Abstract
The UspA1 and UspA2 proteins from Moraxella catarrhalis share antigenic epitopes and are promising vaccine candidates. In this study, the levels and bactericidal activities of antibodies in sera from healthy adults and children toward UspA1 and UspA2 from the O35E strain were measured. Human sera contained antibodies to both proteins, and the levels of immunoglobulin G (IgG) antibodies were age dependent. Adult sera had significantly higher titers of IgG than child sera (P < 0.01). The IgG3 titers to the UspA proteins were higher than the IgG1 titers in the adults’ sera, while the IgG1 titers were higher than the IgG3 titers in the children’s sera (P < 0.05). The IgG antibodies in the sera from 2-month-old children appeared to be maternally derived, since the mean titer was significantly higher than that in sera from 6- to 7-month-old children (P < 0.05). Serum IgA antibodies to both UspA1 and UspA2 were low during the first 7 months of age but thereafter gradually increased along with the IgG titers. Analysis of sera absorbed with UspA1 or UspA2 showed that the antibodies to UspA1 and UspA2 were cross-reactive with each other and associated with serum bactericidal activity. Examination of affinity-purified human antibodies confirmed that naturally acquired antibodies to UspA1 and UspA2 were bactericidal and cross-reactive. These results support using UspA1 and UspA2 in a vaccine to prevent M. catarrhalis infections.
The bacterium Moraxella catarrhalis causes significant morbidity among children. It is a major cause of otitis media (4, 22, 24, 27) and a common cause of persistent cough (17), sinusitis (2, 3), and other respiratory infections (5, 29, 30). Nearly 80% of children are colonized before reaching 2 years of age, and 30 to 50% of healthy toddlers are colonized at any given time (14, 25, 28). In contrast, human beings between the ages of 10 and 55 years and very young infants seldom develop disease and have a carriage rate of 5% or less (10, 11, 13, 28). Antibodies specific for M. catarrhalis antigens have been reported to be present in sera of convalescent humans who have suffered from otitis media and lower respiratory tract infections as well as in normal human sera (9, 15, 16, 18, 20, 26). However, the role of acquired immunity in preventing infections caused by M. catarrhalis has not been established.
Previous studies indicate that sera from convalescent patients recovering from lower respiratory tract infections due to M. catarrhalis contain antibodies to a high-molecular-mass protein named ubiquitous surface protein A (UspA) (18, 19). This protein is considered a promising vaccine candidate because a monoclonal antibody (MAb) (17C7) and polyclonal antibodies made in mice are both bactericidal and protective in the murine pulmonary-clearance model (8, 18, 19). Recent studies, however, have shown that the UspA described in the earlier studies is actually composed of two distinct proteins, UspA1 and UspA2, that share the MAb 17C7-reactive epitope (1). Both UspA1 and UspA2 from the O35E strain have since been purified, and antibodies elicited in mice to one protein have been shown to cross-react with the other by an enzyme-linked immunosorbent assay (ELISA) (21).
To determine if humans have naturally acquired antibodies to UspA1 and UspA2 with biological activity, we examined sera from healthy humans of various ages using both ELISA and a bactericidal assay. It was found that healthy people have naturally acquired antibodies to both UspA1 and UspA2 in their sera and that the levels of these antibodies and their bactericidal capacities were age dependent. The results also indicated that naturally acquired antibodies to UspA1 and UspA2 are biologically functional. These results support the use of these proteins in a vaccine for preventing M. catarrhalis disease.
MATERIALS AND METHODS
Bacteria.
The M. catarrhalis strains O35E and TTA24 were provided by Eric Hansen (University of Texas Southwestern Medical Center, Dallas, Tex.). A strain from the American Type Culture Collection (ATCC 25238) and two clinical isolates from our collection (1230-359 and 216-96) were also used.
Human sera.
Fifty-eight serum samples were collected from a group of 10 children at 2, 4, 6, 7, 15, and 18 months of age, i.e., at the times they received routine childhood immunizations. Individual sera from 26 adults, aged 20 to 55 years, and 15 additional children, aged 18 to 36 months, were also examined in some assays. All sera were provided by the Clinical Group of Wyeth-Lederle Vaccines. They were obtained in the United States from clinically healthy individuals and stored at −70°C. Because the sera were drawn as part of another clinical study, no information on M. catarrhalis colonization or infection of these subjects was collected.
Isolation of UspA1, UspA2, and the 74-kDa protein.
Purified UspA1 and UspA2 were prepared from the O35E strain of M. catarrhalis. The preparations met all the criteria of purity for the proteins described by McMichael et al. (21). Briefly, each protein migrated as a single band with greater than 90% homogeneity at the mobility typical of that protein in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). They both reacted with MAb 17C7 but only with the appropriate protein-specific MAbs (11A6 and 17H4) in Western blots. They had low levels of endotoxin.
