Abstract
Objective
Negative beliefs about medication and vaccine side-effects can spread rapidly through social communication. This has been recently documented with the potential side-effects from the COVID-19 vaccines. We tested if pre-vaccination social communications about side-effects from personal acquaintances, news reports, and social media predict post-vaccination side-effect experiences. Further, as previous research suggests that side-effects can be exacerbated by negative expectations, we assessed if personal expectations mediate the relationships between social communication and side-effect experience.
Method
In a prospective longitudinal survey (N = 551), COVID-19 vaccine side-effect information from three sources—social media posts, news reports, and first-hand accounts from personal acquaintances—as well as side-effect expectations, were self-reported pre-vaccination. Vaccination side-effect experience was assessed post-vaccination.
Results
In multivariate regression analyses, the number of pre-vaccination social media post views (β = 0.17) and impressions of severity conveyed from personal acquaintances (β = 0.42) significantly predicted an increase in pre-vaccination side-effect expectations, and the same variables (βs = 0.11, 0.14, respectively) predicted post-vaccination side-effect experiences. Moreover, pre-vaccination side-effect expectations mediated the relationship between both sources of social communication and experienced side-effects from a COVID-19 vaccination.
Conclusions
This study identifies links between personal acquaintance and social media communications and vaccine side-effect experiences and provides evidence that pre-vaccination expectations account for these relationships. The results suggest that modifying side-effect expectations through these channels may change the side-effects following a COVID-19 vaccination as well as other publicly discussed vaccinations and medications.
Keywords: COVID-19, Vaccine, Media, Side-effects, Nocebo, Expectations
1. Introduction
Vaccines are scientifically derived preparations that stimulate the body's immune response against diseases. Many vaccines have been found to be effective in reducing death, hospitalization, and other harmful consequences from diseases [31]. Vaccine hesitancy, however, remains a persistent barrier to many immunization efforts. This has been observed recently at a global level with vaccinations against COVID-19. One of the most frequent reasons reported for COVID-19 vaccine hesitancy is fears about vaccine side-effects [37]. However, some of this fear might be misplaced, as commonly reported side-effects of COVID-19 vaccination (e.g., headache, fatigue) may not be due solely to the pharmacological properties of the vaccines. An analysis of 12 COVID-19 vaccine clinical trials found that 35% of those in a placebo condition reported at least one side-effect [16], indicating that factors other than the pharmacological properties of the vaccines can contribute to the experience of side-effects. Negative outcome expectations may be exacerbating side-effects [33]. This explanation is supported by evidence from a longitudinal study which found that individuals expecting more side-effects pre-vaccination reported more side-effects post-COVID-19 vaccination [14].
The notion that COVID-19 vaccine side-effects are worsened by psychological factors is consistent with clinical and experimental research on the nocebo effect, in which treatment side-effects are amplified by suggestion and contextual cues [[6], [11]]. For example, providing individuals with verbal warnings about potential side-effects increases side-effect symptoms, including headache, nausea, pain, fatigue, dizziness, appetite changes, and itch [10,15,25,27,32,38,39]. It is believed that nocebo effects are caused by negative expectations resulting from social communication as well as experiential and observational learning [30]. Nocebo effects have been observed in a wide variety of samples and contexts, including side-effects following an influenza vaccination, nausea and fatigue in chemotherapy patients, the worsening of motor performance in patients with Parkinson's disease, unpleasant symptoms from wind turbines, and heightened pain in neuropathic pain patients [6,7,10, 30,42, 45]. Nocebo effects are observed in self-reported outcomes as well as on physiological and neurobiological indices, including changes in activity in cortical and subcortical regions of the brain [36,41].
As expectations of side-effects contribute to vaccine hesitancy, and expectations can increase side-effects via the nocebo effect, strategies to reduce side-effect expectations may improve vaccine responses as well as vaccination uptake. To intervene in this pathway, it is usefulto uncover the sources of the side-effect expectations. In terms of the COVID-19 vaccines, prior research indicates that information about side-effects has spread rapidly through individuals' personal acquaintances, news stories, and social media [23,44]. Further, other research shows that information from social communication can increase nocebo side-effects. For example, side-effects can be induced through verbal communication and social observation in face-to-face interactions [6]. Laboratory and naturalistic studies find that news stories also increase side-effect reports [12,21,43]. Although few studies have tested the influence of social media messages on nocebo effects, many individuals use social media to discuss and learn about vaccine side-effects, or are otherwise exposed to this type of information when using the platform, which is also likely to result in the development of negative side-effect expectations [4,22,35]. Clarifying the links from these different social communication sources and vaccine side-effects is especially critical because many individuals report taking a “wait and see” approach, observing how vaccines affect others before receiving one themselves [17].
