Abstract
Background and Aims:
When colon polyps are removed in the setting of inflammatory bowel disease (IBD) involving the large intestine, biopsy sampling of the flat mucosa surrounding such polyps have been recommended, but there are no data to support this practice.
Methods:
We reviewed endoscopic and pathologic findings in IBD patients who had dysplastic polyps removed and biopsy sampling of the adjacent flat mucosa. We assessed risk for subsequent neoplasia based on the presence or absence of dysplasia in the peri-polyp flat mucosa and based on number and grade of index polypoid lesions. Kaplan-Meier survival analysis was performed.
Results:
Fifty-six IBD patients (68% ulcerative colitis [UC]) underwent 102 colonoscopies, in which 129 dysplastic polyps were resected. Five hundred three biopsy procedures of the surrounding flat mucosa were performed (mean, 3.9 biopsy samples per polyp), of which 16 (3.2%) were dysplastic. Thirty-four patients (21 UC) had follow-up in a median of 1.7 years (range, .02-15) and 147 colonoscopies. The presence of dysplasia in peri-polyp biopsy specimens during index colonoscopy was not associated with risk of developing high-grade dysplasia (HGD) or cancer (Pearson χ2 test = .19). The size and number of dysplastic polyps were not predictive of neoplastic outcomes, but the probability of developing subsequent advanced neoplasia for polypoid low-grade dysplasia was 18%, 29%, and 40% by 1, 3, and 5 years, respectively, and for polypoid HGD was 50%, 60%, and 70% by 1, 3, and 5 years, respectively (hazard ratio, 7.0; standard error, 4.8).
Conclusions:
In patients with IBD-associated colitis, biopsy sampling of the mucosa adjacent to discrete dysplastic polypoid lesions are low yield and do not predict findings in follow-up examinations. However, the grade of dysplasia of the polyp itself is predictive of subsequent advanced neoplasia. (Gastrointest Endosc 2018;87:1304-9.)
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory disorders that affect the colon. Both CD and UC have been shown to confer a greater risk for the development of colorectal cancer compared with the general population, which has been mostly attributed to chronic inflammation.1,2 Prevention of disease-related adverse events such as colorectal cancer is 1 of the main goals of management once induction of remission is achieved, and surveillance colonoscopies are recommended to detect early dysplasia.3,4
Historically, when colonic dysplasia was difficult to visualize, it was recommended that random biopsy samples be obtained throughout the colorectum to systematically sample the at-risk mucosa.3,5–9 It was also considered that given the diffuse nature of colonic inflammation, there was a field effect of molecular changes to neoplastic change. However, studies demonstrated that most dysplasia is visible and that the recommended random biopsy samples are of low yield.10–14
Current guidelines recommend biopsy sampling of the flat mucosa around polyps found within the area of colitis to assess for more diffuse neoplastic changes.15 However, the diagnostic yield of such biopsy specimens has not been assessed, and there is no clear evidence that performing a biopsy sampling on the flat mucosa around polyps changes clinical outcomes. We hypothesized that the diagnostic yield of performing biopsy sampling on the flat mucosa around polyps is low and is not associated with subsequent advanced neoplasia.
METHODS
This is a retrospective study, approved by the Institutional Review Board at the University of Chicago Medicine (protocol 09-393-B). Patients in this study were obtained from the University of Chicago IBD Registry, a tertiary clinical database of all IBD patients evaluated at the University of Chicago. Using standard database management software, we identified all IBD patients who were found to have dysplasia during colonoscopic examinations performed for either screening or surveillance indications at our institution between 2005, when we started using high-definition (HD) colonoscopes (models CFH180AL, CF-HQ190L, and PCF-Q180AL; Olympus America, Melville, NY), and 2014. HD is defined as a resolution of at least 720 active lines of pixels at 24 fps with an aspect ratio of 16:9.16
We manually reviewed all colonoscopic examinations and included all examinations in which at least 1 dysplastic polypoid lesion was identified. Polypoid lesions included in this study must have been in an area of known chronic colitis and were defined as raised, endoscopically distinct lesions (Paris classification 0-I)17 that underwent endoscopic removal. Because this is a study of polypoid lesions, patients with unresectable lesions, “invisible” lesions found only by nontargeted biopsy sampling, masses, or those with colorectal cancer at index colonoscopy were excluded. Our institutional practice for such patients has been to recommend surgery. In addition, baseline demographics, the colitis, and the histologic data were collected.