The 74-kDa protein was prepared from the O35E isolate. The bacteria were grown and harvested as described previously (21). The harvested pellets were resuspended in 50 ml of phosphate buffer (10 mM, pH 6.0) containing 0.1% Triton X-100 (J. T. Baker Inc., Philipsburg, N.J.) with stirring for 1 h at room temperature. Particulates were removed by centrifugation (10,000 × g, 60 min). The supernatant was loaded on an S Sepharose column (2.6 cm [inside diameter] by 2 cm [length]; Pharmacia, Piscataway, N.J.), and eluted with an NaCl step gradient in 10 mM phosphate buffer (pH 6.0) containing 0.1% Triton X-100. Fractions enriched in the 74-kDa protein eluted between 70 and 210 mM NaCl. These were pooled and applied to a hydroxyapatite column (particle size, 40 μm; 1.5 cm [inside diameter] by 5 cm [length]; Bio-Rad Laboratories, Hercules, Calif.). The column was washed with 30 ml of 10 mM phosphate buffer (pH 6.0), and the protein was eluted with a step gradient of phosphate buffer (pH 6.0). The 74-kDa protein eluted between 300 and 500 mM buffer. The column fractions were pooled, concentrated with Centriprep-30 (Amicon, Beverly, Mass.) by centrifugation at 2,000 × g, and passaged over an Ultrogel ACA44 column (2.6 cm [inside diameter] by 100 cm [length]; BioSepra Inc., Marlborough, Mass.) at a flow rate of 1.0 ml of 10 mM sodium phosphate–150 mM NaCl (pH 7.4) buffer (PBS) per min.
Isolation of UspA1- and UspA2-specific antibodies from human plasma.
Antibodies specific for the two proteins were isolated from a pool of two human plasmas obtained from the American Red Cross (Rochester, N.Y.). The antibodies were precipitated from the human plasma by adding ammonium sulfate to 50% saturation. The precipitate was collected by centrifugation and dialyzed against PBS.
A nitrocellulose membrane (5 by 7.5 cm) was incubated with either UspA1, UspA2, or the 74-kDa protein at 0.5 mg/ml in PBS containing 0.1% (vol/vol) Triton X-100 for 1 h at room temperature and washed twice with PBS, and residual binding sites on the membrane were blocked with 5% (wt/vol) dry milk in PBS for 2 h at room temperature. The membrane was then sequentially washed twice with each of the following: PBS, 100 mM glycine (pH 2.5), and finally PBS before being incubated with the dialyzed antibody preparation. After being incubated for 4 h at 4°C, the membrane was washed again with PBS and then with 10 mM Tris buffer (pH 8.0) containing 1 M sodium chloride to remove nonspecifically bound proteins. The bound antibodies were eluted by incubation in 5 ml of 100 mM glycine (pH 2.5) for 2 min with shaking. One milliliter of Tris-HCl (1 M, pH 8.0) was immediately added to the eluate to neutralize the pH. The eluted antibodies were dialyzed against PBS and stored at −20°C.
ELISA.
Antibody titers to the O35E strain and other M. catarrhalis strains were determined by a whole-cell ELISA as previously described with biotin-labeled rabbit anti-human IgG or IgA antibodies (Brookwood Biomedical, Birmingham, Ala.) (8). Antibody titers to UspA1, UspA2, and the 74-kDa protein were determined by a similar method except that the plates were coated with 0.1 μg of purified protein in 100 μl of PBS per well overnight at room temperature. The IgG subclass antibodies to UspA1 or UspA2 were determined with sheep anti-human IgG subclass antibodies conjugated to alkaline phosphatase (The Binding Site Ltd., San Diego, Calif.). The antibody endpoint titer was defined as the highest dilution of serum giving an A415 greater than three times that of the control. The control wells received all treatments except human sera. These typically had absorbance values ranging from 0.03 to 0.06.
The specificities of biotin-labeled rabbit anti-human IgG and IgA antibodies for purified human IgG, IgM, and IgA (Pierce, Rockford, Ill.) were determined against purified human ELISA. No cross-reactivity was found. The assay sensitivities, determined against purified human IgG and IgA by ELISA, were 15 and 60 ng/ml, respectively. Likewise, the specificities of the assays for the human IgG subclass antibodies for purified human myeloma IgG subclass proteins (ICN Biomedicals, Inc., Irvine, Calif.) were confirmed by ELISA and were 15 ng/ml in the IgG1, IgG3, and IgG4 assays and 120 ng/ml in the IgG2 assay. Two serum standards were included to control for assay-to-assay variation.
Complement-dependent bactericidal assay.
The bactericidal activities of the human sera were determined as described previously (8). As in that procedure, the complement reagent was prepared by depleting human serum of antibodies by passage over a protein G column. The highest concentration of serum tested was a 1:50 dilution. In some experiments, the sera were absorbed with UspA1, UspA2, or the 74-kDa protein prior to the assay. The absorption of specific antibodies from these sera was accomplished by adding the purified proteins to a 50-μg/ml final concentration. The final serum dilution for the absorption was 1:10. The mixtures were incubated for 2 h at 4°C, and the precipitate was removed by microcentrifugation. The specific UspA1 and UspA2 antibodies isolated from human plasma were assayed against five M. catarrhalis strains in a similar manner.
SDS-PAGE and immunoblotting.
The SDS-PAGE and Western immunoblotting procedure were described previously (8). Briefly, outer membrane vesicles were resolved in 4 to 20% polyacrylamide gradient gels, transferred to a nitrocellulose membrane, and probed with a 1:1,000 dilution of either the absorbed serum or the affinity-isolated antibodies. A dilution of 1:1,000 of goat anti-human IgG conjugated to alkaline phosphatase (Biosource International, Camarillo, Calif.) was used as the secondary antibody. Both antibody incubations were done for 2 h at 4°C.
