Gain/amplification of chromosome arm 1q21 (1q21 +) is among the most common abnormalities found in multiple myeloma (MM) and is by now almost universally recognized as a poor prognostic factor in MM patients, recently included among the top features of the second revision of the International Staging System (R2-ISS) [1, 2]. 1q21 + can be divided into gain (gain1q) or amplification of 1q21 (amp1q), respectively, if three or more than three copies of 1q21 are detected, and the distinction between these two categories has increasingly become more important since the copy number of 1q21 seems to reflect the genomic instability, proliferation rate, drug resistance, and early progression/death rate of the disease [1, 3, 4]. Nevertheless, the optimal management of 1q21 + patients in the era of novel therapies has yet to be determined [1, 5, 6]. Daratumumab, an anti-CD38 monoclonal antibody, has provided clear demonstration of efficacy in MM patients, even among high-risk (HR) MM patients, and is now licensed for use in relapsed-refractory MM (RRMM) as well as newly diagnosed MM (NDMM) [7, 8]. However, data supporting the effectiveness of adjunctive daratumumab in 1q21 + patients are scarce, since pivotal trials didn’t report outcomes on this patient population, and potential mechanisms of resistance conferred by 1q21 + to daratumumab therapy have been proposed [1]. Interestingly, Hu and colleagues [9] have recently published a retrospective study describing real-world outcomes of 34 NDMM patients harboring 1q21 + , reporting no prognostic role of daratumumab therapy and worse outcomes for amp1q patients compared to gain1q patients. As the authors noted, only one study by Mohan and colleagues [10] had previously investigated the outcomes of 1q21 + patients treated with daratumumab, reporting poor prognosis associated with both 1q21 + and GEP-70 HR status, although patients in that study were heavily pretreated and only 63% of them received daratumumab in combinations with immunomodulatory drugs.
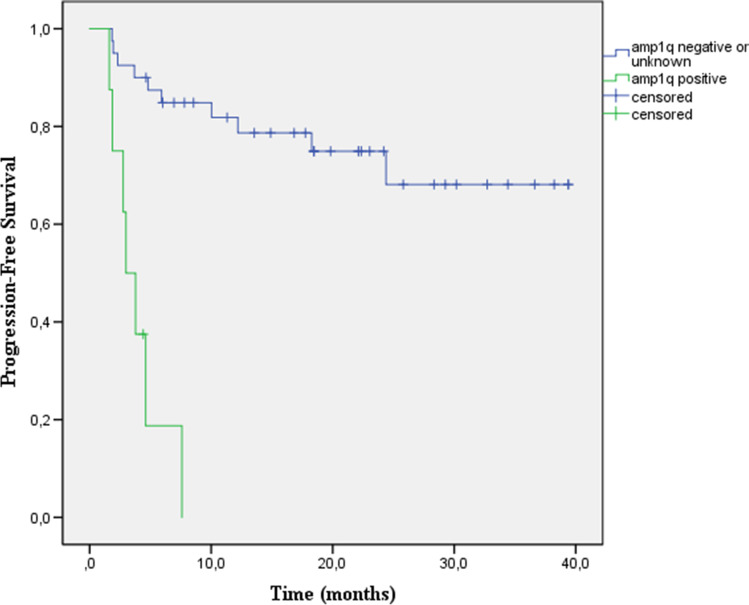
In this letter, we report a retrospective series of 8 consecutive RRMM patients harboring amp1q who received treatment at our institution with daratumumab-based triplet regimens at first (n = 7) or second (n = 1) relapse, with very dismal outcomes (Table S1). Interphase FISH was performed using locus-specific DNA probe 1q21/8p21 dual color FISH (Empire Genomics). The study was approved by local Ethical Committee (Protocol 0,036,084/21). On the data cutoff date 30 November 2021, after a median follow-up of 10.1 months (3.0–17.7), median PFS was 3.0 months (1.6–7.6), only 1 patient achieved very good partial response (VGPR), 7 patients discontinued treatment, all due to disease progression, and 4 have died. By contrast, during the same period, for RRMM patients without amp1q (n = 40) who received daratumumab-based rescues at comparable disease stages, the median PFS was not reached after a median follow-up of 18.4 months (2.3–39.4) (Fig. 1) (Table S2). Our findings support data on poor outcomes associated with daratumumab treatment in amp1q patients, even when daratumumab-based regimens are used very early in the treatment course. We believe that these patients should be considered for novel treatment strategies.