At the University of Chicago, during the time period of this review it has been our institutional practice that polypoid lesions found during colonoscopic examinations in colitis patients are approached in a standard fashion: The polyp is assessed to determine whether it is inflammatory-appearing or not, whether it is endoscopically discrete and resectable, and if it is not inflammatory-appearing and does appear resectable, it is removed. The typical appearance of inflammatory polyps has been described elsewhere and has been applied here as well.18,19 Inflammatory polyps in this study were identified at the endoscopists’ discretion based on classic features of hyperemia, exudative caps, and, in later years, a pit pattern that was similar to surrounding mucosa. The approach to removing the polypoid lesions identified in this study depended on its size and at the discretion of the endoscopists (R.D.C., S.B.H., D.T.R.). There was not a previously defined protocol for obtaining peri-polyp biopsy samples, but it was an agreed approach to such lesions. A minimum of 2 biopsy specimens was obtained in our approach.
It is also the University of Chicago institutional practice to have an expert GI pathologist review all histologic specimens obtained from our IBD patients. If a specimen is determined to contain neoplasia, a second expert GI pathologist reviews the specimen to confirm the diagnosis. Our pathologists use the standard definitions of neoplasia in IBD-related colorectal epithelium as no dysplasia, low-grade dysplasia (LGD), high-grade dysplasia (HGD), indefinite dysplasia (favor positive or favor negative), or adenocarcinoma. In addition, during the time of this review period it was our practice to routinely perform biopsy sampling of the flat mucosa adjacent to polypoid lesions after resection of the lesion and to place such biopsy samples in separate pathology jars appropriately labeled as the flat mucosa surrounding polyp.
In this analysis we characterized polypoid lesions as described above as LGD or HGD. The first colonoscopy with a dysplastic polyp was designated the “index colonoscopy” for a specific patient and in subsequent analysis of follow-up. There were no index lesions of indefinite dysplasia, and, per protocol, cancers were excluded. Biopsy specimens of the flat mucosa were classified in the same manner and subsequently analyzed based on whether they were dysplastic or not. “Diagnostic yield” was defined as the prevalence of confirmed dysplasia in the flat mucosa surrounding the dysplastic polyp.
Patients with follow-up were defined as those who had follow-up colonoscopies at the University of Chicago and who had not had interval colectomy. All subsequent examinations of these patients were reviewed until the endpoint of the data, the patient had surgery, or until an advanced neoplastic lesion (HGD or cancer) was identified. We assessed in a per-patient analysis the risk for subsequent neoplasia in follow-up based on the grade of dysplasia in the index polyp, the number of index dysplastic polyps, the presence or absence of dysplasia in the peri-polyp flat mucosa, and, when applicable, the surgical pathology. Calculation of mean age and mean duration of disease were weighted to adjust for patients who had more than 1 examination or more than 1 polyp. Kaplan-Meier survival analysis was performed.
RESULTS
Assessment of peri-polyp biopsy yield
We identified 56 patients, including 38 (68%) UC and 18 (32%) CD patients, who had 129 dysplastic polypoid lesions in 102 colonoscopies (56 of these examinations were by definition “index colonoscopies”) (Fig. 1). A total of 503 biopsy procedures of the surrounding flat mucosa were performed (average of 3.9 biopsy samples per polyp), of which 16 (3.2%) were dysplastic. All examinations were performed with white light only and not chromoendoscopy. Patient demographics are included in Table 1.
Figure 1.
