Abstract
Background
According to relevant clinical research, dietary and circulating antioxidants vitamin A are connected with the risk of breast, cervical, and ovarian cancer in women. However, there was inconsistency between the findings. We completed this meta-analysis at the right moment to address this contradiction of the problem.
Methods
Web of Science, Embase, and PubMed databases were searched using the proposed search strategy and filtered using the inclusion and exclusion criteria as well as the NOS quality score. As of May 2022, low intake or low concentration was used as a control, and odds ratio (OR) or relative risk (RR) and ninety-five percent confidence intervals (95% CI) were extracted for high intake. Stata 12.0 was used to process the data.
Results
Our meta-analysis included a total of 49 studies, 29 on breast cancer, 10 on ovarian cancer, and 10 on cervical cancer. There were 38 case-control studies included, with 25,363 cases and 42,281 controls; there were 11 cohort studies included, 1,334,176 individuals were followed up, and finally 9496 obtained cancer. The pooled OR value results were as follows: diet or supplements (OR = 0.83, 95% CI 0.76-0.90, I2 = 56.1%) and serum or plasma (OR = 0.96, 95% CI 0.86-1.09, I2 = 29.5%). Subgroup analyses were performed according to cancer type, diet or supplements, serum or plasma, study type, and geographic regions.
Conclusions
In North American and Asian populations, high dietary consumption of vitamin A or supplements decreases the incidence of three cancers in women, with breast and ovarian cancers being more significant. However, high circulating vitamin A concentrations were not significantly connected with the risk of the three malignancies.
1. Introduction
With the development of science and technology and the progress of society, women's health has been paid more and more attention by people. However, the incidence and mortality of malignant tumors have gradually increased and become a major killer endangering human health [1, 2]. Among them, ovarian, breast, and cervical cancer are important cancers that endanger women and bring great pressure to health institutions and medical resources [2–5]. Since this century, medical technology has advanced significantly. Comprehensive treatments have enhanced the efficacy of malignant tumors, but the prognosis is still poor, and early diagnosis is still difficult [6–8]. The etiology of cancer is mainly affected by the environment and living habits. Diet, age, obesity, lack of physical exercise, adverse emotions, HPV infection, and family history are all risk factors for the above tumors [9–11]. Therefore, how to prevent these tumors by changing dietary habits and lifestyle is our research focus.
According to studies, excessive generation of reactive oxygen species (ROS) in the body has been linked to the emergence and progression of a number of chronic and degenerative disorders, including Alzheimer's disease, cardiovascular disease, digestive system diseases, and malignant tumors [12, 13]. Normally, in healthy individuals, antioxidants modulate the concentration of ROS to keep it fluctuating within a certain range [14, 15]. The chance of developing malignant tumors and other disorders rises when antioxidant deficiency results in increased ROS concentration, which increases the body's susceptibility to oxidative stress [15]. Therefore, antioxidants are critical in counteracting oxidative stress and regulating ROS concentration [16]. Antioxidants can be taken through endogenous supplementation or dietary intake. It has been found that some vitamins, including vitamin A and vitamin D, can reduce oxidative stress damage, prevent disease occurrence, and control disease progression [17–19]. Among them, vitamin A is a crucial member of the vitamin family. Vitamin A is a fat-soluble vitamin that involves retinol and retinol derivatives (retinoic acid, retinyl esters, and retinaldehyde) [20, 21]. Vitamin A deficiency is common in the population because it cannot be synthesized by itself in the body and must be actively obtained from food [22, 23]. Vitamin A has antioxidant and gene transcriptional regulation and plays a key role in cell differentiation, growth, proliferation, and immunity [24–26]. Vitamin A insufficiency can cause diseases such as decreased immunity, night blindness, dry skin, and diarrhea [19, 27]. According to related studies, vitamin A insufficiency raises the incidence of certain cancers and deserves our further exploration [23, 28, 29].
Since the new century, with the deepening of research on the role and mechanism of antioxidants, the great medical prospects of antioxidants have been revealed and attention has been paid to the association between antioxidant intake and various cancers [30]. Vitamin A deficiency is correlated to an increased incidence of certain malignancies, such as breast cancer [31], lung cancer [32], skin cancer [33], cervical cancer [34], gastric cancer [35], liver cancer [29], and ovarian cancer [36]. Today, we will look at the link between vitamin A consumption and circulating concentrations and breast, cervical, and ovarian cancer. Some studies suggest that its intake and circulating concentration are negatively correlated with the risk of these tumors [34, 37, 38], and some studies show no significant correlation [39–41]. Thus, we completed the meta-analysis in order to synthesize related research and draw more reliable conclusions to guide cancer prevention and promote women's health.
2. Methods and Materials
2.1. Search Strategy for Literature
Utilizing the Embase, Web of Science, and PubMed English databases, two writers (Han X and Zhao R) independently searched the literature for vitamin A and human breast, cervical, and ovarian cancer risk. The key search terms were as follows: “Vitamin A” or “Retinol” or “All-Trans-Retinol” or “All Trans Retinol” or “Nutrients” paired with “Breast cancer” or “Cervical cancer” or “Ovarian cancer”. The search period was from inception to May 2022. In addition, to avoid possibly overlooked papers, we carefully examined references from pertinent reviews, meta-analyses, and so on. The language included in this study was restricted to English. The third writer resolved the search debate between the two writers.
2.2. Criterion for Inclusion and Exclusion
The following standards were applied to determine inclusion studies: (1) the patients were definitely diagnosed with breast cancer or ovarian or cervical cancer; (2) the types of included studies were prospective cohort studies and case-control studies; (3) the subjects of the studies were human clinical studies, excluding cell and animal trials; (4) the correlations between circulation or dietary vitamin A levels and supplement doses and the incidence of breast, ovarian, and cervical cancer were the connections of interest; and (5) containing data indicating the risk of the disease: odds ratio (OR) or relative risk (RR) and ninety-five percent confidence intervals (95% CI). We applied the following exclusion criteria: (1) reviews or meetings or letters to the editors or abstracts, (2) studies on animals, (3) repeat studies, (4) research into other malignancies, (5) lack of data on RR or OR, and (6) other kinds of vitamins.
2.3. Data Extraction and Quality Assessment
Two writers completed quality assessment and extracted pertinent data on the included research, respectively. The following basic information was included: the first author's name, publishing year, region, age, cancer type, kind of study design, sample size, OR or RR, and 95% CI, covariates involved in correction. The differences between the two writers Han X and Zhao R were discussed and decided by other writers. The NOS score was utilized to judge the quality of each article, and studies with scores more than or equal to 6 points were eligible for inclusion in this meta-analysis.
2.4. Analysis of Data
From the included studies, the cancer risk HR, OR, or RR of the highest intake was extracted one by one against the lowest intake (or the lowest concentration in the circulation). In the original study, prospective cohort studies mostly used RR values or HR values to assess the risk of morbidity and case-control studies mostly used OR values to evaluate the risk of morbidity. Since the differences among the three are small, all risk results are presented as ORs to better combine and calculate the study results. In addition, we performed Q and I2 tests to analyze the heterogeneity of the studies. If the Q-test (PQ), P value < 0.1, or I2 > 50%, indicating significant heterogeneity among the research, a random-effects model was utilized. On the contrary, a fixed-effects model was utilized. Forest plots were used to show the combined outcomes of this meta-analysis, and Begg's funnel plot and Begg's test were used to evaluate the publication bias of the studies. Finally, the stability of the combined data was tested using sensitivity analysis. All the above calculations were analyzed using the Stata 12.0 software for Windows, and P < 0.05 was regarded as statistically significant.
3. Results
3.1. Screening Procedures for Studies Meeting Criteria
Perform related literature search in 3 major databases using the specified search methods: total (n = 2905), including PubMed (n = 1021), Web of Science (n = 988), and Embase (n = 896). Endnote was used to remove duplicates leaving 1189 articles. After reviewing the article titles and abstracts, 1092 were excluded because they were not related to the study subjects, article type, or study purpose. 97 full texts needed to be downloaded, of which 48 were excluded after initial examination because of unqualified article quality or lack of important data. Finally, 49 publications that fully satisfied the inclusion criteria and were of sufficient quality were included in our meta-analysis. Figure 1 depicts the retrieval procedure mentioned previously.
Figure 1.
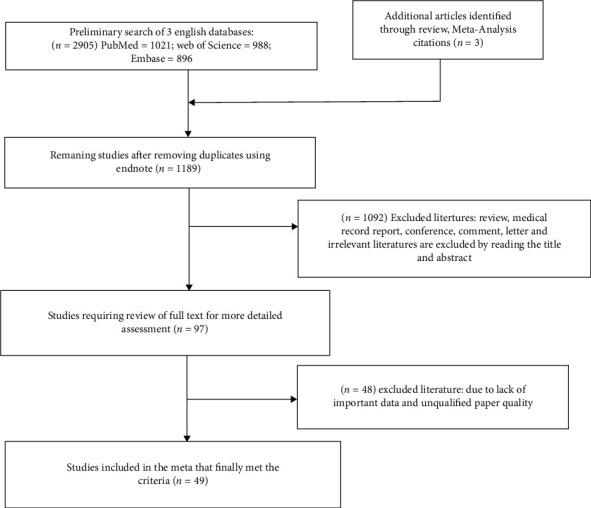
Flow diagram of this meta-analysis.
3.2. Characteristics of the Included Studies
The key features of the 49 studies included in the meta-analysis are shown in Table 1. With regard to breast cancer, 29 studies were included, including 9 cohort studies; 937,252 people were followed up for 5-15 years, and finally 8,146 people had breast cancer; 20 case-control studies, involving 18,915 cases and 27,660 controls; 19 studies on diets or supplements, 6 studies on serum, and 7 studies on plasma. For cervical cancer, 10 studies were included, including 1 cohort study; 299,649 people were followed up for 5-15 years, and finally 1,070 people had cervical cancer; 9 case-control studies involving 2682 cases and 5,305 controls; 9 studies on diets or supplements, 2 studies on serum, and 1 study on plasma. For ovarian cancer, 10 studies were included, including 1 cohort study with 97,275 individuals followed up for 8-15 years resulting in 280 ovarian cancers; 9 case-control studies with 3,766 cases and 9,316 controls; 10 studies on meals or supplements and 1 study on plasma. Studies were published between 1988 and 2021. All included research was adjusted for multivariate covariates, such as age, smoking, sex, drinking, relevant genetic history of cancer, marriage and childbearing history, menstrual history, and energy intake. The NOS was graded on a scale of 6 to 8.
