Abstract
Killing of Staphylococcus aureus and Candida albicans by neutrophils involves adherence of the microorganisms, phagocytosis, and a collaborative action of oxygen reactive species and components of the granules. While a number of intracellular signalling pathways have been proposed to regulate neutrophil responses, the extent to which each pathway contributes to the killing of S. aureus and C. albicans has not been clearly defined. We have therefore examined the effect of blocking one such pathway, the extracellular signal-regulated protein kinase (ERK) cascade, using the specific inhibitor of the mitogen-activated protein kinase/ERK kinase, PD98059, on the ability of human neutrophils to kill S. aureus and C. albicans. Our data demonstrate the presence of ERK2 and a 43-kDa form of ERK but not ERK1 in human neutrophils. Upon stimulation with formyl methionyl leucyl phenylalanine (fMLP), the activities of both ERK2 and the 43-kDa form were stimulated. Despite abrogating the activity of both ERK forms, PD98059 only slightly reduced the ability of neutrophils to kill S. aureus or C. albicans. This is consistent with our finding that PD98059 had no effect on neutrophil adherence or degranulation, although pretreatment of neutrophils with PD98059 inhibited fMLP-stimulated superoxide production by 50%, suggesting that a change in superoxide production per se is not strictly correlated with microbicidal activity. However, fMLP-stimulated chemokinesis was markedly inhibited, while random migration and fMLP-stimulated chemotaxis were partially inhibited, by PD98059. These data demonstrate, for the first time, that the ERK cascade plays only a minor role in the microbicidal activity of neutrophils and that the ERK cascade is involved primarily in regulating neutrophil migration in response to fMLP.
Neutrophils are prominent phagocytic leukocytes of the acute inflammatory response. Under the influence of chemoattractants, these cells rapidly migrate into and are activated at sites of inflammation, resulting in microbial killing. Previous studies have demonstrated that the killing of Staphylococcus aureus and Candida albicans by neutrophils involves adherence of the microorganisms via CR3 receptors, phagocytosis, and the coordinated stimulation of the respiratory burst and degranulation, which results in the release of oxygen-derived reactive species such as H2O2 and granule constituents such as myeloperoxidase into the phagolysosomes (14, 18, 29). While the intracellular signalling pathways which regulate key neutrophil responses have been partly characterized, the extent to which these pathways contribute to the killing of S. aureus and C. albicans remains to be clearly defined. Also, it is not clear how chemoattractants stimulate the migration of neutrophils into sites of infection and inflammation.
The chemotactic peptide, formyl methionyl leucyl phenylalanine (fMLP), and other neutrophil ligands, such as interleukin 8 (IL-8), complement 5a, granulocyte-macrophage colony-stimulating factor, platelet activating factor, phorbol 12-myristate 13-acetate, opsonized zymosan, and leukotriene B4, have all been demonstrated to stimulate the activity of the extracellular signal-regulated protein kinase (ERK) subfamily of the mitogen-activated protein kinase (MAP kinase) (11, 13, 26–28, 30). This implies that the ERK cascade may play an important role in regulating neutrophil function. The activities of ERK1 (44 kDa) and ERK2 (42 kDa) are regulated by a cascade of upstream kinases which include the serine/threonine kinase, Raf-1, and the dual-specificity kinases, MAP kinase/ERK kinase 1 (MEK 1) and MEK 2 (7). Dual phosphorylation of the TEY motif on ERK by MEK results in the activation of ERK. In neutrophils, there are some uncertainties regarding the molecular forms of ERK that are expressed and which forms are activated. While the expression of 40-, 41-, 42-, and 44-kDa forms of ERK have been reported in neutrophils (10, 11, 13, 26), some studies have reported the expression and activation of only one form of ERK (41- or 42-kDa form) in neutrophils (11, 26). Thus, the expression and activation of ERK forms in neutrophils require further characterization.
The aims of the present study were, therefore, to further characterize the expression and activation of ERK forms in neutrophils and to investigate their roles in neutrophil-mediated killing of S. aureus or C. albicans and in fMLP-stimulated migration. Our data show that human neutrophils express ERK2 and a 43-kDa form of ERK, but not ERK1, and that the ERK cascade plays a major role in regulating neutrophil migration but only a minor role in the killing of S. aureus and C. albicans.
MATERIALS AND METHODS
Reagents.
fMLP and general reagents were obtained from Sigma Chemical Company. The MEK inhibitor, PD98059, was obtained from New England Biolabs, Inc., Beverly, Mass. The anti-ACTIVE ERK antibody was obtained from Promega and anti-MAP kinase antibodies, R1 (ERK1-NT) and R2 (ERK1-CT), were from Upstate Biotechnology Inc. Enhanced chemiluminescence solutions and reinforced nitrocellulose were from Dupont-NEN and Schleicher and Schuell, respectively. fMLP and PD98059 were dissolved in dimethyl sulfoxide (DMSO), and the final concentration of the vehicle was ≤0.2% (vol/vol). Control cells received vehicle (up to 0.2%), which did not affect neutrophil responses.
Isolation and incubation of neutrophils.
