS (50% vs 70%) and 2-year freedom from locoregional failure (54% vs 78%), compared with those whose treatment was compliant.9
Early RT plan review may have a positive effect on protocol compliance. For the early intervention review of the TROG 02.02 trial, diagnostic imaging and RT treatment plans for enrolled patients were to be submitted to IROC by the end of the first week of RT.9 Of the 687 patient plans submitted to IROC, changes were recommended in 197. In the 89 patient plans for which IROC recommendations were implemented, all were deemed to be compliant at Trial Management Committee (TMC) review. Conversely, when IROC changes were not implemented, 95 (88%) of 108 evaluable patients had noncompliant plans at TMC review. Some RT plans that initially passed the IROC interventional review were found to be noncompliant at TMC review; however, in 85% of these, the RT plans that were submitted for initial review were incomplete. This analysis emphasizes the value of early RT review resulting in compliant treatment and the importance of completeness of materials submitted.
The COG ACNS1123 study evaluated the outcomes of reduced RT for patients with nonmetastatic NGGCT with a CR/PR to induction chemotherapy. Early RT plan review was performed with the intent of standardizing the volumes and ensuring that dosing to targets and normal tissue constraints were performed per protocol. In addition, a contouring atlas was provided that allowed a visual guide with detailed written guidelines. Of the RT plans that underwent the early interventional review, 4 plans required modifications and all institutions were compliant with those suggested modifications. This resulted in excellent protocol adherence at the time of final central review, with the exception of 2 major deviations. One of the major deviations came from an RT plan that was not submitted on time for the early interventional review, and the wrong dose was prescribed. The second major deviation likely would have also been caught at the initial review, but the institution had only submitted the initial plan, and the deviation regarded the boost volume. The minor deviations were discussed with the study radiation oncologists in advance. Providing access to contouring guidelines and an atlas resulted in no deviations for the whole ventricular volume, which is a complex structure to delineate. Given the low number of deviations, the effect of number of patients treated per institution, type of institution, and RT modality could not be assessed for RT quality. Compliant RT data submission for early interventional review and requiring composite RT plans at that time may reduce major deviations for future trials. The addition of more study-specific radiation oncologists will help facilitate that review, and this is planned for the ACNS2021 (NCT04684368). Submission of appropriate tumor marker data and central review to determine treatment response will also be verified before initiation of RT.
The best 5-year survival outcomes for patients with NGGCT come from COG ACNS0122, which used induction chemotherapy followed by 36 Gy CSI and 54 Gy to the primary tumor/tumor bed.3 However, understanding that CSI can result in significant late effects, studies have evaluated chemotherapy followed by reduced-field RT for patients with NGGCT with localized disease. The COG ACNS1123 study specifically evaluated the outcomes of reduced RT (WVI plus tumor bed boost) for patients with nonmetastatic NGGCT with a CR/PR to induction chemotherapy. The International Society of Pediatric Oncology (SIOP) CNS GCT 96 trial used a different chemotherapy and RT approach for patients with localized disease.5 They treated 116 patients with NGGCT with a regimen of 4 cycles of dose-intense chemotherapy with cisplatin, etoposide, and ifosfamide followed by 54 Gy involved field RT, yielding a 5-year PFS rate of 72% and OS of 82%. There were 30 (26%) cases of progression during or after treatment. The majority of failures included a component of locoregional recurrence/progression (Table 3). None of these were defined in reference to the RT field; however, the conclusion stated that for localized malignant NGGCT, CSI could be avoided without increased relapses outside the RT field.
Table 3.
Induction chemotherapy followed by RT for nonmetastatic NGGCT
| Relapse location and number of patients | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Study | n | RT dose (Gy) and volume | PFS | Local only n (%) | Ventricular n (%) | Distant only n (%) | Local + distant n (%) | Any distant n* (%) |
|
| ||||||||
| COG ACNS 0122 (MO)3 | 79 | 36 CSI + 54 IF boost | 5-y: 92% | 9 (60) | 5 (33) | 1 (7) | 6 (40) | |
| SIOP GCT ‘962 | 116 | 54 IF | 5-y: 72% | 14 (47) “locoregional” | 7 (23) | 6 (20) | 13 (43) | |
| Mayo Clinic4 | 34 | 54–59.4 IF | 10-y: 68% | 4(36) | 3 (27) | 4† (36) | 4(36) | |
| ACNS 1123 | 66 | 30.6 WVI-54 Gy focal boost | 4-y: 88% | 7 (88) | 1 (12) | 8 (100) | ||
Abbreviations: CSI = craniospinal; GCT = germ cell tumor; IF = involved field; n = number of patients; NGGCT = nongerminomatous germ cell tumor; PFS = progression free survival; RT = radiation therapy; SIOP = International Society of Pediatric Oncology; WVI = whole ventricular irradiation.
Distant only and combined.
One additional recurrence was at an unknown location, and these numbers only include those relapsed after local therapy.
The Mayo Clinic published their experience of induction chemotherapy followed by RT for patients with NGGCT.4 Their series included 34 patients with focal and metastatic disease. For most patients with nonmetastatic presentation, focal RT was used as defined by the prechemotherapy primary tumor volume plus a 2-cm margin. A total of 15 patients experienced recurrence at a median of 1.9 (range, 0.6–19.3) years, 12 (80%) of whom had initially received involved field RT (Table 3). The pattern of failure from this smaller series of involved field RT demonstrated that 63% of patients had a locoregional component and 36% had a distant component.
As a comparison, with the use of WVI plus boost volume used in COG ACNS1123, there was only 1 locoregional failure and 100% of patients had a component of distant spinal failure. This pattern suggests that the volume and RT dose is adequate for intracranial control, but it is hard to reconcile this with the fact that the majority of failures (10 of 15) in COG ACNS 0122 were local. Regardless, the finding that all patients who failed had a component of failure in the spine and that the majority had isolated spinal relapse is worthy of attention.
