Abstract
BNT162b2 (Pfizer/BioNTech) is a coronavirus disease 2019 (COVID-19) vaccine containing nucleoside-modified messenger RNA encoding the severe acute respiratory syndrome coronavirus 2 spike glycoprotein. Recently, ocular complications of mRNA vaccines have been reported increasingly frequently. However, immunological adverse events due to mRNA vaccines in real-world settings are not fully known. We herein report the novel development of sarcoidosis manifested as uveitis, bilateral hilar lymphadenopathy, angiotensin-converting enzyme elevation, and epithelioid and giant cell granuloma formation in the lung soon after the first BNT162b2 injection and review the current literature, including three reported cases of sarcoid-like reaction following COVID-19 vaccination.
Keywords: mRNA vaccine, sarcoidosis, COVID-19, uveitis, angiotensin-converting enzyme (ACE)
Introduction
Sarcoidosis is a multisystemic inflammatory disease of unknown cause with a wide range of clinical manifestations (1). The disorder can affect virtually any organ in the body, such as the lungs, lymphatic system, eyes, or a combination of these sites, and is characterized by noncaseating granuloma formation (1). Although the etiology of sarcoidosis remains unclear, many studies have suggested that genetic, host immunologic, and environmental factors interact to cause sarcoidosis (2-4). Several drugs and vaccines have been associated with the development of sarcoidosis or sarcoid-like reaction, a so-called drug-induced sarcoidosis-like reactions (DISR) that is indistinguishable from sarcoidosis (5).
BNT162b2 (Pfizer/BioNTech) is a coronavirus disease 2019 (COVID-19) vaccine containing nucleoside-modified messenger RNA encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein (6). BNT162b2 is 95% effective in preventing COVID-19 and first received emergency use authorization from the Food and Drug Administration (FDA) on December 11, 2020, for COVID-19 prevention in persons ≥16 years old (7). BNT162b2 also had a favorable safety profile characterized by transient mild-to-moderate injection-site pain, fatigue, and headache, with the only notable exception being potentially immune-mediated autoimmune thrombosis and myocarditis (7-9). Recently, ocular complications of FDA-approved mRNA vaccines, including uveitis, optic neuritis, abducens nerve palsy, acute macular neuroretinopathy, central serous retinopathy, and thrombosis, have been reported (10-16). However, the immunological adverse events of mRNA vaccines in real-world settings are not fully known.
We herein report the simultaneous development of uveitis, bilateral hilar lymphadenopathy (BHL), angiotensin-converting enzyme (ACE) elevation, and non-caseating epithelioid and giant cell granuloma formation, all of which are hallmarks of sarcoidosis, soon after the first BNT162b2 injection. We also review the current literature, including three reported cases of sarcoidosis or sarcoid-like reaction following COVID-19 vaccination (17,18).
Case Report
A 61-year-old Japanese man developed a low-grade fever and malaise 3 hours after the first inoculation of the SARS-CoV-2 recombinant mRNA vaccine (BNT162b2; Pfizer/BioNTech) (Fig. 1A). The next day, he noticed discomfort and blurred vision in his right eye. He visited a local ophthalmologist, and right iritis and increased intraocular pressure (IOP; 25 mmHg) were noted, so he was treated with steroid eye drops. He was subsequently referred to the ophthalmology department of our hospital because he had diminished visual acuity and further increased IOP. He was later referred to our department because of an abnormal shadow on chest X-ray (CXR). He was a smoker but had been previously healthy. He had no health problems on annual medical checkups, including CXR. He had no family history of sarcoidosis or autoimmune diseases. He was taking no oral medication or medical herbs.