Antibody cross-reactivity with other bacterial species.
To examine the capacity of the UspA proteins to elicit antibodies toward other species of bacteria, guinea pigs were immunized with 25 μg of the UspA proteins mixed with 50 μg of 3-O-deacylated monophosphoryl lipid A (Ribi ImmunoChem Research, Hamilton, Mont.) and 100 μg of aluminum phosphate. The animals received three immunizations 4 weeks apart and were exsanguinated 2 weeks after the last immunization.
The bacterial species and strains tested for reactivity with antisera were Pseudomonas aeruginosa PA01, Neisseria meningitidis H44176, Neisseria gonorrhoeae LB2, Bordetella pertussis Tohama, Escherichia coli W1-3, and nontypeable Haemophilus influenzae 860295. A suspension of the bacteria was prepared by swabbing the bacteria from agar plates into distilled water, and its absorbance at 600 nm was adjusted to 1.0. One milliliter of this suspension was microcentrifuged for 5 min and resuspended in 0.15 ml of 30 mM Tris–2% (wt/vol) SDS–10% (vol/vol) glycerol–5% (vol/vol) mercaptoethanol–0.0004% (wt/vol) bromphenol blue. The samples were heated for 10 min at 100°C and examined by the SDS-PAGE immunoblotting method described above, except the membrane was probed with the guinea pig anti-UspA sera. The bound guinea pig antibodies were then detected with goat anti-guinea pig IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch, Bar Harbor, Maine).
Statistics.
Statistical analysis was performed on logarithmic transformed titers with JMP software (SAS Institute, Cary, N.C.). To allow transformation, a value of one-half of the lowest dilution of serum was assigned to sera that had no detectable titers. Comparison of IgG levels among the age groups was done by analysis of variance. The relationship between antibody titer and bactericidal titer was determined by logistic regression. A probability of less than 0.05 was considered significant.
RESULTS
Comparison of IgG titers to UspA1 and UspA2 in the sera of children and adults.
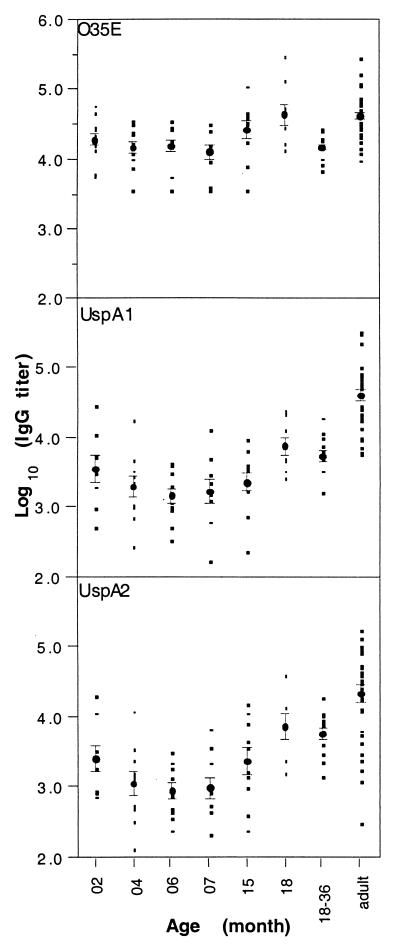
The IgG antibody titers were determined for sera from 10 children collected longitudinally between 2 and 18 months of age. Titers were also determined for sera from a group of 10 children between 18 and 36 months of age as well as from a group of 26 adults. Titrations against whole bacterial cells of the O35E strain, purified UspA1, and purified UspA2 were done by ELISA. An IgG titer to all three antigens was detected in nearly every serum (Fig. 1). The IgG titers to UspA1 and UspA2 exhibited stronger age-dependent variation than the IgG titers to the O35E bacterium. The adult sera contained significantly higher titers of IgG to the purified proteins than the sera from children of any age group (P < 0.01). The sera from the 6- and 7-month-old children had the lowest titers of IgG to the UspA proteins. The mean titer at this age was significantly lower than at 2 months of age (P < 0.05).
FIG. 1.
Levels of IgG to UspA1, UspA2, and M. catarrhalis O35E in normal children’s sera. The data plotted on the ordinate axis are log10-unit-transformed ELISA endpoint titers. The individual titers were plotted by age group. The geometric mean titers and the standard errors for each age group are indicated.
Comparison of IgA titers to UspA1 and UspA2 in the sera of children and adults.
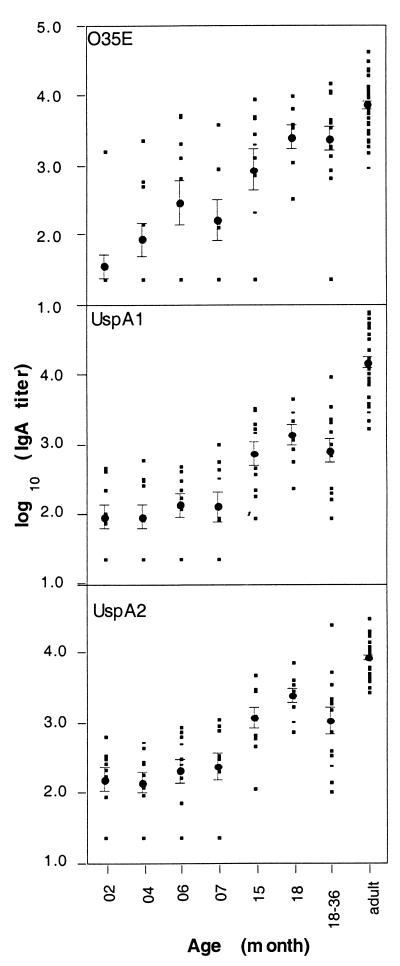
The levels of IgA antibodies to UspA1, UspA2, and O35E bacterial cells were also age dependent (Fig. 2). IgA against UspA1 and UspA2 was detected in the sera of all 26 adults. For children less than 18 months of age, the proportion exhibiting antigen-specific IgA titers increased with age. The mean titers of IgA to UspA1, UspA2, and the O35E bacterium in these sera were low for the first 7 months of age but increased thereafter.