Table 1.
Characteristics of included studies.
| Author Year Country |
Age | Type of cancer | Type of study | Sample size | Diet/serum/plasma/supplements | Dose: highest comparison lowest | OR (95%Cl) | Adjustment for covariates. | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Fernandez-Lazaro et al. [67] 2021 Spain |
Mean age: 35.1 | Breast cancer | Cohort study | 9983/107 | Diet | >2282 vs. <1387 (mcg/d) | 0.94 (0.59–1.50) | Age, smoking, alcohol intake, BMI, height, reproductive history, and family history of breast cancer. | 7 |
| Tamimi et al. [68] 2005 USA |
Mean age: 57 | Breast cancer | Case-control study | 969/969 | Plasma | five-fold vs. one-fold | 0.78 (0.56–1.07) | Age, menstrual history, body mass index, family history of breast disease, reproductive history, alcohol consumption, and smoking. | 7 |
| Maillard et al. [69] 2010 France |
Mean age: 56.8 | Breast cancer | Case-control study | 366/720 | Serum | five-fold vs. one-fold | 0.85 (0.53–1.35) | Alcohol, height, use of menopausal hormones, educational level, age and parity at birth, family history of breast cancer, and history of benign breast disease. | 8 |
| Epplein et al. [70] 2009 USA |
Mean age: 66 | Breast cancer | Case-control study | 286/535 | Plasma | ≥1,188.0 vs ≤842.6 (ng/ml) | 1.13 (0.73–1.76) | Smoking, ethnicity, body mass index, alcohol use, age at first birth, number of full-term pregnancies, age at menarche, and age at menopause. | 8 |
| Bakker et al. [71] 2016 Europe |
Mean age: 50 | Breast cancer | Case-control study | 1502/1502 | Plasma | five-fold vs. one-fold | ER (-): 1.65 (0.97–2.81) ER (+): 1.02 (0.64–1.63) |
BMI, height, age at menarche, reproductive history, smoking, alcohol use, education, energy intake, and blood sampling time. | 8 |
| Longnecker et al. [72] 1997 USA |
65-74 | Breast cancer | Case-control study | 3285/8736 | Diet | ≥12000 vs. <3000 (IU/day) | 0.40 (0.15–1.05) | Age, reproductive history, body mass index, age at menarche, education, family history of breast cancer, smoking, and alcohol consumption. | 6 |
| Sato et al. [73] 2002 USA |
51.3 | Breast cancer | Case-control study | 244/244 | Serum | ≥73.0 vs. <46.2 (μg/dl) | 0.97 (0.53–1.80) | Family history of breast cancer, age at birth, age at menarche, alcohol use, smoking status, body mass index, lactation, and education. | 6 |
| Cho et al. [74] 2003 USA |
25-42 | Breast cancer | Cohort study | 90655/714 | Diet | Median intake 21,916 (IU/day) vs. 5639 (IU/day) | 0.92 (0.72–1.17) | Smoking status, alcohol consumption, body mass index, height, age at menarche, oral contraceptive use, family history of breast cancer, reproductive history, menopausal status, and energy intake. | 6 |
| Zhang et al. [75] 2009 China |
Mean age: 47 | Breast cancer | Case-control study | 438/438 | Diet | four-fold vs. one-fold | 0.31 (0.20–0.48) | Age at menarche, BMI, family history of breast cancer in first degree relatives, history of benign breast disease, physical activity, and smoking. | 7 |
| Do et al. [76] 2003 Korean |
20-69 | Breast cancer | Case-control study | 224/250 | Diet+supplements | ≥2902.72 vs. <1773.41 (RE) | 0.63 (0.39–0.98) | Age at menarche, total menstrual cycle, pregnancy, total number of full-term deliveries, total lactation period, family history of breast cancer, and BMI. | 7 |
| London et al. [77] 1992 USA |
Mean age: 64 | Breast cancer | Case-control study | 377/403 | Diet/serum | Diet: median 10916 vs. 639 (IU/day) Serum: median 2.70 vs. 1.47 (μmol/l) |
Diet: 0.7 (0.4–1.3) Serum: 1.0 (0.6–1.7) |
Age, alcohol intake, age at birth, parity, family history of breast cancer, age at menopause, age at menarche, and weight. | 6 |
| Hulten et al. [78] 2001 Sweden |
49-65 | Breast cancer | Case-control study | 201/290 | Plasma | four-fold vs. one-fold | 0.9 (0.5–1.4) | Age, alcohol intake, parity, family history of breast cancer, age at menopause, age at birth, physical activity, and weight. | 6 |
| Zhang et al. [37] 1999 USA |
33-60 | Breast cancer | Cohort study | 83234/2697 | Diet | Median 17073 vs. 5293 (IU/day) | Premenopausal: 0.82 (0.65–1.04) Postmenopausal: 1.03 (0.89–1.19) |
Age, parity, family history of breast cancer, weight, age at menopause, and use of postmenopausal hormones. | 8 |
| Rohan et al. [79] 1993 Canada |
40-59 | Breast cancer | Case-control study | 519/1182 | Diet/supplements | Diet: five-fold vs. one-fold Supplements: three-fold vs. one-fold |
Diet: 0.80 (0.55–1.17) Supplements: 0.70 (0.42–1.15) |
Age, energy intake, age at menarche, surgical menopause, age at first birth, years of education, family history of breast cancer, and history of benign breast disease. | 6 |
| Kushi et al. [80] 1996 USA |
Mean age: 61 | Breast cancer | Cohort study | 34387/879 | Diet/supplements | Diet: ≥20343 vs. ≤7254 (IU/day) Supplements: >10,000 vs. 0 (IU/day) |
Postmenopausal: Diet: 1.26 (0.88–1.80) Supplements: 0.74 (0.47–1.18) |
Age, energy intake, menstrual history, marriage and childbearing history, body mass index, family history of breast cancer, alcohol consumption, and education. | 7 |
| Negri et al. [81] 1996 Italy |
/ | Breast cancer | Case-control study | 2569/2588 | Diet | ≥2361 vs. ≤706 (μg/day) | 0.73 (0.6–0.9) | Age, weight, family history of breast disease, reproductive history, smoking, alcohol, and energy intake. | 8 |
| Bohlke et al. [82] 1999 USA |
Mean age: 56.4/54.4 | Breast cancer | Case-control study | 820/1548 | Diet | ≥2120.7 vs. ≤659.1 (μg/day) | 0.89 (0.65–1.23) | Age, place of birth, body mass index, reproductive history, menstrual history, and energy intake. | 8 |
| Verhoeven et al. [83] 1997 Netherlands |
55-69 | Breast cancer | Cohort study | 62573/650 | Diet | ≥0.766 vs. ≤0.229 (mg/day) | 1.24 (0.83–1.83) | Age, energy intake, alcohol, family history of breast cancer, menstrual history, and reproductive history. | 8 |
| Dorjgochoo et al. [84] 2009 China |
40-70 | Breast cancer | Case-control study | 365/726 | Plasma | four-fold vs. one-fold | 1.20 (0.80–1.81) | Age, education, occupation, menstrual history, reproductive history, physical exercise, smoking, alcohol consumption, family history of breast cancer, and total energy intake. | 7 |
| Dorgan et al. [85] 1998 USA |
Mean age: 58 | Breast cancer | Case-control study | 105/203 | Serum | ≥2.31 vs. ≤1.67 (μmol/l) | 0.9 (0.4-2.0) | Serum total cholesterol concentration, smoking, and body mass index (kg/m2). | 6 |
| Kabat et al. [86] 2009 USA |
Mean age: 62 | Breast cancer | Cohort study | 5450/190 | Serum | ≥0.65 vs. <0.53 (μg/ml) | 1.00 (0.68–1.48) | Age, education, race, body mass index, reproductive history, menstrual history, alcohol, energy intake, and family history of breast cancer. | 8 |
| Ching et al. [87] 2002 Australia |
Mean age: 54 | Breast cancer | Case-control study | 153/151 | Serum | ≥2.50 vs. ≤1.90 (μmol/l) | 0.53 (0.28–1.01) | Age at menarche, parity, alcohol intake, and total fat intake. | 7 |
| Michels et al. [88] 2001 Swedish |
40-76 | Breast cancer | Cohort study | 508267/1271 | Diet | ≥1.51 vs. ≤0.52 (mg/day) | 1.00 (0.83–1.20) | Age, family history of breast cancer, height, body mass index, education, reproductive history, energy intake, and alcohol, fiber intake. | 8 |
| Hunter et al. [89] 1993 England |
34-59 | Breast cancer | Cohort study | 89494/1439 | Diet+supplements | ≥17640 vs. <6630 (IU/day) | 0.84 (0.71–0.98) | Age, follow-up time, ethnicity, family history of breast cancer, body mass index, education, reproductive history, energy intake, and alcohol. | 8 |
| Mignone et al. [90] 2009 USA |
/ | Breast cancer | Case-control study | 5707/6389 | Diet | five-fold vs. one-fold | 0.84 (0.74–0.94) | Age, status, family history of breast cancer, reproductive history, alcohol use, education, menstrual history, body mass index, smoking, and hormone replacement therapy. | 7 |
| Pantavos et al. [31] 2015 Netherlands |
>55 | Breast cancer | Cohort study | 53209/199 | Diet | / | 0.98 (0.69–1.39) | Age, BMI, educational level, family history of breast cancer, smoking and alcohol consumption, reproductive history, and fiber intake. | 8 |
| Katsouyanni et al. [91] 1988 Greek |
Mean age: 54.7/53.7 | Breast cancer | Case-control study | 120/120 | Diet | ≥12482 vs. <6868 (IU/day) | 0.40 (0.17–0.93) | Age at first birth, menopausal status, age at menarche, age at menopause, residence, marital status, and other significant (10% level) nutrients. | 7 |
| Marubini et al. [92] 1988 Italy |
30-65 | Breast cancer | Case-control study | 214/215 | Diet/serum | five-fold vs. one-fold | Plasma: 2.0 (1.0–4.0) Diet: 0.7 (0.4–1.5) |