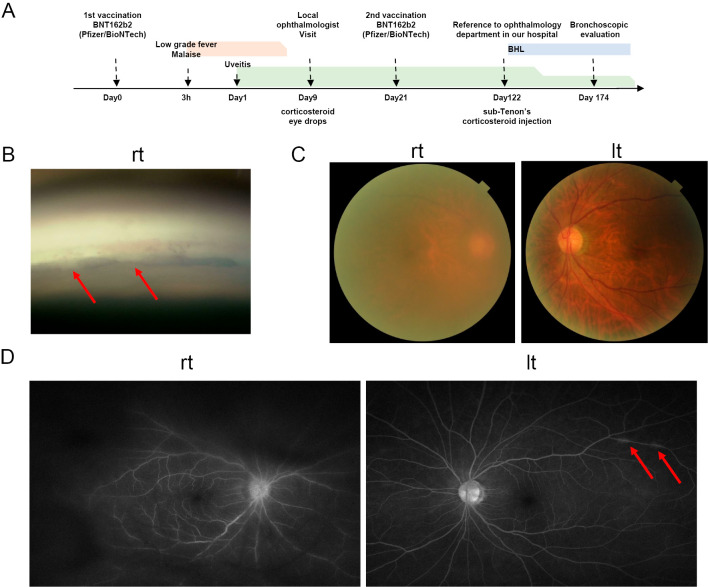
Figure 1.
Timeline and the ocular manifestation of the current case. A: timeline of the current case. B: right corner nodules are indicated by arrows. C: right vitreous turbidity was present. D: left perivenous inflammation is indicated by arrows. rt: right, lt: left, BHL: bilateral hilar lymphadenopathy
An ophthalmology examination revealed that the best-corrected visual acuity had been reduced to 0.3 [oculus dexter (OD)] and 1.5 [oculus sinister (OS)]. The IOP was 40.0 mmHg (OD) and 30.0 mmHg (OS). According to the slit lamp examination, the bilateral corneas were edematous, and multiple mutton fat keratic precipitates were observed in the corneal endothelium. The anterior chamber was noted to have 1+ (OD) cells. By laser flare photometry (LFP), the cell count was 85.6 ph/ms (OD) and 11.0 ph/ms (OS) (normal values 4-6 ph/ms). By gonioscopy, multiple nodules were observed on the trabecular meshwork in OD (Fig. 1B). A fundus examination revealed severe vitreous opacity in OD (Fig. 1C). Fluorescein angiography revealed leakage of dye from the retinal veins and optic disc in OD. In OS, there were some areas of intense hyper-fluorescence due to the leakage of dye from the retinal veins with vasculitis (Fig. 1D). No vascular injury, thrombosis, or neuropathy was observed.
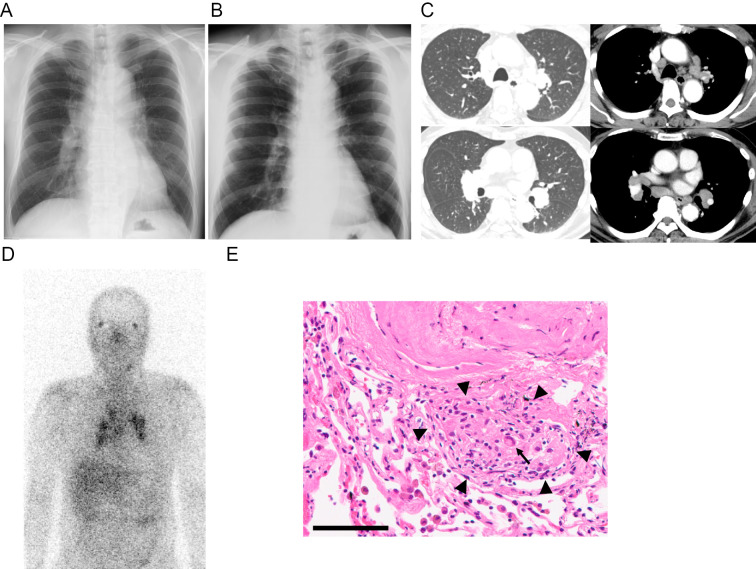
Blood tests showed an increase in soluble interleukine-2 receptor (sIL-2R) (1,455 U/L) and ACE (25.8 U/L). QuantiFERON was negative, and anti-nuclear antibody (ANA) was x40. CXR revealed marked BHL (Fig. 2A) compared to the findings on CXR from the previous year (Fig. 2B). An electrocardiogram and ultrasound cardiography were normal. Chest contrast-enhanced CT (CECT) showed bilateral hilar and mediastinal lymphadenopathy (Fig. 2B). There were no abnormal shadows in the lung fields (Fig. 2B) nor hepatosplenomegaly. Gallium (Ga)-67 scintigraphy showed an increased uptake in the bilateral ocular lesion (panda sign) and bilateral hilar and mediastinal lymph nodes (Fig. 2C). Axillary lymphadenopathies were absent on CECT and on Ga-67 scintigraphy. A bronchoalveolar lavage fluid (BALF) analysis showed mild lymphocytosis (21.5%) and an increased CD4/8 ratio (3.48) (Table 1). Endobronchial ultrasound-guided trans-bronchial nodal aspiration (EBUS-TBNA) of the mediastinal lymph node revealed a non-caseating granuloma (not shown). A transbronchial lung biopsy (TBLB) also detected non-caseating granulomas adjacent to a vessel wall and mild infiltration of lymphocytes in the stromal area (Fig. 2E). No obvious microorganisms, such as acid-fast bacilli, fungi, or viruses, were detected. A nasopharyngeal swab test for SARS-CoV-2 nucleic acid was also negative.