Given the amount of true and misleading COVID-19 vaccine information shared across media and word-of-mouth [5], here we tested if pre-vaccination side-effect information received from personal acquaintances, news stories, and social media posts predict subsequent COVID-19 vaccine side-effect expectations and experiences. We separately asked participants about the amount of exposure (e.g., number of social media posts viewed) and the resulting impressions of aversiveness provided by the exposure sources (e.g., negative impressions formed from social media posts). We measured amount of exposure separately from negative impressions, as individuals are passively exposed to much information in their daily lives. For example, almost 4 in 5 social media users report being indirectly exposed to news-related content while using the platform for other purposes [26]. Although this information may not be consciously attended to, exposure may influence expectations outside of awareness [8]. Incidental exposure to adverse side-effects may be particularly likely, as research finds an attentional bias to negative information [46]. Consequently, we hypothesized that pre-vaccination exposure to and negative impressions from the social sources of information would exacerbate COVID-19 post-vaccination side-effect reports.
It was also hypothesized that pre-vaccination side-effect expectations mediate the link between social communication and vaccine side-effects. Although different explanations have been proposed for nocebo side-effects, a leading account is that cues and communications generate side-effect expectations which, in turn, increase the experience of adverse symptoms [6]. There is limited data supporting expectations as a mediator and existing data arises primarily from laboratory studies in which expectations were induced by direct verbal suggestion from an experimenter [10]. The present study adds uniquely to this literature by testing the mediating role of expectations between three different information sources (i.e., friends, news reports, social media) and vaccine side-effect experience outside of the laboratory.
2. Materials and methods
2.1. Participants and design
Hypotheses were tested using a preregistered (https://osf.io/r6utm) and IRB approved (University of Toledo IRB protocol: 300993) prospective longitudinal data set [14]. Sample size was determined a priori using the Pwr2Ppl package for R [1] with a small to medium effect size of r = 0.2. To obtain power of 0.95 with an α = 0.05, it was determined that 500 participants were needed. A diverse sample of community participants was recruited using the Prolific recruitment platform [29]. Participants were compensated at a rate of $6 per hour for their participation, in line with Prolific's policies.
The present data was collected early in the distribution of the first round of COVID-19 vaccines in the U.S, a time in which there was high uncertainty about vaccine side-effects. All participants completed both a pre-vaccination and a post-vaccination survey. The pre-vaccination survey was open from April 16–28, 2021, and the post-vaccination survey was open from May 21 to July 19, 2021. By constraining the time frame for the completion of Survey 2, the design reduces the potential for memory distortions in side-effect reports following vaccination. The median number of days between Survey 1 and Survey 2 was 39 days. Eligibility criteria for Survey 1 included ≥18 years old, residing in the U.S., having not yet received any COVID-19 vaccination, and planning to receive – or being undecided about receiving – a future COVID-19 vaccination. Informed consent was obtained online by all participants at the start of the survey. Data was collected from 1579 individuals during Survey 1. Eligibility for Survey 2 included completion of Survey 1 and becoming fully vaccinated (i.e., receiving two doses of the two dose Pfizer or Moderna vaccines, or one dose of the single dose Janssen/Johnson & Johnson vaccine) since Survey 1. Proof of vaccination status was verified through information from participants' vaccination cards. In total, 551 participants completed Survey 2. While information regarding vaccination status was not available for those who did not respond to Survey 2, 585 of those who completed Survey 1 indicated having received at least one COVID-19 vaccine dose in the Prolific recruitment system by the close of Survey 2, which gives an approximate 94% retention rate.
2.2. Materials
The pre-vaccination survey assessed self-reported exposures to information regarding COVID-19 vaccine side-effects from personal acquaintances, news stories, and social media. Participants also reported their resulting impressions about side-effects from these sources and their expectations for experiencing side-effects from a COVID-19 vaccine. A post-vaccination survey assessed experience of the same side-effects following the vaccination. All measures are presented in Supplemental Materials. Data and analysis code is available at: https://osf.io/r6utm.
2.2.1. Side-effect expectancies
Side-effect expectancies were measured with a 45-item version of the General Assessment of Expected Side-Effects Scale (GASE-expect; [40,47]). As in prior research (e.g., [28,40]), this scale was expanded from the original 36-item version. Here, nine items relevant to COVID-19 vaccines were added (e.g., injection site pain). Response options were not expected (0), expect mild (1), expect moderate (2), and expect severe (3). As in prior studies, a total side-effect expectation intensity scale (M = 63.43; SD = 15.17) was created by summing responses [28].