Age, triglycerides, and cholesterol. | 7 |
| Rohan et al. [93] 1988 Australia |
20-74 | Breast cancer | Case-control study | 451/451 | Diet | >1445.8 vs. ≤245.6 (μg/day) | 1.19 (0.78–1.82) | Family history of breast cancer, menstrual history, oral contraceptives or not, smoking, and years of education. | 7 |
| González et al. [94] 2011 Europe |
35-70 | Cervical cancer | Cohort study | 299649/1070 | Diet | >949.23 vs. <287.24 (μg/d) | 1.01 (0.83–1.22) | BMI, energy intake, smoking, alcohol use, physical activity, marriage and childbearing history, and education. | 8 |
| Shannon et al. [95] 2002 Thailand |
/ | Cervical cancer | Case-control study | 134/384 | Diet | ≥6000 vs. ≤1400 (IU/day) | 0.32 (0.13–0.83) | Smoking, drinking, HPV infection, body mass index, education, history of sexual intercourse, and reproductive history. | 6 |
| Ghosh et al. [38] 2008 USA |
Mean age: 52 | Cervical cancer | Case-control study | 239/979 | Diet | ≥12768 vs. ≤7420 (IU/day) | 0.47 (0.30–0.73) | Adjusted for age, education level, income, smoking status, body mass index, marriage and childbearing history, family history of cervical cancer, and energy intake. | 6 |
| Kim et al. [96] 2010 Korea |
/ | Cervical cancer | Case-control study | 144/288 | Diet | ≥999 vs. ≤609 (RE/day) | 0.36 (0.19–0.69) | Adjusted for age, smoking status, alcohol consumption status, exercise, family history, body mass index, and human papillomavirus infection status. | 6 |
| Guo et al. [97] 2015 China |
Mean age: 47.4/46.3 | Cervical cancer | Case-control study | 458/742 | Diet/serum | Serum: ≥92 vs. ≤25 (μg/day) Diet: ≥450 vs. ≤80 (μg/day) |
Serum: 0.85 (0.61–1.19) Diet: 1.06 (0.75–1.50) |
Age, body mass index, marital status, education, family history of cancer, HPV infection, passive smoking, current alcohol consumption, calcium supplementation, physical activity, and daily energy intake. | 7 |
| Brock et al. [98] 1988 Australia |
18-65 | Cervical cancer | Case-control study | 117/196 | Plasma/diet | Plasma: >68 vs. ≤45 (μg/dl) Diet: >2248 vs. ≤565 (μg/day) |
Plasma: 0.4 (0.1-1.3) Diet: 1.2 (0.4–3.60) |
Number of sexual partners, age at first intercourse, smoking, use of oral contraceptives, and other listed risk factors (i.e., carotene, vitamin C, folic acid, retinol, and total energy). | 8 |
| Verreau et al. [99] 1989 USA |
Mean age: 44.6/43 | Cervical cancer | Case-control study | 189/227 | Diet | >805 vs. ≤248 (μg/day) | 1.1 (0.5–2.2) | Age, education, smoking, oral contraceptive use, history of cervicovaginal infection, number of sexual partners, and total energy intake. | 7 |
| Slattery et al. [100] 1990 USA |
20-59 | Cervical cancer | Case-control study | 266/408 | Diet | ≥13876 vs. <6383 (IU/day) | 0.71 (0.43–1.2) | Age, education, smoking, alcohol use, family history, and sexual partner. | 7 |
| Potischman et al. [101] 1991 USA |
/ | Cervical cancer | Case-control study | 387/670 | Serum | >56.1 vs. ≤35.9 (μg/dl) | 1.28 (0.8–2.1) | Age, age at first sexual intercourse, number of sexual partners, reproductive history, whether HPV infection, family history, triglyceride, and cholesterol. | 7 |
| Herrero et al. [102] 1991 USA |
Mean age: 46.5 | Cervical cancer | Case-control study | 748/1411 | Diet | >1830 vs. ≤217 (μg/day) | 1.13 (0.8–1.5) | Age, age at first sexual intercourse, number of sexual partners, reproductive history, whether HPV infection, family history, triglyceride, and cholesterol. | 7 |
| Cramer et al. [103] 2001 USA |
/ | Ovarian cancer | Case-control study | 549/516 | Diet | ≥18721 vs. <6478 (IU/day) | 0.77 (0.51–1.18) | Age, study site, marriage and childbearing history, family history, BMI, and energy intake. | 8 |
| Silvera et al. [41] 2006 USA |
Mean age: 48.6 | Ovarian cancer | Case-control study | 487/264 | Diet/diet+supplements | >11,534 vs. <6,589 (IU/d) >11,560 vs. <6,595 (IU/d) |
Diet: 0.77 (0.52–1.14) Diet+supplements: 0.79 (0.53–1.16) |
Age, smoking, alcohol use, menopausal status, BMI, education, and physical activity. | 7 |
| Bertone et al. [104] 2001 USA |
50-79 | Ovarian cancer | Case-control study | 327/3129 | Diet | ≥18000 vs. <4600 (IU/d) | 0.84 (0.57–1.2) | Age, BMI, menstrual history, marriage and childbearing history, family medical history, physical activity, race, religion, smoking, and alcohol. | 6 |
| McCann et al. [105] 2001 USA |
20-87 | Ovarian cancer | Case-control study | 496/1425 | Diet | >14204 vs. ≤5917 (IU/day) | 0.66 (0.45–0.98) | Age, education, region of residence, regularity of menstruation, family history of ovarian cancer, parity, age at menarche, oral contraceptive use, and total energy intake. | 6 |
| Tung et al. [106] 2005 USA |
Mean age: 54.8 | Ovarian cancer | Case-control study | 558/607 | Diet | four-fold vs. one-fold | 0.61 (0.42–0.89) | Age, ethnicity, site, education, oral contraceptive use, parity, and tubal ligation. | 7 |
| Tzonou et al. [107] 1993 Greece |
/ | Ovarian cancer | Case-control study | 189/200 | Diet | ≥11000 vs. <7000 (IU/day) | 0.87 (0.73–1.03) | Age, years of education, parity, age at first birth, menopausal status, and energy intake. | 8 |
| Salazar-Martinez et al. [108] 2002 Mexico |
20-79 | Ovarian cancer | Case-control study | 84/629 | Diet | ≥718 vs. ≤521 (RE) | 0.52 (0.28–0.95) | Age, total energy intake, number of live births, recent changes in weight, physical, activity (METs), and diabetes. | 6 |
| Chang et al. [109] 2007 USA |
/ | Ovarian cancer | Cohort study | 97275/280 | Diet | >695 vs. ≤233 (μg/day) | 1.17 (0.52–2.66) | Ethnicity, total energy intake, parity, oral contraceptive use, strenuous exercise, wine consumption, and menopausal status/hormonal therapy. | 7 |
| Jeong et al. [110] 2009 Korea |
Mean age: 52.8/52.1 | Ovarian cancer | Case-control study | 45/135 | Plasma | >134 vs. ≤23.8 (μg/dl) | 0.45 (0.21–0.98) | Education level, BMI, menopause, number of births, oral contraceptive use, smoking status (former vs. never), and alcohol use. | 7 |
| Bidoli et al. [111] 2001 Italy |
Mean age: 56/57 | Ovarian cancer | Case-control study | 1031/2411 | Diet | five-fold vs. one-fold | 1.1 (0.8–1.4) | Age, site, year of interview, education, body mass index, parity, oral contraceptive use, occupational physical activity, and energy intake. | 8 |
3.3. Association of Diet Consumption of Vitamin A or Supplements with Risk of Three Cancers in Women
In terms of diet or supplements, a sum of 41 sets of data from 37 researches was considered. Forest plot results showed that high intake of dietary vitamin A or supplements significantly (pt = 0.001) reduced a woman's risk of three cancers by 17% (OR = 0.83, 95% CI 0.76-0.90; Figure 2). Because of the high heterogeneity (I2 = 56.1%, P < 0.001), we chose a random-effects model for pooling. We subgroup analyzed the kind of cancer and discovered a significant inverse relationship between high vitamin A consumption and breast cancer incidence (OR = 0.84, 95% CI 0.76-0.93; Figure 3(a)) and ovarian cancers (OR = 0.81, 95% CI 0.72-0.92; Figure 3(a)) and a nonsignificant inverse association with the cervical cancer risk (OR = 0.77, 95% CI 0.59-1.02; Figure 3(a)). In addition, we subgroup analyzed the type of study; cohort studies (OR = 0.96, 95% CI 0.90-1.03; Figure 3(b)) found that a higher vitamin A consumption might lower the incidence of three malignancies marginally but not considerably, and case-control studies (OR = 0.75, 95% CI 0.67-0.83; Figure 3(b)) found that higher consumption can significantly lower the incidence of three cancers. Then, we subgroup analyzed according to diet or supplements, and high dietary vitamin A (OR = 0.83, 95% CI 0.76-0.91; Figure 3(c)) consumption and diet plus supplements (OR = 0.81, 95% CI 0.70-0.93; Figure 3(c)) were all able to significantly reduce the incidence of three cancers in women, and supplements (OR = 0.72, 95% CI 0.51-1.01; Figure 3(c)) lower their risk but not significantly. Finally, we made subgroup analysis based on the geographic region of the study area, including Asia (OR = 0.50, 95% CI 0.29-0.86; Figure 3(d)), Europe (OR = 0.91, 95% CI 0.82-1.01; Figure 3(d)), North America (OR = 0.83, 95% CI 0.75-0.91; Figure 3(d)), and Oceania (OR = 1.19, 95% CI 0.80-1.77; Figure 3(d)). The findings revealed that a higher diet and supplement consumption considerably lowered the incidence of all three malignancies in women in North America and Asia, but no significant association was found in Oceania and Europe.
Figure 2.