Human neutrophils were isolated from the peripheral blood of healthy volunteers by the rapid single-step method of Ferrante and Thong (8). Only preparations of >98% purity were used. These cells were >99% viable as judged by the ability of viable cells to exclude trypan blue. Cells, in Hanks buffered salts solution (HBSS), were incubated in a humidified atmosphere of CO2-air (5:95) in the presence of fMLP and inhibitor for the times indicated.
Preparation of cellular extracts.
Incubations were terminated by removing the incubation medium and washing the cells once with HBSS (4°C). For ERK assays, pelleted cells were sonicated in buffer A (25 mM Tris-HCl [pH 7.5], 2 mM EGTA, 25 mM NaCl, 1 mM Na3VO4, 38 mM p-nitrophenylphosphate, pepstatin A [10 μg/ml], aprotinin [10 μg/ml], leupeptin [10 μg/ml], 0.2 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol) and centrifuged (100,000 × g for 20 min), and the supernatants (termed cytosolic fractions) were collected. The fractions were batch adsorbed onto phenylsepharose CL4B to partially purify ERK (2). After washing the beads with 10% (2×) and 35% (2×) ethylene glycol in buffer A (vol/vol), ERK was eluted with 60% ethylene glycol. Previous studies have demonstrated that phenylsepharose-adsorbed ERK1 and ERK2 are eluted by ethylene glycol at concentrations between 35 and 60% (2). In some experiments, the cytosolic fractions, after the addition of Laemmli buffer, were stored frozen (−20°C) until analyzed by Western blotting as described below.
Assay for ERK activity.
ERK activity was assayed as described previously (15, 17). Briefly, partially purified ERK was added to assay mixture (25 mM Tris-HCl [pH 7.4], 50 mM β-glycerophosphate, myelin basic protein [0.33 mg/ml], 1.5 mM EGTA, 0.1 mM sodium orthovanadate, 10 mM MgCl2, protein kinase A (PKA) peptide inhibitor [10 μg/ml], 40 μM ATP, and 0.1 μCi of [γ32P]ATP), and the mixture was incubated for 20 min at 30°C. Assays were terminated by spotting aliquots of the reaction mixture onto P81 filter paper. After washing with 75 mM orthophosphoric acid, the radioactivity associated with the paper was determined by liquid scintillation spectrometry. There was no detectable PKA activity in phenylsepharose-purified fractions since omission of the PKA peptide inhibitor from the assay mixture did not result in increased phosphorylation of myelin basic protein (reference 2 and data not shown). Since the assay mixture did not contain phospholipids or calcium, it is unlikely that PKC or Ca2+/calmodulin-dependent kinases were responsible for phosphorylating myelin basic protein in these samples. We have also excluded the possibility that contaminating p38, if present in the fractions, contributed to the kinase activity that was detected (16).
Maintenance of WB cells.
WB rat liver epithelial cells were maintained in modified Eagle’s medium which had been supplemented with vitamins, essential and nonessential amino acids, antibiotics, and 10% fetal calf serum as described previously (17). Cells were plated at a density of 0.25 × 106/10-cm-diameter dish and used 4 days after plating. To prepare cellular extracts for Western blotting, cells were sonicated in buffer A and centrifuged and, after the addition of Laemmli buffer, samples were stored at −20°C until use.
Western blotting.
Denatured proteins were separated on 10% polyacrylamide gels and were transferred to nitrocellulose (100 V; 1.5 h). Immediately after transfer, blots were stained with Ponceau S (0.1% in 5% acetic acid) to confirm equal loading of all lanes of the gels. Affinity-purified polyclonal anti-ACTIVE ERK antibody was used to detect dual phosphorylated (active) ERK forms. In some cases, blots were probed with anti-MAP kinase R1 or R2. Immunocomplexes were detected by enhanced chemiluminescence (17).
Fungicidal and bactericidal assays.
These assays were carried out essentially as described previously (20). Briefly, neutrophils (5 × 106), preincubated with PD98059 or DMSO (0.1% vol/vol) for 45 min, were mixed with S. aureus (106 cells of strain NTCL6571; National Collection of Culture Type, London, United Kingdom) or C. albicans (106 cells isolated from a patient within our hospital), pooled human AB serum (10%, vol/vol) and sufficient PD98059 to maintain the concentrations of PD98059 at the desired levels. After gassing with CO2-air (5:95), the tubes were incubated with end-to-end mixing. At the indicated times, aliquots were taken, diluted with sterile water and plated onto blood agar (S. aureus) or Sabouraud agar (C. albicans). After incubation for 24 h (S. aureus) or 48 h (C. albicans) at 37°C, colonies were counted. Neither PD98059 nor DMSO per se affected microbial growth.
Superoxide production.
Superoxide production was measured by monitoring the chemiluminescence resulting from the oxidation of lucigenin (9,9′,-bis-N-methyl-acridinium nitrate). Briefly, 106 cells were preincubated with DMSO (0.1%, vol/vol) or PD98059 for 45 min at 37°C. Lucigenin (250 μM, dissolved in HBSS, and fMLP (50 nM) or DMSO (0.1%, vol/vol) were added, and the resulting chemiluminescence (in millivolts) was recorded with a luminometer (model 1250 or 1251; Bio-Orbit Oy, Turku, Finland) with MultiUse software. Results are expressed as the maximum rate of superoxide production (in millivolts) achieved during a 1-min period.