Fig. 1.
Kaplan–Meier curves showing progression-free survival in the groups stratified by amplification of chromosome arm 1q21
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
EB, MM, GL, LP, RM, and LM treated the patient and wrote the manuscript. FG and GC provided cytogenetic data and contributed to the drafting of the manuscript. All authors approved the final manuscript.
Funding
This work was supported by grants to ML from the “Progetto di Eccellenza Dipartimento MIUR 2017”; by grants to ML from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (IG 20,624–2018); the “Charity Dinner Initiative” in memory of Alberto Fontana for Associazione Italiana Lotta alle Leucemie, Linfoma e Mieloma (AIL)—Sezione “Luciano Pavarotti”— Modena-ONLUS; and the Fondazione Iris Ceramica Group.
Data availability
The data that support the findings of this study are available from the corresponding author [EB], upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the local Ethical Committee (CE protocol no. 0036084/21). Informed consent was obtained from all alive patients for being included in the study.
Competing interests
ML: advisory boards for Novartis, AbbVie, Sanofi, Daiichi-Sankyo, Grifols; honoraria for lectures from Gilead Sci, MSD, Jazz Pharma; travel grant from Gilead Sci.
RM: honoraria from AbbVie, Janssen, Astra Zeneca.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roberto Marasca and Mario Luppi equally contributed to the study.
References
- 1.Bisht K, Walker B, Kumar SK, et al. Chromosomal 1q21 abnormalities in multiple myeloma: a review of translational, clinical research, and therapeutic strategies. Expert Rev Hematol. 2021;14:1099–1114. doi: 10.1080/17474086.2021.1983427. [DOI] [PubMed] [Google Scholar]
- 2.D’Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the international staging system (R2-ISS) for overall survival in multiple myeloma A European myeloma network (EMN) report within the HARMONY project. J Clin Oncol. 2022;21:02614. doi: 10.1200/JCO.21.02614. [DOI] [PubMed] [Google Scholar]
- 3.Hanamura I (2021) Gain/amplification of chromosome arm 1q21 in multiple myeloma. Cancers 13. 10.3390/cancers13020256 [DOI] [PMC free article] [PubMed]
- 4.Schmidt TM, Fonseca R, Usmani SZ. Chromosome 1q21 abnormalities in multiple myeloma. Blood Cancer J. 2021;11:1–11. doi: 10.1038/s41408-021-00474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:1086–1107. doi: 10.1002/ajh.26590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Premkumar V, Pan S, Lentzsch S, Bhutani D (2020) Use of daratumumab in high risk multiple myeloma A meta-analysis. eJHaem 1:267–271. 10.1002/jha2.47 [DOI] [PMC free article] [PubMed]
- 8.Giri S, Grimshaw A, Bal S, et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors. JAMA Oncol. 2020;6:1–8. doi: 10.1001/jamaoncol.2020.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, Wu C-H, Cowan JM, et al. Outcomes of patients with multiple myeloma harboring chromosome 1q gain/amplification in the era of modern therapy. Ann Hematol. 2022;101:369–378. doi: 10.1007/s00277-021-04704-8. [DOI] [PubMed] [Google Scholar]
- 10.Mohan M, Weinhold N, Schinke C, et al. Daratumumab in high-risk relapsed/refractory multiple myeloma patients: adverse effect of chromosome 1q21 gain/amplification and GEP70 status on outcome. Br J Haematol. 2020;189:67–71. doi: 10.1111/bjh.16292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [EB], upon reasonable request.