Abstract
The protozoan parasite Cryptosporidium parvum is an important cause of diarrhea in humans, calves, and other mammals worldwide. No approved vaccines or parasite-specific drugs are currently available for the control of cryptosporidiosis. To effectively immunize against C. parvum, identification and characterization of protective antigens are required. We previously identified CPS-500, a conserved, neutralization-sensitive antigen of C. parvum sporozoites and merozoites defined by monoclonal antibody 18.44. In the present study, the biochemical characteristics and subcellular location of CPS-500 were determined. CPS-500 was chloroform extractable and eluted with acetone and methanol in silicic acid chromatography, consistent with being a polar glycolipid. Following chloroform extraction and silicic acid chromatography, CPS-500 was isolated by high-pressure liquid chromatography for glycosyl analysis, which indicated the presence of mannose and inositol. To identify which component of CPS-500 comprised the neutralization-sensitive epitope recognized by 18.44, the ability of the monoclonal antibody to bind CPS-500 treated with proteases, or with α- or β-glycosidases, was determined. Monoclonal antibody 18.44 did not bind antigen treated with β-d-mannosidase but did bind antigen treated with α-d-mannosidase, other α- or β-glycosidases, or a panel of proteases. These data indicated that the target epitope was dependent on terminal β-d-mannopyranosyl residues. By immunoelectron microscopy, 18.44 binding was localized to the pellicle and an intracytoplasmic tubulovesicular network in sporozoites. Monoclonal antibody 18.44 also bound to antigen deposited and released onto substrate over the course travelled by gliding sporozoites and merozoites. Surface localization, adhesion and release during locomotion, and neutralization sensitivity suggest that CPS-500 may be involved in motility and invasion processes of the infective zoite stages.
Cryptosporidium parvum is an apicomplexan parasite that causes the diarrheal disease cryptosporidiosis in humans and economically important food animals throughout the world (10, 34). Despite progress, prevention and treatment of the disease remain limited by the absence of approved vaccines or immunotherapies and by the lack of safe and effective parasite-specific drugs (6, 24). Because C. parvum infection is controlled by normal immune responses, immunologic strategies for prevention and treatment are being investigated (reviewed in reference 24). Central to such investigations is the structural and functional characterization of candidate target antigens.
Apical organelle and surface-exposed molecules of apicomplexan parasites are involved in the pathogenesis of infection and present rational targets for immunologic intervention (18, 28, 29). We previously reported that monoclonal antibody (MAb) 18.44, prepared against whole C. parvum, binds to sporozoites and merozoites and neutralizes their infectivity in a time-dependent manner (5, 21, 26). The antigen defined by MAb 18.44 has been designated CPS-500. Because this antigen is expressed in both infective life cycle stages, contains one or more neutralization-sensitive epitopes, and is conserved among geographically diverse human and bovine C. parvum isolates (33), it likely has an important biological role. Therefore, CPS-500 is a candidate target antigen for active or passive immunization against cryptosporidiosis. In initial experiments to characterize the antigen, CPS-500 migrated with the dye front in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), eluted in the void volume of a Bio-Gel-A column with an exclusion limit of 500 kDa, was not radiolabelled by biosynthetic incorporation of [35S]methionine, and did not contain iodinatable tyrosine residues (26). In addition, preparative electrophoresis-isolated CPS-500 was weakly immunogenic in mice, rabbits, and hens immunized for preparation of MAbs or polyclonal antibodies (23). These observations, taken together, suggested that CPS-500 was nonproteinaceous, thereby complicating recombinant approaches for its production and characterization. For these reasons, experiments to biochemically characterize CPS-500 and the target epitope recognized by MAb 18.44 were performed. In the present study, CPS-500 was classified as a polar glycolipid based on its chloroform extractability and elution properties in silicic acid chromatography. Most importantly, it was determined that the neutralization-sensitive epitope recognized by MAb 18.44 is dependent on terminal β-d-mannopyranosyl residues based on β-d-mannosidase susceptibility, an observation consistent with the identification of mannose by glycosyl analysis of high-pressure liquid chromatography (HPLC)-isolated CPS-500. A possible function for CPS-500 in the motility of the infective stages is suggested by its immunoelectron microscopic localization to the sporozoite pellicle and its deposition on substrate by viable sporozoites and merozoites during locomotion. We conclude that CPS-500 is a candidate molecular target for immunologic control of cryptosporidiosis. While its glycolipid composition may preclude standard recombinant approaches for subunit production, chemical synthesis of the target epitope or anti-idiotypic antibody approaches may lead to CPS-500-based vaccines for cryptosporidiosis.
MATERIALS AND METHODS
Oocyst, sporozoite, and merozoite isolation.
The Iowa C. parvum isolate (13), used for all experiments, was passaged bimonthly in newborn Cryptosporidium-free Holstein calves to obtain parasite material (27). Oocysts were separated from calf feces by sucrose density gradient centrifugation and stored in 2.5% KCr2O7 (4°C) for up to 2 months prior to use (3). Oocysts were treated with hypochlorite prior to excystation, after which sporozoites were isolated by anion-exchange chromatography (27). Merozoites were isolated by Percoll density gradient centrifugation of intestinal contents from neonatal BALB/c mice at 65 h post-oocyst inoculation as previously described (25).
