Abstract
A key function of monocytes/macrophages (Mφ) is to present antigens to T cells. However, upon interaction with bacteria, Mφ lose their ability to effectively present soluble antigens. This functional loss was associated with alterations in the expression of adhesion molecules and CD14 and a reduction in the uptake of soluble antigen. Recently, we have demonstrated that Salmonella typhi flagella (STF) markedly decrease CD14 expression and are potent inducers of proinflammatory cytokine production by human peripheral blood mononuclear cells (hPBMC). In order to determine whether S. typhi and soluble STF also alter the ability of Mφ to activate T cells to proliferate to antigens and mitogens, hPBMC were cultured in the presence of tetanus toxoid (TT) or phytohemagglutinin (PHA) and either killed whole-cell S. typhi or purified STF protein. Both whole-cell S. typhi and STF suppressed proliferation to PHA and TT. This decreased proliferation was not a result of increased Mφ production of nitric oxide, prostaglandin E2, or oxygen radicals or the release of interleukin-1β, tumor necrosis factor alpha, interleukin-6, or interleukin-10 following exposure to STF. However, the ability to take up soluble antigen, as determined by fluorescein isothiocyanate-labeled dextran uptake, was reduced in cells cultured with STF. Moreover, there was a dramatic reduction in the expression of CD54 on Mφ after exposure to STF. These results indicate that whole-cell S. typhi and STF have the ability to alter in vitro proliferation to soluble antigens and mitogens by affecting Mφ function.
Monocytes/macrophages (Mφ) and other antigen-presenting cells (APC) play crucial roles in the protection of the host from invading pathogens. Among the most important functions of Mφ and other APC is their ability to take up, process, and present antigens to both naive and memory T cells (11, 17, 31, 57, 58). Mechanisms of antigen uptake by these cells include phagocytosis, pinocytosis, and macropinocytosis (11, 31, 58). These processes allow Mφ to take up particulate antigens, such as bacteria, and soluble antigens, including proteins and protein-antibody complexes (31). Once inside the cells, antigens are processed into smaller peptides in specialized compartments, become loaded onto major histocompatibility complex (MHC) antigen molecules, and are transported to the cell membranes, where they become available for presentation to T cells (11, 17, 31, 57, 58). Following the binding of MHC antigen-peptide complexes to T-cell receptors, and in the presence of adhesion molecules that increase APC–T-cell interactions and of secondary signals generated by costimulatory molecules (e.g., CD80, CD86, and CD40) and cytokines (e.g., interleukin 1β [IL-1β]), T lymphocytes become activated and mature into memory and effector cells (15, 50).
Recently, Pryjma et al. (39) showed a reduction in the expression of surface molecules CD14 and CD54 by human Mφ following phagocytosis of many whole-cell bacteria, including Staphylococcus aureus and Salmonella enteritidis. Similarly, Tsuyuguchi et al. (55) demonstrated that human peripheral blood mononuclear cells (hPBMC) incubated with killed complexes of Mycobacterium avium and Mycobacterium intracellulare exhibit decreased CD14 and CD11b expression, which was associated with a reduced ability of the cells to proliferate to both mitogens and the purified protein derivative of tuberculin. Similar results were reported by Gercken et al. (16), who demonstrated that incubating hPBMC with Mycobacterium tuberculosis results in decreased expression of HLA-DR and a reduced ability of hPBMC to proliferate to mitogens and tetanus toxoid (TT). Taken together, these studies suggest that decreases in T-cell proliferative responses observed following bacterial phagocytosis are the result of alterations in Mφ expression of adhesion and costimulatory molecules that affect T-cell–Mφ interactions and antigen presentation.
The human restricted intracellular pathogen Salmonella typhi, the causative agent of typhoid fever, survives within Mφ by a number of mechanisms, including suppression of macrophage activity (21, 60). We have shown recently that upon incubation of hPBMC with S. typhi flagella (STF), there is a rapid production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and IL-1β as well as the production of IL-6, IL-10, and gamma interferon (IFN-γ) (64). Similar to the observations of Pryjma et al. and Tsuyuguchi et al. (39, 55), we also observed a rapid decrease in the expression of CD14 on the Mφ population following exposure to STF (64). Based on these observations, we hypothesized that S. typhi, and in particular STF, may suppress in vitro T-cell proliferation to mitogens and specific antigens, as previously reported for other whole-cell bacteria. To this end, we examined the effects of whole-cell S. typhi or soluble purified STF protein on the ability of hPBMC to proliferate to mitogens and to TT and investigated the mechanisms involved.
MATERIALS AND METHODS
Reagents.
Indomethacin (IM), β2-mercaptoethanol (2-ME), polymyxin B (PMB), bovine serum albumin (BSA), and all reagents used in the Griess reaction were obtained from Sigma Chemical Co. (St. Louis, Mo.). l-Arginine and l-arginine-free medium were obtained from Gibco-BRL (Gaithersburg, Md.). Ng-monomethyl-l-arginine (NMMA) was obtained from Calbiochem-Behring Corporation (La Jolla, Calif.) and used at a concentration of 50 μM. TT was purchased from Wyeth (Marietta, Pa.). Neutralizing monoclonal antibodies to human IL-10 (clone JES3-19F1), TNF-α (clone MAB1), and anti-IL-6 (clone MQ2-13A5) were obtained from Pharmingen (San Diego, Calif.). Neutralizing monoclonal antibody to human IL-1β (clone MAB201) was obtained from R&D Systems (Minneapolis, Minn.). These neutralizing antibodies were used in our studies at concentrations 10- to 100-fold higher than the amounts required to neutralize 50% of the biological activity of the concentrations of the corresponding cytokines present in culture supernatants of hPBMC following exposure to STF (64).
Isolation of PBMC from healthy volunteers.
PBMC were isolated by density gradient centrifugation over lymphocyte separation medium (Organon-Teknika, Durham, N.C.) from healthy volunteers who had or had not been previously vaccinated with the licensed attenuated S. typhi Ty21a vaccine (Swiss Serum and Vaccine Institute, Berne, Switzerland) following the recommended protocol (four oral doses of ∼2 × 109 to 6 × 109 viable CFU/dose over a 7-day period). A total of 15 unvaccinated and 5 vaccinated volunteers were used in these studies. PBMC were aliquoted and frozen in RPMI containing 10% fetal calf serum, and 10% dimethyl sulfoxide with a controlled linear rate freezer apparatus (1°C per min; Planer Biomed, