Abstract
Capsular hyaluronic acid (HA) mediates adhesion of serogroup A strains of Pasteurella multocida to elicited turkey air sac macrophages (TASM). In contrast, freshly isolated turkey peripheral blood monocytes (TPBM) do not bind serogroup A strains. Following culture of TPBM for 6 days in chamber slides, adhesion of the bacteria to TPBM increased gradually. Incubation in chamber slides coated with entactin-collagen IV-laminin (ECL) attachment matrix or exposure to phorbol myristate acetate (PMA) further enhanced the adhesion of P. multocida to TPBM. Addition of HA, but not Arg-Gly-Asp peptide, to TPBM culture inhibited bacterial adherence similarly to the inhibition previously reported for TASM. Exposure of TPBM to monoclonal antibody directed against HA-binding cell surface proteoglycan (CD44) decreased binding of P. multocida. Collectively, these findings indicate that P. multocida adhesion to TPBM is mediated by capsular HA and can be increased by culture on ECL attachment matrix or PMA exposure. Additionally, the findings suggest that the capsular mucopolysaccharide of serogroup A strains of P. multocida recognizes an isoform of CD44 expressed on cultured TPBM.
Pasteurella multocida causes fowl cholera, a widely distributed disease occurring in most poultry-producing countries of the world. Annual losses to the poultry industry were estimated at 200 million U.S. dollars in 1986 (11). Serogroup A strains of P. multocida are the major cause of fowl cholera in turkeys. Survival of P. multocida outside the host and resistance to phagocytosis in nonimmunized birds are associated with the presence of a capsule. With serogroup A strains of P. multocida, the capsule contains hyaluronic acid (HA), an anionic polysaccharide composed of repetitive disaccharidic units of d-glucuronic acid and N-acetyl-d-glucosamine. Invasion by P. multocida is believed to occur through the lymphoid tissues of the respiratory tract, and the bacterial capsule is suspected to play a major role in this event.
Previous studies in our laboratory demonstrated that serogroup A strains of P. multocida adhere to turkey air sac macrophages (TASM) but are not internalized. Although we showed that bacterial adhesion to TASM occurs through specific recognition of capsular HA by a cell surface glycoprotein (10), the host cell receptor was not identified. CD44, an 85-kDa transmembrane glycoprotein found on a variety of cell types, is one of several receptors capable of binding HA. Because CD44 is an HA receptor also associated with lung macrophages (3, 5), we suspected that it might be involved in adhesion of serogroup A strains of P. multocida to TASM. However, with avian species, the number of resident macrophages in the lungs and air sacs is low and recovery by lavage is poor (13). Consequently, our initial attempts to isolate CD44 from TASM failed due to our inability to acquire a sufficient number of cells. This failure prompted a study to determine whether freshly isolated turkey peripheral blood monocytes (TPBM) could replace elicited TASM. We found that serogroup A strains of P. multocida do not adhere to freshly isolated TPBM. Similarly, others have reported that freshly isolated peripheral blood monocytes do not bind soluble HA but that in vitro culture for 8 to 16 h (7), as well as exposure to phorbol myristate acetate (PMA) (8), will increase HA binding. HA is recognized as a major component of the extracellular matrix in animals. Interactions of blood monocyte HA binding proteins with the extracellular matrix have a central role in their tissue-specific migration, differentiation, and function. These HA binding proteins, although binding with higher affinity to HA, also bind fibronectin, collagen (I, II, and IV), and laminin (1, 14, 15).
The goals of this study were to determine whether 6-day culture, exposure to PMA, or culture on entactin-collagen IV-laminin (ECL) attachment matrix of freshly isolated TPBM would increase adhesion of serogroup A P. multocida.
MATERIALS AND METHODS
Animals.
Twelve- to 24-week-old male Beltsville small white turkeys from the closed flock at the National Animal Disease Center were used. Females were not used due to their high serum lipid concentration, which impeded isolation of blood monocytes by centrifugation procedures.
Monoclonal antibodies.
The following monoclonal antibodies purchased from Sigma Chemical Co. (St. Louis, Mo.) were used: mouse anti-human CD44 monoclonal antibody (immunoglobulin G1 [IgG1] isotype derived from hybridoma A3D8), fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD44 monoclonal antibody (mouse IgG1 derived from hybridoma A3D8), and the nonspecific mouse monoclonal IgG (IgG1).
Bacteria.
