Abstract
We have previously shown that different isolates of Chlamydia pneumoniae display heterogeneity in the in vitro stimulation of chemokines and adhesion molecules from infected human endothelial cells. In the present study, we examined the ability of different isolates of C. pneumoniae to promote transendothelial migration of neutrophils and monocytes. Human umbilical vein endothelial cells (HUVEC) were infected with low (<15)-passage C. pneumoniae isolates A-03, PS-32, and BR-393 and high (>40)-passage isolates BAL-16, TW-183, and T-2634, and levels of neutrophil and monocyte transendothelial migration were determined following 24 h of infection. Compared to mock-infected controls, significant increases in neutrophil migration were observed in response to most C. pneumoniae isolates examined (P < 0.001). Levels of monocyte migration were significantly increased in response to TW-183 and T-2634 (P < 0.001). Serial passage (>40 times) of the three low-passage isolates in HEp-2 cell cultures prior to infection of HUVEC generally resulted in the promotion of higher levels of neutrophil and monocyte transendothelial migration. These findings were compatible with differences observed in the extent of interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) stimulation between low- and high-passage A-03, PS-32, and BR-393. As opposed to C. pneumoniae, infection with C. trachomatis L2 caused only a slight increase in neutrophil transendothelial migration, which correlated with the lack of measurable IL-8 levels by this species. However, significant levels of monocyte migration were induced in response to C. trachomatis L2 despite a lack of measurable MCP-1 stimulation. C. trachomatis serovars A and E also failed to induce IL-8 and MCP-1 production in HUVEC. Results from this study indicate that the passage history of C. pneumoniae may play a role in the divergence of stimulatory activities observed among isolates in human endothelial cells. In addition, the differences observed between this organism and C. trachomatis suggest that the upregulation of IL-8 and MCP-1 in endothelial cells may be unique to C. pneumoniae.
Chlamydia pneumoniae causes acute respiratory infections, including sinusitis, bronchitis, and pneumonia (6, 7). Infections with this organism may also become chronic following acute illness, despite appropriate antibiotic therapy (10). A role for C. pneumoniae in chronic inflammatory diseases has been suggested by data showing a propensity for patients with previous respiratory infection to develop asthmatic bronchitis (8, 9) and by the physical and serological evidence implicating an association of this organism with atherosclerosis (18, 19, 22, 24, 28, 32, 34). The recent isolation of C. pneumoniae from human carotid (15) and coronary (23, 29) atheromas provides further support for an association of this bacterium with atherogenesis by demonstrating the presence of viable organisms within lesions.
Atherosclerosis has been defined as an inflammatory disease, mainly the result of injurious stimuli and healing responses of the arterial wall occurring in a hyperlipidemic and dyslipoproteinemic environment (31, 33). Initial damage to the endothelium may be caused by blood flow shear, free radicals, oxidized low-density lipoprotein uptake, inflammatory mediators, infection, immune complexes, or smoking (31). The presence of chemokines such as monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8) within human atheromas implicates their role in atherogenesis (27, 36). Upregulation of MCP-1 by activated endothelial cells may modulate monocyte-macrophage recruitment to the intima (31, 33), while IL-8 may have chemotactic activities for T lymphocytes, neutrophils, and vascular smooth muscle cells (14, 21, 39).
C. pneumoniae has been shown to multiply in vitro in human endothelial cells, aortic smooth muscle cells, and macrophages (3–5, 16). Since the endothelium is central to the recruitment of leukocytes during atherogenesis, studies aimed at the inflammatory activation of endothelial cells by C. pneumoniae may provide a better understanding of the role of this organism in atherosclerosis. We have previously shown that infection of human endothelial cells with C. pneumoniae induces the production of IL-8 and MCP-1 and found heterogeneity in the extent of activation of these chemokines among different isolates (26). The present study investigated the capability of different C. pneumoniae isolates to stimulate the transendothelial migration of neutrophils and monocytes in an in vitro model of the human vascular wall.
MATERIALS AND METHODS
Chlamydia isolates.
C. pneumoniae A-03 (ATCC VR-1452) was isolated from an atheroma of a patient with coronary artery disease (29). C. pneumoniae PS-32 was isolated from a carotid atheroma (15) and was kindly supplied by J. Thomas Grayston, University of Washington, Seattle. Respiratory isolates BAL-16 and T-2634 were provided by Margaret Hammerschlag, State University of New York, Brooklyn. BR-393 was a recent respiratory isolate and a gift from Charlotte Gaydos, The Johns Hopkins University, Baltimore, Md. C. pneumoniae TW-183 was purchased from the Washington Research Foundation, Seattle. All isolates were propagated in HEp-2 cell cultures (ATCC CCL-23) as previously described (26). Low-passage (LP) A-03, PS-32, and BR-393 stock cultures had undergone <15 passages in HEp-2 cells, while the remaining isolates (high-passage [HP] stock cultures) had been passed >40 times before these experiments. HP stock cultures of A-03, PS-32, and BR-393 were prepared as well. Chlamydia trachomatis L2/434 was a gift from James B. Mahoney, McMaster University Regional Virology and Chlamydiology Laboratory, Hamilton, Ontario, Canada. C. trachomatis A/HAR-13 and E/BOUR were kindly provided by Gerald Byrne, University of Wisconsin, Madison.
Endothelial cell cultures.
Human umbilical vein endothelial cells (HUVEC; ATCC 1730-CRL) were cultured in 75-cm2 culture flasks and maintained in Ham’s F12K medium (Sigma, St. Louis, Mo.). Cell medium was supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin-amphotericin B (Fungizone) mix (BioWhittaker, Walkersville, Md.), 30 μg of endothelial cell growth supplement per ml, and 100 μg of heparin (Sigma) per ml.