CPS-500 isolation.
In initial experiments to isolate CPS-500, MAb 18.44-affinity chromatography using CNBr-activated Sepharose, hydrazide Fc linkage matrix, or matrix with carbon chain spacers was unsuccessful (data not shown). Chloroform-methanol extraction of whole C. parvum was then performed to isolate the lipid fraction (7). Prior to extraction, oocysts (1.1 × 109) were excysted, then solubilized in lysis buffer (50 mM Tris, 5 mM EDTA, 5 mM iodoacetamide, 0.1 mM Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK], 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.01 mM leupeptin, 0.01 mM pepstatin A) by five rapid freeze-thaw cycles and sonication (4°C) (25). All glassware used in the subsequent extraction procedure was pretreated with concentrated HCl (12 M), rinsed with distilled water (dH2O), cleaned in PCC-54 detergent (50°C) (Pierce, Rockford, Ill.), rinsed with dH2O, dried (100°C), and siliconized. Standard measures to prevent contamination or degradation of lipids were implemented during all stages of extraction and storage (15). Following solubilization, the excysted oocyst preparation (4 volumes) was combined with glass-distilled chloroform (5 volumes) and methanol (10 volumes) (Burdick & Jackson, Muskegon, Mich.) to form a monophasic solution. After vortexing (30 min at 21°C), chloroform (5 volumes) and deionized water (5 volumes) (NANOpure; Barnstead, Dubuque, Iowa) were added to the monophasic solution (19 volumes) to form a diphasic solution. The diphasic solution was vortexed (for 30 min at 21°C) and then centrifuged (at 2,250 × g), after which the chloroform phase was collected. The methanol-water phase was back-extracted twice with chloroform, and the fractions were collected. Chloroform-extracted fractions and methanol-water fractions were filtered (Teflon membrane; 0.2-μm pore size; Nalge-Nunc, Rochester, N.Y.), dried under N2 (final volume, 0.5 ml), and stored at −80°C prior to analysis. To determine which fraction(s) contained CPS-500, samples of each were examined by dot immunoblot assay (33). In brief, samples were dotted onto nitrocellulose, incubated with MAb 18.44 immunoglobulin G3 (IgG3) or an isotype-matched control MAb of irrelevant specificity (each at 4 μg ml−1), washed, and incubated with affinity-purified alkaline phosphatase-conjugated rabbit anti-mouse IgG3 (Zymed, South San Francisco, Calif.) followed by substrate. To determine the (glyco)protein content of each fraction, samples were resolved in 10 to 20% gradient SDS-PAGE gels under reducing conditions and were silver stained (Bio-Rad, Hercules, Calif.) (28). Molecular weight standards (Bio-Rad) included myosin (200 kDa), phosphorylase B (97.4 kDa), bovine serum albumin (BSA) (66.2 kDa), ovalbumin (45 kDa), carbonic anhydrase (31 kDa), and soybean trypsin inhibitor (21.5 kDa).
Following identification of CPS-500 in the chloroform-extracted fraction, the extract (0.5 ml) was applied to a chloroform-equilibrated 9- by 45-mm BioSil-A silicic acid (Bio-Rad) column, which was then sequentially eluted with chloroform (7 column volumes), acetone (28 column volumes), and methanol (7 column volumes) (15). Eluate fractions were examined for the presence of CPS-500 by dot immunoblot assay as described above. Fractions containing CPS-500 were combined, dried under N2 (final volume, 0.5 ml), and stored at −80°C prior to use. To determine the (glyco)protein content in the combined CPS-500 fractions, samples were resolved in SDS-PAGE and silver stained as described above.
Silicic acid column-isolated CPS-500 was further purified by HPLC as follows (15). A mixture of chloroform (2 parts) and methanol (1 part) was added to N2-dried CPS-500 obtained from 2.6 × 109 excysted oocysts, and the preparation was then injected onto an HPLC (Beckman, Fullerton, Calif.) with a 250- by 7-mm Econosil silica column (10-μm bead size; Alltech Scientific, Deerfield, Ill.). Solvent A was HPLC-grade chloroform, and solvent B was anhydrous methanol (flow rate, 2 ml/min). After an initial 2 min at 0% B, a linear gradient was started, resulting in 100% B at 30 min. Twenty fractions (2 ml each) were collected into anhydrous methanol-cleaned glass tubes over a 40-min elution period. Aliquots (100 μl) of each fraction were dried under N2, reconstituted in phosphate-buffered saline (PBS) containing 0.1% (wt/vol) SDS, sonicated (4°C), and examined for CPS-500 by a dot immunoblot assay as described above. Fraction 16 reacted strongly with MAb 18.44 and was used for glycosyl analysis (described below).
Epitope characterization.
