Objectives:
To compare the efficacy of pulsed electromagnetic field therapy (PEMFT) versus transcutaneous electrical nerve stimulation (TENS) in the treatment of post-herpetic neuralgia of the sciatic nerve.
Methods:
A double-blinded randomized clinical study has included 56 patients (18 males and 38 females). Participants were randomly and equally assigned into 2 groups. Both groups received conventional physical therapy treatment. Moreover, group (A) has an additional TENS, and group (B) had PEMFT. Both modalities were applied once daily, 3 times a week for 20 minutes for 8 successive weeks. Visual analog scale (VAS) and carbamazepine intake (CMI) dose have been assessed before and after interventions.
Results:
There was a significant decrease in VAS and CMI post-treatment in group A and B compared with that pretreatment (P > .001). The percent decrease in VAS and CMI in group A were 72.44% and 69.47% respectively and that for group B was 68.95% and 67.94% respectively. The findings revealed a non-significant difference in VAS and CMI (P > .05) between groups. The Means of VAS and CMI were (2.4 ± 0.78, 204.5 ± 16.76 and 2.67 ± 0.9, 210.57 ± 16.5) in group A and group B respectively. The mean difference for VAS and CMI was (−0.27 and −6.07) between groups post-treatment respectively.
Conclusion:
Both TENS and PEMFT were effective and nearly equivalent in improving the post-herpetic neuralgia of the sciatic nerve as measured by in VAS and CMI. Clinical recommendations should be highlighted to instigate the using of TENS and PEMFT in the management of post-herpetic neuralgia of the sciatic nerve.
Keywords: carbamazepine intake, post-herpetic neuralgia of the sciatic nerve, pulsed electromagnetic field therapy, transcutaneous electrical nerve stimulation, visual analog scale
1. Introduction
Post-herpetic neuralgia (Shingles) results from the varicella zoster virus (VZV) (herpes zoster [HZ]) that outbreaks 1 or more dorsal root ganglia and the corresponding sensory nerves. The incidence of acute HZ is 3.4 cases per 1000 persons per year, increasing by proceeding in age up to 11 cases per 1000.[1]
Post herpetic neuralgia (PHN) diagnosis is initially difficult until the skin eruptions of vesicles and blisters appear, that is preceded by neuropathic pain from second to fourth weeks then the emergence of rash at seventh to 8th week after infection.[2] Drug- resistant neuropathic pain is the most common complication of VZV, this pain resulting from changes in somatosensory processing of central and peripheral nervous system.[3] PHN is the commonest chronic post-infectious neuropathic pain resulting from VZV.[4] Pain is defined as burning or electric shock-like and may be associated with hyperalgesia or allodynia, Periods of randomly occurring day and night burning, stabbing, and shooting pains continue for months or years after the acute stage. The area concerned is hyper-esthetic and non-noxious stimuli may cause painful reaction that may be triggered by light touch, clothing rubbing against the skin, noise, temperature changes, sweating and emotional upsets.[5,6]
Sciatica is a disorder characterized by sciatic nerve pain that travels down a leg. Herniated intervertebral discs are the most frequent cause of sciatica. A spinal nerve that is compressed by the herniated disc and results in a painful, burning feeling in the leg. The prevalence of sciatic pains reported in literature ranges widely, from 1.6% of the general population to 43% of a specific working population. It has been suggested that the varicella-zoster, or chickenpox, virus, may recur in a spinal neuron as a result of irritation. In the area where the nerve is distributed, the virus’s activation results in a blistering rash. Ten percentage of these people may experience post-herpetic neuralgia, a persistent pain that coexists with the irritation of the nerve.[7,8] One-tenth of HZ patients suffer from PHN, which is a painful complication with the highest proportion of 15.56% being found in patients aged 75 to 79 years. Sciatica as a disease with chronic pain, is related to a preexisting HZ virus infection.[9]https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-020-03847-5-ref-CR6]