Capsulated P. multocida P-1059 (serotype A:3) was grown overnight at 37°C on dextrose starch agar (Baltimore Biological Laboratories, Cockeysville, Md.). The bacterial cells were resuspended in RPMI 1640 without sodium bicarbonate and phenol red (Sigma Chemical Co.). The bacterial suspension was adjusted to a density equivalent to that of a no. 1 McFarland nephelometer standard (109 bacteria/ml) with a spectrophotometer (model 35; Perkin-Elmer, Oak Brook, Ill.).
TPBM collection.
A two-step gradient procedure for isolation of monocytes was used as follows. Turkey peripheral blood was collected by venipuncture of the brachycephalic vein into EDTA-treated Vacutainer tubes (Becton Dickinson and Company, Franklin Lakes, N.J.). Pooled blood from three birds was diluted 1:3 with RPMI 1640 containing l-glutamine but lacking sodium bicarbonate (Sigma Chemical Co.). When necessary, penicillin (100 U/ml), streptomycin (50 μg/ml), and fungizone (2 μg/ml) were added (mRPMI). For separation of leukocytes, 6 ml of diluted blood was layered onto 3 ml of density gradient medium (Accu-Prep; specific gravity, 1.077; Accurate Chemical & Scientific Corporation, Westbury, N.Y.) in 13- by 100-mm tubes and the tubes were centrifuged at 800 × g for 20 min at 22°C. The leukocyte layer above the gradient medium was collected and diluted 1:3 with mRPMI. Six milliliters of the diluted suspension was applied to 3 ml of a second density gradient medium (1-Step Monocytes; specific gravity, 1.068; Accurate Chemical & Scientific Corporation) in 13- by 100-mm tubes. The tubes were centrifuged at 600 × g for 20 min at 22°C. After centrifugation, the clear plasma down to within 3 to 4 mm of the interface was discarded. The gradient, including the interface, was removed to a level just above the cell pellet and diluted 1:3 with saline (0.85% NaCl) containing 0.13% EDTA to reduce the density of the solution. The diluted gradient was centrifuged at 600 × g for 10 min at 22°C. The cell pellet containing the monocytes was resuspended in mRPMI supplemented with 10% heat-inactivated fetal bovine serum (ΔmRPMI), and cell counts were made with a cell counter (Nova Cell Track; Alicia Diagnostics, Oveido, Fla.). Cell viability was assessed with propidium iodide dye. Briefly, 25 μl of propidium iodide (0.5 mg/ml) was added to 100 μl of the monocyte suspension. This suspension was gently mixed and incubated for 5 min at 22°C before being spread onto a hemacytometer. Counts were made by light and fluorescence microscopy; dead cells fluoresced orange. Cell viability above 85% was the minimum requirement for all experiments. Isolated monocytes were seeded (5 × 105/cm2) in four-well chamber slides (Lab Tek; Nunc Inc., Naperville, Ill.). Two wells were used for a treatment, and the other two wells of the slide were used as controls.
Adhesion assays.
Expression of P. multocida receptor by cultured TPBM was investigated daily for 6 days by adhesion assays. To perform the assays, TPBM cultures were rinsed with warm (37°C) RPMI supplemented with 10% heat-inactivated fetal bovine serum (ΔRPMI) followed by reincubation for 1 h with warm ΔRPMI. The cultures were rinsed with warm ΔRPMI, and adjusted suspensions of P. multocida were added to a final concentration of 100 bacteria/monocyte. Culture slides were incubated for 1.5 h at 37°C and 5% CO2. The slides were rinsed three times with warm ΔRPMI to remove nonadherent bacteria and stained with Diff Quick (Baxter Healthcare Corporation, McGaw Park, Ill.). Adhesion of P. multocida was evaluated by light microscopy by counting the total number of adherent bacteria on 200 to 400 randomly chosen monocytes in two wells of the four-well chamber slide. The other two wells of the slide were used as controls. All experiments were repeated three times with one replicate per experiment.
Up-regulation of bacterial adhesion.
To determine whether ECL attachment matrix (Promega, Madison, Wis.) would enhance bacterial adhesion, 15 μg of ECL cell attachment matrix/cm2 was added directly onto chamber slides according to the instructions provided by the manufacturer. The wells were seeded with 5 × 105 monocytes/cm2 in ΔmRPMI, and the cultures were incubated for 6 days at 37°C in a 5% CO2 atmosphere. For controls, uncoated chamber slides were treated similarly, as described above.