Neutrophil migration. (i) Under-agarose technique.
Cells were preincubated with PD98059 or DMSO as described above, and excess medium was removed to obtain a cell density of 4 × 107/ml. Neutrophil locomotion was determined as described previously (22) with minor modifications (9). Briefly, sets of three wells (2.5-mm diameter, 3 mm apart) were made in agarose plates (60-mm-diameter culture dishes). To assess the chemotactic response, fMLP (5 μl of 5 × 10−8 M), neutrophils (5 μl), and DMSO (5 μl of 0.1% [vol/vol] DMSO in HBSS) were added to the outer, center, and inner wells, respectively. To determine random migration, DMSO was added to both the outer and inner wells. To assess chemokinesis, cells were stimulated with fMLP for 5 min before being placed in the wells. Control cells were exposed to DMSO. After the addition of cells, the plates were incubated in a humidified atmosphere at 37°C for 90 min and the distances migrated (in millimeters) were measured under an inverted microscope. The maximum concentration of DMSO that the cells were exposed to was 0.2% (vol/vol), which had no effect on migration (data not shown).
(ii) Filter assay.
To assess the effect of PD98059 on the migration of neutrophils across a filter, cells (106), preincubated with PD98059 or DMSO and resuspended in RPMI-1640 (200 μl) that had been supplemented with bovine serum albumin (2%, wt/vol), were placed in filter chambers (Millicell culture plate inserts with 3-μm-pore-size polycarbonate filters; Millipore). The chambers were then placed into wells (24-well plate) that contained RPMI-1640–bovine serum albumin and either fMLP or DMSO. After 10 min at 37°C, the inserts were transferred into another set of wells, and the process was repeated four times. The number of cells that migrated across the filter during each 10-min period was determined by harvesting and counting the cells that had collected on the bottom of the wells.
Statistical analysis.
Where appropriate, differences were analyzed by the unpaired Student two-tailed t test or analysis of variance, followed by Dunnett’s modification for multiple comparisons. Differences were considered significant when P was <0.05.
RESULTS
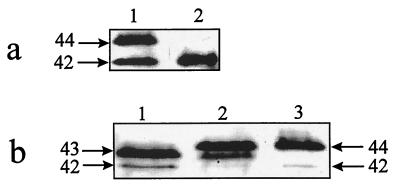
The molecular mass of ERK in neutrophils has been reported to range from 40 to 44 kDa (11, 13, 26–28, 30), as opposed to a 42-kDa form (ERK2) and a 44-kDa form (ERK1) which are commonly observed in other cell types (7). Figure 1 demonstrates that human neutrophils express 42- and 43-kDa forms of ERK as determined by the differential ability of two anti-MAP kinase antibodies, R1 and R2, to detect the 42-, 43-, and 44-kDa forms of ERK/MAP kinase (28b). When the blots were probed with R1, which detects the 42- and 44-kDa forms of ERK (28a), only one band of immunoreactive material that migrated with a relative molecular mass of 42 kDa was detected in the lysate of unstimulated neutrophils (Fig. 1a, lane 2). However, R1 detected 42- and 44-kDa forms of ERK in the lysate of unstimulated WB rat liver epithelial cells (Fig. 1a, lane 1) that express ERK1 and ERK2 (15, 17). When samples from unstimulated neutrophils were probed with R2, which detects the 42-, 43-, and 44-kDa forms of ERK (28a), 42- and 43-kDa forms of ERK were detected (Fig. 1b, lane 1). Both the 42- and the 44-kDa forms of ERK were again observed in WB cells (Fig. 1b, lane 3). Likewise, human monocytes were found to express ERK forms that migrated with relative molecular masses of 42 and 43 kDa (data not shown). The above data demonstrate that human neutrophils, unlike WB cells (15, 17) and other nonhemopoietic cell types (7), express ERK2 and a 43-kDa form of ERK.
FIG. 1.
Human neutrophils express 42- and 43-kDa forms of ERK. Neutrophils (3 × 107 in 10 ml of HBSS) and WB cells (1-by-10-cm dish), stimulated or unstimulated, were sonicated, and the soluble fractions were Western blotted with anti-MAP kinase antibody R1 (a) or R2 (b) as described in Materials and Methods. (a) Lane 1, unstimulated WB cells; lane 2, unstimulated neutrophils. (b) Lane 1, unstimulated neutrophils; lane 2, neutrophils stimulated with fMLP (100 nM; 2 min); lane 3, unstimulated WB cells. Similar results were obtained in a repeat experiment. Molecular masses (in kilodaltons) are indicated.