2.2.2. Side-effect experiences
Side-effect experiences were measured with the same 45 side-effect items used to assess expectations. To measure side-effect experience, the response options were changed to the standard options of the General Assessment of Side-Effects Scale [34]: not experienced (0), mild (1), moderate (2), and severe (3). As in prior research with the GASE (e.g., [9,13,24,34]), a side-effect intensity scale (M = 57.64; SD = 10.94) was created by summing item responses. As two of the COVID-19 vaccines that were available to the U.S. participants required two doses, whereas one vaccine required a single dose, in Survey 2 participants were instructed to report all side-effects they experienced from their entire vaccination experience (one or two doses). This strategy allowed the collection of all pre- and post-vaccination responses in two survey waves.
2.2.3. Social information sources
In the pre-vaccination survey, participants indicated (yes/no) if they had obtained information about COVID-19 vaccine side-effects from each of the three information sources considered in this study: acquaintance reports, news reports, and social media posts. If participants indicated “yes” for a source, they were then asked to estimate the number of information exposures 1 (1 to 5), 2 (6 to 10), 3 (11 to 15), 4 (16 to 20), and 5 (>20). Responses to these two items were used to measure the amount of exposure to each information source. In the analyses examining the amount of vaccine information exposure, participants indicating no exposure to a given source were assigned the value of “0”. To measure impressions of the side-effects from each of the three sources, participants indicating source exposure provided an impression response on a 1 to 4 scale (e.g., I have heard the vaccines have: 1, no side-effects to 4, severe side-effects).
2.3. Statistical analyses
Four multivariate regressions were conducted. In two, the number of exposures to each of the three information sources served as predictors. In the first of these regressions, expected side effects served as the outcome variable, and in the second, experienced vaccine side-effects served as the outcome variable. Two other multivariate regressions were conducted with impressions from each of the three information sources serving as predictors. With the impression variables as predictors, one regression had expected side effects serve as the outcome variable and the other had experienced vaccine side-effects as the outcome variable. Finally, single mediational model analyses were conducted using PROCESS for SPSS [18] to determine if vaccine side-effect expectations mediated the relationship between social information sources and experienced side-effects. Mediational models included a single significant social communication source as the predictor variable, experienced vaccine side effects as the outcome variable, and expected side effects as the mediator. No other variables were entered into the models.
3. Results
Participants were diverse in terms of race, gender, education, income level, and geographic location in the U.S. Of the participants, 53% were women, M age = 32; SD age = 11, age range = 18–71, 69% White, 12% Hispanic, 50% obtained a bachelor's degree education or higher, 45% reported an income above $60,000, and they represented 48 of the U.S. states. In this sample, 56% received the Pfizer-BioNTech vaccine, 33% the Moderna vaccine, and 11% the Janssen/Johnson & Johnson vaccine. See Table 1 for additional demographic information.
Table 1.
Participant Characteristics.
| Characteristics | N = 551a | % |
|---|---|---|
| Age (M = 32; SD = 11; range = 18–71) | ||
| 18 to 24 | 160 | 29.1 |
| 25 to 31 | 158 | 28.8 |
| 32 to 38 | 112 | 20.4 |
| 39 to 45 | 50 | 9.2 |
| 46 to 52 | 32 | 5.8 |
| ≥53 | 37 | 6.7 |
| Sex | ||
| Female | 289 | 52.7 |
| Male | 244 | 44.4 |
| Non-binary | 11 | 2.0 |
| Other-identified | 5 | 0.9 |
| Race/Ethnicity | ||
| White | 380 | 69.0 |
| African American | 29 | 5.3 |
| Arab | 2 | 0.4 |
| Asian | 96 | 17.4 |
| American Indiana/Alaskan Native | 1 | 0.2 |
| Native Hawaiian/other Pacific Islander | 3 | 0.5 |
| More than one race | 31 | 5.6 |
| Hispanic/Latino | 66 | 12.0 |
| Education | ||
| Up to high school diploma | 65 | 11.8 |
| Some college | 154 | 28.1 |
| Associate degree | 57 | 10.4 |
| Bachelor degree | 219 | 39.9 |
| Master/professional/doctoral degree | 54 | 9.8 |
| Income | ||
| ≤$19,999 | 71 | 13.0 |
| $20,000 to $39,999 | 92 | 16.8 |
| $40,000 to $59,999 | 110 | 20.0 |
| $60,000 to $79,999 | 100 | 18.2 |
| $80,000 to $99,999 | 62 | 11.3 |
| $100,000 to $150,000 | 74 | 13.5 |
| ≥$150,000 | 39 | 7.1 |
| US states of participant residency | 48 | 96.0 |
| Most common side-effects expected | ||
| Headache | 457 | 82.9 |
| Fatigue | 442 | 80.4 |
| Pain at injection site | 432 | 78.4 |
| Most common side-effects reported | ||
| Pain at injection site | 448 | 81.3 |
| Fatigue | 400 | 72.6 |
| Headache | 334 | 60.6 |
| Vaccine Type | ||
| Pfizer-BioNTech | 311 | 56.4 |
| Moderna | 182 | 33.1 |
| Jansen/Johnson & Johnson | 58 | 10.5 |
| Contracted COVID-19 | ||
| No | 386 | 70.2 |
| Unsure | 57 | 10.4 |
| Yes (unconfirmed) | 64 | 11.6 |
| Yes (confirmed) | 43 | 7.8 |
Note. 3 participants declined to provide their race and income information, 2 declined to report age, gender, and education, and 1 declined to report COVID-19 infection history.