For leukocyte migration studies, HUVEC were seeded in 6.5-mm Transwell-Clear inserts (Corning Costar, Cambridge, Mass.) at a density of 4 × 104 cells/insert and allowed to reach confluency for 2 days before infection. The formation of confluent monolayers was verified by silver nitrate stain and microscopic examination under a 400× objective (35). Intercellular junction integrity of the endothelial monolayer was also assessed by measuring the permeability to bovine serum albumin as described elsewhere (13). For the IL-8 and MCP-1 experiments, HUVEC were seeded in gelatin-coated 24-well plates at a density of 2 × 105 cells/well and allowed to adhere overnight prior to infection. The cell number and viability of HUVEC were not significantly diminished following the described incubation periods, as determined by direct counting of trypsinized cells with a hemacytometer and by trypan blue dye exclusion, respectively.
Infection protocol.
HUVEC monolayers in 6.5-mm transwells or 24-well plates were infected separately with each isolate of C. pneumoniae or C. trachomatis suspended in inoculation medium (Iscove’s minimal essential medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1% [vol/vol] nonessential amino acids, 10 mM HEPES, 4 mg of glucose per ml [pH 7.5], 10 μg of gentamicin per ml, and 25 μg of vancomycin per ml). Cells grown in 6.5-mm transwells received 4 × 104 inclusion-forming units (IFU) per well, while cells grown in 24-well plates were inoculated with 2 × 105 IFU per well, resulting in a multiplicity of infection of 1:1 for each case. In addition to infection with viable chlamydiae, HUVEC were infected with organisms that had been previously inactivated by heat (90°C for 30 min) or UV light (12-h exposure to a model UVSL-25 Mineralight UV lamp at a distance of 2 cm).
For the transendothelial migration experiments, infection was performed by incubation for 2 h at 37°C in 5% CO2. Following incubation, the medium was aspirated, cell monolayers were washed with Hanks’ balanced salt solution (HBSS), and fresh inoculation medium was added to the cultures. For the chemokine experiments, infection was performed by centrifugation as described previously (26). Since the C. pneumoniae inoculum may have contained remnants of HEp-2 cells, mock-infected controls (HUVEC treated with crude lysates of HEp-2 cells) were processed in the same manner as infected cells. Uninfected, mock-infected, and infected cells were incubated for 24 h at 37°C in 5% CO2 before migration, and chemokine assays were performed. The response of HUVEC to stimulation with 500 U of human recombinant tumor necrosis factor alpha (TNF-α; Promega, Madison, Wis.) per ml was used as a positive control.
To examine the growth of C. pneumoniae and C. trachomatis in HUVEC, infected cells were scraped at 48 h postinfection, resuspended in inoculation medium, titrated, and inoculated in fresh HEp-2 monolayers. Growth titers were obtained from replicate wells and expressed as IFU/milliliter. Viability of HUVEC during infection was determined by trypan blue dye exclusion analysis.
Isolation of human neutrophils and monocytes.
Neutrophils and peripheral blood mononuclear cells (PBMC) were isolated from venous blood of healthy adults by dextran sedimentation and density centrifugation in a Percoll gradient as described previously (11). Neutrophil and PBMC fractions were collected separately, resuspended in Krebs Ringer solution (120 mM NaCl, 4.8 mM KCl, 5.5 mM dextrose, 3.12 mM NaH2PO4, 12.48 mM Na2HPO4 [pH 7.4]), and centrifuged at 300 × g. Neutrophils were treated for 30 s with 0.2% NaCl solution to lyse contaminating erythrocytes and washed twice in Krebs Ringer solution. Purity of isolated neutrophils was >98%, as determined by Diff-Quik staining (Baxter, Miami, Fla.). PBMC were washed twice with ice-cold HBSS-HEPES (HBSS supplemented with 20 mM HEPES and 1% penicillin-streptomycin-amphotericin B [Fungizone] mix) and seeded in six-well plates at a density of 2 × 106 cells/well. PBMC were maintained in HBSS-HEPES with 0.1% heat-inactivated autologous serum for 2 h at 37°C in 5% CO2. Following incubation, nonadherent cells were removed by aspiration and several washing steps. Adherent monocytes were removed by gently scraping with a rubber policeman and washed twice with HBSS-HEPES before the transendothelial migration assays. Monocytes obtained by this method were >95% pure by α-naphthyl acetate esterase staining (Sigma).
Neutrophil and monocyte transendothelial migration assays.
Before the migration assays were performed, the medium from the upper and lower chambers of the transwells was removed and endothelial monolayers were washed three times with HBSS. Neutrophils or monocytes were subsequently added to the upper chambers at a density of 4 × 105 cells/insert, and fresh medium was added to the lower chambers. Neutrophils and monocytes were coincubated with uninfected, mock-infected, infected, or TNF-α-treated HUVEC at 37°C for 30 and 60 min, respectively. Following incubation, the medium from both chambers was aspirated, the upper chamber was washed three times with HBSS, the endothelial monolayer was removed with a cotton swab, and the polycarbonate membrane was stained with Diff-Quik (Baxter). Transendothelial migration of neutrophils or monocytes was assessed by light microscopic examination of the underside of the polycarbonate membrane under a 1,000× objective. The average number of cells from a total of 50 high-power fields (HPF) was determined to present the data as number of neutrophils or monocytes per HPF.
For antibody inhibition experiments, HUVEC were preincubated for 1 h with 10 and 20 μg per ml of anti-IL-8 or anti-MCP-1 monoclonal antibodies (R&D Systems, Minneapolis, Minn.) before the transendothelial migration assays. Following incubation, neutrophils or monocytes were added to the appropriate wells, and transmigration was allowed as described above, with the antibodies present throughout the experiments.