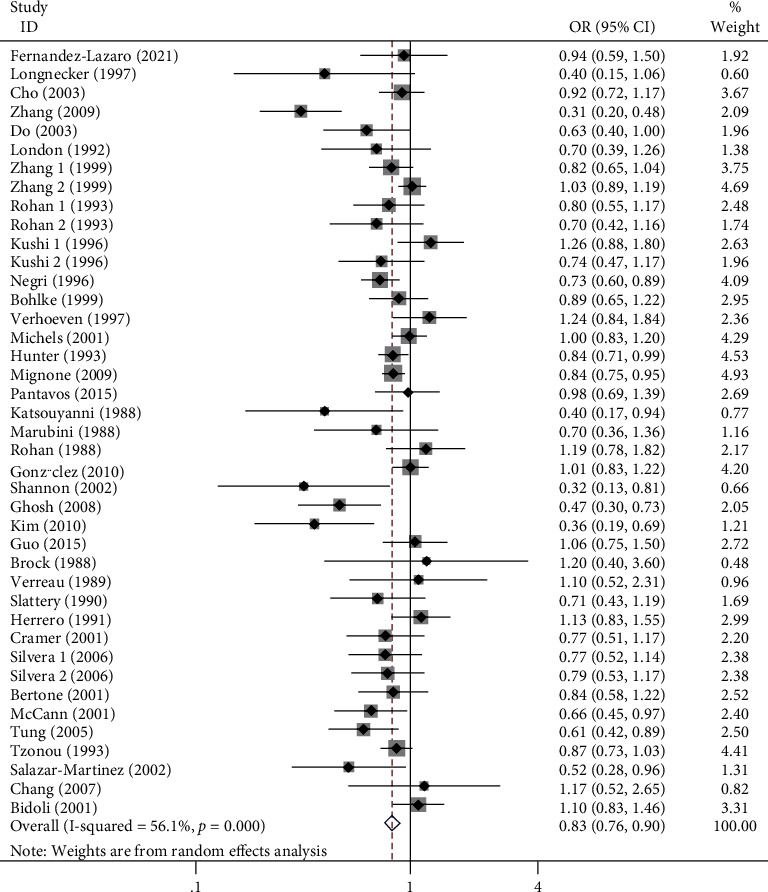
Forest plot of high dietary intake of vitamin A or supplements and risk of breast, cervical, and ovarian cancer in women.
Figure 3.
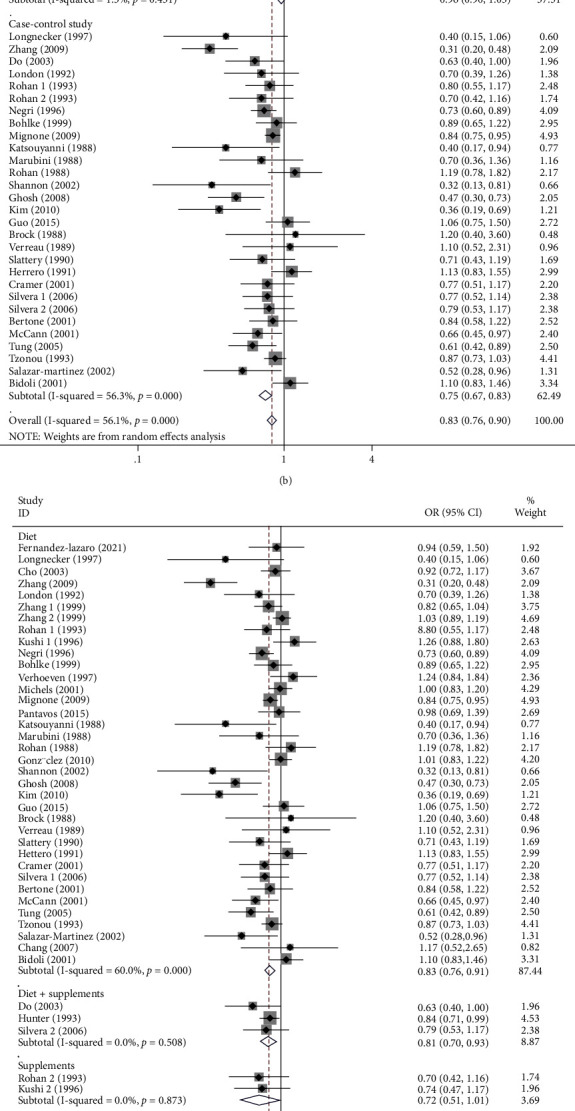
Subgroup analysis of high dietary vitamin A or supplement intake and risk of breast, cervical, and ovarian cancer in women. (a) Subgroup analysis of tumor types; (b) subgroup analysis of study types; (c) subgroup analysis of dietary and supplements; (d) subgroup analysis of geographical location.
3.4. The Relationship between Circulating Vitamin A Concentrations and the Risk of Three Malignancies in Women
Regarding circulation, we included a total of 16 studies with 17 sets of data. Forest plot results showed that high concentrations of circulating vitamin A (OR = 0.96, 95% CI 0.86-1.09, pt = 0.552; Figure 4) were not associated with cancer risk in women. Since there was no significant heterogeneity (I2 = 29.5%, P = 0.122), we chose the fixed-effects model to combine effect sizes. Our subgroup analysis by types of malignant tumor found that high concentrations of circulating vitamin A were not significantly associated with the incidence of breast (OR = 1.00, 95% CI 0.87-1.14; Figure 5(a)) and cervical malignant tumor (OR = 0.93, 95% CI 0.71-1.12; Figure 5(a)). Furthermore, we subgroup analyzed by type of study, and the case-control studies (OR = 0.96, 95% CI 0.85-1.09; Figure 5(b)) found that high concentrations of circulating vitamin A were not significantly associated with cancer incidence in women. Then, we subgroup analyzed serum or plasma concentrations and found no significant association between high serum vitamin A concentrations (OR = 0.92, 95% CI 0.78-1.19; Figure 5(c)) and high plasma vitamin A concentrations (OR = 1.01, 95% CI 0.86-1.19; Figure 5(c)) and the risk of malignant tumor in women. Finally, we performed a subgroup analysis according to geographic location at the study site, and circulating vitamin A concentrations were not associated with the risk of these three cancers in North American (OR = 0.97, 95% CI 0.82-1.16; Figure 5(d)), European (OR = 1.12, 95% CI 0.89-1.42; Figure 5(d)), and Asian women (OR = 0.90, 95% CI 0.71-1.15; Figure 5(d)) but were significantly negatively associated with the incidence of these three cancers in Oceania women (OR = 0.50, 95% CI 0.28-0.89; Figure 5(d)). Table 2 summarizes the above meta-analysis findings.
Figure 4.
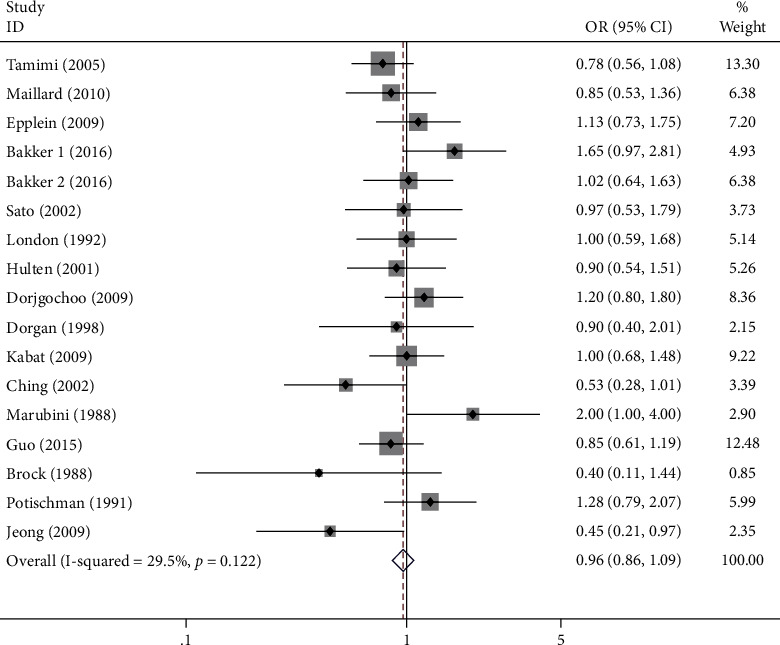
Forest plot of circulating high levels of vitamin A and risk of breast, cervical, and ovarian cancer in women.
Figure 5.
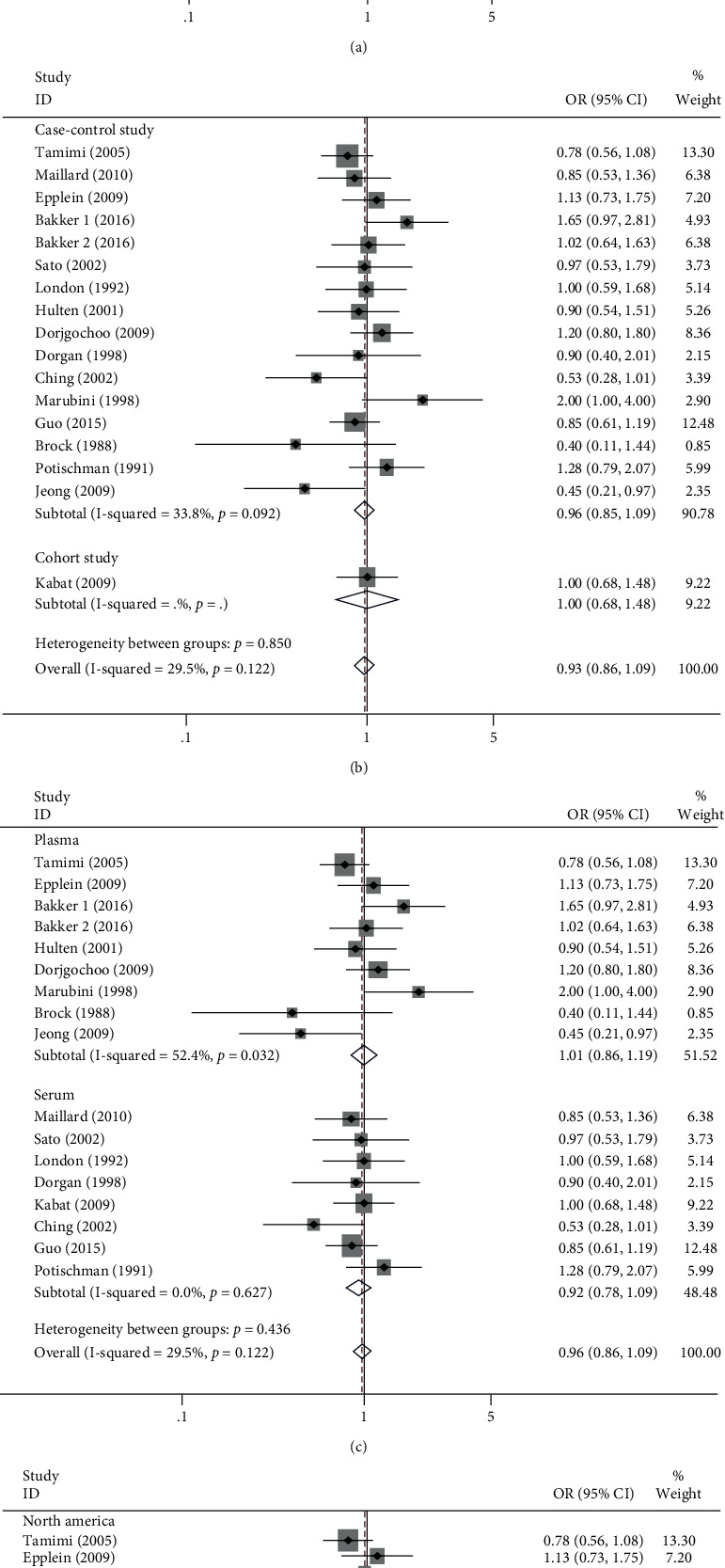
Subgroup analysis of circulating high levels of vitamin A and risk of breast, cervical, and ovarian cancer. (a) Subgroup analysis of tumor types; (b) subgroup analysis of study types; (c) subgroup analysis of serum and plasma; (d) subgroup analysis of geographical location.