To test our hypotheses, four multivariate regressions were conducted (see Table 2 ). A first set tested if the number of exposures to each of the three information sources predicted (a) expected and (b) experienced vaccine side-effects. These analyses found the number of social media posts about COVID-19 vaccine side-effects viewed significantly predicted both pre-vaccination side-effect expectations, t(547) = 3.92, p < .001, β = 0.17, 95% CI[0.03, 0.09], and post-vaccination side-effect experiences, t(547) = 2.49, p = .013, β = 0.11, 95% CI[0.01, 0.05]. A second set of regressions tested if impressions from the three sources predicted (a) expected and (b) experienced vaccine side-effects. Both impressions from personal acquaintances, t(311) = 8.19, p < .001, β = 0.42, 95% CI[0.17, 0.19)] and news stories, t(311) = 3.01, p = .003, β = 0.17, 95% CI[0.03, 0.14], were significant predictors of pre-vaccination side-effect expectations, but only impressions from personal acquaintances predicted post-vaccination side-effects, t(311) = 2.40, p = .017, β = 0.14, 95% CI[0.03, 0.09].
Table 2.
Four regression models with exposure to and impressions from social sources as predictors of COVID-19 side-effect expectations and experiences.
| b | SE | 95 %CIs | β | t | p | |
|---|---|---|---|---|---|---|
| DV: Expected Side-Effects | ||||||
| Model 1: Exposure | ||||||
| Personal Contacts | 0.003 | 0.02 | (−0.04, 0.04) | 0.01 | 0.14 | 0.892 |
| News Reports | 0.004 | 0.01 | (−0.01, 0.02) | 0.04 | 0.81 | 0.419 |
| Social Media Posts | 0.06 | 0.02 | (0.03, 0.09) | 0.17 | 3.92 | < 0.001 |
| Model 2: Impressions | ||||||
| Personal Contacts | 0.22 | 0.03 | (0.17, 0.28) | 0.42 | 8.19 | < 0.001 |
| News Reports | 0.08 | 0.03 | (0.03, 0.14) | 0.17 | 3.01 | 0.003 |
| Social Media Posts | 0.04 | 0.03 | (−0.02, 0.10) | 0.07 | 1.30 | 0.195 |
| DV: Experienced Side-Effects | ||||||
| Model 3: Exposure | ||||||
| Personal Contacts | 0.001 | 0.01 | (−0.03, 0.03) | 0.01 | 0.10 | 0.917 |
| News Reports | −0.004 | 0.004 | (−0.01, 0.003) | −0.05 | −1.13 | 0.259 |
| Social Media Posts | 0.03 | 0.01 | (0.01, 0.05) | 0.11 | 2.49 | 0.013 |
| Model 4: Impressions | ||||||
| Personal Contacts | 0.06 | 0.02 | (0.01, 0.10) | 0.14 | 2.40 | 0.017 |
| News Reports | 0.003 | 0.02 | (−0.04, 0.05) | 0.01 | 0.11 | 0.914 |
| Social Media Posts | 0.01 | 0.03 | (−0.04, 0.06) | 0.03 | 0.42 | 0.672 |
Note. Exposure scores are participant estimates of the number of source information exposures, none = 0, 1 (1 to 5), 2 (6 to 10), 3 (11 to 15), 4 (16 to 20), and 5 (>20). Impressions scores are ratings from 1 (no side-effects) to 4 (severe side-effects) for each information source. Expected side-effect scores are a sum of 45-items on the pre-vaccination expect-GASE and experienced side-effects are the sum of 45-items on the post-vaccination GASE.