To determine whether PMA (Sigma Chemical Co.) would increase bacterial adhesion, cultured TPBM were exposed to 15 ng of PMA/ml of ΔmRPMI for 12 min at 37°C and 5% CO2 on days 1 and 5 of incubation. Following exposure, the culture medium was removed and replaced by warm ΔmRPMI. On day 6, 5 h before adhesion assays were performed, TPBM were reexposed to PMA as before.
Adherence inhibition studies.
Cell surface proteoglycans recognize specific amino acid sequences or extracellular polysaccharides of the extracellular matrix. We previously showed that capsulated P. multocida adhesion to elicited TASM occurs through specific recognition of the capsular polysaccharide by a cell surface glycoprotein (10). Others have shown that adhesion molecules can also bind to a specific amino acid sequence of extracellular matrix components, such as the Arg-Gly-Asp peptide of laminin, fibronectin, and collagen (9). To determine whether the P. multocida receptor on cultured TPBM is the same as that previously described for TASM, 6-day-old TPBM were incubated with 2.5 mg of HA (Sigma Chemical Co.)/ml of ΔRPMI or 1 mg of Arg-Gly-Asp peptide (Sigma Chemical Co.)/ml of ΔRPMI for 1.5 h at 37°C and 5% CO2. The culture fluid was discarded, the chamber slides were gently rinsed with warm ΔRPMI, and the TPBM were used in adhesion assays.
To determine whether CD44 functions as a receptor for P. multocida on cultured TPBM, 6-day-old monocytes were exposed for 30 min to 30 μg of monoclonal anti-CD44/5 × 105 TPBM in 1 ml of warm RPMI at 37°C and 5% CO2 before adhesion assays were done. Antibodies were not removed during these assays. As a control, isotype-matched nonspecific mouse monoclonal IgG1 was used under the same conditions described above.
Fluorescence microscopy.
Fluorescence microscopy was used to confirm CD44 expression by TPBM as follows: (i) freshly isolated TPBM and 6-day-old cultured TPBM were washed three times in warm RPMI; (ii) the TPBM were fixed for 5 min in methanol at 4°C, dehydrated for 5 min in acetone at 4°C, and air dried; (iii) nonspecific binding sites on the TPBM (4 × 106 cells) were blocked for 3 h at 22°C with a 1:25 dilution of mouse isotype-matched control IgG1 in phosphate-buffered saline (PBS; pH 8); (iv) the slides were washed three times with PBS; (v) the TPBM were labeled with a 1:25 dilution of FITC-conjugated anti-human CD44 in PBS for 3 h at 22°C; (vi) the culture slides were washed three times with PBS and rinsed with distilled H2O; and (vii) following the washes, coverslips were immediately mounted with mounting medium (Vector Laboratories, Inc., Burlingame, Calif.). To determine whether false-positive reactions might arise due to monoclonal antibody Fc segments binding to monocyte Fc receptors, FITC-labeled anti-CD44 F(ab′)2 was also tested for 6-day-cultured TPBM as described above but starting at step v. The Fc portion of the anti-CD44 IgG was removed with the Immunopure F(ab′)2 kit from Pierce (Rockford, Ill.) according to the instructions provided by the manufacturer. Preparation fluorescence was observed with a BX50 microscope equipped with a reflected fluorescent light attachment and a U-MNIBA filter cube (Olympus Optical Co., Tokyo, Japan).
Statistical analysis.
Because the distribution was clearly skewed, the median rather than the mean of the observations was used for each experiment. The median value is a better summary statistic of the response. The means were calculated from the median values of three repeated experiments. A standard two-sample t test was used to compare treatment and control groups. For the kinetics of adhesion experiments, a two-way analysis of variance with blocking was used.
RESULTS
TPBM collection.
Cells isolated by the two-step gradient procedure consisted of monocytes as the main adherent cell population, with small numbers of thrombocytes and nonadherent lymphocytes. Heterophils and erythrocytes were excluded from the monocyte fraction, and lymphocytes were removed by rinsing the slides after the adherence of monocytes.
TPBM cultures and bacterial adhesion.