Table 2.
Metaresults of dietary or supplements and circulating vitamin A and risk of breast, cervical, and ovarian cancer in women.
| Studies (n) | OR | 95% CI | P value | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I 2 | P value | ||||||
| Sum of three cancers (dietary or supplements) | 37 | 0.83 | 0.76-0.90 | 0.001 | Random | 56.1% | 0.001 |
| Breast cancer (dietary or supplements) | 19 | 0.84 | 0.76-0.93 | 0.001 | Random | 59.9% | 0.001 |
| Cervical cancer (dietary or supplements) | 9 | 0.77 | 0.59-1.12 | 0.071 | Random | 69.6% | 0.001 |
| Ovarian cancer (dietary or supplements) | 9 | 0.81 | 0.72-0.92 | 0.001 | Random | 20.7% | 0.253 |
| Sum of three cancers (circulating) | 16 | 0.96 | 0.86-1.09 | 0.552 | Fix | 29.5% | 0.122 |
| Breast cancer (circulating) | 12 | 1.00 | 0.87-1.14 | 0.944 | Fix | 20.2% | 0.239 |
| Cervical cancer (circulating) | 3 | 0.93 | 0.71-1.22 | 0.616 | Fix | 44.8% | 0.163 |
| Ovarian cancer (circulating) | 1 | 0.45 | 0.21-0.97 | 0.042 | — | — | — |
3.5. Publication Bias and Sensitivity Analysis
In order to determine if there was publication bias in the combined data, we utilized Begg's funnel plot and Begg's test. Firstly, the combined results of diet and supplement and risk of cancer were evaluated. Begg's funnel plot seemed to have slight asymmetry (Figure 6(a)). The result of Begg's test was Pr > |z| = 0.005. Since Pr > |z| < 0.05, it further indicated that there might be some publication bias in our study results. The test of the combined results of circulating vitamin A concentration and cancer risk showed that Begg's funnel plot was basically symmetrical (Figure 6(b)), and Begg's test result was Pr > |z| = 0.773. Since Pr > |z| > 0.05, there was no publication bias. Finally, we performed a sensitivity analysis of the findings (Figures 6(c) and 6(d)), and by removing each study, the pooled OR fluctuated within a certain range without particularly obvious changes, indicating that our findings were more stable.
Figure 6.
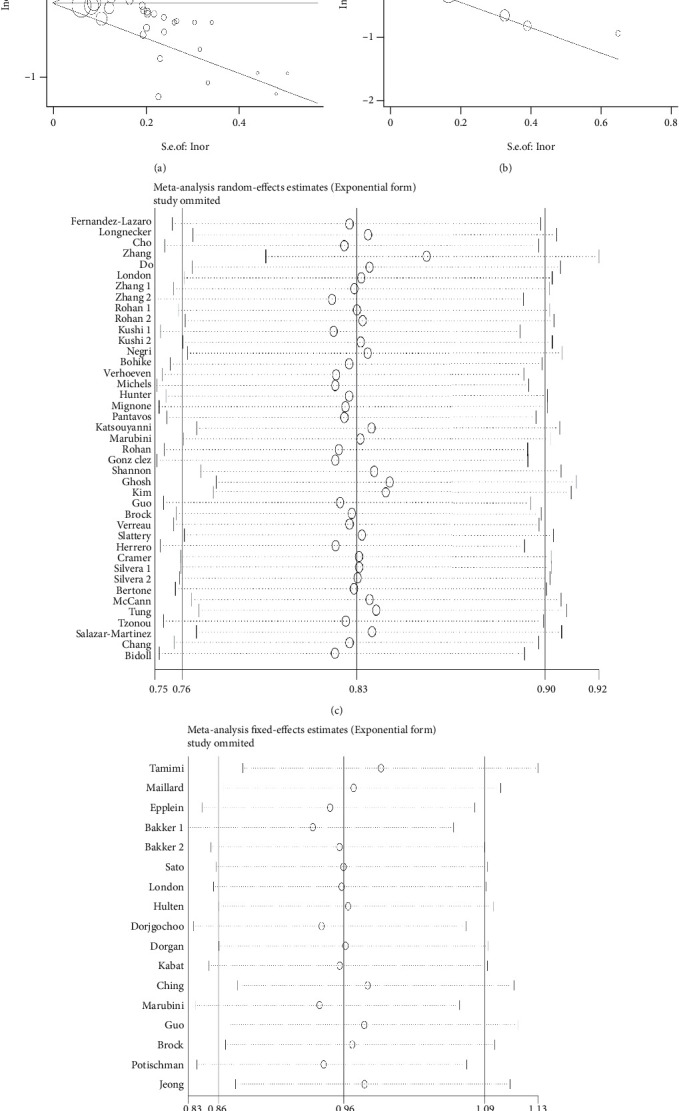
Begg's funnel plot and sensitivity analysis for publication bias test. (a) Begg's plot of combined results of diet or supplements; (b) Begg's funnel plot of pooled circulating results; (c) sensitivity analysis of combined results of diet or supplements; (d) sensitivity analysis of pooled circulating results.
4. Discussion
ROS are metabolic byproducts produced by tissues and cells as they consume ingested oxygen [42]. According to earlier research, ROS is directly linked to the initiation and growth of malignant tumors, and tumor cells create more ROS than normal cells do. Increased ROS levels can lead to DNA, protein, and lipid damage [43, 44]. As a signaling molecule in cancer, ROS has the effects of antiapoptosis, promoting cancer cell metastasis and angiogenesis, and even blocking differentiation [45]. ROS accumulation can damage DNA and lead to genomic instability in cancer, increasing the resistance to chemotherapeutic drugs and the chance of cancer recurrence [46]. The main function of antioxidants is to reduce ROS levels, prevent excessive ROS accumulation, and maintain a dynamic balance between oxidation and reduction [45]. Antioxidants are divided into two main classes, one endogenous and the other exogenous. Endogenous antioxidants are represented by glutathione, which prevents DNA damage and plays an important role in preventing tumor initiation [47, 48]. Exogenous antioxidants are mainly antioxidant substances that people supplement through diet or drugs, of which the vitamin family plays an important role, such as vitamins A, C, and E. We used the meta-analysis to investigate the effect of vitamin A on the risk of the three major cancers in women and to clarify its potential anticancer value.
Vitamin A is a fat-soluble micronutrient and a powerful antioxidant that controls oxidative stress and retards cancer progression [27]. It is crucial for protecting vision, maintaining the integrity of the skin and mucous membranes, maintaining and promoting immunity, promoting growth and development, and maintaining reproductive function [49, 50]. Despite being abundantly found in meat products and a wide range of vegetables and fruits, vitamin A is still insufficient in a significant portion of the global population [51, 52]. Therefore, it is critical to investigate the relationship between vitamin A consumption and the risk of breast, cervical, and ovarian cancer in women, which has significant public health implications. We pooled clinical studies of dietary vitamin A or supplements and circulating vitamin A and the risk of these three cancers by including a total of 49 studies through an extensive search. Further subgroup analysis was done in accordance with the type of cancer, type of study, geographical location, and type of diet or circulation.
Our findings show that high intake of dietary vitamin A and supplements can considerably lower the incidence of three cancers in women. We then subgroup analyzed according to cancer type, study type, geographical location, and diet or supplement. High vitamin A consumption reduces the incidence of ovarian and breast cancer considerably, and the same conclusion can be drawn for both diet and diet plus supplements. Although the cohort study results showed no significant correlation between the two, it may be due to loss to follow-up bias and confounding bias. High vitamin A intake in Asia and North America can greatly lower the risk of these three cancers. Furthermore, in terms of circulating vitamin A, the findings showed that high amounts of circulating vitamin A were not significantly connected with the incidence of the three cancers in women. All results of the subgroup analysis were consistent, and no association was found between them. In summary, higher dietary vitamin A and supplements can considerably lower the incidence of these three cancers and deserve deeper exploration. However, there is no correlation between plasma or serum vitamin A concentrations and the risk of the disease, which is worthy of further verification of this conclusion.
We will further discuss the underlying mechanisms and associated evidence for vitamin A (retinol) in the prevention of cervical, breast, and ovarian cancer. In the past century, retinol and its derivatives have attracted much attention due to their wide range of physiological functions, which can affect cell differentiation, proliferation, and apoptosis, further affect individual growth and aging, and play a vital physiological role in a variety of biological activities [53]. The occurrence and development of tumors are closely related to the dysregulation of retinoid signaling pathway [54]. It has been shown that the bioavailability of intracellular retinoids is regulated by specific cytoplasmic retinol and retinoic acid-binding proteins (CRBPs and CRABPs) [55, 56]. In breast, ovarian, and nasopharyngeal carcinomas, CRBP-1 downregulation is linked to malignant phenotypes [55]. In vitro, CRBP-1 reexpression improved retinol sensitivity while decreasing the viability of ovarian cancer cells [57]. Vitamin A derivatives are crucial cell-permeable signaling agents that activate nuclear receptors to control gene expression [58]. It has also been shown that there is a synergistic effect between carotenoids and vitamin A and chemotherapy agents to promote breast cancer cell apoptosis and inhibit breast cancer cell proliferation [59, 60]. Therefore, retinol derivatives have great potential for the treatment and prevention of malignant tumors and deserve further study of their role in clinical practice, especially as a combination therapy with chemotherapeutic drugs.