There are several pharmacological alternatives to alleviate pain in PHN however; side effects could have dramatic effects particularly in long term use and elderly people.[10] Both pulsed electromagnetic field (PEMF) therapy[11] and transcutaneous electrical nerve (TENS) stimulation are non-pharmacological approaches that are used primarily to treat common painful conditions without obvious side effects specially when used correctly.[12]
PEMF is a physical therapy modality used for the treatment of many debilitating disorders such as the musculoskeletal[13–15] and neurological[16–18] disorders. PEMF has been shown to increase the permeability of the cell membrane, increase blood circulation, oxygen supply, and increase production of adenosine tri-phosphate. In addition, it promotes the healing cycle by increasing the epithelialization of the damaged tissues, strengthening fibroblastic, osteoplastic, anti-inflammatory, analgesic effects and stimulating bone healing activities.[19]
TENS is a noninvasive, self- administered, non-pharmacological modality that has been used to treat several painful conditions. TENS can be used on its own or in conjunction with medicine to reduce the dosage. Nerve stimulation is provided by applying surface electrodes to the nerve fiber distribution. The mechanism for pain relief was believed to be produced through central and peripheral mechanisms. It activates the release of endogenous opioids, modifies electrical transmission and dilates the blood vessels, reducing neuropathic pain,[20–22] another method proposed here is the old pain gait theory. where, stimulation of the skin via the dorsal columns leads to activation of non-noxious, low threshold afferents (A–b fibers) only but not noxious afferents (A–d and C fibers) at the same time. A–b afferents activity limits the transmission of information related to pain through the spinal cord and brainstem.[19]
Previous studies proved the role of TENS for the treatment of PHN,[23–25] although there are numerous studies investigated the effect of PEMFS on different painful conditions,[11,13–17] Due to the shortage of research to investigate the effect of PEMFS on PHN, specifically, according to PubMed database and Google scholar search, English language only, the current study is a novel randomized controlled trial – to the best knowledge of the authors- to investigate the effect of PEMFS on PHN in comparison to the pre-investigated TENS application.
2. Materials and Methods
2.1. Study design
This research was a double blind randomized clinical trial (patient & assessor), was conducted from March 2018 to October 2019, was clinically registered with ID of NCT 04488835, and was ethically approved by the committee of faculty of physical Therapy, Cairo University (P.T. REC/012/002/745). Patients were recruited from Kasr Al-Aini outpatient dermatology department, neurology department and Faculty of physical therapy outpatient clinic. Each participant signed an informed consent form according to the ethical principles of the Declaration of Helsinki.
2.2. Participants
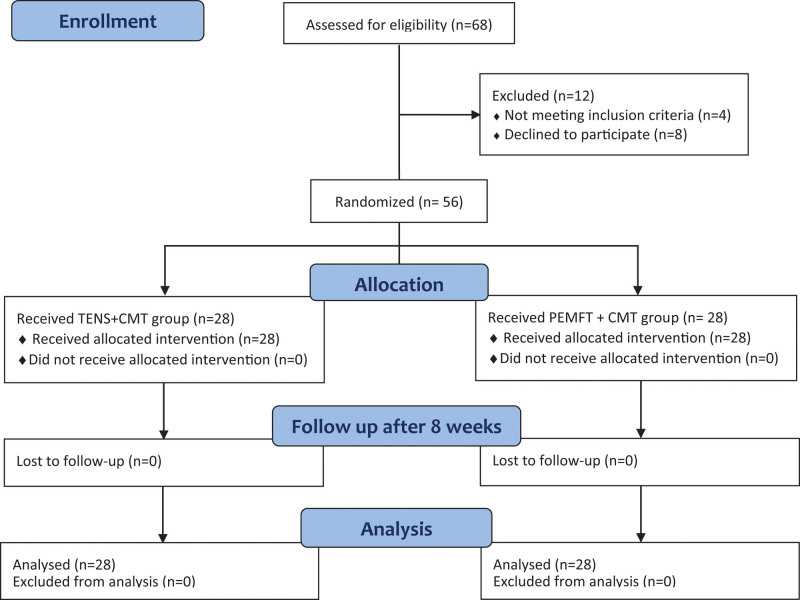
Out of 68 patients assessed for eligibility by trained neurologists, 56 patients with mean age 32.93 ± 5.16 years (18 males and 38 females) who had post-herpetic neuralgia of the sciatic nerve, participated in the study. Figure 1 shows the flow of patients throughout the study. Inclusion criteria for the patients; ages ranged from 30 to 50 years, history of HZ more than 90 days and VAS is ≥ 5 out on a scale from 0 to 10, and all patients were on pain medication “Carbamazepine.” Assessment of pretreatment amount of carbamazepine intake (CMI) were applied to all patients. Exclusion was applied to uncooperative behavior (i.e., the patient did not provide a proper response), and intellectual disability to complete the self-evaluation scale, VAS, patients with seizure disorders, patients with pacemakers, Pregnant female, patients with metabolic or endocrinal diseases.