The ability of MAb 18.44 to bind CPS-500 treated with proteases (Sigma, St. Louis, Mo.), sodium periodate, or glycosidases was determined by a dot immunoblot assay as described above. For pepsin, trypsin, or papain treatment, purified sporozoites (7.5 × 107; 95 μg of protein [bicinchoninic acid assay; Pierce]) were solubilized (in 100 μl of PBS containing 0.5% [wt/vol] SDS and 0.5% [wt/vol] octylglucoside) and incubated with pepsin (0.85 U/μg of sporozoite protein for 1 h at 38°C; pH 4.7), trypsin (3.4 U/μg of sporozoite protein for 12 h at 38°C; pH 7.6), or papain (2.1 U/μg of sporozoite protein, with 0.05% [wt/vol] cysteine, for 12 h at 25°C; pH 6.2). Sporozoites were also solubilized in borate buffer (0.1 M sodium borate, 0.03% [wt/vol] CaCl2, 0.5% [wt/vol] SDS, 0.5% [wt/vol] octylglucoside) and incubated with pronase (0.001 U/μg of sporozoite protein, for 12 h at 38°C; pH 7.0), or they were solubilized in TNE buffer (0.01 M Tris, 0.1 M NaCl, 0.001 M EDTA, 0.5% [wt/vol] SDS, 0.5% [wt/vol] octylglucoside) and incubated with proteinase K (0.004 U/μg of sporozoite protein, for 1 h at 50°C; pH 7.0). For each treatment, control sporozoite protein was incubated identically in buffer lacking protease. Following incubation, protease inhibitors (1.0 mM PMSF and 0.1 mM TLCK) were added to each preparation. For periodate treatment, silicic acid column-isolated CPS-500 (from 5 × 107 excysted oocysts/treatment) was reconstituted in PBS containing 0.1% (wt/vol) SDS, dotted onto nitrocellulose, and incubated with sodium periodate (1 to 10 mM) or control buffer lacking periodate (36). For glycosidase treatment, silicic acid column-isolated CPS-500 (from 107 excysted oocysts/treatment) was incubated (at 37°C for 12 h; pH 7.5) in PBS with or without a glycosidase preparation from Turbo cornutus (final concentration, 25 mg ml−1) (ICN, Costa Mesa, Calif.) containing α-l-fucosidase, β-xylosidase, α- and β-mannosidase, α- and β-glucosidase, α- and β-galactosidase, α- and β-N-acetylglucosaminidase, and α- and β-N-acetylgalactosaminidase. Following observations that MAb 18.44 did not bind CPS-500 after incubation with mixed glycosidases from T. cornutus, treatments were repeated with component glycosidases (Sigma) as follows. Silicic acid column-isolated CPS-500 (from 107 excysted oocysts/treatment) was incubated (at 37°C for 12 h) in buffer (50 mM sodium acetate, pH 4.5) with or without (i) a combination of α-d-mannosidase (1 U), α-d-galactosidase (0.1 U), α-l-fucosidase (0.01 U), and α-d-glucosidase (1 U) or (ii) a combination of β-d-mannosidase (0.5 U), β-d-xylosidase (0.05 U), and β-d-glucosidase (1 U). Following observations that MAb 18.44 bound to CPS-500 which had been treated with the α-glycosidase combination, but not with the β-glycosidase combination, treatments were repeated with component β-glycosidases as follows. Silicic acid column-isolated CPS-500 was incubated as described above in buffer with or without β-d-mannosidase (0.5 U), β-d-xylosidase (0.05 U), or β-d-glucosidase (1 U) to determine which enzyme altered the epitope recognized by MAb 18.44.
Glycosyl analysis.
The glycosyl composition of whole C. parvum (106 excysted oocysts) and HPLC-isolated CPS-500 (fraction 16; 10% [vol/vol]) was determined by methanolysis, re-N-acetylation, trimethysilation, and gas chromatography-mass spectrometry (GC-MS) (model 5970; Hewlett-Packard, Avondale, Pa.) (8). To minimize the introduction of any contaminating sugars, reaction vessels (500-μl Reacti-Vials; Pierce) were preconditioned (at 70°C for 3 h) with methanolic HCl (3 M; 0.5 ml) and methyl acetate (125 μl). Positive displacement glass capillary tube pipettors and sterile Eppendorf pipettors were used for all organic and aqueous reagents, respectively. Samples and standards were derivatized as described elsewhere (8) except that scyllo-inositol was used as the internal standard. GC-MS detection and quantitation of derivatized samples was performed by using the selective ion monitoring mode at m/z 204 (hexoses, pentoses, and 6-deoxyhexoses), 173 (N-acetyl amino sugars), and 318 (inositols).
Electron microscopy.
To localize CPS-500 ultrastructurally, sporozoites were examined by immunoelectron microscopy using MAb 18.44 as follows. Purified sporozoites (5 × 107) were suspended (for 25 min at 4°C) in an isotonic phosphate-buffered fixative (40 mM NaH2PO4 · H2O–160 mM Na2HPO4 containing 2% [wt/vol] formaldehyde, 0.5% [vol/vol] glutaraldehyde, and 1% [wt/vol] tannic acid), washed with phosphate buffer, and centrifuged (at 1,850 × g for 5 min) into agarose. The sample was then dehydrated through a series of ethanol solutions (30 to 100% ethanol) while the temperature was progressively lowered (4 to −20°C), and it was embedded (−20°C) in LR White resin. Sections were mounted on nickel grids, blocked (with 0.1% [vol/vol] Tween 20), incubated (for 30 min at 37°C) with protein A-purified MAb 18.44 or an isotype-matched control MAb (each at 50 μg ml−1), washed, incubated with affinity-purified rabbit anti-mouse IgG (Zymed), washed, and incubated with affinity-purified colloidal gold-conjugated goat anti-rabbit IgG (5 nm; electron microscopy grade; Zymed). Following additional washing, sections were postfixed (4% [wt/vol] formaldehyde–1% [vol/vol] glutaraldehyde) and stained with a saturated solution of uranyl acetate. Samples were observed and photographed with a Philips 420 transmission electron microscope at 80 kV.