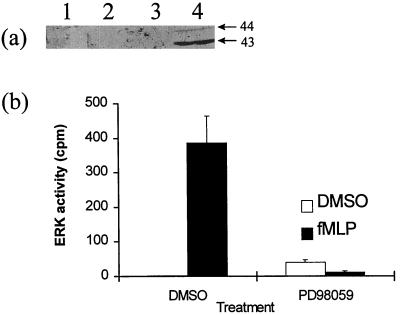
Incubation of neutrophils with fMLP for 1 min resulted in a retardation in the mobility of both ERK2 and the 43-kDa form of ERK (Fig. 1b, lane 2), suggesting that both forms of ERK were activated. When the blots were probed with the anti-ACTIVE ERK antibody, which detects dual phosphorylated and hence the active forms of ERK, two immunoreactive bands which migrated with apparent relative molecular masses of approximately 43 and 44 kDa were detected (Fig. 2a, lane 4). Our studies with HeLa cells, U937 cells, and peripheral blood monocytes have demonstrated that the appearance of dual phosphorylated ERK forms was closely correlated with increases in kinase activity and with retardation in the electrophoretic mobility of ERK forms (Fig. 1 and data not shown). The intensity of the 44-kDa band was less than that of the 43-kDa band. This was likely to be due to a lower immunoreactivity of the phosphorylated 43-kDa ERK with the antibody rather than a differential activation of the two forms of ERK, since the densities of the bands which displayed reduced electrophoretic mobility were similar to each other (Fig. 1b, lane 2). The anti-ACTIVE ERK antibody did not detect any immunoreactive material in control cells (Fig. 2a, lane 1). The above data not only confirm that fMLP stimulated the dual phosphorylation (and hence activation) of ERK but also provide evidence that both ERK2 and a 43-kDa form of ERK are activated. No attempts were made to immunoprecipitate the 43-kDa form of ERK with commercially available anti-ERK1 antibody and assay for the activity of ERK1 since this antibody, available from two different sources (Transduction Laboratory and Santa Cruz Biotechnology), also reacts strongly with ERK2.
FIG. 2.
Inhibition of fMLP-stimulated ERK phosphorylation and activity by PD98059. Neutrophils were preincubated with PD98059 (50 μM) or DMSO (0.1%, vol/vol) for 45 min. fMLP (100 nM) was added, and cells were incubated for 1 min. Soluble fractions were prepared, and samples were either Western blotted (a) or used for kinase activity assays (b) as described in Materials and Methods. The maximum amount of DMSO was 0.2% (vol/vol), which did not affect ERK phosphorylation or activity. (a) Lane 1, control; lane 2, PD98059; lane 3, fMLP with PD98059; lane 4, fMLP. Molecular masses (in kilodaltons) are shown. (b) Kinase activity (mean of duplicate assays [error bars show range]) in partially purified ERK fractions. Results are representative of three separate experiments, each with cells from a different donor.
Preincubation of neutrophils with the specific antagonist of MEK activation, PD98059 (50 μM), totally prevented fMLP from causing the appearance of dual phosphorylated ERK (Fig. 2a, lane 3). PD98059, at 50 μM, was found not to inhibit the activity of each of 18 purified kinases tested in vitro (1). These included PKC, Ca2+/calmodulin-dependent kinases, and kinases in related MAP kinase cascades (1). The film was deliberately overexposed to exclude any low-level remnant phosphorylation in the presence of PD98059. To confirm that PD98059 blocked the activation of ERK by fMLP, phenylsepharose-purified samples were assayed for kinase activity. Peptide-stimulated kinase activity was totally abrogated by PD98059 (Fig. 2b). The Western blot and kinase assay data, therefore, demonstrate that PD98059 (50 μM) completely blocked the activation of the ERK cascade by fMLP.
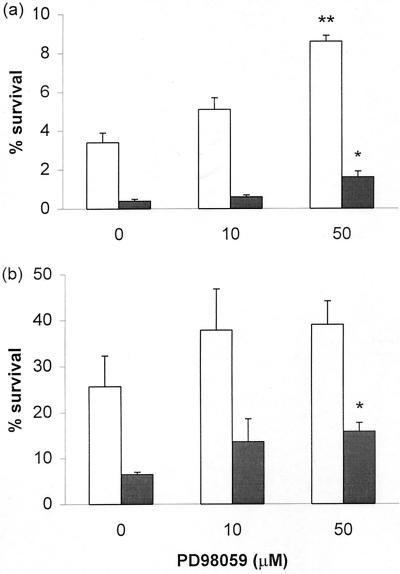
To determine whether blockade of the ERK cascade would impair the ability of neutrophils to kill S. aureus and C. albicans, neutrophils were preincubated with PD98059 for 45 min before being challenged with the bacteria or fungi. While the inhibitor per se did not affect the growth of the microorganisms (data not shown), the ability of neutrophils to kill bacteria or fungi was suppressed by PD98059 (0.01 < P < 0.05), albeit only modestly (Fig. 3).
FIG. 3.