Chemokine measurements.
Levels of IL-8 and MCP-1 were measured from the supernatants of uninfected, mock-infected, infected, and TNF-α-treated HUVEC by commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). The ELISAs were performed according to the manufacturer’s instructions.
Data analysis.
Raw data of experimental groups from the transendothelial migration and chemokine assays were subjected to an unpaired analysis of variance with the Tukey-HSD multiple-comparison test. A P of <0.05 was used as the alpha value to determine statistical significance for all analyses.
RESULTS
Transendothelial migration of neutrophils and monocytes in response to infection with C. pneumoniae.
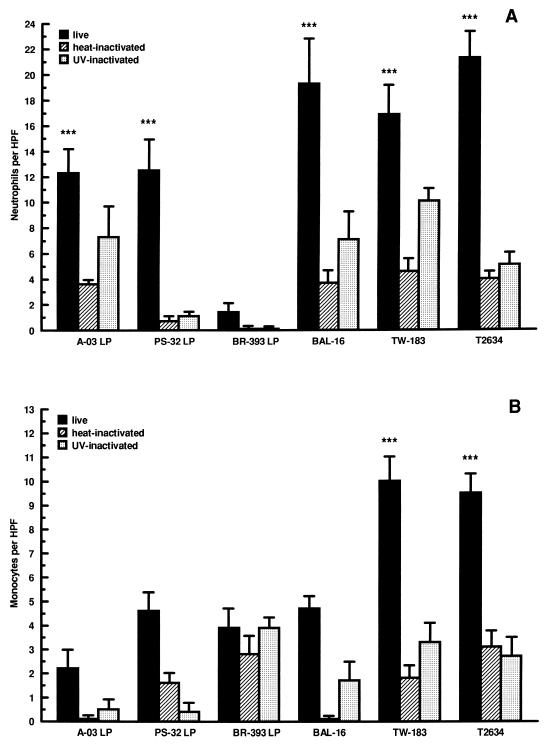
We examined the abilities of different isolates of C. pneumoniae to stimulate the migration of neutrophils and monocytes across infected endothelial cells. HUVEC were infected with LP isolates A-03, PS-32, and BR-393 or HP isolates BAL-16, TW-183, and T-2634, and levels of migration were determined following 24 h of infection. As shown in Fig. 1, the extent of neutrophil and monocyte migration stimulated by C. pneumoniae infection was dependent on the isolate examined. Significant increases in neutrophil transendothelial migration were observed in response to most C. pneumoniae isolates compared to mock-infected controls. These increases were approximately threefold for A-03 LP and PS-32 LP, fourfold for BAL-16 and TW-183, and fivefold for T-2634 (P < 0.001) (Fig. 1A). Little increase was observed in response to BR-393 LP. Use of heat-inactivated C. pneumoniae caused a significant reduction in the stimulation of neutrophil transendothelial migration by most isolates compared to live organisms (P < 0.05). The response to UV-inactivated C. pneumoniae was decreased by an average of 50% for most isolates and not found to be statistically significant compared to viable bacteria for A-03-LP, BAL-16, and TW-183.
FIG. 1.
Extent of neutrophil and monocyte transendothelial migration in response to different isolates of C. pneumoniae. HUVEC monolayers cultured in transwells were inoculated separately with LP isolates A-03, PS-32, and BR-393 or with HP isolates BAL-16, TW-183, and T-2634 at a multiplicity of infection of 1:1. Equivalent inoculum concentrations of each isolate were inactivated by heat or UV light before infection. Levels of neutrophil (A) and monocyte (B) transendothelial migration were determined following 24 h of incubation (see Materials and Methods). Bars indicate means ± standard errors of the means of four separate experiments. In each experiment, triplicate wells were assayed separately for each experimental variable. Data have been normalized to mock-infected levels of neutrophil and monocyte migration (5.9 ± 0.6 and 2.9 ± 0.4 cells/HPF, respectively). Levels from uninfected cells were 4.9 ± 0.7 cells/HPF for neutrophils and 2.6 ± 0.3 cells/HPF for monocytes. The response to HUVEC treated with 500 U of TNF-α per ml, as a positive control, increased to 34.3 ± 5.2 cells/HPF for neutrophils and 10.6 ± 0.5 cells/HPF for monocytes. ∗∗∗, P < 0.001.
As depicted in Fig. 1B, only isolates TW-183 and T-2634 stimulated significant levels of monocyte migration compared to mock-infected controls. This response was approximately fourfold higher than for the mock-infected controls (P < 0.001) and was significantly lower after heat and UV light inactivation (P < 0.05 compared to viable bacteria). The remaining four isolates (A-03 LP, PS-32 LP, BR-393 LP, and BAL-16) induced only a moderate increase in monocyte transendothelial migration above the level for mock-infected cells which was not statistically significant (P > 0.05).
Effects of passage history on the stimulation of neutrophil and monocyte transendothelial migration by C. pneumoniae.