Figure 1.
Flowchart showing the progress of subjects at each stage of the clinical trial.
2.3. Intervention
Both groups receive treatment intervention 3 times per week for 8 successive weeks. Patients were randomly assigned into 2 equal groups. Group (A): 28 patients received conventional physical therapy treatment in addition to the TENS. Group (B): 28 patients received plus the pulsed electromagnetic field therapy (PEMFT).
2.4. Instrumentations
TENS unit: Progoo TENS Unit and EMS Muscle Stimulator Pain Relief FDA Cleared” Universal Product Code “778957044094.” The device contains dual channels, 4 low impedance self-adhesive electrodes and triple A batteries. High frequency conventional TENS was used in this study with frequency 100 Hz, pulse duration 200 µs and intensity according to the patient’s tolerance, for 20 minutes once daily.[20]
The pulsed electromagnetic unit was Quattro PRO; ASA, Italy is a magnetic field therapy with a serial number (00001543). The device consisted of a controlled generator mounted on a movable frame for easy movement. Field power of up to 85 Gauss in emission. The device is consisted of a motorized bed and solenoids, which should be connected to main electrical supply 230 V at a frequency of 50 or 60 Hz connected to the earth. The generated pulsed magnetic field up to 100 Hz, intensity varied according to the type of solenoid and region to be applied on, for 20 minutes once daily.[21–24]
2.5. Treatment procedures
Treatment was applied once daily, 3 sessions per week for 8 weeks, for 40 minutes which was the total period of treatment for all patients. Both groups (A) and (B) received the same conventional physical therapy treatment protocol in the form of Infra-red (Solmed IV) – Meden Inmed Poland) for the gluteal and hamstring regions, Patients were instructed that they were expected to feel “comfortable \mild warmth” to avoid harming of the skin by too much heat. The distance of the skin to the source of heat is vertically 60 to 75 cm, while the patient in prone lying position, for 15 minutes.[25] Then 5 minutes passive stretching exercises for the hamstring while the patient in supine lying position with sustained time of stretching to about 6 seconds. Moreover, all patients received the same medical care (Carbamazepine tablets, Tegretol CR 200, Novartis, Egypt).[26,27] Treatment dose was regulated under consultation of patients treating neurologist.
2.6. TENS protocol
TENS was applied for group (A) only. While the patient was in a relaxed prone lying position TENS was applied unilaterally over the affected side from up to down alongside the sciatic nerve pathway in a linear pathway across the back of the leg starting at the level of L5-S1where the first electrode was applied (erector-spinae motor point) level paravertebrally (channel one), second electrode where applied over upper motor point of gluteus maximus (the tender buttock), (channel one). Third electrode were applied at the midpoint between ischial tuberosity and greater trochanter at level of buttock and posterior upper thigh (channel two). Finally, TENS fourth electrode were applied superior to popliteal crease (channel two). Surface electrodes were mounted in previous 4 points by adhesive tape to prevent its displacement.[28]
2.7. PEMFS protocol
Group (B) received PEMFS. While the patient was relaxed in prone lying position, the solenoid was adjusted to be over the course of the sciatic nerve starting at the gluteal and thigh area, with frequency of 50 Hz and intensity of 20 G for 20 minutes, programme number (4) of device was used for the patients, which is the weakest programme with a soothing north polarity of the magnetic pulses with frequency of 1.6 Hz. Both TENS and PEMFT were applied once daily, 3 times per week for an 8-week period of treatment.[24]
2.8. Outcome measures
Primary outcome of the current study was VAS which measure pain intensity. Secondary outcome measure was the estimation of the dose that were measured at base line then after 8 weeks of treatment.