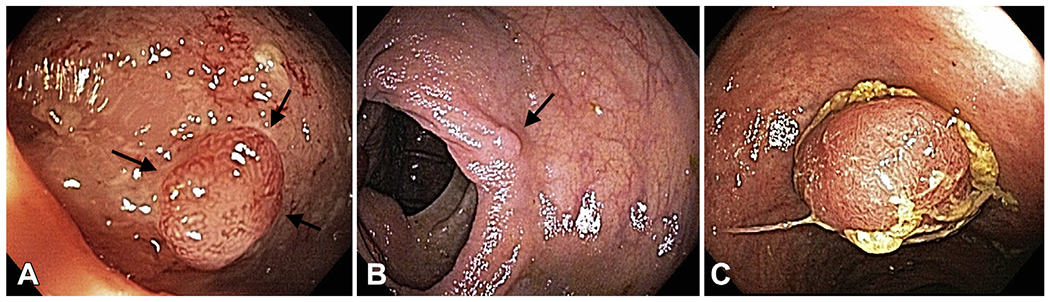
Examples of polypoid dysplasia from this study. A, A 44-year-old man with 2-mm polyp with low-grade dysplasia and surrounding moderately active ulcerative colitis in distal transverse colon. B, A 59-year-old woman with 1-mm polyp with low-grade dysplasia and surrounding quiescent ulcerative colitis in ascending colon. C, A 66-year-old man with 12-mm polypoid low-grade and high-grade dysplasia in ascending colon and surrounding mildly active colitis.
TABLE 1.
Demographics of inflammatory bowel disease patients with dysplastic polypoid lesions (n = 56)
| Value | |
|---|---|
| Sex, male | 38 (68) |
|
| |
| Inflammatory bowel disease diagnosis | |
|
| |
| Ulcerative colitis | 38 (68) |
|
| |
| Crohn’s disease | 18 (32) |
|
| |
| Number of colonoscopies | 102 |
|
| |
| Mean age at time of index colonoscopy (weighted), y | 56.7 |
|
| |
| Mean duration of disease at time of index colonoscopy (weighted), y | 18.8 |
Values are n (%), unless otherwise defined.
Follow-up cohort
Thirty-four patients, including 21 (62%) UC and 13 (38%) CD patients, had additional follow-up that included 147 colonoscopies in a median of 1.7 years (range, .02-15), which includes 46 examinations in the assessment group (102 from the assessment group above, minus the 56 index exams). The index polyps in these follow-up patients included 25 LGD polyps and 12 HGD polyps (3 patients had 2 polyps each). The mean size of polyp in the index examination was 5.1 mm (standard deviation, 2.4 mm). Seven patients went to surgery without additional follow-up colonoscopy, 11 patients had follow-up and surgery, and 16 patients who had follow-up colonoscopies and did not have surgery. Of those patients who had follow-up, 37 lesions were found. Seventeen patients had LGD in follow-up, 7 patients had HGD, and 4 patients had an adenocarcinoma. Four patients had no dysplasia during follow-up (Table 2).
TABLE 2.
Grade of neoplasia in follow-up based on index grade of polyp in chronic colitis
| Grade index | No dysplasia | LGD | HGD | Cancer | Total |
|---|---|---|---|---|---|
| LGD | 4 (18.2%) | 15 (68.2%) | 2 (9.1%) | 1 (4.6%) | 22 (100%) |
|
| |||||
| HGD | 2 (16.7%) | 2 (16.7%) | 5 (41.7%) | 3 (25.0%) | 12 (100%) |
|
| |||||
| Total | 6 (17.7%) | 17 (50.0%) | 7 (20.6%) | 4 (11.8%) | 34 (100%) |
LGD, Low-grade dysplasia; HGD, high-grade dysplasia.
The presence or absence of dysplasia in peri-polyp biopsy samples during index colonoscopy was not associated with the risk of developing HGD or cancer (log rank test, P = .20). In addition, size and number of index dysplastic lesions were not associated with subsequent advanced neoplasia. For each millimeter increase in polyp size, the hazard ratio was 1.10 (95% confidence interval, .74-1.66; log rank test P = .62). The total number of dysplastic polyps in the index colonoscopy was not associated with risk of subsequent HGD or cancer (log rank test, P = .37) (Table 3).
TABLE 3.