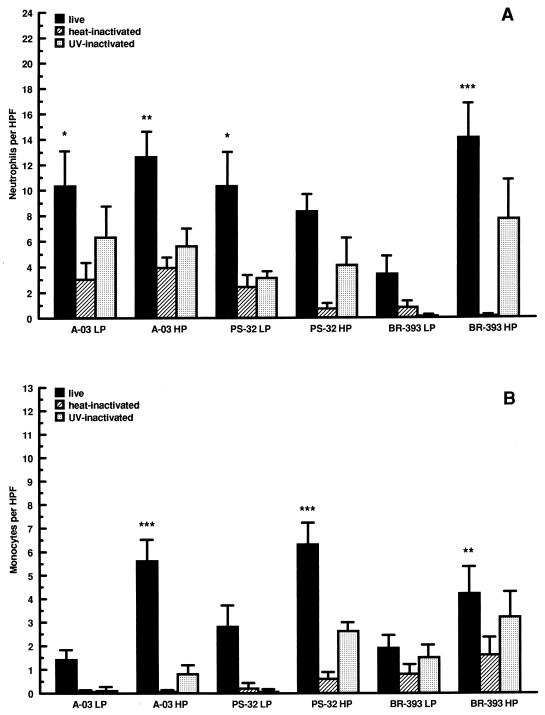
To investigate whether the number of passages in HEp-2 cell cultures influenced the ability of C. pneumoniae to stimulate leukocyte transendothelial migration, levels of neutrophil and monocyte migration were compared among LP and HP isolates A-03, PS-32, and BR-393. As shown in Fig. 2A, HP isolates A-03 and BR-393 induced higher levels of neutrophil migration than did LP isolates. For BR-393, the difference in levels of neutrophil migration stimulated by LP and HP stocks was approximately threefold and found to be statistically significant (P < 0.001). In contrast, serial passage of PS-32 in HEp-2 cells did not result in the promotion of higher levels of neutrophil migration compared to the LP isolate. Following heat and UV light treatment, stimulation of neutrophil transendothelial migration decreased by averages of 60 and 40%, respectively, compared to live organisms for LP and HP A-03, PS-32, and BR-393.
FIG. 2.
Effects of passage history on the stimulation of neutrophil and monocyte transendothelial migration by C. pneumoniae. HUVEC monolayers cultured in transwells were inoculated with LP or HP C. pneumoniae A-03, PS-32, or BR-393 at a multiplicity of infection of 1:1. Equivalent inoculum concentrations of each isolate were inactivated by heat or UV light before infection. LP isolates were passaged <15 times and HP isolates were passaged >40 times in HEp-2 cell cultures prior infection of HUVEC. Levels of neutrophil (A) and monocyte (B) transendothelial migration were determined following 24 h of incubation (see Materials and Methods). Bars indicate means ± standard errors of the means of three separate experiments. In each experiment, triplicate wells were assayed separately for each experimental variable. Data have been normalized to mock-infected levels of neutrophil and monocyte migration (3.9 ± 0.7 and 3.3 ± 0.8 cells/HPF, respectively). Levels from uninfected cells were 3.9 ± 1.1 cells/HPF for neutrophils and 3.5 ± 0.9 cells/HPF for monocytes. The response to HUVEC treated with 500 U of TNF-α per ml, as a positive control, increased to 20.6 ± 4.9 cells/HPF for neutrophils and 14.7 ± 1.6 cells/HPF for monocytes. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
As depicted in Fig. 2B, serial passage of LP A-03, PS-32, and BR-393 in culture resulted in significant increases of monocyte transendothelial migration compared to mock-infected controls. These increases were approximately threefold for HP A-03 and PS-32 (P < 0.001) and twofold for HP BR-393 (P < 0.05). A significant difference in the levels of monocyte migration between LP and HP isolates was observed only for A-03 (P < 0.001). Compared to live bacteria, heat inactivation inhibited the stimulation of monocyte migration by approximately 90% for LP and HP isolates. Similar results were observed for UV-inactivated A-03, while an average decrease of 75% was obtained with UV-inactivated PS-32 and BR-393.
Effects of passage history on the induction of chemokines by C. pneumoniae in HUVEC.
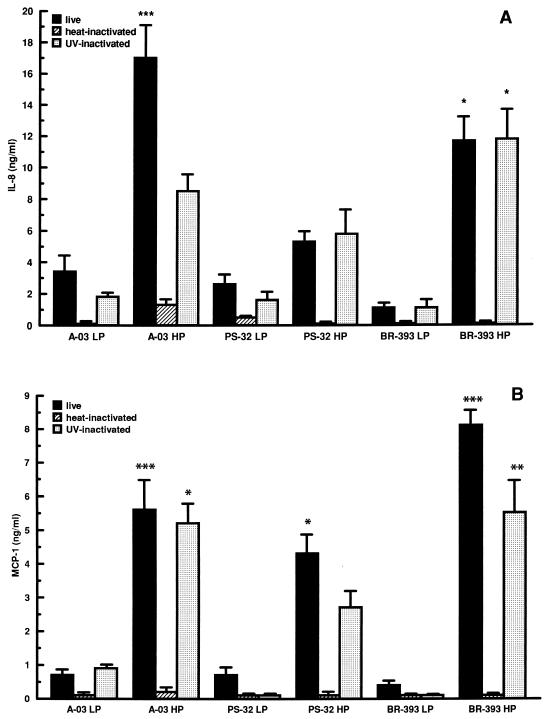
To investigate whether differences in the extent of neutrophil and monocyte migration stimulated by LP and HP C. pneumoniae isolates A-03, PS-32, and BR-393 correlated with differences in chemokine induction, we determined the levels of IL-8 and MCP-1 in infected HUVEC. As depicted in Fig. 3, serial passage of A-03, PS-32, and BR-393 resulted in higher levels of IL-8 and MCP-1 stimulation compared to LP isolates. Levels of IL-8 stimulated by LP and HP isolates differed significantly, fivefold for A-03 (P < 0.001) and ninefold for BR-393 (P < 0.05) (Fig. 3A). Differences in MCP-1 levels between LP and HP isolates were approximately 6-fold for A-03 (P < 0.001), 5-fold for PS-32 (P < 0.05), and 13-fold for BR-393 (P < 0.001) (Fig. 3B). Heat inactivation of all C. pneumoniae isolates examined abrogated the induction of IL-8 and MCP-1. Following UV inactivation, the stimulation of IL-8 by LP and HP C. pneumoniae was reduced by approximately 50% for A-03 and remained virtually unchanged for PS-32 and BR-393 compared to viable bacteria. Secretion of MCP-1 in response to PS-32 and BR-393 decreased by an average of 60% for both LP and HP isolates with UV inactivation, while no effect was observed for A-03.