Figure 2.
Radiological and histopathologic findings of hilar and mediastinal lymphadenopathy. A: Chest X-ray (CXR) revealed marked bilateral hilar lymphadenopathy (BHL) on respiratory consultation. B: Normal CXR findings from the previous year. C: Contrast-enhanced chest computed tomography showed bilateral hilar and mediastinal lymphadenopathy. There were no remarkable findings in the lung parenchyma. D: Gallium (Ga)-67 scintigraphy showed an increased uptake in the ocular lesion (so-called panda sign) and bilateral hilar and mediastinal lymph nodes. E: Histopathological findings of a trans-bronchial lung biopsy (TBLB). A representative image of Hematoxylin and Eosin staining. A small non-caseating granuloma that consisted of tightly packed epithelioid cells (arrowheads), and a few admixed multinucleated giant cells (an arrow) and lymphocytes. Scale bar: 100 μm.
Table 1.
Results of Bronchoalveolar Lavage Fluid (BALF) and Endobronchial Ultrasound-guided Trans-bronchial Nodal Aspiration (EBUS-TBNA).
| BALF | Mediastinal lymph node | |||
|---|---|---|---|---|
| Cytology | Class 2 | Cytology | Class 2 | |
| Total cells | 161.5 | ×105 | Granulomatous inflammation | |
| Mac | 76.3 | % | ||
| Lym | 21.5 | % | ||
| Neutro | 2.2 | % | ||
| CD4/8 | 3.48 | |||
| Acid-fast bacilli | Negative | |||
| TB-PCR | Negative | |||
| MAC-PCR | Negative | |||
| Culture | Normal flora | |||
Mac: macrophages, Lym: lymphocytes, Neutro: neutrophiles, TB: tuberculosis, MAC: mycobacterium avium complex, PCR: polymerase chain reaction
Based on these findings, our diagnosis was uveitis and BHL due to sarcoidosis following administration of BNT162b2, a modified mRNA vaccine. For the aggravated uveitis, a subcapsular injection of steroids was administered, and the ocular symptoms and IOP decreased. Systemic oral corticosteroids were not required, as the respiratory symptoms and thoracic lesions had not progressed.
Discussion
We encountered a case involving the novel development of sarcoidosis following the administration of BNT162b2 requiring topical steroid injection therapy. This case is unique in that uveitis, BHL, ACE elevation, and noncaseating epithelioid granuloma formation, all of which are hallmarks of typical sarcoidosis, were detected.
Sarcoid-like reactions due to influenza, Bacille Calmette-Guerin (BCG), and herpes zoster virus vaccines have been reported (5), and thus far, three cases of sarcoidosis or sarcoidosis-like reaction following COVID-19 vaccination have been described (Table 2) (17,18). In the first case, a 44-year-old man showed abnormal FDG accumulation in the axial and mediastinal lymph nodes on fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT after BNT162b2 vaccination, and non-caseating epithelioid cell granulomas were detected in mediastinal lymphadenopathy by EBUS-TBNA (17). Two other cases diagnosed as Lofgren's syndrome presented with ankle periarthritis, rash, and BHL after COVID-19 vaccination have been reported (18). The second case was a 21-year-old previously healthy Caucasian woman. She received ChAdOx1 (AstraZeneca) for the first injection and CX-024414 (Moderna) for the second injection and developed a skin rash and ankle periarthritis following the second inoculation. The third case was a 28-year-old previously healthy Caucasian man. He developed a skin rash, ankle periarthritis, and BHL 28 days after the first inoculation of ChAdOx1. The second and third cases were treated with systemic corticosteroids, and disease remission was achieved in both. There is also a report of existing neurosarcoidosis symptoms that worsened following mRNA vaccination (8).