Several meta-analyses have previously been conducted to investigate the link between dietary or circulating vitamin A and the incidence of breast, ovarian, or cervical cancer. In 2011, Fulan et al. did a meta-analysis of diet vitamin A intake and breast cancer incidence, discovering that high vitamin A intake lowers the incidence of breast cancer by 17%, which is similar to our findings [61]. The results of Hu et al.'s meta-analysis on the relationship between plasma vitamin A and the incidence of breast malignant tumor and our findings are in agreement. They discovered no association between plasma vitamin A and the incidence of breast malignant tumor [40]. Zhang et al. conducted a meta-analysis of vitamin A and cervical cancer incidence in 2012 and found that high diet consumption of vitamin A was able to lower the incidence of cervical cancer, and the conclusion was consistent with ours. However, they discovered a substantial negative relationship between the incidence of cervical cancer and circulating vitamin A concentrations, while we did not find an association between the two, possibly due to the bias caused by the small number of articles they included [34]. Wang published a meta-analysis of diet vitamin A and ovarian cancer incidence in 2020, which indicated a substantial inverse association between dietary vitamin A and ovarian cancer incidence, which agrees with our study [36]. In conclusion, our study's findings are almost consistent compared with the previous ones, but more literature in recent years is included, which is more persuasive.
We discovered heterogeneity in the combined data of dietary and vitamin A supplements with the risk of the three cancers in women (I2 = 56.1%, P < 0.001), while there was no apparent heterogeneity in the combined data of circulating vitamin A (I2 = 29.5%, P = 0.122). In meta-analysis, heterogeneity is inescapable, and it is our crucial responsibility to identify and investigate its causes. Firstly, when the heterogeneity is large, we choose the random-effects model for effect size combination; secondly, we determine the source of heterogeneity through detailed subgroup analysis. For diet and supplements, we subgroup analyzed by tumor type and found that the heterogeneity of ovarian cancer combined was low, and the heterogeneity of cervical cancer and breast cancer was high; we subgroup analyzed by study type and found that the heterogeneity of the cohort study combined was low, while the heterogeneity of case-control study was high; we subgroup analyzed by diet or supplements and found that the heterogeneity of diet combined was high, while the heterogeneity of supplement was low; finally, we conducted a subgroup analysis by geographic location and discovered that the heterogeneity was high in Asia and low in North America, Oceania, and Europe. Furthermore, various objective factors, such as ethnicity, nutritional structure, economic development status, and HPV vaccination status, may enhance heterogeneity. This may be followed by heterogeneity due to nonuniform methods and details of the study itself and inconsistency between diet and vitamin A concentration assessment tools or scales. The inconsistency in the specific dose limit for high intake and low intake is an important cause of heterogeneity. Therefore, strict and unified inclusion and exclusion criteria, large multicenter randomized controlled trials, or cohort studies are needed to more clarify the relationship between vitamin A and the three cancers in women. We tested the studies used for our meta-analysis for bias by Begg's tests and Begg's funnel plots and found that there may be some publication bias. The reason may be that we only searched the English language database and not the databases of other language kinds, resulting in some studies not being included. It is also possible that there were some unpublished results that we could not retrieve and include. As a result, our findings should be interpreted with care.
Our meta-analysis has several strengths. First, we examined for the first time the relationship between vitamin A intake and the incidence of three forms of malignant tumors in women, taking into account not only dietary but also supplements, as well as serum and plasma; second, we examined the references of relevant meta-analyses and reviews on the basis of searching databases to prevent missing studies as much as possible; third, our meta-analysis includes 49 papers with a large number of controls and cases to acquire more credible results; fourth, we conducted an in-depth subgroup analysis based on type of cancer, type of study, diet or supplements, serum or plasma, and geographical location; and fifth, all of the selected researches were adjusted for multivariate covariates to reduce confounding bias.
We acknowledge that this meta-analysis has the following insufficiencies. Firstly, the language of the included research was confined to English, which may cause bias; secondly, the studies had some publication bias, although we explored the source of bias; thirdly, although the included studies were adjusted for covariates, there may still be the effect of other confounders; fourthly, although the specific dose or concentration ingested in each study was stated, a meta-analysis of dose response was not performed; and fifthly, the food and vitamin A conversion scales or tools were not uniform, and the specific dose of high and low intake was different and not uniform.
A high vitamin A consumption can lower the incidence of cervical, breast, and ovarian cancer. Although we recommend that women prevent these three cancers by appropriately increasing vitamin A, we must explain the toxic side effects of huge doses. Acute poisoning occurs when adults take more than 1 million IU of vitamin A each time and children take more than 300,000 IU each time. High levels of vitamin A (100,000-200,000 IU) is frequently used in relevant clinical trials, which raises our concerns about the toxicity of excessive intake of vitamin A. Cases have been reported in which excessive intake of vitamin A can lead to acute toxic reactions, including headache, nausea, vomiting, diarrhea, blurred vision, depression and anxiety, and other symptoms of nerve damage [62–65]. In addition, studies have shown teratogenic effects in pregnant women ingesting large amounts of vitamin A [66]. Therefore, within the range of acceptable doses, we advise adequately increasing vitamin A consumption, particularly by increasing dietary intake of fruits, vegetables, and meat food.
5. Conclusion
In North American and Asian populations, higher dietary consumption of vitamin A or supplements lowers the incidence of three cancers in women, with breast and ovarian cancers being more significant. However, high circulating vitamin A concentrations were not significantly linked to the incidence of the three malignancies. Moderate increases in dietary vitamin A intake or supplements are recommended to prevent the development of these cancers in high-risk groups for these three cancers. From this point of view, vitamin A, as one of the typical antioxidants, has a promising medical application, and multicenter prospective cohort studies or large randomized controlled trials are encouraged to verify the above conclusions.
Acknowledgments
Our study was supported by the following projects: the 2021 Central-Guided Local Science and Technology Development Fund (ZYYDDFFZZJ-1), the Gansu Key Laboratory of Molecular Diagnosis and Precision Treatment of Surgical Tumors (18JR2RA033), the Key Laboratory of Gastrointestinal Cancer Diagnosis and Treatment of National Health Commission (2019PT320005), the Key Talent Project of Gansu Province of the Organization Department of Gansu Provincial Party Committee (2020RCXM076), and the Guiding Plan for Scientific and Technological Development of Lanzhou (2019-ZD-102).
Data Availability
Data resulting from this study may be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors affirm that their study does not present a conflict of interest.
Authors' Contributions
Xiaoyong Han and Rangyin Zhao designed this research; Yongfeng Wang and Bin Liu conducted the literature search; Xiaohong Chen and Shixun Ma gathered the necessary data; Miao Yu and Dongzhi Zhang made the statistical analyses; Xiaoyong Han and Rangyin Zhao wrote the article; and Haizhong Ma and Hui Cai checked the paper. Xiaoyong Han and Rangyin Zhao contributed equally to this work.
References
- 1.Bray F., Laversanne M., Weiderpass E., Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer . 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R. L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians . 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Jones M. R., Kamara D., Karlan B. Y., Pharoah P. D. P., Gayther S. A. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecologic Oncology . 2017;147(3):705–713. doi: 10.1016/j.ygyno.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R. L., Miller K. D., Fuchs H. E., Jemal A. Cancer statistics, 2022. CA: a Cancer Journal for Clinicians . 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Sun L., Legood R., dos-Santos-Silva I., Gaiha S. M., Sadique Z. Global treatment costs of breast cancer by stage: a systematic review. PLoS One . 2018;13(11, article e0207993) doi: 10.1371/journal.pone.0207993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2017. CA: a Cancer Journal for Clinicians . 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 7.Yu S., Li W., Tang L., et al. Depression in breast cancer patients: immunopathogenesis and immunotherapy. Cancer Letters . 2022;536, article 215648 doi: 10.1016/j.canlet.2022.215648. [DOI] [PubMed] [Google Scholar]
- 8.Karamali N., Ebrahimnezhad S., Khaleghi Moghadam R., Daneshfar N., Rezaiemanesh A. HRD1 in human malignant neoplasms: molecular mechanisms and novel therapeutic strategy for cancer. Life Sciences . 2022;301, article 120620 doi: 10.1016/j.lfs.2022.120620. [DOI] [PubMed] [Google Scholar]
- 9.Rock C. L., Thomson C. A., Sullivan K. R., et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA: a Cancer Journal for Clinicians . 2022;72(3):230–262. doi: 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 10.Greathouse K. L., Wyatt M., Johnson A. J., et al. Diet-microbiome interactions in cancer treatment: opportunities and challenges for precision nutrition in cancer. Neoplasia . 2022;29, article 100800 doi: 10.1016/j.neo.2022.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lester S. P., Kaur A. S., Vegunta S. Association between lifestyle changes, mammographic breast density, and breast cancer. The Oncologist . 2022;27(7):548–554. doi: 10.1093/oncolo/oyac084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo L., Zhou T., Pannell B. K., Ziegler A. C., Best T. M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiologica (Oxford, England) . 2015;214(3):329–348. doi: 10.1111/apha.12515. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Ren Z., Zhang J., et al. Role of ROS and nutritional antioxidants in human diseases. Frontiers in Physiology . 2018;9:p. 477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman H. J., Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nature Reviews. Drug Discovery . 2021;20(9):689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes J. D., Dinkova-Kostova A. T., Tew K. D. Oxidative stress in cancer. Cancer Cell . 2020;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaunig J. E. Oxidative stress and cancer. Current Pharmaceutical Design . 2018;24(40):4771–4778. doi: 10.2174/1381612825666190215121712. [DOI] [PubMed] [Google Scholar]