FIG. 2.
Levels of IgA to UspA1, UspA2, and M. catarrhalis O35E in normal children’s sera. For details, see the text and the legend of Fig. 1.
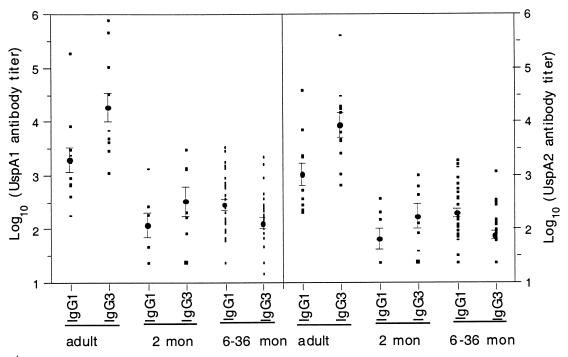
Age-dependent subclass distribution of IgG antibodies to UspA1 and UspA2.
The titers of IgG subclass antibodies to the UspA1 and UspA2 antigens were determined for sera from 10 adults and 35 children. The subclass distribution was found to be age dependent. The predominant antibody subclasses to the UspA1 and UspA2 antigens in most sera were IgG1 and IgG3. The IgG2 and IgG4 titers were either undetectable or extremely low. Therefore, only the results for the IgG1 and IgG3 subclasses are reported (Fig. 3). The IgG3 titers against UspA1 or UspA2 in the adult sera were significantly higher than the IgG1 titers (P < 0.05). The same trend was seen for the sera from the 2-month-old children, but the difference between IgG1 and IgG3 titers did not reach statistical significance, which may have been due to a smaller sample size. Sera from the children between 6 and 36 months of age had IgG1 and IgG3 subclass profiles opposite from those seen for adults and 2-month-old children. The mean titers of IgG1 were significantly higher than the titers of IgG3 to both antigens in these children’s sera (P < 0.05).
FIG. 3.
Distribution of IgG1 and IgG3 antibodies to UspA1 and UspA2 in normal human sera. The left panel shows titers to UspA1, and the right panel shows titers to UspA2. mon, month.
Bactericidal activity.
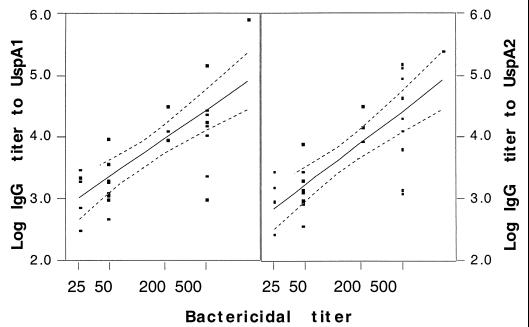
The bactericidal titers of 17 sera from individuals of different age groups against bacteria grown on Mueller-Hinton agar are shown in Table 1. All the adult sera and three of five sera from the 2-month-old children with high titers of IgG to the UspA proteins had strong bactericidal activity. Sera from 6-month-old children had the least bactericidal activity. All five sera from this age group had a marginal bactericidal titer of 50, the lowest dilution assayed. The bactericidal activity of the sera from 18- to 36-month-old children was highly varied, with titers ranging from less than 50 to 500. There was a significant linear relationship (P < 0.01) between the bactericidal titers and the IgG antibody titers against both UspA1 and UspA2 by logistic regression analysis (Fig. 4).
TABLE 1.
Levels of IgG antibodies to UspA1 and UspA2 and bactericidal activities in normal human sera
| Persona | Age | ELISA IgG titerb
|
BC titerc | |
|---|---|---|---|---|
| UspA1 | UspA2 | |||
| 1 | 2 mo | 17,127 | 6,268 | 500 |
| 6 mo | 4,273 | 1,363 | 50 | |
| 15 mo | 798 | 250 | <50 | |
| 2 | 2 mo | 12,078 | 12,244 | 500 |
| 6 mo | 1,357 | 878 | 50 | |
| 18 mo | 14,041 | 14,488 | 200 | |
| 3 | 2 mo | 30,283 | 20,362 | 500 |
| 6 mo | 1,077 | 1,947 | 50 | |
| 18 mo | 2,478 | 1,475 | <50 | |
| 4 | 2 mo | 2,086 | 869 | <50 |
| 6 mo | 530 | 802 | 50 | |
| 18 mo | 9,767 | 8,591 | 200 | |
| 5 | 2 mo | 3,233 | 2,655 | <50 |
| 6 mo | 2,246 | 360 | 50 | |
| 18 mo | 26,693 | 43,703 | 500 | |
| 6 | 1.5–3 yr | 4,036 | 2,686 | 50 |
| 7 | 1.5–3 yr | 2,037 | 1,251 | 50 |
| 8 | 1.5–3 yr | 341 | 251 | <50 |
| 9 | 1.5–3 yr | 2,538 | 1,200 | 500 |
| 10 | 1.5–3 yr | 1,078 | 1,370 | 500 |
| 11 | 1.5–3 yr | 1,265 | 953 | 50 |
| 12 | Adult | 161,750 | 87,180 | 450 |
| 13 | Adult | 873,680 | 248,290 | 1,350 |
| 14 | Adult | 154,650 | 146,900 | 450 |
| 15 | Adult | 10,330 | 7,860 | 50 |
| 16 | Adult | 35,780 | 31,230 | 150 |
| 17 | Adult | 19,130 | 132,200 | 450 |
Three consecutive samples from persons 1 through 5 were collected at the stated ages.