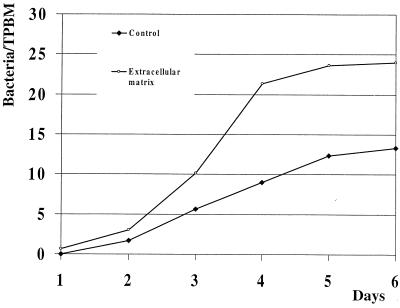
Due to meniscus formation by the media in the chamber slide wells, monocytes and bacteria tended to accumulate at the edges of the wells. Consequently, uniformly distributed monolayers were seen in the corners of wells but not at their centers. For each adhesion assay, the median number of bacteria per TPBM was determined from counts made on 200 to 400 monocytes located along a diagonal line from corner to corner of two wells, using 63× magnification with oil. In each experiment, the blood from three birds was pooled. Because of nonuniform repartition of monocytes in a well and the use of pooled blood, the distribution of the observations was skewed. Nevertheless, adhesion of encapsulated P-1059 cells to cultured TPBM increased daily during the 6-day observation period to reach a maximum by day 6 (P < 0.05 [Fig. 1]). However, when data from each experiment was considered individually, maximum adhesion occurred at day 4 in experiment 1 and day 6 in experiments 2 and 3. Adhesion was significantly enhanced (P < 0.05 [Fig. 1]) when the chamber slides were coated with ECL attachment matrix.
FIG. 1.
Kinetics of P. multocida adhesion to cultured TPBM. The value at each time point is the mean of the median numbers of bacteria per monocyte from three experiments (one replicate per experiment).
Influence of PMA on bacterial adhesion.
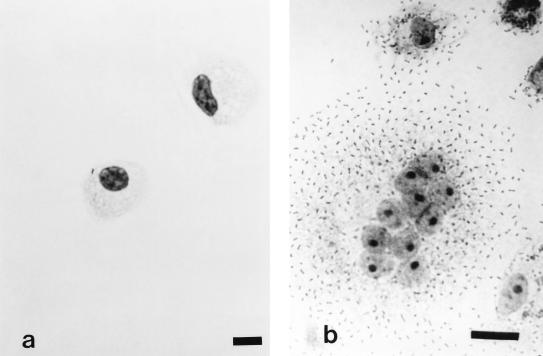
Treatment of TPBM cultures with PMA, a diacylglycerol analogue, resulted in significantly increased bacterial adherence to monocytes (Table 1). Light microscopic examination showed that treated monocytes increased in size and were often multinucleated compared to controls (Fig. 2). Based upon this observation, counts of bacteria per monocyte were determined by dividing the total number of bacteria per monocyte by the number of nuclei within the observed cell. This condition was applied for each of the experimental trials. Although bacterial adhesion was up-regulated, the bacteria were not internalized, as determined by a double-fluorescence technique (10).
TABLE 1.
Influence of various treatments (HA, PMA, and anti-CD44) on in vitro adhesion of P. multocida to cultured TPBM
| Treatment | Median no. of P. multocida/TPBMa | P valueb | Effect of treatment |
|---|---|---|---|
| HA | 1 | 0.0003 | Adhesion inhibition |
| None | 17.5 | ||
| PMA | 29.67 | 0.021 | Increased adhesion |
| None | 7.33 | ||
| Anti-CD44 | 3.33 | 0.0404 | Adhesion inhibition |
| None | 25.67 |
Average median value of bacteria per monocyte for three experiments (one replicate per experiment).
Probability that treatment results in a highly significant difference. Results are statistically different when P values are less than 0.05.
FIG. 2.
Diff Quick stain of encapsulated P. multocida adhering to cultured TPBM. (a) Day 1, control (bar = 9.1 μm); (b) day 6, after exposure to PMA (bar = 18.2 μm).
Adhesion inhibition studies.
Treatment of cultured TPBM with HA or anti-human CD44 monoclonal antibody inhibited adherence of the encapsulated bacteria (Table 1). However, Arg-Gly-Asp peptide treatment or nonspecific isotype-matched mouse IgG1 (control) did not (data not shown).
Fluorescence studies.
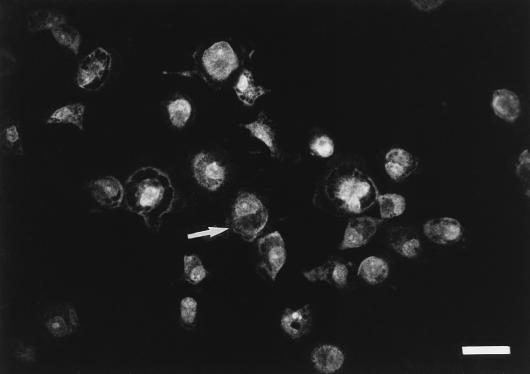
The membranes and cytoplasms of freshly isolated TPBM were weakly stained by FITC-labeled anti-human CD44, whereas 6-day-old cultured TPBM were homogeneously and intensely stained. Incubation with isotype-matched control IgG1 did not suppress fluorescence. Although removal of the Fc portion from the specific antibody did not abolish fluorescence, the intensity of the reaction was reduced and photobleaching increased (Fig. 3).