FIG. 3.
Effects of passage history on the induction of IL-8 and MCP-1 by C. pneumoniae in HUVEC. HUVEC monolayers were inoculated with LP or HP C. pneumoniae A-03, PS-32, or BR-393 at a multiplicity of infection of 1:1. Equivalent inoculum concentrations of each isolate were inactivated by heat or UV light before infection. LP isolates were passaged <15 times and HP isolates were passaged >40 times in HEp-2 cell cultures prior to infection. Levels of IL-8 (A) and MCP-1 (B) from culture supernatants were measured following 24 h of incubation by ELISA. Bars indicate means ± standard errors of the means of three separate experiments. In each experiment, duplicate wells were assayed separately for each experimental variable. Data have been normalized to mock-infected levels of IL-8 (0.2 ± 0.02 ng/ml) and MCP-1 (0.2 ± 0.04 ng/ml). Concentrations of IL-8 from uninfected HUVEC were 0.15 ± 0.03 ng/ml, while treatment of cells with 500 U of human recombinant TNF-α per ml, used as a positive control, increased IL-8 production to 11.2 ± 0.7 ng/ml. Levels of MCP-1 from uninfected cells were 0.12 ± 0.04 ng/ml, while TNF-α-treated HUVEC secreted 12.4 ± 1.1 ng of MCP-1 per ml.
Role of IL-8 and MCP-1 on the stimulation of neutrophil and monocyte transendothelial migration by C. pneumoniae.
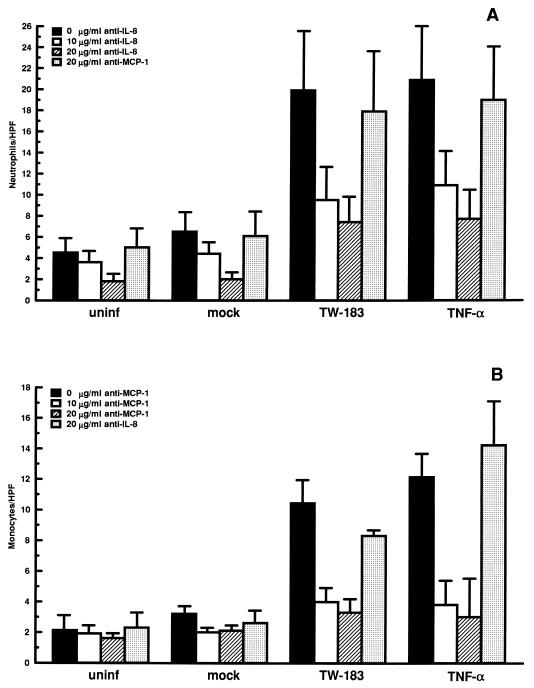
To examine whether IL-8 and MCP-1 were the major chemotactic factors involved in the stimulation of neutrophil and monocyte transendothelial migration by C. pneumoniae, blocking experiments were performed with anti-IL-8 and anti-MCP-1 antibodies. As shown in Fig. 4A, the addition of increasing concentrations of anti-IL-8 to infected HUVEC resulted in a dose-dependent inhibition of neutrophil transendothelial migration, with a significant decrease seen with 20 μg of antibody per ml compared to untreated cells (P < 0.05). Similarly, significant decreases in monocyte migration were observed following anti-MCP-1 treatment of C. pneumoniae-infected HUVEC at both 10 and 20 μg of antibody per ml (P < 0.05) (Fig. 4B). To ensure the specificity of the monoclonal antibodies, anti-MCP-1 and anti-IL-8 at 20 μg per ml were tested for the ability to inhibit neutrophil and monocyte transendothelial migration, respectively, during the blocking experiments. As shown in Fig. 4, antibodies to MCP-1 and/or IL-8 did not cause significant reductions in neutrophil or monocyte migration compared to C. pneumoniae-infected cells or TNF-α-treated cells that received no antibody.
FIG. 4.
Role of IL-8 and MCP-1 in the stimulation of neutrophil and monocyte transendothelial migration by C. pneumoniae. HUVEC monolayers cultured in transwells were inoculated with live C. pneumoniae TW-183 at a multiplicity of infection of 1:1. Mock-infected cells were treated with a suspension of lysed HEp-2 cells. Following 24 h of incubation, cells were preincubated with 10 and 20 μg of anti-IL-8 or anti-MCP-1 monoclonal antibodies per ml for 1 h before the migration assays. Transmigration of neutrophils (A) or monocytes (B) was allowed as described in the text, with the antibodies present throughout the experiments. To ensure the specificity of the monoclonal antibodies, anti-MCP-1 and anti-IL-8 at 20 μg/ml were tested for the ability to inhibit neutrophil (A) and monocyte (B) transendothelial migration, respectively. Bars indicate the means ± standard errors of the means of three separate experiments. In each experiment, triplicate wells were assayed separately for each experimental variable.
Levels of neutrophil and monocyte migration across HUVEC infected with C. trachomatis compared to C. pneumoniae.
To investigate whether the stimulation of neutrophil and monocyte transendothelial migration was a feature of other Chlamydia species, we performed experiments with C. trachomatis L2 and compared the results to those for C. pneumoniae TW-183 (Table 1). Significant differences in the levels of neutrophil and monocyte transendothelial migration stimulated by C. pneumoniae TW-183 compared to C. trachomatis L2 were observed (P < 0.001). Contrary to results for C. pneumoniae TW-183, we observed only a slight increase in neutrophil migration above the level for mock-infected controls when HUVEC were infected with C. trachomatis L2. As opposed to this low neutrophil migration response, levels of monocyte transendothelial migration were significantly above the mock-infected cell level with C. trachomatis L2 (P < 0.05) and decreased by approximately 30% following UV and heat inactivation.
TABLE 1.
Transendothelial migration of neutrophils and monocytes in response to infection with C. trachomatis and C. pneumoniae
| Treatmenta | Level of transendothelial migration (cells/HPF) ofb:
|
|
|---|---|---|
| Neutrophils | Monocytes | |
| Mock | 5.9 ± 0.6 | 2.9 ± 0.4 |
| C. trachomatis L2 | ||
| Live | 9.4 ± 1.7 | 7.0 ± 1.6* |
| Heat inactivated | 8.0 ± 1.6 | 4.9 ± 0.9 |
| UV inactivated | 12.0 ± 1.3 | 4.8 ± 0.6 |
| C. pneumoniae TW-183 | ||
| Live | 22.8 ± 3.2** | 12.9 ± 1.2*** |
| Heat inactivated | 10.5 ± 1.1 | 4.7 ± 0.6 |
| UV inactivated | 16.0 ± 1.1** | 6.2 ± 1.1 |
HUVEC monolayers cultured in transwells were inoculated with C. trachomatis L2 or C. pneumoniae TW-183 at a multiplicity of infection of 1:1. Equivalent inoculum concentrations of each strain were inactivated by heat or UV light before infection (see Materials and Methods). Mock-infected cells were treated with a suspension of lysed HEp-2 cells.
Levels of neutrophil and monocyte transendothelial migration were determined following 24 h of incubation as described in Materials and Methods. Data represent means ± standard errors of the means of three separate experiments. In each experiment, triplicate wells were assayed separately for each condition for neutrophil and monocyte migration. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Secretion of IL-8 and MCP-1 by HUVEC infected with C. trachomatis compared to C. pneumoniae.
To examine whether the differences in the extent of neutrophil and monocyte transendothelial migration between C. trachomatis L2 and C. pneumoniae TW-183 correlated with differences in the induction of IL-8 and MCP-1, we measured levels of these chemokines in infected HUVEC after 24 h of incubation. As depicted in Table 2, contrary to C. pneumoniae TW-183, C. trachomatis L2 failed to stimulate IL-8 and MCP-1 production from HUVEC. In addition to L2, C. trachomatis serovars A and E did not induce secretion of these proteins from infected cells.
TABLE 2.
Secretion of IL-8 and MCP-1 by HUVEC infected with C. trachomatis and C. pneumoniae
| Treatmenta | Concn (ng/ml)b
|
|
|---|---|---|
| IL-8 | MCP-1 | |
| Mock | 0.32 ± 0.11 | 0.3 ± 0.08 |
| C. trachomatis | ||
| Serovar L2 | 0.16 ± 0.05 | 0.3 ± 0.17 |
| Serovar A | 0.13 ± 0.02 | 0.28 ± 0.05 |
| Serovar E | 0.08 ± 0.04 | 0.28 ± 0.09 |
| C. pneumoniae TW-183 | 20.4 ± 0.9* | 5.0 ± 1.3* |
HUVEC monolayers were inoculated with C. trachomatis serovars L2, A, and E or C. pneumoniae TW-183 at a multiplicity of infection of 1:1. Mock-infected cells were treated with a suspension of lysed HEp-2 cells.
Levels of IL-8 and MCP-1 from culture supernatants were measured following 24 h of incubation by ELISA. Data indicate the means ± standard errors of the means of three separate experiments. In each experiment, duplicate wells were assayed separately for each condition for IL-8 and MCP-1 production. *, P < 0.001.
Comparative replication of C. pneumoniae and C. trachomatis in HUVEC.
HUVEC supported replication of all isolates of C. pneumoniae examined (both LP and HP), as determined at 48 h postinfection. Growth titers ranged from 6.3 × 103 to 11.5 × 103 IFU/ml for the transendothelial migration experiments and 6.5 × 103 to 9.8 × 103 IFU/ml for the chemokine experiments. Average growth titers of C. trachomatis L2, A, and E were significantly higher than C. pneumoniae titers (in migration experiments, 4.6 × 106 IFU/ml [P < 0.001]; in chemokine experiments, 1.7 × 106 IFU/ml [P < 0.001]).
Lack of a cytotoxic effect following Chlamydia infection in HUVEC.
C. pneumoniae and C. trachomatis had similar effects on viability of HUVEC after 24 h of infection (>80%), as determined by trypan blue dye exclusion analysis. To further examine whether infection was causing a cytotoxic effect in HUVEC, costimulation experiments were performed with Chlamydia species and TNF-α. HUVEC which were initially infected with C. pneumoniae or C. trachomatis for 24 h were equally capable of responding to exogenous TNF-α (500 U/ml for 24 h) and secreted significant levels of IL-8 and MCP-1 compared to controls. Concentrations of IL-8 and MCP-1 from costimulated cells ranged from 6 to 10 ng/ml and 4 to 7 ng/ml, respectively.