Follow-up of neoplasia based on number of dysplastic polyps
| Total no. of polyps | Grade in follow-up |
||||
|---|---|---|---|---|---|
| No dysplasia | LGD | HGD | Cancer | Total | |
| 1 | 3 | 11 | 5 | 3 | 22 |
|
| |||||
| 2 | 2 | 2 | 1 | 1 | 6 |
|
| |||||
| 3 | 1 | 2 | 1 | 0 | 4 |
|
| |||||
| 4 | 0 | 2 | 0 | 0 | 2 |
LGD, Low-grade dysplasia; HGD, high-grade dysplasia.
On the other hand, the grade of dysplastic polyp was associated with subsequent advanced neoplasia (log rank test, P = .0009). Of the patients with polypoid LGD, the probability of developing subsequent HGD or cancer was 5% by 1 year, 13% by 3 years, and 23% by 5 years. Of patients with polypoid HGD the probability of developing subsequent HGD or cancer was 49% by 1 year, 60% by 3 years, and 70% by 5 years (hazard ratio, 7.0; 95% confidence interval, 1.8-26.6) (Fig. 2).
Figure 2.
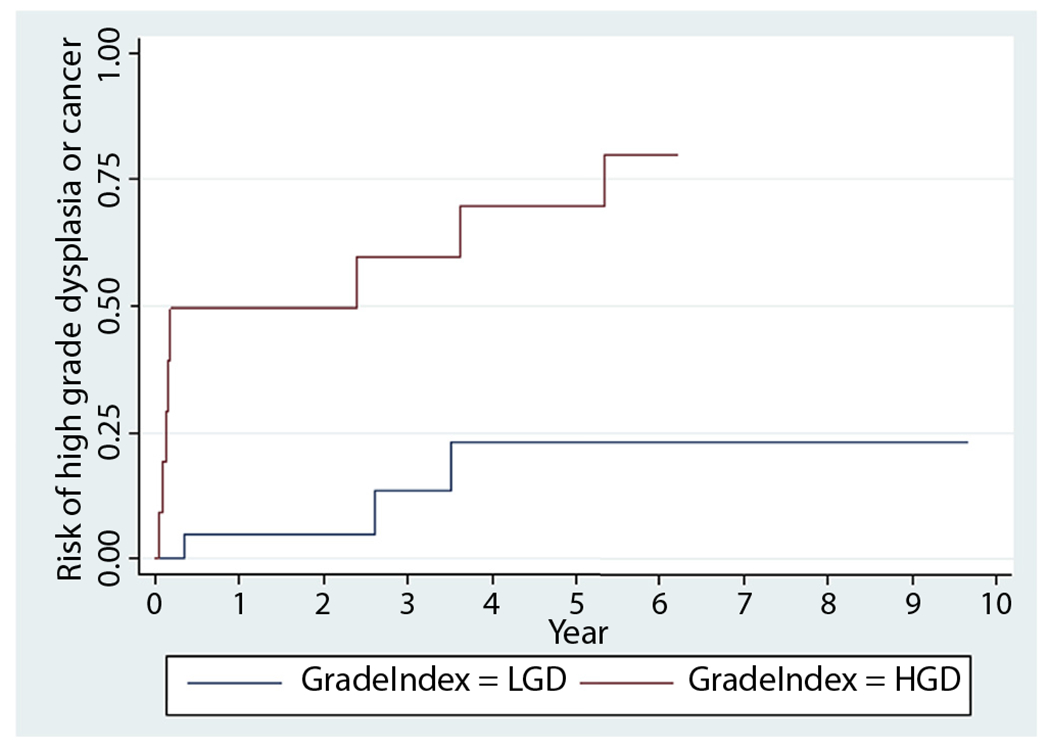
Kaplan-Meier survival analysis of high-grade dysplasia or cancer after index low-grade or high-grade polypoid dysplasia. LGD, Low-grade dysplasia; HGD, high-grade dysplasia.
DISCUSSION
This study answers an important question regarding the management of polypoid dysplasia in patients with chronic colitis. We found that while using HD colonoscopes, obtaining biopsy specimens of the mucosa adjacent to discrete polypoid lesions was low yield and, importantly, did not predict findings in follow-up examinations. Instead, we confirmed that the finding most predictive for future advanced neoplasia was the grade of dysplasia in the polyps themselves, with polypoid HGD having a 7-fold increased risk for subsequent advanced neoplasia (HGD or cancer) compared with polypoid LGD.