ELISA endpoint titers to purified UspA1 or UspA2 from the O35E strain were determined as the highest dilution of serum giving an A415 greater than three times the background.
Bactericidal (BC) titers assayed against the O35E strain. The children’s sera were tested at dilutions of 1:50, 1:100, and 1:500. The adults’ sera were tested at dilutions of 1:50, 1:150, 1:450, and 1:1,350. Bactericidal titer was determined as the highest dilution of serum resulting in the killing of 50% or more of the bacteria relative to that of the control. Bacteria incubated with test serum and heat-inactivated complement serum gave titers of <50.
FIG. 4.
Relationship of serum IgG titers to UspA1 (left panel) and UspA2 (right panel) with the bactericidal titer against the O35E strain as determined by logistic regression (P < 0.05). The solid lines indicate the linear relationship between the IgG titer and the bactericidal titer. The broken lines represent the 95% confidence intervals of the linear fit.
Bactericidal activities of sera absorbed with purified UspA1 or UspA2.
Because normal human sera contain antibodies to numerous antigens of M. catarrhalis, an absorption method was used to determine the contribution of UspA1- and UspA2-specific antibodies toward bactericidal activity. Six adult sera were absorbed with purified UspA1 and UspA2, and the change in ELISA reactivity to UspA proteins was determined. The same sera were also absorbed with the 74-kDa protein, another outer membrane protein from the O35E isolate, to show that the method of preparation of the antibodies did not affect the results. To control for changes in volume during the absorption procedure, a volume of saline equivalent to that containing the antigens was added to the sera, and all samples were treated similarly thereafter. A reduction in ELISA titer was seen for all the sera after absorption with UspA1 or UspA2 but not after absorption with the 74-kDa protein (Table 2). Furthermore, absorption with one UspA protein resulted in a reduction in the titer of IgG to the other. The levels of reduction in UspA2 reactivity were of the same degree regardless of whether the absorbent was UspA1 or UspA2. In contrast, there was less reduction in UspA1 reactivity after absorption with UspA2 than after absorption with UspA1. This result was consistent with the antibodies to UspA1 and UspA2 being partially cross-reactive.
TABLE 2.
ELISA titers of adult sera before and after absorptiona
| Absorbent | IgG titer to UspA1 in sample:
|
IgG titer to UspA2 in sample:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Noneb | 161,750 | 873,680 | 154,650 | 10,330 | 35,780 | 19,130 | 87,180 | 248,290 | 146,900 | 7,860 | 31,230 | 13,200 |
| UspA1 | 2,450 | 2,210 | 3,160 | 1,650 | <500 | 3,010 | 2,800 | 2,120 | 2,700 | 2,220 | <500 | <500 |
| UspA2 | 42,620 | 90,150 | 33,570 | 6,420 | 3,490 | 4,130 | <500 | 1,820 | 3,010 | 2,960 | <500 | <500 |
| 74-kDa protein | 137,530 | 517,970 | 208,240 | 25,930 | 20,570 | 37,570 | 64,760 | 84,590 | 208,240 | 25,930 | 20,570 | 37,570 |
For the absorption, an aliquot of adult serum was diluted and mixed with purified UspA1 or UspA2 from the O35E strain to a final 50-μg/ml protein concentration and a final 1:10 serum dilution. The mixtures were incubated at 4°C for 2 h, and precipitates were removed by microcentrifugation. The titers of IgG to the UspA1 and UspA2 proteins are endpoint titers starting at a serum dilution of 1:500.
Saline was added to these samples in place of purified protein (at an equivalent volume), and samples were treated the same thereafter.
The bactericidal titers of the absorbed sera are shown in Table 3. Absorption with either UspA1 or UspA2 resulted in a nearly complete loss of bactericidal activity (titer, <50) for all six sera when these samples were assayed against the O35E strain, the strain from which the purified proteins were made. The bactericidal activities of the absorbed sera were reduced at least threefold when these sera were assayed against the heterologous strain 1230-359. Absorption with UspA1 in three of six samples resulted in a reduction in the bactericidal titer against the heterologous strain greater than that produced by absorption with UspA2. This result was consistent with the reductions in ELISA titers to UspA1 after absorption with the two proteins. In contrast, absorption with the 74-kDa protein did not result in a decrease in bactericidal activity. Absorption with a mixture of UspA1 and UspA2 did not result in any further reduction in the bactericidal activity than that with UspA1 alone (data not shown). These results indicated that antibodies specific to the UspA proteins were the major source of the bactericidal activity against M. catarrhalis in adult sera.