FIG. 3.
Fluorescence of cultured turkey blood monocytes with FITC-labeled monoclonal anti-human CD44 F(ab′)2. The arrow shows cytoplasmic fluorescence staining of the monocyte. Bar = 25.2 μm.
DISCUSSION
The respiratory systems of avian species differ markedly from those of mammals. After entering the lung, air does not terminate in an alveolus. Air continues through the lung to the thoracic and peritoneal air sacs of the bird, thereby allowing gas exchanges at both inspiration and expiration. Although air exchange efficiency is very high, the defense mechanisms against airborne pathogens are suggested to be poorly developed due to the paucity of resident lung and air sac macrophages (13).
In turkeys, pneumonia and airsaculitis are common features of acute as well as chronic cases of fowl cholera. It is believed that damaged air sac epithelium is one portal of entry for P. multocida in naturally occurring cases of fowl cholera. Ficken and Barnes (4) reported that experimental inoculation of air sacs with P. multocida is followed by an acute, marked heterophilic exudation, with macrophage accumulation occurring to a lesser extent later in the inflammatory reaction. However, nonopsonic phagocytosis by these macrophages was not observed and adhesion of P. multocida to the macrophages was not described. Although we previously described P. multocida adherence to elicited air sac macrophages (10), we found that adherence did not occur with freshly isolated peripheral blood monocytes. Because elicited air sac macrophages most likely arise from peripheral blood monocytes, expression of HA receptor could be a hallmark of differentiation or activation of these phagocytes.
Bacterial adhesion was stimulated by culturing monocytes in vitro for 6 days. Others have shown and our own experience indicates that the presence of serum is required for monocyte activation. In this study, however, heat-inactivated serum did not induce opsonization-mediated adhesion of the bacteria. Adhesion was not blocked by exposure of monocytes to the Arg-Gly-Asp peptide but was significantly decreased after treatment with HA. This evidence strongly suggests that adhesion of P. multocida to TASM as well as cultured TPBM is mediated by the mucopolysaccharide of the bacterial capsule.
HA is a major component of the host extracellular matrix, as well as the principal capsular component of serogroup A strains of P. multocida. Macromolecules of the extracellular matrix are believed to be involved in the regulation of various cellular functions, including cell adhesion, motility, growth, and differentiation in vitro (6). These functions are mediated by HA interacting with cell surface proteoglycans expressed on various cell types, of which macrophages are one. Although these proteoglycans bind with high affinity to HA, they can also bind to fibronectin, collagen (I, II, and IV), and laminin. We hypothesized that culturing TPBM on ECL extracellular matrix would enhance expression of HA-binding protein and therefore increase bacterial adhesion. Our findings confirmed this hypothesis.
CD44H, the hematopoietic isoform of CD44, is a major protein expressed on human monocytes and lymphocytes. In the murine thymoma cell line BW5147, the cytoplasmic domain of CD44 interacts with ankyrin of the cytoskeleton, and this interaction is essential for HA binding. This event is mediated by protein kinase C (2). PMA is a diacylglycerol analogue, and diacylglycerol is known to trigger the activation and translocation of protein kinase C. We suspected that CD44 was the receptor for P. multocida on cultured TPBM and that triggering protein kinase C with PMA would enhance expression of monocytic CD44 or activate a non-HA binding CD44 receptor into its HA binding configuration. In this study, we found that bacterial adhesion was enhanced after exposure to PMA. Fluorescence microscopy clearly demonstrated enhanced expression of CD44 by TPBM cultured for 6 days compared to that of freshly isolated TPBM.
In conclusion, we have shown that P. multocida adhesion to TPBM is mediated by capsular HA and can be enhanced by culture on ECL attachment matrix or PMA exposure. Recognition of the capsular mucopolysaccharide is due to a monocytic CD44 isoform present on cultured TPBM. Recently, CD44 was described as a receptor for adherence of group A Streptococcus (12). Our report is the first describing CD44 as a receptor for adherence of gram-negative bacteria. Recognition of CD44, a membrane glycoprotein involved in cell-matrix interactions, may be used by P. multocida to invade the host tissue. The isolation of this receptor from activated turkey blood monocytes awaits further study.