- 17.Young M. R. I., Xiong Y. Influence of vitamin D on cancer risk and treatment: why the variability? Trends in Cancer Research . 2018;13:43–53. [PMC free article] [PubMed] [Google Scholar]
- 18.Bakalova R., Zhelev Z., Miller T., Aoki I., Higashi T. New potential biomarker for stratification of patients for pharmacological vitamin C in adjuvant settings of cancer therapy. Redox Biology . 2020;28, article 101357 doi: 10.1016/j.redox.2019.101357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao A. V., Rao L. G. Carotenoids and human health. Pharmacological Research . 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Li C., Imai M., Matsuura T., Hasegawa S., Yamasaki M., Takahashi N. Inhibitory effects of retinol are greater than retinoic acid on the growth and adhesion of human refractory cancer cells. Biological & Pharmaceutical Bulletin . 2016;39(4):636–640. doi: 10.1248/bpb.b15-00794. [DOI] [PubMed] [Google Scholar]
- 21.Murillo A. G., Fernandez M. L. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Advances in Nutrition . 2016;7(1):14–24. doi: 10.3945/an.115.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bushue N., Wan Y. J. Retinoid pathway and cancer therapeutics. Advanced Drug Delivery Reviews . 2010;62(13):1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi N., Saito D., Hasegawa S., Yamasaki M., Imai M. Vitamin A in health care: suppression of growth and induction of differentiation in cancer cells by vitamin A and its derivatives and their mechanisms of action. Pharmacology & Therapeutics . 2022;230, article 107942 doi: 10.1016/j.pharmthera.2021.107942. [DOI] [PubMed] [Google Scholar]
- 24.Dao D. Q., Ngo T. C., Thong N. M., Nam P. C. Is vitamin A an antioxidant or a pro-oxidant? The Journal of Physical Chemistry. B . 2017;121(40):9348–9357. doi: 10.1021/acs.jpcb.7b07065. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z., Liu Y., Qi G., Brand D., Zheng S. Role of vitamin A in the immune system. Journal of Clinical Medicine . 2018;7(9):p. 258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddikuzzaman, Grace V. M. B. Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacology and Immunotoxicology . 2013;35(1):164–173. doi: 10.3109/08923973.2012.736520. [DOI] [PubMed] [Google Scholar]
- 27.Kim J. A., Jang J. H., Lee S. Y. An updated comprehensive review on vitamin A and carotenoids in breast cancer: mechanisms, genetics, assessment, current evidence, and future clinical implications. Nutrients . 2021;13(9):p. 3162. doi: 10.3390/nu13093162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filik L. Vitamin A and gastric cancer risk. Gastric Cancer . 2012;15(3):p. 343. doi: 10.1007/s10120-011-0137-y. author reply 344. [DOI] [PubMed] [Google Scholar]
- 29.Lan Q. Y., Zhang Y. J., Liao G. C., et al. The association between dietary vitamin A and carotenes and the risk of primary liver cancer: a case-control study. Nutrients . 2016;8(10):p. 624. doi: 10.3390/nu8100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X., Cheng J., Wang X. Dietary antioxidants: potential anticancer agents. Nutrition and Cancer . 2017;69(4):521–533. doi: 10.1080/01635581.2017.1299872. [DOI] [PubMed] [Google Scholar]
- 31.Pantavos A., Ruiter R., Feskens E. F., et al. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: the Rotterdam study. International Journal of Cancer . 2015;136(9):2178–2186. doi: 10.1002/ijc.29249. [DOI] [PubMed] [Google Scholar]
- 32.Yu N., Su X., Wang Z., Dai B., Kang J. Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: a meta-analysis of 19 publications. Nutrients . 2015;7(11):9309–9324. doi: 10.3390/nu7115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J., Park M. K., Li W. Q., Qureshi A. A., Cho E. Association of vitamin A intake with cutaneous squamous cell carcinoma risk in the United States. JAMA Dermatology . 2019;155(11):1260–1268. doi: 10.1001/jamadermatol.2019.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Dai B., Zhang B., Wang Z. Vitamin A and risk of cervical cancer: a meta-analysis. Gynecologic Oncology . 2012;124(2):366–373. doi: 10.1016/j.ygyno.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki M., Doi Y., Ikeda F., et al. Dietary vitamin A intake and incidence of gastric cancer in a general Japanese population: the Hisayama study. Gastric Cancer . 2012;15(2):162–169. doi: 10.1007/s10120-011-0092-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q., He C. Dietary vitamin A intake and the risk of ovarian cancer: a meta-analysis. Bioscience Reports . 2020;40(4) doi: 10.1042/BSR20193979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S., Hunter D. J., Forman M. R., et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. Journal of the National Cancer Institute . 1999;91(6):547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh C., Baker J. A., Moysich K. B., Rivera R., Brasure J. R., McCann S. E. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutrition and Cancer . 2008;60(3):331–341. doi: 10.1080/01635580701861769. [DOI] [PubMed] [Google Scholar]
- 39.He J., Gu Y., Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clinical Breast Cancer . 2018;18(6):e1389–e1400. doi: 10.1016/j.clbc.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Hu F., Wu Z., Li G., et al. The plasma level of retinol, vitamins A, C and α-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. Journal of Cancer Research and Clinical Oncology . 2015;141(4):601–614. doi: 10.1007/s00432-014-1852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro Silvera S. A., Jain M., Howe G. R., Miller A. B., Rohan T. E. Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiology, Biomarkers & Prevention . 2006;15(2):395–397. doi: 10.1158/1055-9965.EPI-05-0835. [DOI] [PubMed] [Google Scholar]
- 42.Giorgio M., Trinei M., Migliaccio E., Pelicci P. G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nature Reviews. Molecular Cell Biology . 2007;8(9):722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 43.Liou G. Y., Storz P. Reactive oxygen species in cancer. Free Radical Research . 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy K., Wu Y., Meitzler J. L., et al. NADPH oxidases and cancer. Clinical Science (London, England) . 2015;128(12):863–875. doi: 10.1042/CS20140542. [DOI] [PubMed] [Google Scholar]
- 45.Gorrini C., Harris I. S., Mak T. W. Modulation of oxidative stress as an anticancer strategy. Nature Reviews. Drug Discovery . 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 46.Pelicano H., Carney D., Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resistance Updates . 2004;7(2):97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Harris I. S., DeNicola G. M. The complex interplay between antioxidants and ROS in cancer. Trends in Cell Biology . 2020;30(6):440–451. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Winterbourn C. C., Hampton M. B. Thiol chemistry and specificity in redox signaling. Free Radical Biology & Medicine . 2008;45(5):549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Wołoszynowska-Fraser M. U., Kouchmeshky A., McCaffery P. Vitamin A and retinoic acid in cognition and cognitive disease. Annual Review of Nutrition . 2020;40(1):247–272. doi: 10.1146/annurev-nutr-122319-034227. [DOI] [PubMed] [Google Scholar]
- 50.Meléndez-Martínez A. J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Molecular Nutrition & Food Research . 2019;63(15, article e1801045) doi: 10.1002/mnfr.201801045. [DOI] [PubMed] [Google Scholar]
- 51.Wiseman E. M., Bar-El Dadon S., Reifen R. The vicious cycle of vitamin a deficiency: a review. Critical Reviews in Food Science and Nutrition . 2017;57(17):3703–3714. doi: 10.1080/10408398.2016.1160362. [DOI] [PubMed] [Google Scholar]
- 52.Low J. W., Mwanga R. O. M., Andrade M., Carey E., Ball A. M. Tackling vitamin A deficiency with biofortified sweetpotato in sub-Saharan Africa. Global Food Security . 2017;14:23–30. doi: 10.1016/j.gfs.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.di Masi A., Leboffe L., de Marinis E., et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Molecular Aspects of Medicine . 2015;41:1–115. doi: 10.1016/j.mam.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Tang X. H., Gudas L. J. Retinoids, retinoic acid receptors, and cancer. Annual Review of Pathology . 2011;6(1):345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 55.Doldo E., Costanza G., Ferlosio A., et al. CRBP-1 expression in ovarian cancer: a potential therapeutic target. Anticancer Research . 2014;34(7):3303–3312. [PubMed] [Google Scholar]
- 56.Orlandi A., Ferlosio A., Ciucci A., et al. Cellular retinol binding protein-1 expression in endometrial hyperplasia and carcinoma: diagnostic and possible therapeutic implications. Modern Pathology . 2006;19(6):797–803. doi: 10.1038/modpathol.3800586. [DOI] [PubMed] [Google Scholar]
- 57.Doldo E., Costanza G., Agostinelli S., et al. Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. BioMed Research International . 2015;2015:14. doi: 10.1155/2015/624627.624627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarapcsák S., Szalóki G., Telbisz Á., et al. Interactions of retinoids with the ABC transporters P-glycoprotein and breast cancer resistance protein. Scientific Reports . 2017;7(1, article 41376) doi: 10.1038/srep41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boccardo F., Canobbio L., Resasco M., Decensi A. U., Pastorino G., Brema F. Phase II study of tamoxifen and high-dose retinyl acetate in patients with advanced breast cancer. Journal of Cancer Research and Clinical Oncology . 1990;116(5):503–506. doi: 10.1007/BF01613002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koay D. C., Zerillo C., Narayan M., Harris L. N., DiGiovanna M. P. Anti-tumor effects of retinoids combined with trastuzumab or tamoxifen in breast cancer cells: induction of apoptosis by retinoid/trastuzumab combinations. Breast Cancer Research . 2010;12(4):p. R62. doi: 10.1186/bcr2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fulan H., Changxing J., Baina W. Y., et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes & Control . 2011;22(10):1383–1396. doi: 10.1007/s10552-011-9811-y. [DOI] [PubMed] [Google Scholar]
- 62.Allen L. H., Haskell M. Estimating the potential for vitamin A toxicity in women and young children. The Journal of Nutrition . 2002;132(9):2907s–2919s. doi: 10.1093/jn/132.9.2907S. [DOI] [PubMed] [Google Scholar]
- 63.Lam H. S., Chow C. M., Poon W. T., et al. Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics . 2006;118(2):820–824. doi: 10.1542/peds.2006-0167. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira M. R. The neurotoxic effects of vitamin A and retinoids. Anais da Academia Brasileira de Ciências . 2015;87(Supplement 2):1361–1373. doi: 10.1590/0001-3765201520140677. [DOI] [PubMed] [Google Scholar]
- 65.WHO. Geneva: World Health Organization; 2011. Guidelines Approved by the Guidelines Review Committee, in Guideline: vitamin A supplementation in infants and children 6–59 months of age. [PubMed] [Google Scholar]