Evaluation of the SIOP GCT experience revealed that isolated elevation of HCGβ was not associated with prognosis. However, 31 of 86 (36%) with isolated AFP elevation or combined elevation with HCGb relapsed.5 They also confirmed a worse 5-year PFS for a high AFP group (>1000 ng/mL) of 32%, compared with 76% for all other AFP levels (≤1000 ng/ mL). The Mayo Clinic series also found that elevated AFP in general was a poor prognostic variable in that all patients who developed spinal drop metastases had both CSF and serum elevations of AFP at presentation.4 Only 1 of our patients with relapse had normal markers at presentation. The majority had elevations of both HCGβ and AFP; however, there was no statistical association detected with PFS.
Seventeen of 27 (63%) relapsed patients on the SIOP-CNS-GCT-96 protocol died of disease, which suggests that salvage regimens need to be improved. An analysis of UK and German patients who were all initially treated as per the SIOP-CNS-GCT-96 protocol were evaluated for outcomes of treatment at time of relapse.1 This series included 32 relapsed patients with malignant NGGCT treated with curative intent, resulting in 5-year OS of 9% for all patients, 0% for those treated with standard-dose chemotherapy, and 14% for those treated with high-dose chemotherapy and autologous stem cell rescue, respectively. The only relapsed patients with NGGCT who survived were those whose relapse was limited to elevated HCGβ. Two smaller published series evaluating high-dose chemotherapy and autologous stem cell rescue for relapsed patients with NGGCT describe better outcomes,11,12 but these smaller series were more heterogeneous in their patient populations with respect to frontline treatment and may be less reliable. These data suggest that perhaps the risks of more comprehensive radiation may be worth considering because of the lack of effective salvage options. These results and the pattern of failure reported previously from ACNS1123 have influenced ACNS2021 which is the next COG localized NGGCT trial, which offers treatment with induction chemotherapy followed by response evaluation and whole ventricular plus spinal canal irradiation.
Conclusions
Early RT plan review and providing an online contouring atlas resulted in excellent protocol adherence at the time of final central review for this prospective trial. The very limited deviations did not account for the failures. Patterns of failure suggest the spine is at risk for recurrence for patients with localized NGGCT who receive reduced RT after achieving a CR/PR to induction chemotherapy. Although the survival data are encouraging, the fact that all 8 failures include relapse in the spine is difficult to ignore. COG ACNS2021, an intergroup NCTN phase 2 trial of chemotherapy followed by response-based whole ventricular and spinal canal irradiation for patients with localized nongerminomatous CNS germ cell tumor reintroduces radiation to the spine with the reduced dose of 30.6 Gy but keeps the brain volume to the whole ventricular volume rather than returning to the inclusion of the whole brain/CSI used in ACNS0122. This study opened in June 2021 for children and young adults up to age 30 and is currently enrolling patients.
Acknowledgments
Part of this study was presented at the 2018 American Society for Radiation Oncology (ASTRO) annual meeting and at the 2020 International Symposium on Pediatric Neuro-Oncology (ISPNO).
The work in this paper was supported by the Children’s Oncology Group NCTN Operations Center grant U10CA180886, NCTN Statistics & Data Center grant U10CA180899, and by St. Baldrick’s Foundation.
Footnotes
Disclosures: none.
Data sharing statement:
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Murray MJ, Bailey S, Heinemann K, et al. Treatment and outcomes of UK and German patients with relapsed intracranial germ cell tumors following uniform first-line therapy. Int J Cancer 2017;141:621–635. [DOI] [PubMed] [Google Scholar]
- 2.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics 1994;25:26–32. [DOI] [PubMed] [Google Scholar]
- 3.Goldman S, Bouffet E, Fisher PG, et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a children’s oncology group study. J Clin Oncol 2015;33:2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breen WG, Blanchard MJ, Rao AN, Daniels DJ, Buckner JC, Laack NNI. Optimal radiotherapy target volumes in intracranial nongerminomatous germ cell tumors: Long-term institutional experience with chemotherapy, surgery, and dose- and field-adapted radiotherapy. Pediatr Blood Cancer 2017;64. [DOI] [PubMed] [Google Scholar]
- 5.Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumorslessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol 2017;19:1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol 1997;32:71–80. [DOI] [PubMed] [Google Scholar]
- 7.Fangusaro J, Wu S, MacDonald S, et al. Phase II trial of response-based radiation therapy for patients with localized CNS nongerminomatous germ cell tumors: a children’s oncology group study. J Clin Oncol 2019;37:3283–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DS, Lim DH, Kim IH, et al. Upfront chemotherapy followed by response adaptive radiotherapy for intracranial germinoma: Prospective multicenter cohort study. Radiother Oncol 2019;138:180–186. [DOI] [PubMed] [Google Scholar]
- 9.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol 2010;28:2996–3001. [DOI] [PubMed] [Google Scholar]
- 10.Parzuchowski A, Bush R, Pei Q, et al. Patterns of involved-field radiation therapy protocol deviations in pediatric versus adolescent and young adults with Hodgkin lymphoma: A report from the children’s oncology group AHOD0031. Int J Radiat Oncol Biol Phys 2018;100:1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek HJ, Park HJ, Sung KW, et al. Myeloablative chemotherapy and autologous stem cell transplantation in patients with relapsed or progressed central nervous system germ cell tumors: Results of Korean Society of Pediatric Neuro-Oncology (KSPNO) S-053 study. J Neurooncol 2013;114:329–338. [DOI] [PubMed] [Google Scholar]
- 12.Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol 2004;22:1934–1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.