Table 2.
Summary of the Current Case and 3 Reported Cases of Sarcoidosis-like-reaction after COVID-19 Vaccination.
| Case | Type of Vaccine | Age | Sex | Race | Onset | Clinical manifestations | Laboratory findings | Radiographical findings | Histopathological findings | Treatment and outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1st & 2nd: BNT162b2 (Pfizer/BioNTech) |
44 | M | N.D. | A few days after 1st injection | Not reported | N.D. | CT, FDG-PET: axillary and mediastinal lymphadenopathy | EBUS-TBNA: sarcoidal-type granulomatous inflammation | N.D. | (17) |
| 2 | 1st: ChAdOx1 (AstraZeneca) 2nd: CX-024414 (Morena) |
21 | F | Caucasian | 3 weeks after 2nd injection | Löfgren’s syndrome ・skin rash ・ankle joint pain |
CRP: 0.028 mg/dL, ACE: normal |
CXR: not remarkable | N.D. | Ibuprofen: not effective, PSL 20 mg/day: effective | (18) |
| 3 | 1st: ChAdOx1 2nd: N.D. |
28 | M | Caucasian | 28 days after 1st injection | Löfgren's syndrome ・skin rash ・ankle joint pain ・BHL |
CRP: 0.022 mg/dL, ESR: 80 mm/h, sIL-2R: 854 U/L, D-dimer: elevated, ACE: normal |
CXR & CT: BHL and fine nodular shadow in lung parenchyma, PAE | N.D. | PSL 20 mg/day: effective, anticoagulant: effective | (18) |
| 4 | 1st & 2nd: BNT162b2 |
61 | M | Japanese | One day after 1st injection | Low grade fever malaise uveitis | CRP: 0.08 mg/mL, sIL-2R: 1,455 U/L, ACE: 25.8 U/L |
CXR & CT: BHL, Ga-67 scintigraphy: increased uptake in ocular lesion and bilateral hilar and mediastinal lymph nodes | TBLB, EBUS-TBNA: non-caseating granuloma with giant cells | Topical steroid: effective | Current case |
M: male, F: female, CT: computed tomography, CXR: chest X ray, FDG-PET: [18F] fluorodeoxyglucose-positron emission tomography, CRP: c-reactive protein, ESR: erythrocyte sediment rate, sIL2-R: soluble interleukin 2 receptor, ACE: angiotensin converting enzyme, BHL: bilateral hilar lymphadenopathy, PAE: pulmonary artery embolism, TBLB: trans-bronchial lung biopsy, Ga-67 scintigraphy:gallium-67 scintigraphy, EBUS-TBNA: endobronchial ultrasound-guided trans-bronchial needle aspiration, PSL: predonisolone, N.D.: not detailed
The clinical manifestation of the current case is typical for sarcoidosis, and the temporal relationship is compatible with reported cases (16-18). The causal relationship between mRNA vaccination and the onset of sarcoidosis in the current case may be considered “possible causality” under the classification of the World Health Organization (WHO) Adverse Drug Terminology (19) and “possible” according to the Naranjo criteria (20). According to a recent review, the average time from COVID-19 vaccination to the onset of uveitis is 8.0±8.6 days (minimum 1 day, maximum 30 days) (16). The onsets in each of the three reported cases of sarcoidosis or sarcoid-like reaction following mRNA vaccination were a few days, three weeks and four weeks (17,18). In the present case, uveitis developed one day after the first inoculation of BNT162b2, but BHL was detected 122 days after the first inoculation. Thoracic lesions may have developed before that time, since the exact time of the onset is unknown. In our case, bilateral ocular manifestation developed (Fig. 1B-D). Ocular sarcoidosis frequently develops bilaterally (21). Hasseb et al recently reviewed 34 cases of uveitis following COVID-19 vaccination and reported that 29.4% occurring after mRNA vaccination were bilateral (16). Taken together, these findings suggest that mRNA vaccination triggered the onset of sarcoidosis. Since there are few reports on sarcoidosis following mRNA vaccination, it will be necessary to accumulate similar cases in the future.