ACKNOWLEDGMENT
We thank Zhaohui Wu, Department of Statistics, Iowa State University, Ames, for assistance with statistical analysis.
REFERENCES
- 1.Bussey H. Cell shape determination: a pivotal role for Rho. Science. 1996;272:224–225. doi: 10.1126/science.272.5259.224. [DOI] [PubMed] [Google Scholar]
- 2.Chopra R K, Holbrook N J, Powers D C, McCoy M T, Adler W H, Nagel J E. Interleukin-2, interleukin-2 receptor, and interferon-gamma synthesis and mRNA expression in phorbol myristate acetate and calcium ionophore A23187-stimulated T cells from elderly humans. Clin Immunol Immunopathol. 1989;53:297–308. doi: 10.1016/0090-1229(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 3.Culty M, O’Mara T E, Underhill C B, Yeager H, Jr, Swartz R P. Hyaluronan receptor (CD44) expression and function in human peripheral blood monocytes and alveolar macrophages. J Leukoc Biol. 1994;56:605–611. doi: 10.1002/jlb.56.5.605. [DOI] [PubMed] [Google Scholar]
- 4.Ficken M D, Barnes H J. Acute airsacculitis in turkeys inoculated with Pasteurella multocida. Vet Pathol. 1989;26:231–237. doi: 10.1177/030098588902600307. [DOI] [PubMed] [Google Scholar]
- 5.Green S J, Tarone G, Underhill C B. Distribution of hyaluronate and hyaluronate receptors in the adult lung. J Cell Sci. 1988;89:145–156. doi: 10.1242/jcs.90.1.145. [DOI] [PubMed] [Google Scholar]
- 6.Hay E D. Glycosaminoglycans in morphogenesis. In: Hay E D, editor. Cell biology of extracellular matrix. New York, N.Y: Plenum Press; 1991. pp. 259–294. [Google Scholar]
- 7.Levesque M C, Haynes B F. In vitro culture of human peripheral blood monocytes induces hyaluronan binding and up-regulates monocytes variant CD44 isoform expression. J Immunol. 1996;156:1557–1565. [PubMed] [Google Scholar]
- 8.Liao H X, Levesque M C, Patton K, Bergamo B, Jones D, Moody M A, Telen M J, Haynes B F. Regulation of human CD44H and CD44E isoform binding to hyaluronan by phorbol myristate acetate and anti-CD44 monoclonal and polyclonal antibodies. J Immunol. 1993;151:6490–6499. [PubMed] [Google Scholar]
- 9.Lin E C, Ratnikov B I, Tsai P M, Carron C P, Myers D M, Barbas III C F, Smith J W. Identification of a region in the integrin beta 3 subunit that confers ligand binding specificity. J Biol Chem. 1997;272:23912–23920. doi: 10.1074/jbc.272.38.23912. [DOI] [PubMed] [Google Scholar]
- 10.Pruimboom I M, Rimler R B, Ackermann M R, Brogden K A. Capsular hyaluronic acid-mediated adhesion of Pasteurella multocida to turkey air sac macrophages. Avian Dis. 1996;40:887–893. [PubMed] [Google Scholar]
- 11.Rhoades K R, Rimler R B. Fowl cholera. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. London, United Kingdom: Academic Press Ltd.; 1989. pp. 95–113. [Google Scholar]
- 12.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. J Clin Investig. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth T E, Siegel P B, Veit H. Cellular defense of the avian respiratory tract: paucity of free-residing macrophages in the normal chicken. Avian Dis. 1986;30:67–75. [PubMed] [Google Scholar]
- 14.Turley E, Moore D. Hyaluronate binding proteins also bind to fibronectin, laminin and collagen. Biochem Biophys Res Commun. 1984;121:808–814. doi: 10.1016/0006-291x(84)90750-2. [DOI] [PubMed] [Google Scholar]
- 15.Turley E, Brassel P, Moore D. A hyaluronate-binding protein shows a partial and temporally regulated codistribution with actin on locomoting chick heart fibroblasts. Exp Cell Res. 1990;187:243–249. doi: 10.1016/0014-4827(90)90087-q. [DOI] [PubMed] [Google Scholar]