TABLE 3.
Bactericidal titers of adult human sera after absorption with three M. catarrhalis outer membrane proteinsa
| Absorbent | Bactericidal titer to the O35E strain in serum:
|
Bactericidal titer to the 1230-359 strain in serum:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Noneb | 450 | 1,350 | 450 | 50 | 150 | 450 | 450 | 4,050 | 1,350 | 150 | 150 | 450 |
| UspA1 | <50 | <50 | <50 | <50 | <50 | <50 | 50 | 150 | <50 | <50 | 50 | 150 |
| UspA2 | <50 | 150 | <50 | <50 | <50 | <50 | 150 | 1,350 | 450 | <50 | 50 | 50 |
| 74-kDa protein | 450 | 1,350 | 450 | 50 | 50 | 150 | 450 | 4,050 | 1,350 | 150 | 150 | 450 |
Sera are those described in Table 2. The bactericidal titers to both the O35E and 1230-359 strains were determined with threefold dilutions of the sera starting at 1:50. The purified UspA1 and UspA2 proteins were prepared from the O35E strain.
Saline was added to samples in place of purified protein (at an equivalent volume), and samples were treated the same thereafter.
The antibody titers and bactericidal activities of children’s sera absorbed with the proteins was also examined. This absorption was done differently because of the limited volumes of sera available from the children, and they were absorbed with a mixture of the UspA1 and UspA2 proteins. The absorption resulted in the complete loss or a significant reduction in bactericidal activity for four of seven sera; i.e., titers dropped from 200 and 500 to <50 and 50. Three of these were from 2-month-old children, and one was from an 18-month-old child. The three other sera were from 15- and 18-month-old children. The remaining three sera had marginal titers of 50 both before and after absorption. In an ELISA all the sera exhibited reductions in the levels of antibodies to the UspA proteins after absorption. This indicates that while antibodies specific for the UspA1 and UspA2 proteins are present in these children’s sera, they may not be bactericidal.
Affinity-purified antibodies to UspA1 and UspA2.
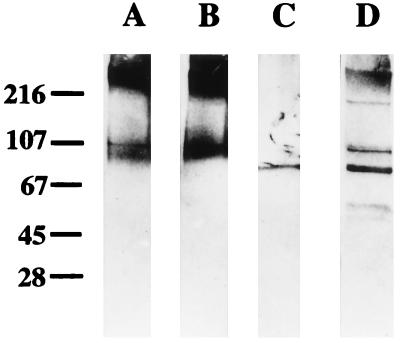
To confirm their cross-reactivities and bactericidal activities, antibodies to UspA1 or UspA2 from adult plasma were isolated by an affinity purification procedure. The purified antibodies reacted specifically with the UspA1 and UspA2 proteins but not with non-UspA proteins in the O35E lysates in a Western blot assay (Fig. 5). The isolated antibodies to one UspA protein reacted with each other with almost equivalent endpoint titers by ELISA (data not shown). The antibody preparations exhibited similar levels of reactivity with five M. catarrhalis strains in both the whole-cell ELISA and bactericidal assays. The ELISA antibody titers ranged from 8,495 to 30,843, while the bactericidal titers against the same strains ranged between 400 and 800.
FIG. 5.
Western blot showing specificities of affinity-isolated human antibodies against whole-bacterial-cell lysate of strain O35E. The antibodies were isolated as described in the text and diluted 1:1,000 for probing the Western blot. Lane A, antibodies isolated with UspA1; lane B, antibodies isolated with UspA2; lane C, antibodies isolated with the 74-kDa protein; lane D, starting serum pool. The mobilities and antigenic cross-reactivities of UspA1 and UspA2 were reported previously (21). The molecular mass markers (in kilodaltons) are indicated at left.
Cross-reactivities of antibodies with other bacterial species.
Since clinical information related to M. catarrhalis infection was not collected in this study, what induced the antibodies to UspA1 or UspA2 is unknown. When antibodies made against the UspA proteins in guinea pigs were tested for reactivity with other bacterial species, including P. aeruginosa, N. meningitidis, N. gonorrhoeae, B. pertussis, E. coli, and nontypeable H. influenzae by Western blot analysis, no reactivity was detected. This result suggests that the antibodies detected in this study were elicited as a specific response to the UspA antigens of M. catarrhalis, which is consistent with the high rate of colonization and the endemic nature of M. catarrhalis in human populations.
Since the affinity-isolated antibodies to the two UspA proteins were cross-reactive, it could not be determined whether the human antibodies were elicited by one or both proteins. It seemed clear that the shared sequence between these two proteins was the main target of the bactericidal antibodies. This supposition was supported by the absorption data, MAb data (1), and data from mice immunized with the two proteins (8).
DISCUSSION
While previous studies have examined human sera for the presence of antibodies to M. catarrhalis or its surface-exposed antigens, none have focused on reactivity to UspA1 or UspA2 in sera. It is clear that several other antigens elicit antibodies; however, the present study suggests that antibodies to these are present in lower levels and have less bactericidal activity than those to the UspA proteins. Further, the level of the IgG antibodies to UspA1 and UspA2 in normal human sera are age dependent. This study points out the importance of the UspA antigens in the immune response, but a more definitive, prospective study needs to be done before any correlation with protection can be made for this response.