- 66.Hathcock J. N., Hattan D. G., Jenkins M. Y., McDonald J. T., Sundaresan P. R., Wilkening V. L. Evaluation of vitamin A toxicity. The American Journal of Clinical Nutrition . 1990;52(2):183–202. doi: 10.1093/ajcn/52.2.183. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez-Lazaro C. I., Martínez-González M. Á., Aguilera-Buenosvinos I., et al. Dietary antioxidant vitamins and minerals and breast cancer risk: prospective results from the SUN cohort. Antioxidants (Basel) . 2021;10(3):p. 340. doi: 10.3390/antiox10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamimi R. M., Hankinson S. E., Campos H., et al. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. American Journal of Epidemiology . 2005;161(2):153–160. doi: 10.1093/aje/kwi030. [DOI] [PubMed] [Google Scholar]
- 69.Maillard V., Kuriki K., Lefebvre B., et al. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N-EPIC study. International Journal of Cancer . 2010;127(5):1188–1196. doi: 10.1002/ijc.25138. [DOI] [PubMed] [Google Scholar]
- 70.Epplein M., Shvetsov Y. B., Wilkens L. R., et al. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the multiethnic cohort study: a nested case-control study. Breast Cancer Research . 2009;11(4):p. R49. doi: 10.1186/bcr2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakker M. F., Peeters P. H. M., Klaasen V. M., et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European prospective investigation into cancer and nutrition cohort. The American Journal of Clinical Nutrition . 2016;103(2):454–464. doi: 10.3945/ajcn.114.101659. [DOI] [PubMed] [Google Scholar]
- 72.Longnecker M. P., Newcomb P. A., Mittendorf R., Greenberg E. R., Willett W. C. Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention . 1997;6(11):887–892. [PubMed] [Google Scholar]
- 73.Sato R., Helzlsouer K. J., Alberg A. J., Hoffman S. C., Norkus E. P., George W. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention . 2002;11(5):451–457. [PubMed] [Google Scholar]
- 74.Cho E., Spiegelman D., Hunter D. J., et al. Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention . 2003;12(8):713–720. [PubMed] [Google Scholar]
- 75.Zhang C. X., Ho S. C., Chen Y. M., Fu J. H., Cheng S. Z., Lin F. Y. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. International Journal of Cancer . 2009;125(1):181–188. doi: 10.1002/ijc.24358. [DOI] [PubMed] [Google Scholar]
- 76.Do M. H., Lee S. S., Jung P. J., Lee M. H. Intake of dietary fat and vitamin in relation to breast cancer risk in Korean women: a case-control study. Journal of Korean Medical Science . 2003;18(4):534–540. doi: 10.3346/jkms.2003.18.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.London S. J., Stein E. A., Henderson I. C., et al. Carotenoids, retinol, and vitamin E and risk of proliferative benign breast disease and breast cancer. Cancer Causes & Control . 1992;3(6):503–512. doi: 10.1007/BF00052746. [DOI] [PubMed] [Google Scholar]
- 78.Hultén K., van Kappel A. L., Winkvist A., et al. Carotenoids, alpha-tocopherols, and retinol in plasma and breast cancer risk in northern Sweden. Cancer Causes & Control . 2001;12(6):529–537. doi: 10.1023/A:1011271222153. [DOI] [PubMed] [Google Scholar]
- 79.Rohan T. E., Howe G. R., Friedenreich C. M., Jain M., Miller A. B. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes & Control . 1993;4(1):29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 80.Kushi L. H., Fee R. M., Sellers T. A., Zheng W., Folsom A. R. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. American Journal of Epidemiology . 1996;144(2):165–174. doi: 10.1093/oxfordjournals.aje.a008904. [DOI] [PubMed] [Google Scholar]
- 81.Negri E., la Vecchia C., Franceschi S., et al. Intake of selected micronutrients and the risk of breast cancer. International Journal of Cancer . 1996;65(2):140–144. doi: 10.1002/(SICI)1097-0215(19960117)65:2<140::AID-IJC3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 82.Bohlke K., Spiegelman D., Trichopoulou A., Katsouyanni K., Trichopoulos D. Vitamins A, C and E and the risk of breast cancer: results from a case- control study in Greece. British Journal of Cancer . 1999;79(1):23–29. doi: 10.1038/sj.bjc.6690006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verhoeven D. T., Assen N., Goldbohm R. A., et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. British Journal of Cancer . 1997;75(1):149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dorjgochoo T., Gao Y. T., Chow W. H., et al. Plasma carotenoids, tocopherols, retinol and breast cancer risk: results from the Shanghai Women Health Study (SWHS) Breast Cancer Research and Treatment . 2009;117(2):381–389. doi: 10.1007/s10549-008-0270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dorgan J. F., Sowell A., Swanson C. A., et al. Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States) Cancer Causes & Control . 1998;9(1):89–97. doi: 10.1023/A:1008857521992. [DOI] [PubMed] [Google Scholar]
- 86.Kabat G. C., Kim M., Adams-Campbell L. L., et al. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. The American Journal of Clinical Nutrition . 2009;90(1):162–169. doi: 10.3945/ajcn.2009.27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ching S., Ingram D., Hahnel R., Beilby J., Rossi E. Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. The Journal of Nutrition . 2002;132(2):303–306. doi: 10.1093/jn/132.2.303. [DOI] [PubMed] [Google Scholar]
- 88.Michels K. B., Holmberg L., Bergkvist L., Ljung H., Bruce Å., Wolk A. Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. International Journal of Cancer . 2001;91(4):563–567. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1079>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 89.Hunter D. J., Manson J. A. E., Colditz G. A., et al. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. The New England Journal of Medicine . 1993;329(4):234–240. doi: 10.1056/NEJM199307223290403. [DOI] [PubMed] [Google Scholar]
- 90.Mignone L. I., Giovannucci E., Newcomb P. A., et al. Dietary carotenoids and the risk of invasive breast cancer. International Journal of Cancer . 2009;124(12):2929–2937. doi: 10.1002/ijc.24334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katsouyanni K., Trichopoulos D., Willett W., et al. Risk of breast cancer among Greek women in relation to nutrient intake. Cancer . 1988;61(1):181–185. doi: 10.1002/1097-0142(19880101)61:1<181::AID-CNCR2820610130>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 92.Marubini E., Decarli A., Mazzoleni C., et al. The relationship of dietary intake and serum levels of retinol and beta-carotene with breast cancer. Results of a case-control study. Cancer . 1988;61(1):173–180. doi: 10.1002/1097-0142(19880101)61:1<173::AID-CNCR2820610129>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 93.Rohan T. E., McMichael A. J., Baghurst P. A. A population-based case-control study of diet and breast cancer in Australia. American Journal of Epidemiology . 1988;128(3):478–489. doi: 10.1093/oxfordjournals.aje.a114996. [DOI] [PubMed] [Google Scholar]
- 94.González C. A., Travier N., Luján-Barroso L., et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. International Journal of Cancer . 2011;129(2):449–459. doi: 10.1002/ijc.25679. [DOI] [PubMed] [Google Scholar]
- 95.Shannon J., Thomas D. B., Ray R. M., et al. Dietary risk factors for invasive and in-situ cervical carcinomas in Bangkok, Thailand. Cancer Causes Control . 2002;13(8):691–699. doi: 10.1023/A:1020289618161. [DOI] [PubMed] [Google Scholar]
- 96.Kim J., Kim M. K., Lee J. K., et al. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: a case-control study in Korea. Nutrition and Cancer . 2010;62(2):181–189. doi: 10.1080/01635580903305326. [DOI] [PubMed] [Google Scholar]
- 97.Guo L., Zhu H., Lin C., et al. Associations between antioxidant vitamins and the risk of invasive cervical cancer in Chinese women: a case-control study. Scientific Reports . 2015;5(1, article 13607) doi: 10.1038/srep13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brock K. E., Berry G., Mock P. A., MacLennan R., Truswell A. S., Brinton L. A. Nutrients in diet and plasma and risk of in situ cervical cancer. Journal of the National Cancer Institute . 1988;80(8):580–585. doi: 10.1093/jnci/80.8.580. [DOI] [PubMed] [Google Scholar]
- 99.Verreault R., Chu J., Mandelson M., Shy K. A case-control study of diet and invasive cervical cancer. International Journal of Cancer . 1989;43(6):1050–1054. doi: 10.1002/ijc.2910430616. [DOI] [PubMed] [Google Scholar]
- 100.Slattery M. L., Abbott T. M., Overall J. C., Jr., et al. Dietary vitamins A, C, and E and selenium as risk factors for cervical cancer. Epidemiology . 1990;1(1):8–15. doi: 10.1097/00001648-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Potischman N., Herrero R., Brinton L. A., et al. A case-control study of nutrient status and invasive cervical cancer: II. Serologic indicators. American Journal of Epidemiology . 1991;134(11):1347–1355. doi: 10.1093/oxfordjournals.aje.a116037. [DOI] [PubMed] [Google Scholar]
- 102.Herrero R., Potischman N., Brinton L. A., et al. A case-control study of nutrient status and invasive cervical cancer: I. Dietary indicators. American Journal of Epidemiology . 1991;134(11):1335–1346. doi: 10.1093/oxfordjournals.aje.a116036. [DOI] [PubMed] [Google Scholar]
- 103.Cramer D. W., Kuper H., Harlow B. L., Titus-Ernstoff L. Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. International Journal of Cancer . 2001;94(1):128–134. doi: 10.1002/ijc.1435. [DOI] [PubMed] [Google Scholar]
- 104.Bertone E. R., Hankinson S. E., Newcomb P. A., et al. A population-based case-control study of carotenoid and vitamin A intake and ovarian cancer (United States) Cancer Causes & Control . 2001;12(1):83–90. doi: 10.1023/A:1008985015927. [DOI] [PubMed] [Google Scholar]
- 105.McCann S. E., Moysich K. B., Mettlin C. Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutrition and Cancer . 2001;39(1):19–28. doi: 10.1207/S15327914nc391_3. [DOI] [PubMed] [Google Scholar]
- 106.Tung K. H., Wilkens L. R., Wu A. H., et al. Association of dietary vitamin A, carotenoids, and other antioxidants with the risk of ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention . 2005;14(3):669–676. doi: 10.1158/1055-9965.EPI-04-0550. [DOI] [PubMed] [Google Scholar]
- 107.Tzonou A., Hsieh C. C., Polychronopoulou A., et al. Diet and ovarian cancer: a case-control study in Greece. International Journal of Cancer . 1993;55(3):411–414. doi: 10.1002/ijc.2910550314. [DOI] [PubMed] [Google Scholar]
- 108.Salazar-Martinez E., Lazcano-Ponce E. C., Lira-Lira G. G., Escudero-de los Rios P., Hernandez-Avila M. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology . 2002;63(2):151–157. doi: 10.1159/000063814. [DOI] [PubMed] [Google Scholar]
- 109.Chang E. T., Lee V. S., Canchola A. J., et al. Diet and risk of ovarian cancer in the California teachers study cohort. American Journal of Epidemiology . 2007;165(7):802–813. doi: 10.1093/aje/kwk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jeong N. H., Song E. S., Lee J. M., et al. Plasma carotenoids, retinol and tocopherol levels and the risk of ovarian cancer. Acta Obstetricia et Gynecologica Scandinavica . 2009;88(4):457–462. doi: 10.1080/00016340902807215. [DOI] [PubMed] [Google Scholar]
- 111.Bidoli E., la Vecchia C., Talamini R., et al. Micronutrients and ovarian cancer: a case-control study in Italy. Annals of Oncology . 2001;12(11):1589–1593. doi: 10.1023/A:1013124112542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data resulting from this study may be obtained from the corresponding author upon reasonable request.