The precise mechanism underlying the development of sarcoidosis in the present patient is unknown; however, several hypotheses have been proposed concerning the pathogenesis of the development of sarcoidosis in this case. First, mRNA vaccinations have directly caused sarcoidosis. Second, mRNA vaccination can cause the host immune system to become more susceptible to the development of sarcoidosis. Third, mRNA vaccination can aggravate subclinical sarcoidosis (5), as mRNA vaccines stimulate the innate immunity through endosolic and cytoplasmic nucleic acid receptors, such as toll-like receptors (TLRs) 3, 7, 8, and 9, and components of the inflammasome, including retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and nucleotide-binding oligomerization domain-containing protein 2 (NOD2) (22,23). These mechanisms are known to play important roles in the development of sarcoidosis (1,2). Further investigations will be required to evaluate the mechanisms underlying the COVID-19 vaccine-related onset of sarcoidosis.
In summary, we reported the novel development of sarcoidosis after COVID-19 mRNA vaccination. This case is unique in that uveitis, BHL, ACE elevation, and noncaseating epithelioid granuloma formation, all of which are hallmarks of typical sarcoidosis, were detected. Vaccination is a very effective strategy for preventing SARS-CoV-2 infection and has more benefits than risks. Physicians should keep in mind that sarcoidosis may occur as a de novo immunological reaction that develops in multiple organs following SARS-CoV-2 mRNA vaccination.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by a Grant-in-Aid for Early-Career Scientists from the Ministry of Education, Sciences, Sports, and Technology (MEXT), Japan, to T. Numakura (21K16106).
Acknowledgements
We thank Mr. Brent K Bell for his reading the manuscript.
References
- 1.Drent M, Crouser ED, Grunewald J. Challenges of sarcoidosis and its management. N Engl J Med 385: 1018-1032, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y, Müller-Quernheim J. Sarcoidosis. Lancet 383: 1155-1167, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol 49: 6-18, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Tamada T, Nara M, Murakami K, et al. The clinical features of patients with sarcoidosis and malignant diseases in Japan. Intern Med 60: 209-216, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra A, Nautiyal A, Kalkanis A, Judson MA. Drug-induced sarcoidosis-like reactions. Chest 154: 664-677, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol 21: 73-82, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med 384: 643-649, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watad A, De Marco G, Mahajna H, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mrna/dna SARS-CoV-2 vaccination. Vaccines 9: 1-23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med 385: 2132-2139, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler N, Mendez Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep 23: 101136, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects: real or coincidence? J Ophthalmic Vis Res 16: 490-501, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heydari-Kamjani M, Vante I, Uppal P, Demory Beckler M, Kesselman MM. Uveitis sarcoidosis presumably initiated after administration of Shingrix vaccine. Cureus 11: 10-12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocular Immunol Inflamm 29: 1216-1224, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after BNT162b2 vaccination: is this just a coincidence? Int J Infect Dis 110: 95-97, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye (Basingstoke) 34: 2352-2353, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haseeb AA, Solyman O, Abushanab MM, Abo Obaia AS, Elhusseiny AM. Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines 10: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauckneht M, Aloè T, Tagliabue E, et al. Beyond COVID-19 vaccination-associated pitfalls on [18F]fluorodeoxyglucose (FDG) PET: a case of a concomitant sarcoidosis. Eur J Nucl Med Mol Imaging 48: 2661-2662, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rademacher J-g, Tampe B. First report of two cases of Löfgren's syndrome after SARS-CoV-2 vaccination-coincidence or causality? Vaccines 9: 1313, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kathleen Holloway, Terry Green. Drug and therapeutics committees: a practical guide. Kathleen Holloway, Ed. World Health Organization, Geneva, 2003. [Google Scholar]
- 20.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30: 239-245, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki M, Smith JR, Takase H, Kaburaki T, Acharya NR, Rao NA. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol 103: 1418, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 17: 261-279, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21: 195-197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]