Our data indicate that most children have serum IgG antibodies to the UspA proteins at 2 months of age but that the level varies from individual to individual. The IgG subclass profile for the sera from these infants was similar to that for the sera from adults, and their antibodies had bactericidal activity. The absorption experiments indicated that the majority of the bactericidal antibodies in these sera were directed against the UspA1 and UspA2 proteins. These results suggest that the IgG antibodies detected in the 2-month-old children are of maternal origin, which is consistent with the report that umbilical cord serum contains high titers of antibodies to an extract of M. catarrhalis whole cells (12).
Because of the small number of subjects and since clinical data were not collected in this study, it could not be determined whether maternal antibodies against UspA, although bactericidal in vitro, are protective in young children. However, at 2 months of age, the children had significantly higher titers of serum IgG antibodies to the UspA proteins than children at 15 to 18 months of age and only a few had IgA antibodies to M. catarrhalis. If serum IgA reflects prior mucosal exposure to the bacterium, then most children are not infected by M. catarrhalis in the first few months of age. One reason may be that maternal antibodies protect them from infection at this age. This possibility is consistent with the findings that very young infants seldom carry this bacterium and that they do not develop M. catarrhalis disease during the first month or two of life (13). It will require further investigation to determine whether this low susceptibility of young children is due to the presence of maternal antibodies to M. catarrhalis or to other reasons, such as the lack of proper bacterial receptors on host cells.
Children may become susceptible to M. catarrhalis infection when maternal antibodies wane. In this study, the sera from 6- to 7-month-old children had the lowest levels of IgG antibodies to the UspA proteins and barely detectable bactericidal titers toward M. catarrhalis. By 15 months of age, nearly all children had serum IgA antibodies to the UspA proteins and the levels of IgA antibodies had significantly increased along with the levels of IgG antibodies and bactericidal activity when their sera were compared with those of children of 6 to 7 months of age. This suggested that these children had been exposed to the bacterium and mounted an antibody response. The UspA-specific IgG antibodies in the older children’s sera had additional characteristics that distinguished them from the antibodies of the 2-month-old children. First, the IgG1 antibody titer was significantly higher than the IgG3 antibody titer in older children’s sera. The opposite was true for the 2-month-old children (Fig. 2). Second, bactericidal activity was detected in most sera from 2-month-old children, while bactericidal activity was barely detectable in the sera from children of 6 months or older. The low antibody levels and the low bactericidal activities seen in the sera of children between 6 and 18 months of age are consistent with the epidemiological finding that children at this age have the highest rate of colonization and highest incidence of M. catarrhalis disease (3, 12, 20, 24, 26, 27).
Adults, a population usually resistant to M. catarrhalis infections (7, 13), were consistently found to have higher levels of IgG antibodies to the UspA proteins as well as higher levels of serum bactericidal activity than children. The bactericidal activity of the adult sera was clearly antibody mediated since Ig-depleted sera had no activity (9), and the antibodies isolated from adult plasma exhibited complement-dependent bactericidal activity. The antibodies isolated from adult human sera to UspA1 or UspA2 from a single isolate exhibited killing against all tested strains. Thus, humans develop bactericidal antibodies toward the conserved epitopes of the UspA proteins in response to natural infections.
In all adult samples, the IgG antibodies were predominantly of the IgG1 and IgG3 subclasses, with IgG3 being the most predominant. This finding is consistent with the findings of previous reports that the IgG3 subclass is a major constituent of the immune response to M. catarrhalis in adults and children greater than 4 years of age but not in younger children (6, 16). Of the four IgG subclasses in humans, IgG3 constitutes only a minor component of the total Ig in serum. However, the IgG3 antibody has the highest affinity for C1q. Binding of this complement component is the initial step in the classic complement pathway leading to elimination of the bacterium by both complement-dependent killing and opsono-phagocytosis (23). Since IgG3 antibody is efficiently transferred across the placenta, it may also confer protective immunity to infants. The data from this study confirm that IgG3 antibody to the UspA proteins is an important component of the immune response to natural infection and has in vitro biological activity. Whether this antibody contributes to protection in vivo and accounts for the low levels of disease susceptibility in adults and young infants remains to be determined.
In summary, this study demonstrated that antibodies to the two UspA proteins are present in nearly all of the individuals of this particular human population, regardless of age. Both the levels and subclass distribution of these antibodies, however, were age dependent. IgG antibodies against UspA1 and UspA2 were cross-reactive and are a major source of serum bactericidal activity in adults. The levels of these antibodies and serum bactericidal activity appear to correlate with age-dependent resistance to M. catarrhalis infection. This study suggests that the humoral response to the UspA proteins is critical for protecting people from M. catarrhalis infections. Additional studies need to be done to confirm this suggestion and whether vaccination with these proteins can provide protection.
ACKNOWLEDGMENTS
We thank Ross Fredenburg for providing the purified UspA1 and UspA2 proteins, Ih Chang for performing the statistical analyses, and Rob Smith for preparing the photographs.