Inhibition of neutrophil antimicrobial activity by PD98059. Neutrophils were preincubated with PD98059 or DMSO for 45 min before being incubated with bacteria (a) or fungus (b) for 30 min (open bar) or 60 min (shaded bar). Microbes were inoculated onto culture plates. After 24 or 48 h, the number of colonies was scored and microbicidal activity was determined as described in Materials and Methods. Results (means of four separate experiments [error bars, standard errors of the means]) are expressed as percent survival. The significance of difference between the absence and presence of inhibitor is shown as follows: ∗, P < 0.05; ∗∗, P < 0.01.
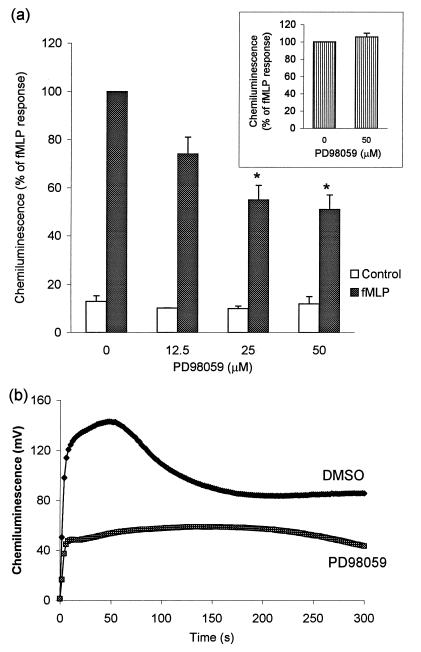
We next examined the effects of PD98059 on some of the responses of neutrophils which are necessary for killing S. aureus and C. albicans. Pretreatment of neutrophils with PD98059 dose-dependently inhibited fMLP-stimulated respiratory burst, and maximum inhibition (∼50%) was achieved with PD98059 at 25 μM (Fig. 4a). The effect of PD98059 was reversible, since the ability of the cells to produce superoxide was totally restored after washing with HBSS (Fig. 4a, inset). PD98059 only slightly reduced the initial rate of fMLP-stimulated superoxide production (Fig. 4b). However, the major effect of the inhibitor was on the maximum rate of superoxide production. In contrast, fMLP-stimulated degranulation, determined as described previously (5), either in the presence or absence of cytochalasin B, was not inhibited by PD98059 (data not shown). PD98059, up to 50 μM, also failed to inhibit the adherence of neutrophils to plastic surfaces (assayed as described in reference 5; data not shown).
FIG. 4.
Inhibition of fMLP-stimulated respiratory burst by PD98059. Neutrophils (106/ml) were preincubated with PD98059 or DMSO for 45 min. (a) After the addition of fMLP, superoxide production was determined as described in Materials and Methods. In some experiments (inset), preincubated cells were washed and allowed to recover before being stimulated with fMLP. Results (means of three determinations [error bars, standard errors of the means]), representative of three to six experiments, are expressed as a percentage of the peak rate of chemiluminescence observed in the presence of fMLP but in the absence of PD98059. (b) Kinetics of fMLP-stimulated chemiluminescence, representative of three replicates from control (DMSO) or PD98059-pretreated cells. Similar results were obtained in three to six other repeat experiments. ∗, significance of difference between the absence or presence of PD98059 (P < 0.05).
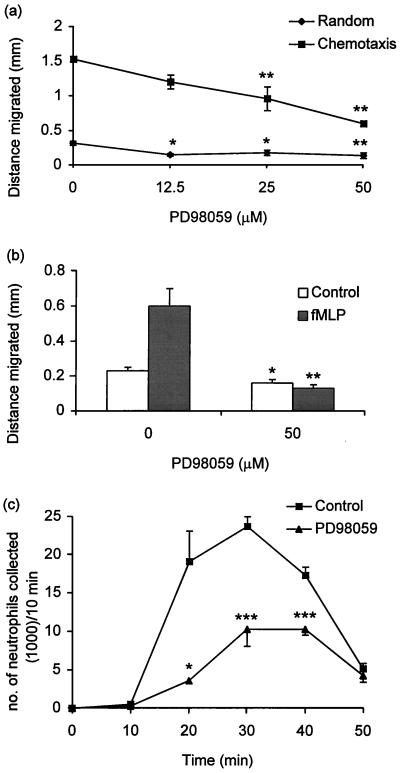
The ability of neutrophils to migrate was inhibited in a dose-dependent manner by PD98059 (Fig. 5). Thus, random migration and chemotaxis, assessed by the under-agarose technique, were inhibited ≥50% by PD98059 (Fig. 5a). The greatest inhibitory effect of PD98059 was on chemokinesis. Not only did PD98059 abrogate chemokinesis, but it also reduced migration to a level which was not dissimilar to that observed with control cells that had been pretreated with PD98059 (Fig. 5b). Chemotaxis, assessed by migration across a 3-μm-pore-size polycarbonate filter, was also inhibited by PD98059 (Fig. 5c). The number of neutrophils that migrated across the filter reached a maximum during the 20- to 30-min incubation period and declined thereafter. In the absence of fMLP, the number of cells that had migrated across the filter was 15 to 20% of that observed in the presence of fMLP.
FIG. 5.