Our study lends some support to the recently published consensus statement by the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendation group, which included among their statements the recommendation that patients who have polypoid dysplasia completely resected could continue with colonoscopic surveillance rather than proceed to colectomy.20 Although there was 100% agreement by the authors of this statement, they acknowledged that the level of evidence for this recommendation was of very low quality.20 This consensus statement was based on the intuitive understanding by participants that polypoid dysplasia can be managed by endoscopic resection and that advances in imaging equipment enabled active surveillance rather than colectomy. It was also based on small retrospective studies that described safe follow-up of patients who had polyps resected.21–25 It is notable that in this analysis, polypoid HGD had a high risk of subsequent cancer (49% by 1 year, 60% by 3 years, and 70% by 5 years), implying that endoscopists should carefully consider these findings in their decisions for follow-up and in their discussions with patients.
The prior recommendation to perform colectomy when any dysplasia was identified was based on the appreciation of the limits of existing endoscopic technologies, and the prior findings that dysplasia in chronic colitis may be part of a broader “field effect” that would mean that if dysplasia was found in 1 part of the colon, it was statistically likely that there would be dysplasia in other parts of the colon that was not seen. More worrisome was the concern that when dysplasia was found in 1 part of the colon, there was a defined risk of a synchronous cancer in other parts of the colorectum.9,26,27 Similar concerns led to the recommendation (also without evidence) to perform biopsy sampling adjacent to polypoid lesions to confirm complete resection of the polyps, despite the absence of evidence to support this practice.15
Advances in imaging technology and widespread incorporation of surveillance colonoscopy has led in recent years to changed beliefs and understanding about these recommendations. First is the understanding that polypoid dysplasia found in chronic colitis has a different prognosis from dysplasia, which has a nonpolypoid or flat morphology. This has been seen in several retrospective studies demonstrating that patients with polypoid dysplasia who do not proceed to colectomy have a lower likelihood of developing subsequent advanced neoplasia than patients who have dysplasia found on nontargeted (“random”) biopsy sampling.21–25,28,29 Second is the appreciation that HD colonoscopes are able to visualize the mucosa at a higher level of resolution and therefore the likelihood of “missed” dysplasia or cancer is very low. In a recent study reviewing this and using colectomy as the criterion standard for detection of dysplasia and cancer, no cancers were missed by white-light HD colonoscopy.30 Third is the increasing appreciation for the low yield of “random” (nontargeted) biopsy sampling in colitis.10,12,13 Finally, the practical reality is that compliance with the recommendation for “peri-polyp biopsy sampling” is probably poor. This is further supported by prior assessments of other components of the cancer prevention guidelines, in which gastroenterologists’ compliance with surveillance recommendations was poor.31,32
There are several strengths to this study. It is the first study to describe the yield of performing peri-polyp biopsy sampling in patients with chronic colitis in the era of HD colonoscopes. In addition, it is a strength that this study includes patients with UC and with CD. The study was performed in an established expert center for IBD, which included experienced IBD endoscopists and GI pathologists. The approach to polypoid dysplasia and peri-polyp biopsy sampling was consistent among the participants, because it had been standardized institutionally. Finally, the present study followed clinical outcomes for up to 15 years, with findings that are consistent with prior publications of polypoid dysplasia.