REFERENCES
- 1.Aebi C, LaFontaine E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis strain O35E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamberger D M. Antimicrobial treatment of sinusitis. Semin Respir Infect. 1991;6:77–84. [PubMed] [Google Scholar]
- 3.Bluestone C D. Otitis media and sinusitis in children: role of Branhamella catarrhalis. Drugs. 1986;31(Suppl. 3):132–141. doi: 10.2165/00003495-198600313-00029. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone C D, Stephenson J S, Martin L M. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11:S7–S11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Boyle F M, Georghiou P R, Tilse M H, McCormack J G. Branhamella (Moraxella) catarrhalis: pathogenic significance in respiratory infections. Med J Aust. 1991;154:592–596. doi: 10.5694/j.1326-5377.1991.tb121219.x. [DOI] [PubMed] [Google Scholar]
- 6.Carson R T, McDonald D F, Kehoe M A, Calvert J E. Influence of Gm allotype on the IgG subclass response to streptococcal M protein and outer membrane proteins of Moraxella catarrhalis. Immunology. 1994;83:107–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen D, McMichael J C, VanDerMeid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen J J, Renneberg J, Bruun B, Forsgren A. Serum antibody response to proteins of Moraxella (Branhamella) catarrhalis in patients with lower respiratory tract infection. Clin Diagn Lab Immunol. 1995;2:14–17. doi: 10.1128/cdli.2.1.14-17.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiGiovanni C, Riley T V, Hoyne G F, Yeo R, Cooksey P. Respiratory infections due to Branhamella catarrhalis: epidemiological data from Western Australia. Epidemiol Infect. 1987;99:445–453. doi: 10.1017/s0950268800067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejlertsen T. Pharyngeal carriage of Moraxella (Branhamella) catarrhalis in healthy adults. Eur J Clin Microbiol Infect Dis. 1991;10:89. doi: 10.1007/BF01964414. [DOI] [PubMed] [Google Scholar]
- 12.Ejlertsen T, Thisted E, Ostergaard P A, Renneberg J. Maternal antibodies and acquired serological response to Moraxella catarrhalis in children determined by an enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1994;1:464–468. doi: 10.1128/cdli.1.4.464-468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejlertsen T, Thisten E, Ebbesen F, Olesen B, Renneberg J. Branhamella catarrhalis in children and adults. A study of prevalence, time of colonization, and association with upper and lower respiratory tract infections. J Infect. 1994;29:23–31. doi: 10.1016/s0163-4453(94)94979-4. [DOI] [PubMed] [Google Scholar]
- 14.Faden H, Harabuchi Y, Hong J J, Pediatrics T W. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169:1312–1317. doi: 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 15.Faden H, Hong J, Murphy T. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect Immun. 1992;60:3824–3829. doi: 10.1128/iai.60.9.3824-3829.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldblatt D, Turner M W, Levinsky R J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 17.Gottfarb P, Brauner A. Children with persistent cough—outcome with treatment and role of Moraxella catarrhalis. Scand J Infect Dis. 1994;26:545–551. doi: 10.3109/00365549409011812. [DOI] [PubMed] [Google Scholar]
- 18.Helminen M E, Beach R, Maciver I, Jarosik G, Hansen E J, Leinonen M. Human immune response against outer membrane proteins of Moraxella (Branhamella) catarrhalis determined by immunoblotting and enzyme immunoassay. Clin Diagn Lab Immunol. 1995;2:35–39. doi: 10.1128/cdli.2.1.35-39.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 20.Leinonen M, Luotonen J, Herva E, Valkonen K, Mäkelä P H. Preliminary serological evidence for a pathogenic role of Branhamella catarrhalis. J Infect Dis. 1981;144:570–574. doi: 10.1093/infdis/144.6.570. [DOI] [PubMed] [Google Scholar]
- 21.McMichael J C, Fiske M J, Fredenburg R A, Chakravarti D N, VanDerMeid K R, Barniak V, Caplan J, Bortell E, Baker S, Arumugham R, Chen D. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–4381. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen M J, Baldwin C D, Swank P R, Pannu A K, Johnson D L, Howie V M. Relation of infant feeding practices, cigarette smoke exposure, and group child care to the onset and duration of otitis media with effusion in the first two years of life. J Pediatr. 1993;123:702–711. doi: 10.1016/s0022-3476(05)80843-1. [DOI] [PubMed] [Google Scholar]
- 23.Roitt I, Brostoff J, Male D. Immunology. New York, N.Y: Gowere Medical Publishing; 1985. [Google Scholar]
- 24.Ruuskanen O, Heikkinen T. Otitis media: etiology and diagnosis. Pediatr Infect Dis J. 1994;13:S23–S26. [PubMed] [Google Scholar]
- 25.Sehgal S C, Alshaimy I. Moraxella catarrhalis in upper respiratory tract of healthy Yemeni children/adults and paediatric patients: detection and significance. Infection. 1994;22:193–196. doi: 10.1007/BF01716701. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teele D W, Klein J O, Rosner B the Greater Boston Otitis Media Study Group. Epidemiology of otitis media during the first five years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Vaneechoutte M, Verschraegen G, Claeys G, Weise B, Van den Abeele A M. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28:2674–2680. doi: 10.1128/jcm.28.12.2674-2680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wald E R, Milmoe G J, Bowen A D, Ledesma-Medina J, Salamon N, Bluestone C D. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 30.Wald E R, Reilly J S, Casselbrant M. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104:297–302. doi: 10.1016/s0022-3476(84)81018-5. [DOI] [PubMed] [Google Scholar]