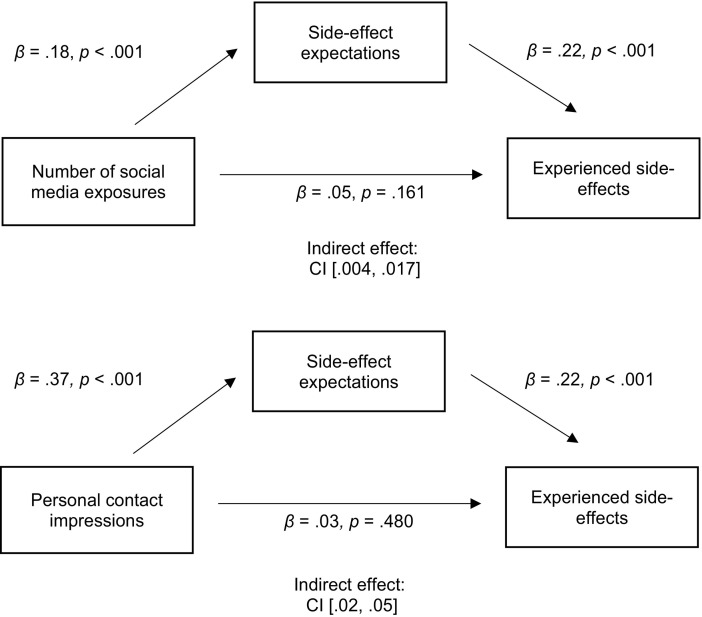
As shown in Fig. 1 , mediational analyses (PROCESS for SPSS; [18]) indicated that vaccine side-effect expectations mediated the relationship from social media exposure, (indirect effect: CI[0.004, 0.017]) and personal acquaintance impressions (indirect effect: CI[0.02, 0.05]) to experienced side-effects.
Fig. 1.
Side-effect expectations mediate the relationship between the number of social media exposures and impressions from personal contacts and experienced side-effects.
Note: The values in the model are standardized betas weights. The beta weights between the predictor and outcome variables in the diagrams are with the mediator in the models (the c’ path). The beta weights for the direct paths without the mediator variable in the models (the c path) are: β = 0.10, p = .020, for the number of social media exposures model, and β = 0.12, p = .007, for the personal contact impressions model.
Additional analyses are presented in Supplemental Materials. Specifically, the reported results remained the same when controlling for age, vaccine type, COVID-19 history, and pre-vaccination baseline symptomatology. We also tested worry about COVID-19 vaccine side-effects as an alternative mediator and found that, although side-effect worry was related to exposure and impressions from personal acquaintances and social media, it was not related to post-vaccination side-effect experience.
4. Discussion
Negative information about vaccine and drug side-effects can be transmitted rapidly through different forms of social communication. Using a sample of 551 community members, the present study established links between two self-reported social information sources and increases in COVID-19 vaccine side-effect intensity. Further, the results provide evidence for a mechanism to account for these relationships. Specifically, in a prospective longitudinal study, both the number of pre-vaccination social media exposures related to COVID-19 vaccine side-effects and impressions about side-effects stemming from personal acquaintances predicted an increase in post-vaccination side-effect experiences. Further, consistent with the literature on nocebo effects, the association between the social media exposure and impressions from personal acquaintances variables were mediated by pre-vaccination side-effect expectations.
The results indicated two different social communication variables predict COVID-19 vaccine side effects. This is not the first study to show that factors beyond the pharmacological properties of the vaccines predict side effect reports. Previous studies indicate, for example, that COVID-19 vaccination side effect experiences vary with factors including, age, sex, and vaccine type [3]. The present results extend such findings by establishing connections between social messaging and COVID-19 vaccine side effects. Whereas recent studies have found use of social media predicts willingness to become vaccinated [19], the present study finds that contact from both personal acquaintances and social media predict increased vaccine side effect intensity.
The finding that the number of social media exposures, rather than the impressions formed, predicts side-effects is consistent with the notion that social media can influence individuals outside of their awareness [26]. Further, exposure to and impressions from news stories did not predict either side-effect expectations or experienced side-effects. This could be explained by the involvement of close others in both social media and personal acquaintance information, which often receives greater consideration than information about unfamiliar individuals [2], such as that portrayed in news reports. However, additional research is certainly required to ascertain how and when impressions and exposures from social communications impact vaccine and treatment side-effect reports.
The present findings add novel data to the literature on nocebo side-effects. As noted at the outset, expectations are theorized to be a mechanism underlying nocebo side-effects. There is limited data, however, supporting expectations as a mediator and a majority of the data that does exist arrives from studies in which expectations were directly induced by an experimenter's verbal suggestion [10]. The present study adds to the current database by providing longitudinal evidence, collected in an ecologically valid context, that expectations mediate the relationship between personal contacts and social media posts and vaccine side-effect experience. Further, these are the first data showing social media communications change side-effect reports through personal expectations.