Pain intensity was measured using VAS at the beginning (first record) then after 2 months (second final record). The VAS consists of a line, usually 100 mm long, the ends of which are categorized as pain extremes (e.g., no pain to severe unbearable pain). Patient was asked to mark on the line at the point, which better reflects his pain experience between 2 “no pain” to “worst pain,” then the operator measured the distance in centimeters from the zero “no pain.”[29–31]
Estimation of the CMI has been used to measure improvements in the sciatic nerve’s post-herpetic neuralgia. starting with a maximum dose of 1200 mg per day. Patients were instructed to decrease the daily dose of Carbamazepine as required according to their pain perception. All patients were instructed to record the time and number of Carbamazepine daily tablets they took during the whole period of the study. All the above parameters (VAS and CMI) were calculated 2 times; the baseline record taken before the study started, the final record was taken after 8 weeks after the research began.[6,32,33]
2.9. Sample size
Calculation of sample size was performed prior to the study using G*POWER statistical software (version 3.1.9.2; Franz Faul, Universitat Kiel, Germany) and showed that the minimum required sample size for this study was 26 subjects per group. Calculations were made using α = 0.05, power: 80% and effect size = 0.8, the primary outcome measure was the VAS, and allocation ratio N2/N1 = 1.
2.10. Randomization
Allocation was done using sealed, sequentially numbered opaque envelopes. Randomization was generated by the second author who was not involved in data collection. The first and fourth authors opened the envelopes and proceeded with treatment according to group allocation. At the beginning and at the end of the treatment period patients were assessed by the third author who was blinded to their allocations.
2.11. Statistical analysis
Descriptive statistics and Unpaired t test were conducted for comparison of subject characteristics between both groups. Chi-squared test was used for comparison of sex distribution between groups. Normal distribution of data was checked using the Shapiro–Wilk test. Levene’s test for homogeneity of variances was conducted to ensure the homogeneity between groups. Unpaired t test was conducted to compare the mean values of VAS and CMI between groups. Paired t test was conducted for comparison between pre and post treatment in each group. The level of significance for all statistical tests was set at P < .05. All statistical analysis was conducted through the statistical package for social studies (SPSS) version 22 for windows (IBM SPSS, Armonk, NY).
3. Results
3.1. Subject characteristics
Table 1 shows the subject characteristics of the group A and B. There was no significant difference between groups in the mean age, weight, height and BMI (P > .05). Also, there was no significant difference in sex distribution between groups (P = .56).
Table 1.
Comparison of subject characteristics between group A and B.
| ± SD | MD | t- value | P-value | ||
|---|---|---|---|---|---|
| Group A | Group B | ||||
| Age (yrs) | 32.92 ± 5.31 | 33.1 ± 4.28 | −0.18 | −0.14 | .89 |
| Weight (kg) | 75.57 ± 4.18 | 74.21 ± 3.83 | 1.36 | 1.26 | .21 |
| Height (cm) | 168.46 ± 4.63 | 167.5 ± 3.42 | 0.96 | 0.88 | .38 |
| BMI (kg/m²) | 26.7 ± 2.13 | 26.48 ± 1.82 | 0.22 | 0.38 | .7 |
| Males/females | 8/20 | 10/18 | (χ2 = 0.32) | .56 | |
BMI = body mass index, MD = mean difference, P-value = probability value, SD = standard deviation, x̄ = Mean, χ2 = Chi squared value.
3.2. Effect of treatment on VAS and CMI
There was a significant decrease in VAS and CMI post treatment in the group A and B compared with that pretreatment (P ˂ .001). The percent of decrease in VAS and CMI in the group A were 72.44% and 69.47% respectively and that for group B were 68.95% and 67.94% respectively (Table 2). There was no significant difference in the VAS and CMI between both groups pretreatment (P > .05). Comparison between the groups A and B post treatment revealed a non-significant difference in VAS and CMI (P > .05) (Table 2).
Table 2.
Mean differences of VAS and CMI pre and post treatment of the group A and B.
| Group A | Group B | MD | t- value | P-value | |
|---|---|---|---|---|---|
| ± SD | ± SD | ||||
| VAS | |||||
| Pre treatment | 8.71 ± 0.93 | 8.6 ± 0.78 | 0.11 | 0.46 | .64 |
| Post treatment | 2.4 ± 0.78 | 2.67 ± 0.9 | −0.27 | −1.26 | .21 |
| MD | 6.31 | 5.93 | |||
| % of change | 72.44 | 68.95 | |||
| t-value | 29.76 | 24.07 | |||
| P-value | ˂.001 | ˂.001 | |||
| CMI (mg) | |||||
| Pre treatment | 669.82 ± 37.56 | 656.78 ± 40.64 | 13.04 | 1.24 | .21 |
| Post treatment | 204.5 ± 16.76 | 210.57 ± 16.5 | −6.07 | −1.36 | .17 |
| MD | 465.32 | 446.21 | |||
| % of change | 69.47 | 67.94 | |||
| t-value | 68.56 | 51.39 | |||
| P-value | ˂.001 | ˂.001 |
CMI = carbamazepine intake, MD = mean difference, P-value = probability value, SD = standard deviation, VAS = visual analog scale, = Mean.
4. Discussion
The main purpose of the current study was to compare the effect of TENS and PEMFS in management of post-herpetic neuralgia of the sciatic nerve. Findings of the current study showed that both treatment techniques were statistically significant in improving post-herpetic neuropathic pain in an almost equivalent manner.
Neuropathic pain is 1 of the greatest pain management challenges; it is a tremendous cause of health care demand and expense to patients and the community. Neuropathic pain refers to pain caused by peripheral or central damage to the nervous system, that may include pain syndromes such as trigeminal neuralgia; severe diabetic neuropathies; post-stroke pain; and phantom limb. Post-herpetic neuralgia (PHN) is a condition of neuropathic pain which follow an acute HZ (shingles) attack. The severity of PHN pain is frequently sufficient to fully disturb the lives of otherwise healthy individuals and is often incompatible with traditional methods of treatment.[3,5,6]
An active-controlled study compared a combination of transcutaneous electronic nerve stimulation (TENS) with carbamazepine (up to 1000 mg daily) plus clomipramine (up to 75 mg daily) with in 29 participants over 8 weeks. The authors reported that drug combination was superior to TENS in terms of pain relief in 30% to 50% reduction in pain intensity in patients received medication.[34] Carbamazepine is thought to function through blocking voltage-sensitive sodium channels, which means less of those channels are available to open, making brain cells (less likely to fire) less excitable.[35]
Although these therapies can partially relieve pain, undesirable side effects and high risks of complications may result in as impaired motor and mental function that limit their clinical use. Therefore, safe and noninvasive therapies with almost no side effects are desperately needed.[36,37]
TENS was used for many types of painful conditions, particularly neuropathic pain.[12,38] Proper application of TENS has been found to relieve multiple neuropathic pain disorders such as carpal tunnel syndrome, diabetic neuropathy,[17,20]sciatica, radiculopathy and post-herpetic neuralgia (PHN).[39,40] One study analyzed the effect of TENS as a preventive measure to post herpetic neuralgic pain compared to antiviral drug administration to the controls. results revealed that in acute phase of the disease TENS has no unusual impact that is different from the control group. Nonetheless, following the acute stage, most patients in the study group did not experience PHN.[41] The results of this study showed some agreement to the current study; however, the stage of the disease was different which was acute in the previous study (72 hours after the appearance of rash) while it was chronic in the current study (more than 90 days after HZ affection). In a recent systemic review article conducted about different treatment options for PHN, TENS application was conducted in 4 different studies; 2 randomized controlled trials (RCT) investigated the effect of TENS in combination with medication for treatment of PHN in the facial area. Both studied used high frequency TENS for 30 minutes per day for a period of 4 to 8 weeks.[42]
One study showed that the combination of TENS with oral pregabalin reduced VAS scores, persistent pain intensity scores short-form McGill Pain Questionnaire total scores, and sleep interference, after 4 weeks.[23] The other RCT investigated the effect of TENS plus subcutaneous injection of cobalamin alone or with lidocaine. The results of this study showed improvement in pain intensity mean allodynia, paresthesia scores, and quality of life.[24] The third randomized controlled trial investigated the effect of TENS (Tennant modulator, Biohealth, Germany) a self-controlled electronic neuroadaptive regulation device on PHN. The results were significant after 1 week of treatment.[25] The fourth trial concerned with the prevention of PHN and was discussed before.[43] Results of these previous studies supported the findings of the current study, which make TENS a preferable, noninvasive, affordable modality with no side effects more than medications and other invasive modalities.[39,42]
There are several mechanisms were recommended to explain the analgesic effect of PEMFT on pain. Anti-inflammatory effect was 1 of the first suggestion of its analgesic effect. It was suggested that PEMFT could significantly reduce pro-inflammatory cytokines in humans. In addition, it has anti-inflammatory activities in many musculoskeletal and neurological tissues by restoring plasma membrane calcium ATPase activity.[44–47]
Another mechanism explaining the analgesic effect of PEMFT was suggested by Putowski et al,[18] who reported that the intracellular movement of ions due to electromagnetic field causes hyperpolarization of the cell membrane and increase in cell metabolism. Thus, improving blood supply to the tissues and oxygen partial pressure.[18]Consequently, it accelerates bone-healing, improvement of central and peripheral nervous system disorders, circulatory disorders, inflammation, and certain skin conditions. It was suggested also that electromagnetic field stimulation could elevate threshold of pain sensitivity and activate anticoagulation system. It stimulates production of opioid peptides, activates Merkel cells, Mast cells, improve vascularization of the sarcoplasmic reticulum, and increase the electric activities of different types of tissues. Thus, provide analgesic effect and decrease tissue edema.[11]
This study has clinical significance based on its results, both TENS and PEMFT were valuable tools and nearly equivalent in improving the post-herpetic neuralgia of the sciatic nerve as manifested by the highly significant decrease in VAS and CMI. On the other hand, this study was limited by the absence of follow up due to limited time and funding. Further studies are needed to investigate different modes of TENS and PEMFT on the treatment of post-herpetic neuralgia, also PHN-specific assessment methods (e.g., the Zoster Brief Pain Inventory) could be used in addition to VAS and CMI.
5. Conclusion
Current study finding supported that both techniques as methods to control pain in cases of PHN of the sciatic nerve, were effective almost equally in improving VAS score and reduction of the dose of CMI post treatment compared to before treatment.
Acknowledgments
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R145), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors express their gratitude to all patients for their trust and help in making this study possible.
Author contributions
Conceptualization: Marwa M. Eid, Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi, Hadaya M. Eladl, Heba A. Bahey El-Deen.
Data curation: Marwa M. Eid, Nashwa Sayed Hamed, Hadaya M. Eladl, Heba A. Bahey El-Deen.
Formal analysis: Marwa M. Eid, Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi, Hadaya M. Eladl, Heba A. Bahey El-Deen.
Funding acquisition: Safaa Mostafa Elkholi, Heba A. Bahey El-Deen.
Investigation: Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi.
Methodology: Marwa M. Eid, Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi, Hadaya M. Eladl, Heba A. Bahey El-Deen.
Project administration: Marwa M. Eid, Walid Kamal Abdelbasset.
Resources: Safaa Mostafa Elkholi.
Software: Heba A. Bahey El-Deen.
Supervision: Marwa M. Eid, Walid Kamal Abdelbasset.
Validation: Hadaya M. Eladl.
Visualization: Hadaya M. Eladl.
Writing – original draft: Marwa M. Eid, Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi, Hadaya M. Eladl, Heba A. Bahey El-Deen
Writing – review & editing: Marwa M. Eid, Nashwa Sayed Hamed, Walid Kamal Abdelbasset, Safaa Mostafa Elkholi, Hadaya M. Eladl, Heba A. Bahey El-Deen.
Abbreviations:
- CMI =
- carbamazepine intake
- HZ =
- herpes zoster
- PEMFT =
- pulsed electromagnetic field therapy
- PHN =
- post herpetic neuralgia
- TENS =
- transcutaneous electrical nerve stimulation
- VAS =
- visual analog scale
- VZV =
- varicella zoster virus
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Ethical approval was obtained by the ethical committee of the Faculty of Physical Therapy, Cairo University (P.T.REC/012/002745).
Princess Nourah bint Abdulrahman University.
The authors have no conflicts of interest to disclose.
How to cite this article: Eid MM, Hamed NS, Abdelbasset WK, Elkholi SM, Eladl HM, Bahey El-Deen HA. A comparative study between transcutaneous electrical nerve stimulation and pulsed electromagnetic field therapy in the management of post-herpetic neuralgia of the sciatic nerve. Medicine 2022;101:44(e31433).
Contributor Information
Marwa M. Eid, Email: alaaamr50@yahoo.com.
Nashwa Sayed Hamed, Email: nashwahamed@gmail.com.
Safaa Mostafa Elkholi, Email: smelkholi@pnu.edu.sa.
Hadaya M. Eladl, Email: hd_mos@yahoo.com.
Heba A. Bahey El-Deen, Email: heba_bahey@yahoo.com.
References
- [1].Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371:1526–33. [DOI] [PubMed] [Google Scholar]
- [2].Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20:17. [DOI] [PubMed] [Google Scholar]
- [3].Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. [Erratum, Mayo Clin Proc 2008;83:255]. [DOI] [PubMed] [Google Scholar]
- [5].Saguil A, Kane S, Mercado M, et al. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96:656–63. [PubMed] [Google Scholar]
- [6].Jeon YH. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain. 2015;28:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clarke JA, van Tulder MW, Blomberg SE, et al. Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev. 2007;2:CD003010. [DOI] [PubMed] [Google Scholar]
- [8].Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976). 2008;33:2464–72. [DOI] [PubMed] [Google Scholar]
- [9].Ke DS, Hsu CY, Lin CL, et al. Herpes zoster in patients with sciatica. BMC Musculoskelet Disord. 2020;21:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grahame-Smith DG, Aronson JK. Oxford textbook of clinical pharmacology and drug therapy. 2nd Edition. Oxford: Oxford University Press. 1992. [Google Scholar]
- [11].Pawluk W. Pain management with pulsed electromagnetic field (PEMF) treatment. In Am Pain Soc. 2003;20:23–32. [Google Scholar]
- [12].Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother. 2011;11:735–53. [DOI] [PubMed] [Google Scholar]
- [13].Abdulla FA, Alsaadi S, Sadat-Ali MI, et al. Effects of pulsed low-frequency magnetic field therapy on pain intensity in patients with musculoskeletal chronic low back pain: study protocol for a randomised double-blind placebo-controlled trial. BMJ Open. 2019;9:e024650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andrade R, Duarte H, Pereira R, et al. Pulsed electromagnetic field therapy effectiveness in low back pain: a systematic review of randomized controlled trials. Porto Biomed J. 2016;1:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hulme J, Robinson V, DeBie R, et al. Electromagnetic fields for the treatment of osteoarthritis. Cochrane Database Syst Rev. 2002;1:CD003523. [DOI] [PubMed] [Google Scholar]
- [16].Weintraub MI, Cole SP. Pulsed magnetic field therapy in refractory neuropathic pain secondary to peripheral neuropathy: electrodiagnostic parameters--pilot study. Neurorehabil Neural Repair. 2004;18:42–6. [DOI] [PubMed] [Google Scholar]
- [17].Stein C, Eibel B, Sbruzzi G, et al. Electrical stimulation and electromagnetic field use in patients with diabetic neuropathy: systematic review and meta-analysis. Braz J Phys Ther. 2013;17:93–104. [DOI] [PubMed] [Google Scholar]
- [18].Putowski M, Piróg M, Podgórniak M, et al. The use of electromagnetic radiation in the physiotherapy. EJMT. 2016;2:53–8. [Google Scholar]
- [19].Barnes FS, Greenebaum B. Handbook of Biological Effects of Electromagnetic Fields-Two Volume Set. Boca Raton, FL: CRC Press. 2018. [Google Scholar]
- [20].NaderiNabi B, Sedighinejad A, Haghighi M, et al. Comparison of transcutaneous electrical nerve stimulation and pulsed radiofrequency sympathectomy for treating painful diabetic neuropathy. Anesth Pain Med. 2015;5:e29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ing MR, Hellreich PD, Johnson DW, et al. Transcutaneous electrical nerve stimulation for chronic post-herpetic neuralgia. Int J Dermatol. 2015;54:476–80. [DOI] [PubMed] [Google Scholar]
- [22].Mirkovic VB, Banjac L, Dasic Z, et al. Non-pharmacological treatment of diabetic polyneuropathy by pulse electromagnetic field. Healthmed. 2012;6:1291–5. [Google Scholar]
- [23].Mohamed RA, Abdallah GA, Abdeen HA, et al. Influence of low level laser therapy versus pulsed electromagnetic field on diabetic peripheral neuropathy. PhysTher Rehab. 2017;4:17. [Google Scholar]
- [24].Suszyński K, Marcol W, Górka D. Physiotherapeutic techniques used in the management of patients with peripheral nerve injuries. Neural Regen Res. 2015;10:1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Usman Z, Maharaj SS, Kaka B. Effects of combination therapy and infrared radiation on pain, physical function, and quality of life in subjects with knee osteoarthritis: a randomized controlled study. Hong Kong Physiother J. 2019;39:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Souza JB, Carqueja CL, Baptista AF. Physical rehabilitation to treat neuropathic pain. Revista Dor. 2016;17:85–90. [Google Scholar]
- [27].Akyuz G, Kenis O. Physical therapy modalities and rehabilitation techniques in the management of neuropathic pain. Am J Phys Med Rehabil. 2014;93:253–9. [DOI] [PubMed] [Google Scholar]
- [28].Ottoson D, Lundeberg T. Pain treatment by transcutaneous electrical nerve stimulation (TENS): a practical manual. New York: Springer-Verlag. 1988. [Google Scholar]
- [29].Gibson W, Wand BM, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;9:CD011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Younger J, McCue R, Mackey S. Pain outcomes: A brief review of instruments and techniques. Curr Pain Headache Rep. 2009;13:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2:e088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Friesen KJ, Falk J, Alessi-Severini S, et al. Price of pain: population-based cohort burden of disease analysis of medication cost of herpes zoster and postherpetic neuralgia. J Pain Res. 2016;9:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dworkin RH, Gnann JW, Jr, Oaklander AL, et al. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9(1 Suppl 1):S37–44. [DOI] [PubMed] [Google Scholar]
- [34].Gerson GR, Jones RB, Luscombe DK. Studies on the concomitant use of carbamazepine and clomipramine for the relief of post-herpetic neuralgia. Postgrad Med J. 1977;53:104–9. [PubMed] [Google Scholar]
- [35].Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev. 2011;1:CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rall TW, Schleifer LS. Drugs effective in the therapy of the epilepsies. In: Goodman LS, Gilman A, Rall TW, Nies AS, Taylor P, eds. The pharmacological basis of therapeutics. 8th ed. Toronto: McGraw-Hill. 1992. 436–62. Schulz 1994. [Google Scholar]
- [37].Sweetman S., ed. Martindale: the complete drug reference. 4th ed. London: Pharmaceutical Press. 2005; 353–358. [Google Scholar]
- [38].Gozani SN. Remote analgesic effects of conventional transcutaneous electrical nerve stimulation: a scientific and clinical review with a focus on chronic pain. J Pain Res. 2019;12:3185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stepanović A, Kolšek M, Kersnik J, et al. Prevention of post-herpetic neuralgia using transcutaneous electrical nerve stimulation. Wien Klin Wochenschr. 2015;127:369–74. [DOI] [PubMed] [Google Scholar]
- [40].Lin CS, Lin YC, Lao HC, et al. Interventional treatments for postherpetic neuralgia: a systematic review. Pain Physician. 2019;22:209–28. [PubMed] [Google Scholar]
- [41].Barbarisi M, Pace MC, Passavanti MB, et al. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clin J Pain. 2010;26:567–72. [DOI] [PubMed] [Google Scholar]
- [42].Xu G, Xu G, Feng Y, et al. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: A single-center randomized controlled trial. Am J Phys Med Rehabil. 2014;93:287–98. [DOI] [PubMed] [Google Scholar]
- [43].Tanelian DL, Brose WG. Neuropathic pain can be relieved by drugs that are use-dependent sodium channel blockers: lidocaine, carbamazepine, and mexiletine. Anesthesiology. 1991;74:949–51. [DOI] [PubMed] [Google Scholar]
- [44].Niv D, Maltsman-Tseikhin A. Postherpetic neuralgia: the never-ending challenge. Pain Pract. 2005;5:327–40. [DOI] [PubMed] [Google Scholar]
- [45].PEMF therapy for shingles and postherpetic neuralgia (PHN): Dr Pawluk. DrPawluk.com. 2019. Available at: www.drpawluk.com/blog/pemf-device-therapy-to-reduce-pain-during-and-after-shingles/.
- [46].Chan AK, Tang X, Mummaneni NV, et al. Pulsed electromagnetic fields reduce acute inflammation in the injured rat-tail intervertebral disc. JOR Spine. 2019;2:e1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Beaulieu K, Beland P, Pinard M, et al. Effect of pulsed electromagnetic field therapy on experimental pain: a double-blind, randomized study in healthy young adults. Electromagn Biol Med. 2016;35:237–44. [DOI] [PubMed] [Google Scholar]