Despite the strengths, this study does have some limitations. First, it is retrospective, small in size, and only included a single center. It is subject to the challenges of chart review, missing data, and potential for observational bias. It is also possible that patients who were not sent immediately to colectomy and therefore followed-up in this study are likely to represent a cohort who were lower risk for poor outcomes based on findings not included in this review, like degree of inflammation or prior disease severity. Despite this, the quality of our records was excellent, and the single-center location of the study provides more consistency in practice and histologic interpretation. Second, although the 3 co-investigator endoscopists (R.D.C., S.B.H., D.T.R.) had similar approaches to management and we do believe that the peri-polyp biopsy specimens were obtained in standard fashion, it is certainly possible that some of these specimens were not obtained in this manner. If so, it would potentially underestimate the yield of these findings. Third, we did not have adequate data to further characterize the technique of resection and the morphology of the polypoid lesions, either by the Paris classification or by the proposed “modified Paris classification” of the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendation.20 Fourth, given the small size of this study, we were unable to assess potential differences between patients with Crohn’s colitis and those with UC or other variables that may be predictive such as size of the index lesion. Finally, although chromoendoscopy was performed in some follow-up examinations, we did not assess the utility of chromoendoscopy for similar size/power reasons.
The findings of this study support a practice of performing polypectomy for polypoid lesions that are discrete and can be removed completely, followed by active surveillance, with the above-mentioned considerations regarding HGD in polypoid lesions. However, we believe that performing biopsy sampling of the flat mucosa adjacent to the polyp to assess for a field effect does not enhance clinical decision-making, both because of the low yield seen in this study and also because of the high yield of performing follow-up examinations, which identified subsequent neoplasia and allowed for additional interventions. It is also important to note that neither the number nor the size of dysplastic polyps found during index colonoscopy was predictive of subsequent neoplasia. Although this may seem somewhat counterintuitive and may be underpowered, it suggests that when discrete and resectable, polypoid dysplasia can be managed endoscopically. By design, this analysis excluded patients with unresectable dysplastic lesions or neoplasia that was found only on nontargeted biopsy sampling (so-called invisible dysplasia). The published recommendations and our institutional practice for such higher-risk patients have been to recommend surgical management.
Therefore, based on these findings we recommend that clinicians stratify follow-up of their patients based on the grade of dysplasia in a polyp. For example, a chronic colitis patient with polypoid HGD should have shorter interval follow-up or be referred for colectomy, compared with a patient with polypoid LGD who might have a more relaxed follow-up interval. Such intervals for follow-up have not been defined in any prospective study, so including these risk factors can aid clinicians and patients in decisionmaking. The role for subsequent chromoendoscopy in higher risk patients has been studied, and the limited data do suggest an additional yield in performing dye spray colonoscopy in patients with prior dysplastic polypoid and nonpolypoid lesions.33,34
There is still a role for performing additional biopsy samples after polypectomy in patients with chronic colitis. This would include assessment for completion polypectomy when the lesion has been removed piecemeal or is of uncertain extent otherwise, to assess if the lesion is in the field of colitis, or to assess for histologic activity of inflammation to adjust therapy or consider a separate risk for subsequent neoplasia.35,36
In conclusion, we have shown that performing biopsy sampling adjacent to polypoid dysplasia in colitis is of low yield and unnecessary in the era of enhanced visualization. We also confirm prior studies that demonstrate the high risk of subsequent advanced neoplasia based on the grade of dysplasia of polypoid lesions. This study adds to the body of evidence to support the evolving approach to dysplasia management in chronic colitis.