Inhibition of neutrophil migration by PD98059. Cells (4 × 107/ml) were preincubated with PD98059 or DMSO for 45 min. (a) With the under-agarose technique, cells were placed in wells at a density of 2 × 105/5 μl, and chemotaxis and random migration were determined as described in Materials and Methods. (b) For chemokinesis, cells were incubated with fMLP for 5 min before being placed in wells. (c) In some experiments, chemotaxis was determined by migration across a polycarbonate filter as described in Materials and Methods and the number of neutrophils that had migrated across the filter was scored every 10 min. Results (means [n = 5 to 8] [error bars, standard errors of the means]) are representative of five separate experiments with cells from different donors. The significance of difference between the absence and presence of inhibitor is shown as follows: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
DISCUSSION
Previous studies estimated the molecular mass of ERK in neutrophils to range from 40 to 44 kDa (11, 13, 26–28, 30), with one study claiming a 41-kDa form of ERK as ERK1 (11) instead of the 44-kDa form of ERK (7). While one study documented the expression of 40-, 42-, and 44-kDa forms of ERK in neutrophils (10), other studies have reported the presence and activation of only a 41-kDa form (11) or a 42-kDa form (26) of ERK in neutrophils. Mono-Q fast-performance liquid chromatography fractionation of lysates from activated neutrophils consistently revealed the presence of only one peak of kinase activity (references 3 and 26 and unpublished data) as opposed to the widely reported two peaks (ERK1 and ERK2) which are detected in other cell types (2, 7). These observations hint at the possibility that the ERK forms that are expressed in neutrophils may differ from the widely reported ERK1 (44 kDa) and ERK2 (42 kDa) (7). By using two anti-ERK MAP kinase antibodies, R1 and R2, which have slightly different specificities, the present study provides evidence that neutrophils express ERK2 and a 43-kDa form of ERK. ERK1 was not detected. Although some studies have demonstrated the expression and activation of 42- and 44-kDa forms of ERK in neutrophils (13), these relied on the ability of only one anti-ERK antibody to detect the ERK forms, and the electrophoretic mobility of the larger ERK form was not compared with that from a cell type that expresses ERK1. The present study is the only one, to date, which has compared the expression of ERK in human neutrophils with that in a cell type that has previously been shown to express ERK1 and ERK2 by Western blot analysis and by Mono-Q fast-performance liquid chromatography fractionation (15, 17). Our data also demonstrate that the activities of both ERK2 and the 43-kDa form of ERK were stimulated in activated neutrophils. The lack of specificity of commercially available anti-ERK1 antibodies (23a, 28a) prevented us from using anti-ERK1 antibodies to immunoprecipitate and assay for the activity of the 43-kDa form of ERK. However, dual phosphorylation (and hence activation) of the 43-kDa form of ERK was demonstrated with the anti-ACTIVE ERK antibody. Activation of ERK2 and the 43-kDa form of ERK was further confirmed by the reduction in electrophoretic mobility of both the 42- and 43-kDa forms of ERK in fMLP-stimulated cells. These data argue against ERK1 being expressed or activated in human neutrophils and being involved in the regulation of neutrophil responses.
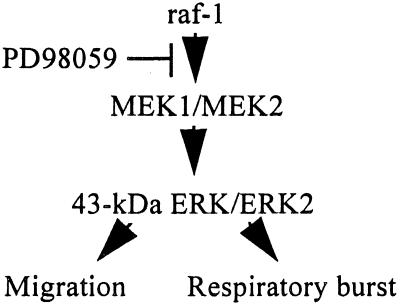
Currently, the extent to which the ERK cascade plays a role in regulating the killing of microorganisms such as S. aureus and C. albicans by neutrophils is not known. We have utilized the specific antagonist of MEK activation, PD98059, to answer the question. This inhibitor has been reported to specifically prevent MEK activation (Fig. 6), and hence ERK activation, by preventing the phosphorylation of MEK1 and MEK2 by Raf-1 or MEK kinase (1). In IL-8-stimulated neutrophils, PD98059 was found to only inhibit the activity of ERK, while the activity of p38, Ca2+ mobilization, and the viability of neutrophils were not affected (19). Our data on the inhibition of ERK activity and dual phosphorylation by PD98059 are in close agreement with the findings of Suchard et al. (25) that PD98059 inhibited ERK activity by 85 to 90% in human neutrophils.
FIG. 6.
ERK cascade and its role in regulating fMLP-stimulated responses.
Previous studies from our laboratory and those of others have demonstrated that oxidative mechanisms are involved in the killing of S. aureus and C. albicans (4, 6, 18, 20, 24). Hence, the concerted actions of reactive oxygen species and components of the granules such as myeloperoxidase are needed (12, 31). The observed lack of a major effect of PD98059 on microbicidal activity is consistent with a lack of effect of PD98059 on neutrophil adherence and degranulation, although the respiratory burst was inhibited by approximately 50%. This suggests that the respiratory burst would have to be inhibited by greater than 50% in order to cause a substantial reduction in bactericidal and fungicidal activity. Using neutrophils from an X91-variant chronic granulomatous disease patient, Bu-Ghanim et al. (6) previously demonstrated that an NADPH oxidase activity of 12% of normal was associated with grossly impaired bactericidal activity (6). The present study therefore demonstrates that the ERK cascade per se plays only a minor role in modulating the ability of neutrophils to kill S. aureus and C. albicans and suggests that this signalling pathway is of no biological significance in the microbicidal activity of neutrophils.