The current results identify personal acquaintances and social media as predictors of side effect expectations and experiences. Information from these two sources of social communication could be modifiable, and therefore messages from the sources should serve as points of intervention to decrease vaccine hesitancy and vaccine side-effects. Prior research has uncovered strategies that show promise in addressing negative expectations and reducing the nocebo effect, including thoughtful clinical information framing, reducing the negative impact of media coverage, and educating people about the nocebo effect [10,20,42]. Educational strategies, for example, could be provided that explain how best to talk about vaccine side effects to lessen the likelihood of nocebo effects. Other strategies, such as changing the message framing used by medically-relevant social media outlets, could be implemented in an effort to improve the publics' experience of COVID-19 vaccines and perhaps curb vaccination hesitancy.
4.1. Strengths and limitations
This study provides novel evidence for the role of socially transmitted expectations in vaccine side-effects and has notable strengths, including use of a prospective longitudinal design with a national community sample, separate assessments of information source exposure and impressions, a test of the mechanistic-based variable (expectation), a comprehensive assessment of side-effects. To our knowledge, this is the first longitudinal study to directly compare the predictive ability of different social information sources on side-effects and to identify that these relationships are mediated by expectations. The study has notable limitations, including the reliance on a U.S. only sample and the use of self-report measures of source exposure, which could be subject to recall bias. Additionally, side effect experiences were reported retrospectively in this longitudinal study, and therefore memory biases may have influenced reporting. As social information sources can overlap, future research should further separate social media, personal acquaintances, and news reports, and the extent to which prior beliefs may interact with social communication. The data was also collected in the early stages of the COVID-19 pandemic, and it may be that the links between social communication and vaccine side effects changed as the pandemic progressed. Additionally, the social information source and personal expectation data were measured cross-sectionally in Survey 1. As such, although we theorize that social information altered expectations, the data do not allow us to establish the directionality of that relationship. Finally, experimental designs are needed to establish the causal links between social information sources and vaccine side-effect expectations and experience.
5. Conclusions
This prospective longitudinal study provides novel evidence that personal acquaintance and social media communications may alter side effect expectations and vaccine side-effect experiences. The results also add support to the perspective that personal expectations are a mechanism by which social communications produce nocebo side-effects. It may be possible to modify social communications and reduce side-effects from publicly discussed vaccinations and medications.
Author contributions
Data acquisition, analysis, and interpretation by AG, KC, EJ, and KF. Manuscript drafted by KC, AG, WT, and KF. All authors contributed to concept, design, and revisions for intellectual content.
Declaration of Competing Interest
The authors declare no conflicts of interest. The research was supported by grant # DP200101748 by the Australian Research Council to Dr. Ben Colagiuri.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2022.111081.
Appendix A. Supplementary data
Supplementary material
References
- 1.Aberson C. 2nd ed. Routledge; 2019. Applied Power Analysis for the Behavioral Sciences. [Google Scholar]
- 2.Andersen S., Chen S., Miranda R. Significant others and the self. Self Identity. 2002;1:159–168. [Google Scholar]
- 3.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open. 2021;4(12):e2140364. doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X., Faviez C., Schuck S., Lillo-Le-Louët A., Texier N., Dahamna B., Burgun A. Mining patients’ narratives in social media for pharmacovigilance: adverse effects and misuse of methylphenidate. Front. Pharmacol. 2018;9:541. doi: 10.3389/fphar.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinelli M., Quattrociocchi W., Galeazzi A., Valensise C.M., Brugnoli E., Schmidt A.L., Zola P., Zollo F., Scala A. The COVID-19 social media infodemic. Sci. Rep. 2020;10(1):16598. doi: 10.1038/s41598-020-73510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colloca L., Barsky A.J. Placebo and nocebo effects. N. Engl. J. Med. 2020;382(6):554–561. doi: 10.1056/NEJMra1907805. [DOI] [PubMed] [Google Scholar]