Abbreviations:
- CD
Crohn’s disease
- HD
high definition
- HGD
high-grade dysplasia
- IBD
inflammatory bowel disease
- LGD
low-grade dysplasia
- UC
ulcerative colitis
Footnotes
DISCLOSURE: All authors disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med 2015;372:1441–52. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 2005;11:314–21. [DOI] [PubMed] [Google Scholar]
- 5.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–89. [DOI] [PubMed] [Google Scholar]
- 6.Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:746–74; 774.e1-4; quiz e12-3. [DOI] [PubMed] [Google Scholar]
- 7.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738–45. [DOI] [PubMed] [Google Scholar]
- 8.Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc 2006;63:558–65. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein C, Shanahan F, Weinstein W. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 1994;343:71–4. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DT, Rothe JA, Hetzel JT, et al. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc 2007;65:998–1004. [DOI] [PubMed] [Google Scholar]
- 11.Blonski W, Kundu R, Furth EF, et al. High-grade dysplastic adenoma-like mass lesions are not an indication for colectomy in patients with ulcerative colitis. Scand J Gastroenterol 2008;43:817–20. [DOI] [PubMed] [Google Scholar]
- 12.Rutter MD, Saunders BP, Wilkinson KH, et al. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc 2004;60:334–9. [DOI] [PubMed] [Google Scholar]
- 13.Van Den Broek FJ, Stokkers PC, Reitsma JB, et al. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol 2014;109:715–22. [DOI] [PubMed] [Google Scholar]
- 14.Blonski W, Kundu R, Lewis J, et al. Is dysplasia visible during surveillance colonoscopy in patients with ulcerative colitis? Scand J Gastroenterol 2008;43:698–703. [DOI] [PubMed] [Google Scholar]
- 15.Friedman S, Odze RD, Farraye FA. Management of neoplastic polyps in inflammatory bowel disease. Inflamm Bowel Dis 2003;9:260–6. [DOI] [PubMed] [Google Scholar]
- 16.Hurbis-Cherrier M. The digital video system. In: Francis T, editor. Voice and vision: a creative approach to narrative film and DV production. Oxford, UK: Taylor & Francis; 2007. p. 169–77. [Google Scholar]
- 17.Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [DOI] [PubMed] [Google Scholar]
- 18.Riddell R, Goldman H, Ransohoff D, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983;14:931–68. [DOI] [PubMed] [Google Scholar]
- 19.Farraye F, Waye J, Moscandrew M, et al. Variability in the diagnosis and management of adenoma-like and non-adenoma-like dysplasia-associated lesions or masses in inflammatory bowel disease: an Internet-based study. Gastrointest Endosc 2007;66:519–29. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc 2015;81:489–501. [DOI] [PubMed] [Google Scholar]
- 21.Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology 1991;100:1241–8. [PubMed] [Google Scholar]
- 22.Odze RD, Farraye FA, Hecht JL, et al. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 2004;2:534–41. [DOI] [PubMed] [Google Scholar]
- 23.Rubin PH, Friedman S, Harpaz N, et al. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology 1999;117:1295–300. [DOI] [PubMed] [Google Scholar]
- 24.Medlicott SA, Jewell LD, Price L, et al. Conservative management of small adenomata in ulcerative colitis. Am J Gastroenterol 1997;92:2094–8. [PubMed] [Google Scholar]
- 25.Torres C, Antonioli D, Odze RD. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow-up study of 89 polyps from 59 patients. Am J Surg Pathol 1998;22:275–84. [DOI] [PubMed] [Google Scholar]
- 26.Connell WR, Lennard-Jones JE, Williams CB, et al. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology 1994;107:934–44. [DOI] [PubMed] [Google Scholar]
- 27.Ullman T, Croog V, Harpaz N, et al. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 2003;125:1311–9. [DOI] [PubMed] [Google Scholar]
- 28.Pekow JR, Hetzel JT, Rothe JA, et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis 2010;16:1352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackstone MO. DALM in ulcerative colitis cancer surveillance. Gastroenterology 1986;91:266–7. [DOI] [PubMed] [Google Scholar]
- 30.Krugliak Cleveland N, Colman RJ, Rodriquez D, et al. Surveillance of IBD using high definition colonoscopes does not miss adenocarcinoma in patients with low-grade dysplasia. Inflamm Bowel Dis 2016;22:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernstein CN, Weinstein WM, Levine DS, et al. Physicians’ perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis. Am J Gastroenterol 1995;90:2106–14. [PubMed] [Google Scholar]
- 32.Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc 2000;51:123–8. [DOI] [PubMed] [Google Scholar]
- 33.Deepak P, Hanson GJ, Fletcher JG, et al. Incremental diagnostic yield of chromoendoscopy and outcomes in inflammatory bowel disease patients with a history of colorectal dysplasia on white-light endoscopy. Gastrointest Endosc 2016;83:1005–12. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DT, Krugliak Cleveland N, et al. Outcomes of colitis-associated dysplasia after referral from the community to a tertiary center. Gastrointest Endosc 2016;84:1078–9. [DOI] [PubMed] [Google Scholar]
- 35.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol 2013;11:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–9. [DOI] [PubMed] [Google Scholar]