Exoantigen characterization.
To determine if CPS-500 is an exoantigen, two assays were performed. First, viable sporozoites or merozoites in PBS containing 0.5% (wt/vol) BSA were applied to poly-l-lysine-coated multiwell glass slides, incubated (at 37°C for 15 min), air dried, and gently heat fixed (4). Slides were processed for an indirect immunofluorescence assay (IFA) using MAb 18.44, the C. parvum P23-reactive MAb 7D10 (20), or isotype-matched control MAbs; then they were examined by epifluorescence microscopy. In a second assay, viable sporozoites (2 × 107 in Hank’s balanced salt solution) were incubated in suspension (under 10% CO2 for 1.5 h at 37°C), then centrifuged (at 5,000 × g at 4°C for 30 min) to separate sporozoites and incubation medium. Pelleted sporozoites were solubilized in lysis buffer containing 1% (wt/vol) octylglucoside by freeze-thawing and sonication (4°C). Following removal of insoluble material (at 100,000 × g at 4°C for 45 min), the soluble fractions from sporozoites and incubation medium were examined for the presence of CPS-500 by a dot immunoblot assay as described above.
RESULTS
CPS-500 is a chloroform-extractable glycolipid.
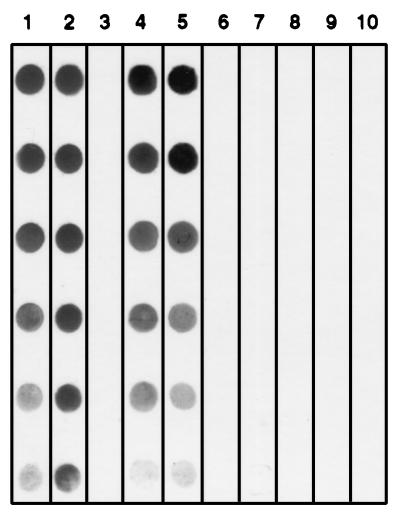
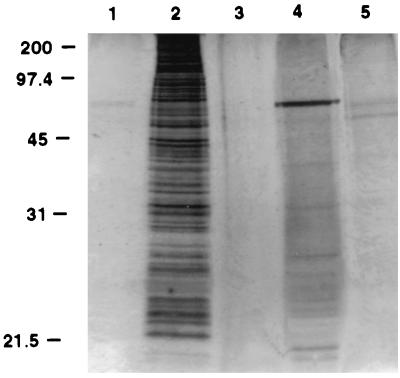
On the basis of the inability to purify CPS-500 by MAb 18.44 affinity chromatography, and of previous observations suggesting that the molecule was nonproteinaceous (26), the lipid fraction from whole C. parvum was extracted and examined. CPS-500 was identified in chloroform extracts, but not in the methanol-water fraction (Fig. 1). In silicic acid chromatography of the chloroform extract, CPS-500 eluted in acetone and methanol (Fig. 1), suggesting a polar glycolipid composition (15). Chloroform-extracted, silicic acid chromatography-isolated CPS-500 was relatively free of protein and glycoprotein contamination (Fig. 2) and was considered appropriate for use in characterizing the epitope recognized by MAb 18.44. The preparation was further purified by HPLC to characterize the glycosyl composition of CPS-500. Glycosyl analysis was first performed on excysted oocyst preparations to obtain general knowledge of the sugar composition in whole C. parvum and for comparison to that in HPLC-isolated CPS-500. A substantial amount of N-acetylgalactosamine was identified in whole C. parvum (Table 1). Other sugars expected in eucaryotic organisms were also identified (Table 1) (35). In HPLC-isolated CPS-500 (fraction 16), mannose and inositol (∼1:1) were identified as the major sugars (Table 1). Small amounts of glucose and galactose were also detected. In contrast to whole C. parvum, no N-acetylgalactosamine was identified.
FIG. 1.
Dot immunoblot demonstrating chloroform extractability and isolation of CPS-500 from whole C. parvum. Shown are an excysted oocyst preparation before extraction (lanes 1 and 6), chloroform (lanes 2 and 7) and methanol (lanes 3 and 8) extracts, and chloroform extract following successive silicic acid chromatography (lanes 4 and 9) and HPLC (lanes 5 and 10) isolation steps. Lanes 1 through 5 were probed with MAb 18.44, and lanes 6 through 10 were probed with isotype control MAb. Serial twofold dilutions of each preparation are dotted from top to bottom.
FIG. 2.
Silver-stained SDS-PAGE gel of CPS-500 at successive stages of isolation from whole C. parvum, demonstrating relative freedom from protein and glycoprotein contamination. Shown are an excysted oocyst preparation (5 × 106 oocysts) prior to chloroform-methanol extraction (lane 2), the methanol-water-extracted phase (lane 4), and the chloroform-extracted phase before (lane 5) and after (lane 3) silicic acid chromatography. Lane 1 was loaded with sample buffer to identify silver stain artifacts.
TABLE 1.
Glycosyl composition of whole C. parvum and HPLC-isolated CPS-500
| Glycosyl residue | Mol% of glycosyl residue in:
|
|
|---|---|---|
| C. parvum oocysts | CPS-500 | |
| Mannose | 18 | 43 |
| Galactose | 9 | 5 |
| Glucose | 61 | 11 |
| N-Acetylglucosamine | 1 | 0 |
| N-Acetylgalactosamine | 9 | 0 |
| Inositol | 2 | 41 |
β-d-Mannose comprises a neutralization-sensitive CPS-500 epitope.
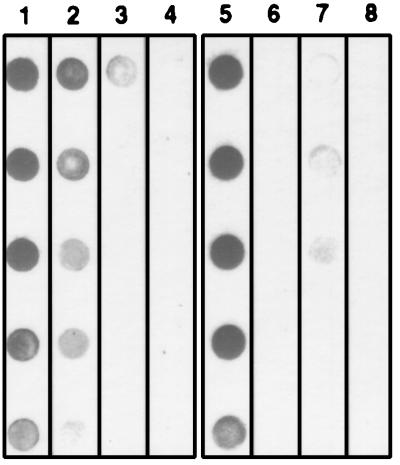
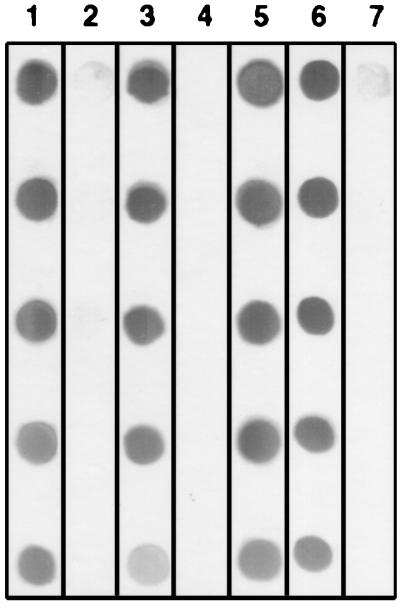
Treatment of solubilized sporozoites with individual proteases did not affect the binding of MAb 18.44 to CPS-500 (data not shown). When CPS-500 was treated with periodate, mixed glycosidases from T. cornutus, or mixed β-glycosidases, the binding of MAb 18.44 was substantially reduced or eliminated (Fig. 3 and 4). Treatment with mixed α-glycosidases did not affect the binding of MAb 18.44 (Fig. 4). When glycosidase treatments were repeated by using individual enzymes present in the mixed preparations, β-d-mannosidase, but not α-d-mannosidase or other glycosidases, eliminated the binding of MAb 18.44 to CPS-500 (Fig. 4). These findings are consistent with glycosyl composition data and indicated that CPS-500 contains a terminal β-d-mannopyranosyl residue essential for recognition by MAb 18.44.
FIG. 3.
Dot immunoblot detection of the CPS-500 epitope recognized by MAb 18.44 before (lanes 1 and 5) and after treatment with 1 mM (lane 2), 5 mM (lane 3), or 10 mM (lane 4) periodate or with mixed glycosidases from T. cornutus (lane 7). Lane 6 (untreated CPS-500) and lane 8 (CPS-500 treated with mixed glycosidases) were probed with an isotype control MAb. Serial twofold dilutions of CPS-500 are dotted from top to bottom.
FIG. 4.
Dot immunoblot detection of the CPS-500 epitope recognized by MAb 18.44 before (lane 1) and after treatment with mixed α-glycosidases (lane 3), mixed β-glycosidases (lane 4), β-d-glucosidase (lane 5), β-d-xylosidase (lane 6), or β-d-mannosidase (lane 7). Lane 2 contains untreated CPS-500 probed with an isotype control MAb. Serial twofold dilutions of CPS-500 are dotted from top to bottom.
CPS-500 exposed on the pellicle surface is bound and released onto substrate during sporozoite and merozoite motility.
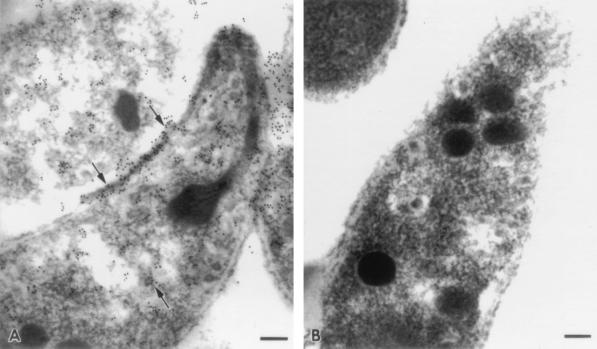
Although the glycolipid composition of CPS-500 precluded optimal fixation and processing for ultrastructural studies, immunoelectron microscopy localized MAb 18.44 binding to the sporozoite pellicle (Fig. 5). This finding is consistent with the binding of MAb 18.44 to viable sporozoites (26) and merozoites (5) in an IFA, and it confirmed surface exposure of the target epitope. MAb 18.44 binding was also observed in a tubulovesicular pattern in the sporozoite cytoplasm but was not localized to any discernible organelle (Fig. 5).
FIG. 5.
Immunoelectron photomicrographs of sporozoites probed with MAb 18.44 (A) or an isotype-matched control MAb (B). Note immunogold labelling of the sporozoite pellicle and intracytoplasmic material by MAb 18.44 (arrows). Bars, 0.1 μm.
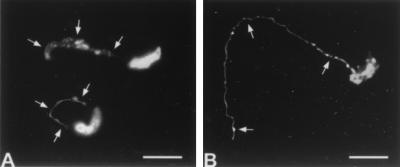
In an IFA, MAb 18.44 and the P23-reactive MAb 7D10 detected prominent trails of antigen deposited on substrate over the path travelled by motile sporozoites (Fig. 6) and merozoites (data not shown). Sporozoite antigen deposits detected by each MAb were morphologically similar (Fig. 6). Following incubation of viable sporozoites in suspension, CPS-500 was detected by MAb 18.44 in immunoblots of the sporozoite fraction but not in the soluble phase of incubation medium (data not shown).
FIG. 6.
Immunofluorescence reactivity of MAb 18.44 (A) and MAb 7D10 (B) with sporozoites. Note MAb detection of CPS-500 (A) and P23 (B) antigen deposits emanating from the posterior sporozoite and demarcating the gliding path (arrows). Bars, 5 μm.
DISCUSSION
In the present study, the biochemical properties, subcellular location, and possible function of the neutralization-sensitive C. parvum zoite antigen CPS-500 were characterized. Information related to these subjects was essential to ongoing investigations on the utility of this antigen for passive or active immunoprophylaxis against cryptosporidiosis (24). Other studies have reported that the antigenic composition of C. parvum is biochemically complex (17, 19) and suggested that parasite carbohydrates or glycoconjugates are important targets of the humoral immune response (5, 14, 17, 22, 26, 28, 32). Accordingly, the general biochemical composition of CPS-500 was determined, and the specific target epitope recognized by MAb 18.44 was characterized. This was of paramount interest because of the ability of MAb 18.44 to neutralize the infectivity of both sporozoites and merozoites (5, 26) and to significantly reduce C. parvum infection in oocyst-challenged mice when coadministered with other neutralizing MAb (21). In addition, conservation of the target epitope among different C. parvum isolates (33), and in both infectious stages (5, 26), suggests an important biological role. Finally, hyperimmune bovine polyclonal antibodies, which neutralize C. parvum sporozoite infectivity and significantly reduce infection in oocyst-challenged mice, recognize sporozoite and merozoite antigens comigrating with CPS-500 in SDS-PAGE (25). This observation suggests that cattle are able to respond to CPS-500 when immunized with whole C. parvum; thereby CPS-500 satisfies a fundamental criterion for consideration as a vaccine candidate.
Initial biochemical characterization of CPS-500 indicated a glycolipid composition based on chloroform extractability, and susceptibility to periodate and mixed glycosidases. Following HPLC isolation of CPS-500 from chloroform-extracted, silicic acid chromatography-enriched preparations, glycosyl analysis identified mannose and inositol as the predominant residues, suggesting that the antigen may be a mannosylated inositol. Glucose and galactose were also identified, but in much smaller amounts than mannose or inositol, suggesting that they may not be components of CPS-500. Relatively small amounts of native CPS-500 were recoverable for analysis in the present study. Isolation of CPS-500 in quantity will be required to determine its complete molecular structure and whether inositol, glucose, and galactose are components.
In studies to characterize the neutralization-sensitive CPS-500 epitope recognized by MAb 18.44, resistance to serine-, thiol-, acid-, and metalloproteases confirmed and extended previous observations that the epitope was not likely peptide dependent (26). A carbohydrate-dependent epitope was initially suggested by the IgG3 isotype of MAb 18.44, in that this isotype often predominates in murine antibody responses to carbohydrate epitopes (11). The rationale for determining if the epitope contained mannose was twofold. First, glycosyl analysis identified mannose as a major sugar in CPS-500. Second, a mixed-glycosidase preparation which contained α- and β-mannosidases destroyed the epitope. Subsequent studies using individual glycosidases of defined specificity led to the critical observation that the epitope was susceptible to β-d-mannosidase but not to α-d-mannosidase or other glycosidases. This finding provided unequivocal evidence that the target epitope was dependent on terminal β-d-mannopyranosyl residues. Importantly, because glycoconjugates in mammals are essentially devoid of terminal β-d-mannopyranosyl residues (35), the CPS-500 epitope composition identified herein may facilitate targeted disruption of a parasite-specific molecule known to have a role in the pathogenesis of infection. In relation to this, the weak immunogenicity of native CPS-500 observed in immunized animals may be accounted for by its biochemical properties (1) identified in the present study. Indeed, when coupled to methylated BSA or keyhole limpet hemocyanin, CPS-500 was highly immunogenic based on antibody responses in immunized mice and hens (23). Further, orally administered CPS-500-reactive mouse MAbs, and polyclonal monospecific hen egg yolk antibodies derived from animals immunized with carrier protein-coupled CPS-500, provided highly significant passive protection against C. parvum oocyst challenge in mice (23).