- 7.Crichton F., Petrie K.J. Health complaints and wind turbines: the efficacy of explaining the nocebo response to reduce symptom reporting. Environ. Res. 2015;140:449–455. doi: 10.1016/j.envres.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Custers R., Aarts H., Oikawa M., Elliot A. The nonconscious road to perceptions of performance: achievement priming augments outcome expectancies and experienced self-agency. J. Exp. Soc. Psychol. 2009;45:1200–1208. [Google Scholar]
- 9.Doering B.K., Nestoriuc Y., Barsky A.J., Glaesmer H., Brähler E., Rief W. Is somatosensory amplification a risk factor for an increased report of side effects? Reference data from the German general population. J. Psychosom. Res. 2015;79:492–497. doi: 10.1016/j.jpsychores.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Faasse K. Nocebo effects in health psychology. Aust. Psychol. 2019;54(6):453–465. [Google Scholar]
- 11.Faasse K., Huynh A., Pearson S., Geers A.L., Helfer S.G., Colagiuri B. The influence of side effect information framing on nocebo effects. Ann. Behav. Med. 2019;53:621–629. doi: 10.1093/abm/kay071. [DOI] [PubMed] [Google Scholar]
- 12.Faasse K., Gamble G., Cundy T., Petrie K.J. Impact of television coverage on the number and type of symptoms reported during a health scare: a retrospective pre–post observational study. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez A., Kirsch I., Noël L., Rodondi P.Y., Kaptchuk T.J., Suter M.R., et al. A test of positive suggestions about side effects as a way of enhancing the analgesic response to NSAIDs. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0209851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geers A.L., Clemens K.S., Faasse K., Colagiuri B., Webster R., Vase L., Sieg M., Jason E., Colloca L. Psychosocial factors predict COVID-19 vaccine side effects. Psychother. Psychosom. 2022;91(2):136–138. doi: 10.1159/000519853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geers A.L., Close S.R., Caplandies F., Vase L. A positive mood induction for reducing the formation of nocebo effects from side effect information. Ann. Behav. Med. 2019;11:999–1008. doi: 10.1093/abm/kaz005. [DOI] [PubMed] [Google Scholar]
- 16.Haas J.W., Bender F.L., Ballou S., Kelley J.M., Wilhelm M., Miller F.G., Rief W., Kaptchuk T.J. Frequency of adverse events in the placebo arms of COVID-19 vaccine trials: a systematic review and Meta-analysis. JAMA Netw. Open. 2022;5(1):e2143955. doi: 10.1001/jamanetworkopen.2021.43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamel L., Kirzinger A., Lopes L., Kearney A., Sparks G., Brodie M. KFF; 2021. KFF COVID-19 Vaccine Monitor: January 2021 - Vaccine Hesitancy.https://www.kff.org/report-section/kff-covid-19-vaccine-monitor-january-2021-vaccine-hesitancy/ [Google Scholar]
- 18.Hayes A.F. 3rd ed. Guilford Press; 2022. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- 19.Jennings W., Stoker G., Bunting H., Valgarðsson V.O., Gaskell J., Devine D., Mills M.C. Lack of trust, conspiracy beliefs, and social media use predict COVID-19 vaccine hesitancy. Vaccines. 2021;9(6):593. doi: 10.3390/vaccines9060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinstäuber M., Colgan S., Petrie K.J. Changing understanding, perceptions, pain relief of and preference for generic medicines with patient education: an experimental intervention study. Res. Soc. Adm. Pharm. 2021;17(7):1288–1299. doi: 10.1016/j.sapharm.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Köteles F., Tarján E., Berkes T. Artificial concerns. Effects of a commercial advertisement on modern health worries & sympathetic activation. Mentálhigiéné És Pszichoszomatika. 2016;17:61–79. [Google Scholar]
- 22.Lentzen M.P., Huebenthal V., Kaiser R., Kreppel M., Zoeller J.E., Zirk M. A retrospective analysis of social media posts pertaining to COVID-19 vaccination side effects. Vaccine. 2022;40(1):43–51. doi: 10.1016/j.vaccine.2021.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach K.J., Salas Reyes R., Pentz B., Taylor J., Costa C.A., Cruz S.G., Thomas K.E., Arnott J.C., Donald R., Klenk, N. News media coverage of COVID-19 public health and policy information. Humanities and Social Sciences Communications. 2021;8(1):220. [Google Scholar]
- 24.MacKrill K., Groom K.M., Petrie K.J. The effect of symptom-tracking apps on symptom reporting. Br. J. Health Psychol. 2020;25:1074–1085. doi: 10.1111/bjhp.12459. [DOI] [PubMed] [Google Scholar]
- 25.Mao A., Barnes K., Sharpe L., Geers A.L., Helfer S.G., Faasse K., Colagiuri B. Using positive attribute framing to attenuate nocebo side effects: a cybersickness study. Ann. Behav. Med. 2021;55(8):769–778. doi: 10.1093/abm/kaaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell A., Kiley J., Gottfried J., Guskin E. The Role of News on Facebook. 2013. https://www.pewresearch.org/journalism/2013/10/24/the-role-of-news-on-facebook/