DISCUSSION
A number of different methods have been developed to study the interactions of leukocytes and the endothelium, with the purpose of simulating the luminal face of a venular vessel wall during an inflammatory process. In the present study, HUVEC were cultured in Transwell-Clear polycarbonate microporous cell culture inserts, which provide independent access to both apical and basolateral sides of the cell monolayer. Using this system, we demonstrated that C. pneumoniae promotes the transendothelial migration of neutrophils and monocytes. In addition, heterogeneity of stimulatory activities was observed among different isolates of this organism, and this correlated with differences in the number of passages in HEp-2 cell culture. Overall, HP isolates TW-183 and T-2634 stimulated higher levels of neutrophil and monocyte transendothelial migration compared to LP isolates A-03, PS-32, and BR-393. Serial passage of the LP isolates in HEp-2 cell cultures prior infection of HUVEC generally resulted in higher levels of stimulation of neutrophil and monocyte transendothelial migration. This event correlated with the induction of the neutrophil- and monocyte-specific chemokines IL-8 and MCP-1. As in the leukocyte migration experiments, the extent of IL-8 and MCP-1 secretion elicited by C. pneumoniae A-03, PS-32, and BR-393 was dependent on the number of passages in culture before infection of endothelial cells. All C. pneumoniae isolates examined in this study exhibited similar replication patterns within HUVEC, and it remains unclear whether the divergence observed among LP and HP isolates reflects intrinsic differences. Possibly, adaptation to HEp-2 cell cultures of recently isolated C. pneumoniae strains results in the acquisition or loss of specific features that may be important in the activation of endothelial cells in vitro.
Infection of HUVEC with C. trachomatis L2 caused only a slight increase in neutrophil transendothelial migration above the level for mock-infected controls. These results were consistent with IL-8 measurements since this serovar, in addition to C. trachomatis serovars A and E, failed to stimulate production of this chemokine from infected HUVEC. Substantial levels of monocyte migration were observed in response to L2 compared to mock-infected controls, although not at the same extent as C. pneumoniae, despite a lack of measurable MCP-1 stimulation. These findings may reflect differences in sensitivities between the MCP-1 and monocyte migration assays. Alternatively, chemotactic factors other than MCP-1 may be involved in the monocyte migration response to C. trachomatis L2.
The absence of IL-8 and MCP-1 stimulation by C. trachomatis L2, A, and E was not a consequence of lack of replication in HUVEC, since growth titers that were significantly higher than C. pneumoniae-infected cells were recovered after 48 h of incubation. In addition, significant differences in cell viability between C. pneumoniae and C. trachomatis were not observed, as determined by trypan blue dye exclusion analysis or the ability of infected HUVEC to respond to exogenous TNF-α. These findings suggest that between these two Chlamydia species, the upregulation of IL-8 and MCP-1 in endothelial cells may be unique to C. pneumoniae. The possibility exists, however, that the observed differences among species presented herein are due to the cell type used, since previous work has shown induction of IL-8 by C. trachomatis L2 in epithelial (30) and monocytic (1) cells.
Results from the blocking antibody experiments suggest that the induction of IL-8 and MCP-1 by C. pneumoniae may be the major stimulus responsible for the promotion of neutrophil and monocyte transendothelial migration. Effects of heat and UV inactivation show that viability of this organism is required to stimulate an optimal leukocyte transmigration response. A trend for UV light treatment to reduce the migration of neutrophils and monocytes to a lower extent compared to heat treatment correlated with the chemokine experiments, where UV-inactivated organisms retained a partial ability to stimulate IL-8 and MCP-1 production. Lack of activation of these chemokines by heat-inactivated bacteria has been shown previously (26) and suggests that chlamydial lipopolysaccharide may not be essential in eliciting this response from HUVEC. Alternatively, activation of IL-8 and MCP-1 from these cells may involve a surface-associated molecule in C. pneumoniae, such as an outer membrane protein, which is altered by heat but not by UV light treatment.
There is limited information regarding the pathogenesis of C. pneumoniae infections in humans, and most data have been derived from animal models. Lung pathology following infection in mice is characterized by inflammation with polymorphonuclear leukocyte infiltration in the early stage and mononuclear cell infiltration in the late stage (37). Dissemination of C. pneumoniae to the spleen (25, 38) and aorta (25) has been shown following intranasal inoculation of mice, and recent evidence has demonstrated that this organism is capable of inducing arterial inflammatory lesions resembling atherosclerosis in the aortas of infected rabbits (2, 20). The mechanisms that elicit these responses have not been studied in detail. In addition to eliciting the expression of chemokines and adhesion molecules in vitro (17, 26), previous work has shown that C. pneumoniae is capable of activating human endothelial cells to a proinflammatory phenotype by enhancing the production of tissue factor (3). Furthermore, C. pneumoniae is a potent inducer of cytokines such as TNF-α, IL-1, and IL-6 in human monocytic cells (12). These events presumably would play important roles in the immunological response to infection, but they may also promote deleterious effects and contribute to tissue damage.
In summary, C. pneumoniae causes activation of chemokines in human endothelial cells and promotes the recruitment of leukocytes in vitro. These events may correlate with the pathology described in vivo in animal models of respiratory infection and atherosclerosis. Differences in passage history among isolates of this organism may play a significant role in the divergence of stimulatory activities. For this reason, special consideration should be given to the number of passages in culture of recently isolated C. pneumoniae strains prior to their examination in biological systems.
ACKNOWLEDGMENT
We thank Terri Manning, Division of Nephrology, University of Louisville, for technical assistance in the isolation of neutrophils and monocytes from venous blood of healthy volunteers.