Immunoelectron microscopic localization of CPS-500 to the sporozoite pellicle and IFA identification of antigen bound and released onto substrate by gliding sporozoites and merozoites suggest that CPS-500 has a role in the motility of the infective stages. Previous studies have shown that sporozoites deposit surface antigens of 15 (GP15) (12, 31), 23 (P23) (4, 9, 31), and 38 or >900 (12) kDa on substrate or epithelial-cell monolayers during gliding motility in vitro. Deposition of GP15 and P23, antigens which are known to express neutralization-sensitive epitopes (2, 9, 20, 32), was detected in these studies by using MAbs specific for each antigen, including C6B6 (P23). Antigen deposits were morphologically similar to those described previously for the circumsporozoite protein of Plasmodium sp. (30), suggesting that specific C. parvum surface molecules may be involved in motility and invasion. In the present study, the P23-reactive MAb 7D10 detected antigen deposited by motile sporozoites and merozoites. We previously reported that MAb 7D10, prepared against C6B6 affinity chromatography-isolated native P23, significantly reduces C. parvum infection in mice and recognizes a neutralization-sensitive epitope which is different from that defined by MAb C6B6 (20). Therefore, recognition of a second distinct epitope by MAb 7D10 in P23 deposits further supports the hypothesis that surface antigen binding and release during infective-stage motility in vitro has probable biological relevance in vivo. Deposition of surface antigens other than the preceding by motile C. parvum sporozoites has not been previously reported. The results presented herein demonstrate that CPS-500, or a portion thereof containing the neutralization-sensitive epitope recognized by MAb 18.44, is bound and released onto substrate by motile sporozoites and, additionally, by merozoites. This finding is consistent with the surface location of CPS-500 and suggests a functional role for the molecule. Indeed, if the binding and release of CPS-500, P23, and GP15 are required for zoite motility and invasion, a probable mechanism for the neutralizing activity of MAbs against these antigens would be suggested.
With regard to the foregoing, we recently reported the identification of an ∼1,300-kDa apical and surface glycoprotein of C. parvum zoites designated CSL (28). CSL is a soluble exoantigen released into the incubation medium by sporozoites, and it contains a repetitive carbohydrate-dependent epitope recognized by MAbs which elicit the circumsporozoite precipitate-like reaction (28). MAbs which elicit this reaction neutralize sporozoite infectivity in vitro and provide passive protection against oocyst challenge in vivo (16, 28). On the basis of the characteristics of CSL and the inability of 18.44, 7D10, or other neutralizing MAbs against CPS-500 and P23 to elicit the circumsporozoite precipitate-like reaction (24), it was of interest to determine if CPS-500 is a soluble exoantigen. Although CPS-500 was bound and released during zoite motility and was identified in sporozoites after incubation in suspension, it was not detected in the incubation medium. These findings suggest that CPS-500, unlike CSL, is not a soluble exoantigen and that its release may require adhesion to a substrate during zoite motility. Alternatively, if CPS-500 is constitutively released by sporozoites, lack of detection in the incubation medium may relate to its glycolipid composition and immiscibility with the aqueous phase used for immunoblots. The relationships between C. parvum zoite surface molecules deposited during motility, released as soluble exoantigens, or bound by MAbs in the circumsporozoite precipitate-like reaction remain to be defined.
In this report we have characterized the biochemical properties and possible function of CPS-500, the neutralization-sensitive C. parvum zoite antigen defined by MAb 18.44. Of most significance, β-d-mannose has been identified as the specific sugar conferring carbohydrate dependency on the target epitope recognized by MAb 18.44. These findings now provide the rationale for determining the complete molecular structure of CPS-500 and for investigating the utility of chemically synthesized β-mannosides or anti-idiotype-18.44 antibodies in vaccination strategies against cryptosporidiosis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 30223 from the National Institutes of Health, U.S. Department of Agriculture NRICGP grant 94-37204-0496, and funds from the Agricultural Experiment Station, Colorado State University.
We thank Beth A. Auerbach for excellent technical assistance and preparation of figures, and David L. Bentley for valuable advice on ultrastructural localization of glycolipid antigens.