- 27.Neukirch N., Colagiuri B. The placebo effect, sleep difficulty, and side effects: a balanced placebo model. J. Behav. Med. 2015;38:273–283. doi: 10.1007/s10865-014-9590-5. [DOI] [PubMed] [Google Scholar]
- 28.Nestoriuc Y., Von Blanckenburg P., Schuricht F., Barsky A.J., Hadji P., Albert U.S., Rief W. Is it best to expect the worst? Influence of patients’ side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann. Oncol. 2016;27(10):1909–1915. doi: 10.1093/annonc/mdw266. [DOI] [PubMed] [Google Scholar]
- 29.Palan S., Schitter C. Prolific.Ac—a subject pool for online experiments. J. Behav. Exp. Financ. 2018;17:22–27. [Google Scholar]
- 30.Petrie K.J., Rief W. Psychobiological mechanisms of placebo and nocebo effects: pathways to improve treatments and reduce side effects. Annu. Rev. Psychol. 2019;70(1):599–625. doi: 10.1146/annurev-psych-010418-102907. [DOI] [PubMed] [Google Scholar]
- 31.Plotkin S.A. Vaccines: past, present and future. Nat. Med. 2005;11(4):S5–S11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollo A., Carlino E., Vase L., Benedetti F. Preventing motor training through nocebo suggestions. Eur. J. Appl. Physiol. 2012;112(11):3893–3903. doi: 10.1007/s00421-012-2333-9. [DOI] [PubMed] [Google Scholar]
- 33.Rief W. Fear of adverse effects and COVID-19 vaccine hesitancy: recommendations of the treatment expectation expert group. JAMA Health Forum. 2021;2(4):e210804. doi: 10.1001/jamahealthforum.2021.0804. [DOI] [PubMed] [Google Scholar]
- 34.Rief W., Glombiewski J.A., Barsky A.J. 2009. Generic Assessment of Side Effects: GASE. [Google Scholar]
- 35.Saha K., Torous J., Kiciman E., De Choudhury M. Understanding side effects of antidepressants: large-scale longitudinal study on social media data. JMIR mental health. 2021;8(3) doi: 10.2196/26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schedlowski M., Enck P., Rief W., Bingel U. Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol. Rev. 2015;67(3):697–730. doi: 10.1124/pr.114.009423. [DOI] [PubMed] [Google Scholar]
- 37.Solís Arce J.S., Warren S.S., Meriggi N.F., Scacco A., McMurry N., Voors M., Syunyaev G., Malik A.A., Omer S.B. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021;27(8):1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tinnermann A., Geuter S., Sprenger C., Finsterbusch J., Büchel C. Interactions between brain and spinal cord mediate value effects in nocebo hyperalgesia. Science. 2017;358(6359):105–108. doi: 10.1126/science.aan1221. [DOI] [PubMed] [Google Scholar]
- 39.van Laarhoven A.I., Vogelaar M.L., Wilder-Smith O.H., van Riel P.L., van de Kerkhof P.C., Kraaimaat F.W., Evers A.W. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. PAIN. 2011;152(7):1486–1494. doi: 10.1016/j.pain.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 40.von Blanckenburg P., Schuricht F., Albert U.S., Rief W., Nestoriuc Y. Optimizing expectations to prevent side effects and enhance quality of life in breast cancer patients undergoing endocrine therapy: study protocol of a randomized controlled trial. BMC Cancer. 2013;13(1):1–10. doi: 10.1186/1471-2407-13-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wager T.D., Atlas L.Y. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci. 2015;16(7):403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster R.K., Weinman J., Rubin G.J. A systematic review of factors that contribute to nocebo effects. Health Psychol. 2016;35(12):1334–1355. doi: 10.1037/hea0000416. [DOI] [PubMed] [Google Scholar]
- 43.Witthöft M., Rubin G.J. Are media warnings about the adverse health effects of modern life self-fulfilling? An experimental study on idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF) J. Psychosom. Res. 2013;74(3):206–212. doi: 10.1016/j.jpsychores.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Yasir A., Hu X., Ahmad M., Rauf A., Shi J., Ali Nasir S. Modeling impact of word of mouth and E-government on online social presence during COVID-19 outbreak: a multi-mediation approach. Int. J. Environ. Res. Public Health. 2020;17(8):2954. doi: 10.3390/ijerph17082954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor A.M., Pennie R.A., Dales R.E. Framing effects on expectations, decisions, and side effects experienced: The case of influenza immunization. J. Clin. Epidemiol. 1996;49(11):1271–1276. doi: 10.1016/s0895-4356(96)00177-1. [DOI] [PubMed] [Google Scholar]
- 46.Greville-Harris M., Dieppe P. Bad is more powerful than good: the nocebo response in medical consultations. Amer. J. Med. 2015;128(2):126–129. doi: 10.1016/j.amjmed.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 47.Pan Y., Kinitz T., Stapic M., Nestoriuc Y. Minimizing drug adverse events by Informing about the nocebo effect—An experimental study. Front. Psychiatry. 2019;10:504. doi: 10.3389/fpsyt.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material