Our data demonstrate that the ERK cascade is involved in regulating neutrophil migration. While random migration and chemotaxis were partially inhibited by PD98059, chemokinesis was totally abrogated. A direct effect of PD98059 on random migration raises the possibility that, in addition to being a signalling pathway which mediates some of the actions of fMLP, the MEK/ERK cascade also directly regulates the migratory machinery. Previous studies have suggested that the ERK cascade participates in outside-in signalling, which is initiated by the engagement of integrins by the extracellular matrix or adhesion molecules (21). This dual site requirement for the ERK cascade is consistent with the observation that PD98059 not only abrogated chemokinesis but it reduced migration to levels which were less than those observed in control cells that had not been pretreated with PD98059. PD98059 also inhibited fMLP-stimulated migration of neutrophils across polycarbonate filters. In this assay, fMLP was found to only transiently increase the number of migrating neutrophils. This could be due to a gradual diffusion of fMLP into the chambers with time and hence caused a reduction in the chemotactic gradient across the filters. Consistent with this suggestion, slow diffusion of Giemsa stain into the chambers was observed when the chambers were placed in wells that contained this stain (unpublished data). The incomplete suppression of chemotaxis by PD98059 suggests that mechanisms other than the ERK cascade are also involved in regulating fMLP-stimulated chemotaxis. This is consistent with the observation that fMLP-stimulated chemotaxis was also partially inhibited by SB203580, a specific inhibitor of p38 MAP kinase (reference 23 and our unpublished data).
The ability of PD98059 to inhibit chemotaxis may be chemokine specific. Thus, in contrast to fMLP-stimulated chemotaxis, IL-8-stimulated chemotaxis was found not to be inhibited by PD98059 (19). This suggests that ERK-dependent and ERK-independent mechanisms are involved in modulating chemotaxis and that different mechanisms may be utilized by different chemoattractants.
In summary, the present study suggests that the ERK cascade plays only a minor role in modulating the killing of S. aureus and C. candida by neutrophils. However, our data suggest that the ERK cascade is involved in regulating fMLP-stimulated respiratory burst activity and the migration of neutrophils in response to fMLP (Fig. 6).
ACKNOWLEDGMENTS
This work was supported by funds from the National Health and Medical Research Council of Australia, the Channel 7 Children’s Research Foundation, and the Women’s and Children’s Hospital Research Foundation.
We thank David Goh and Anne-Marie Tan for technical assistance.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Anderson N G, Maller J L, Tonks N K, Sturgill T W. Requirement for the integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 3.Avdi N, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by formyl-methionyl-leucyl-phenylalanine in human neutrophils. J Biol Chem. 1996;271:33598–33606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 4.Baehner R L. The growth and development of chronic granulomatous disease. In: Bellanti J A, Dayton D H, editors. The phagocytic cell in host resistance. New York, N.Y: Raven Press; 1975. pp. 173–200. [Google Scholar]
- 5.Bates E J, Ferrante A, Harvey D P, Poulos A. Polyunsaturated fatty acids increase neutrophil adherence and integrin receptor expression. J Leukoc Biol. 1992;53:420–426. doi: 10.1002/jlb.53.4.420. [DOI] [PubMed] [Google Scholar]
- 6.Bu-Ghanim H N, Segal A W, Keep N H, Casimir C M. Molecular analysis of three cases of X91-variant chronic granulomatous disease. Blood. 1995;86:3575–3582. [PubMed] [Google Scholar]
- 7.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante A, Thong Y H. Separation of mononuclear and polymorphonuclear leukocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48:81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante A, Beard L J, Thong Y H. Early decay of human neutrophil chemotactic responsiveness following isolation from peripheral blood. Clin Exp Immunol. 1980;39:532–537. [PMC free article] [PubMed] [Google Scholar]
- 10.Fouda S I, Molski T F, Ashour M S, Sha’afi R I. Effect of lipopolysaccharide on mitogen-activated protein kinase and cytosolic phospholipase A2. Biochem J. 1995;308:815–822. doi: 10.1042/bj3080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinstein S, Furuya W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J Biol Chem. 1992;267:18122–18125. [PubMed] [Google Scholar]
- 12.Hampton M B, Kettle A J, Winterbourn C C. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazan I, Dana R, Granot Y, Levy R. Cytosolic phospholipase A2 and its mode of activation in human neutrophils by opsonised zymosan: correlation between 42/44 kDa mitogen-activated protein kinase, cytosolic phospholipase A2 and NADPH oxidase. Biochem J. 1997;326:867–876. doi: 10.1042/bj3260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henson P M, Henson J E, Fittschen C, Kimani G, Bratton D L, Riches D W H. Phagocytic cells: degranulation and secretion. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation. Basic principles and clinical correlates. New York, N.Y: Raven Press; 1988. pp. 363–390. [Google Scholar]