REFERENCES
- 1.Alving C R. Immune reactions of lipids and lipid model membranes. In: Sela M, editor. Antigens. Vol. 4. London, United Kingdom: Academic Press; 1981. [Google Scholar]
- 2.Arrowood M J, Mead J R, Mahrt J L, Sterling C R. Effects of immune colostrum and orally administered antisporozoite monoclonal antibodies on the outcome of Cryptosporidium parvum infections in neonatal mice. Infect Immun. 1989;57:2283–2288. doi: 10.1128/iai.57.8.2283-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 4.Arrowood M J, Sterling C R, Healey M C. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J Parasitol. 1991;77:315–317. [PubMed] [Google Scholar]
- 5.Bjorneby J M, Riggs M W, Perryman L E. Cryptosporidium parvum merozoites share neutralization-sensitive epitopes with sporozoites. J Immunol. 1990;145:298–304. [PubMed] [Google Scholar]
- 6.Blagburn B L, Soave R. Prophylaxis and chemotherapy. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 111–123. [Google Scholar]
- 7.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Chambers R E, Clamp J R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971;125:1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enriquez F J, Riggs M W. Role of immunoglobulin A monoclonal antibodies against P23 in controlling murine Cryptosporidium parvum infection. Infect Immun. 1998;66:4469–4473. doi: 10.1128/iai.66.9.4469-4473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayer R, Speer C A, Dubey J P. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–33. [Google Scholar]
- 11.Goding J W. Monoclonal antibodies: principles and practice. London, United Kingdom: Academic Press; 1996. p. 83. [Google Scholar]
- 12.Gut J, Nelson R G. Cryptosporidium parvum sporozoites deposit trails of 11A5 antigen during gliding locomotion and shed 11A5 antigen during invasion of MDCK cells in vitro. J Eukaryot Microbiol. 1994;41:42S–43S. [PubMed] [Google Scholar]
- 13.Heine J, Pohlenz J F L, Moon H W, Woode G N. Enteric lesions and diarrhea in gnotobiotic calves monoinfected with Cryptosporidium species. J Infect Dis. 1984;150:768–775. doi: 10.1093/infdis/150.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins M C, Fayer R. Cloning and expression of cDNA encoding an antigenic Cryptosporidium parvum protein. Mol Biochem Parasitol. 1995;71:149–152. doi: 10.1016/0166-6851(95)00050-b. [DOI] [PubMed] [Google Scholar]
- 15.Kates M. Techniques of lipidology: isolation, analysis, and identification of lipids. In: Burdon R H, van Knippenberg P H, editors. Laboratory techniques in biochemistry and molecular biology. Amsterdam, The Netherlands: Elsevier; 1986. pp. 80–109. [Google Scholar]
- 16.Langer R C, Riggs M W. Neutralizing monoclonal antibody protects against Cryptosporidium parvum infection in vitro by inhibiting sporozoite attachment and invasion. J Eukaryot Microbiol. 1996;43:76S–77S. doi: 10.1111/j.1550-7408.1996.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 17.Luft B J, Payne D, Woodmansee D, Kim C W. Characterization of the Cryptosporidium antigens from sporulated oocysts of Cryptosporidium parvum. Infect Immun. 1987;55:2436–2441. doi: 10.1128/iai.55.10.2436-2441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lumb R, Smith K, O’Donoghue P J, Lanser J A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue cultures. Parasitol Res. 1988;74:531–536. doi: 10.1007/BF00531630. [DOI] [PubMed] [Google Scholar]
- 19.Mitschler R R, Welti R, Upton S J. A comparative study of lipid compositions of Cryptosporidium parvum (Apicomplexa) and Madin-Darby bovine kidney cells. J Eukaryot Microbiol. 1994;41:8–12. doi: 10.1111/j.1550-7408.1994.tb05927.x. [DOI] [PubMed] [Google Scholar]
- 20.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 21.Perryman L E, Riggs M W, Mason P H, Fayer R. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–259. doi: 10.1128/iai.58.1.257-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen C, Gut J, Doyle P S, Crabb J H, Nelson R G, Leech J H. Characterization of a >900,000-MrCryptosporidium parvum sporozoite glycoprotein recognized by protective hyperimmune bovine colostral immunoglobulin. Infect Immun. 1992;60:5132–5138. doi: 10.1128/iai.60.12.5132-5138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggs, M. W. Unpublished data.
- 24.Riggs M W. Immunology: host response and development of passive immunotherapy and vaccines. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 130–154. [Google Scholar]
- 25.Riggs M W, Cama V A, Leary H L, Jr, Sterling C R. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect Immun. 1994;62:1927–1939. doi: 10.1128/iai.62.5.1927-1939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riggs M W, McGuire T C, Mason P H, Perryman L E. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J Immunol. 1989;143:1340–1345. [PubMed] [Google Scholar]
- 27.Riggs M W, Perryman L E. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987;55:2081–2087. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 29.Sam-Yellowe T Y. Rhoptry organelles of the Apicomplexa: their role in host cell invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 30.Stewart M J, Vanderberg J P. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool. 1988;35:389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 31.Tilley M, Upton S J. Both CP15 and CP25 are left as trails behind gliding sporozoites of Cryptosporidium parvum (Apicomplexa) FEMS Microbiol Lett. 1994;120:275–278. doi: 10.1111/j.1574-6968.1994.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 32.Tilley M, Upton S J, Fayer R, Barta J R, Chrisp C E, Freed P S, Blagburn B L, Anderson B C, Barnard S M. Identification of a 15-kilodalton surface glycoprotein on sporozoites of Cryptosporidium parvum. Infect Immun. 1991;59:1002–1007. doi: 10.1128/iai.59.3.1002-1007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl E W, O’Connor R M, Perryman L E, Riggs M W. Neutralization-sensitive epitopes are conserved among geographically diverse isolates of Cryptosporidium parvum. Infect Immun. 1992;60:1703–1706. doi: 10.1128/iai.60.4.1703-1706.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungar B L P. Cryptosporidiosis in humans (Homo sapiens) In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 60–82. [Google Scholar]
- 35.Vliegenthart J F G, Montreuil J. Primary structure of glycoprotein glycans. In: Montreuil J, Vliegenthart J F G, editors. Glycoproteins. Amsterdam, The Netherlands: Elsevier; 1995. pp. 13–28. [Google Scholar]
- 36.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]