REFERENCES
- 1.Bianchi A, Dosquet C, Henry S, Couderc M-C, Ferchal F, Scieux C. Chlamydia trachomatis growth stimulates interleukin-8 production by human monocytic U-937 cells. Infect Immun. 1997;65:2434–2436. doi: 10.1128/iai.65.6.2434-2436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong I W, Chiu B, Viira E, Fong M W, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fryer R H, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Investig Med. 1994;45:168–174. [PubMed] [Google Scholar]
- 4.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godzik K L, O’Brien E R, Wang S K, Kuo C-C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grayston J T, Wang S P, Kuo C-C, Campbell L A. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur J Clin Microbiol Infect Dis. 1989;8:191–202. doi: 10.1007/BF01965260. [DOI] [PubMed] [Google Scholar]
- 7.Grayston J T. Chlamydia pneumoniae strain TWAR pneumonia. Annu Rev Med. 1992;43:317–323. doi: 10.1146/annurev.me.43.020192.001533. [DOI] [PubMed] [Google Scholar]
- 8.Hahn D L, Dodge R W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 9.Hahn D L, Bukstein D, Luskin A, Zeitz H. Evidence for Chlamydia pneumoniae infection in steroid-dependent asthma. Ann Allergy Asthma Immunol. 1998;80:45–49. doi: 10.1016/S1081-1206(10)62938-9. [DOI] [PubMed] [Google Scholar]
- 10.Hammerschlag M R, Chirgwin K, Roblin P M, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–182. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 11.Haslett C, Guthrie L A, Kopaniak M M, Johnston J, Henson P M. Modulation of multiple neutrophil functions by preparative methods of trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemman M, Susa M, Simnacher V, Marre R, Essig A. Growth of Chlamydia pneumoniae induces cytokine production and expression of CD14 in a human monocytic cell line. Infect Immun. 1996;64:4872–4875. doi: 10.1128/iai.64.11.4872-4875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennig B, Shasby D M, Fulton A B, Spector A A. Exposure to free fatty acids increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4:489–497. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- 14.Hennig B, Chow C K. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radical Biol Med. 1988;4:99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 15.Jackson L A, Campbell L A, Kuo C-C, Rodriguez D I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 16.Kaukoranta-Tolvanen S S, Laitinen K, Saikku P, Leinonen M. Chlamydia pneumoniae multiplies in human endothelial cells in vitro. Microb Pathog. 1994;16:313–319. doi: 10.1006/mpat.1994.1032. [DOI] [PubMed] [Google Scholar]
- 17.Kaukoranta-Tolvanen S S E, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 18.Kuo C-C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 19.Kuo C-C, Gown A M, Benditt E P, Grayston J T. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen K, Laurila A, Pyhala L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen C G, Anderson A O, Apella E, Oppenheim J J, Matsushima K. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-α, LPS, and IL-1β. Science. 1989;243:1464–1466. [Google Scholar]
- 22.Linnanmäki E, Leinonen M, Mattila K, Nieminen M S, Valtonen V, Saikku P. Chlamydia pneumoniae-specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 23.Maass M, Bartels C, Engel P M, Mamat U, Sievers H-H. Endovascular presence of viable Chlamydia pneumoniae is a common phenomenon in coronary artery disease. J Am Coll Cardiol. 1998;31:827–832. doi: 10.1016/s0735-1097(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 24.Melnick S L, Shahar E, Folsom A R, Grayston J T, Sorlie P D, Wang S-P, Szklo M. Past infection by Chlamydia pneumoniae strain TWAR and asymptomatic carotid atherosclerosis. Am J Med. 1993;95:499–504. doi: 10.1016/0002-9343(93)90332-j. [DOI] [PubMed] [Google Scholar]
- 25.Moazed T C, Kuo C-C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 26.Molestina R E, Dean D, Miller R D, Ramirez J A, Summersgill J T. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect Immun. 1998;66:1370–1376. doi: 10.1128/iai.66.4.1370-1376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelken N A, Coughlin S R, Gordon D, Wilcox J N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Investig. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puolakkainen M, Kuo C-C, Shor A, Wang S-P, Grayston J T, Campbell L A. Serological response to Chlamydia pneumoniae in adults with coronary arterial fatty streaks and fibrolipid plaques. J Clin Microbiol. 1993;31:2212–2214. doi: 10.1128/jcm.31.8.2212-2214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez J A, Ahkee S, Summersgill J T, Ganzel B L, Ogden L L, Quinn T C, Gaydos C A, Bobo L L, Hammerschlag M R, Roblin P M, LeBar W, Grayston J T, Kuo C-C, Campbell L A, Patton D L, Dean D, Schachter J. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y-X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 32.Saikku P, Mattila K, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz, C. J., A. J. Valente, E. A. Sprague, J. L. Kelley, and R. M. Nerem. The pathogenesis of atherosclerosis: an overview. Clin. Cardiol. 14:I-1–I-16. [DOI] [PubMed]
- 34.Shor A, Kuo C-C, Patton D L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. South Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 35.Smeets E F, von Asmuth E J U, van der Linden C J, Leeuwenberg J F M, Buurman W A. A comparison of substrates for human umbilical vein cell culture. Biotech Histochem. 1992;67:241–250. doi: 10.3109/10520299209110072. [DOI] [PubMed] [Google Scholar]
- 36.Wang N, Tabas I, Winchester R, Ravalli S, Rabani L E, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;271:8837–8842. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z-P, Kuo C-C, Grayston J T. A mouse model of Chlamydia pneumoniae strain TWAR pneumonitis. Infect Immun. 1993;61:2037–2040. doi: 10.1128/iai.61.5.2037-2040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z-P, Kuo C-C, Grayston J T. Systemic dissemination of Chlamydia pneumoniae following intranasal inoculation in mice. J Infect Dis. 1995;171:736–738. doi: 10.1093/infdis/171.3.736. [DOI] [PubMed] [Google Scholar]
- 39.Yue T-L, Wang X, Sung C-P, Olson B, McKenna P J, Gu J-L, Feuerstein G Z. Interleukin-8: a mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]