- 15.Hii C S T, Ferrante A, Edwards Y, Huang Z H, Hartfield P J, Rathjen D A, Poulos A, Murray A W. Activation of mitogen-activated protein kinase by arachidonic acid in rat liver epithelial WB cells by a protein kinase C-dependent mechanism. J Biol Chem. 1995;270:4201–4204. doi: 10.1074/jbc.270.9.4201. [DOI] [PubMed] [Google Scholar]
- 16.Hii C S T, Huang Z H, Bilney A, Costabile M, Murray A W, Rathjen D A, Der C J, Ferrante A. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells, HL60 promyelocytic leukaemic cells and human neutrophils: evidence for cell-type specific activation of MAP kinases. J Biol Chem. 1998;273:19277–19282. doi: 10.1074/jbc.273.30.19277. [DOI] [PubMed] [Google Scholar]
- 17.Hii C S T, Oh S Y, Schmidt S, Clark K J, Murray A W. Lysophosphatidic acid inhibits gap junctional communication and stimulates phosphorylation of connexin 43 in WB cells: possible involvement of the mitogen-activated protein kinase cascade. Biochem J. 1994;303:475–479. doi: 10.1042/bj3030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klebanoff S J. Phagocytic cells: products of oxygen metabolism. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation. Basic principles and clinical correlates. New York, N.Y: Raven Press; 1988. pp. 391–444. [Google Scholar]
- 19.Knall C, Worthen G S, Johnson G L. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independently of mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowanko I C, Ferrante A, Clemente G, Kumaratilake L M. Tumor necrosis factor primes neutrophils to kill Staphylococcus aureus by an oxygen-dependent mechanism and Plasmodium falciparum by an oxygen-independent mechanism. Infect Immun. 1996;64:3435–3437. doi: 10.1128/iai.64.8.3435-3437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto S, Teramoto H, Coso O A, Gutland J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signalling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson R D, Quie P G, Simmons R L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975;115:1650–1656. [PubMed] [Google Scholar]
- 23.Nick J A, Avdi N J, Young S K, Knall C, Gerwins P, Johnson G L, Worthen G S. Common and distinct intracellular signalling pathways in human neutrophils utilised by platelet activating factor and fMLP. J Clin Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Santa Cruz Biotechnology. /99. Santa Cruz Biotechnology catalog. Santa Cruz, Calif: Santa Cruz Biotechnology; 1998. pp. 262–263. [Google Scholar]
- 24.Stein D K, Malawista S E, Van-Blaricom G, Wysong D, Diamond R D. Cytoplasts generate oxidants but require added neutrophil granule constituents for fungicidal activity against Candida albicans hyphae. J Infect Dis. 1995;172:511–520. doi: 10.1093/infdis/172.2.511. [DOI] [PubMed] [Google Scholar]
- 25.Suchard S J, Mansfield P J, Boxer L A, Shayman J A. Mitogen-activated protein kinase activation during IgG-dependent phagocytosis in human neutrophils: inhibition by ceramide. J Immunol. 1997;158:4961–4967. [PubMed] [Google Scholar]
- 26.Thompson H L, Shiroo M, Saklatvala J. The chemotactic factor N-formyl methionyl leucyl phenylalanine activates microtubule-associated protein 2 (MAP) kinase and a MAP kinase kinase in polymorphonuclear leukocytes. Biochem J. 1993;290:483–488. doi: 10.1042/bj2900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson H L, Marshall C J, Saklatvala J. Characterisation of two different forms of mitogen-activated protein kinase kinase induced in polymorphonuclear leukocytes following stimulation by N-formyl methionyl-leucyl-phenylalanine or granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1994;269:9486–9492. [PubMed] [Google Scholar]
- 28.Torres M, Hall F L, O’Neil K. Stimulation of human neutrophils with fMLP induced tyrosine phosphorylation and activation of 2 distinct mitogen-activated protein kinases. J Immunol. 1993;150:1563–1577. [PubMed] [Google Scholar]
- 28a.Transduction Laboratory. Transduction Laboratory catalog. Lexington, Ky: Transduction Laboratory; 1998. [Google Scholar]
- 28b.Upstate Biotechnology Inc. Upstate Biotechnology Inc. specification sheet. Lake Placid, N.Y: Upstate Biotechnology Inc.; 1996. pp. 98–99. [Google Scholar]
- 29.Unkeless J C, Wright S D. Phagocytic cells: Fcγ and complement receptors. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation. Basic principles and clinical correlates. New York, N.Y: Raven Press; 1988. pp. 343–362. [Google Scholar]
- 30.Van Lint J V, Van Damme J, Billiau A, Merlevede W, Vandenheede J R. Interleukin-8 activates microtubule-associated protein 2 kinase (ERK1) in human neutrophils. Mol Cell Biochem. 1993;127/128:171–177. doi: 10.1007/BF01076768. [DOI] [PubMed] [Google Scholar]
- 31.Wagner D K, Collins-Lech C, Sohnle P G. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect Immun. 1986;51:731–735. doi: 10.1128/iai.51.3.731-735.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]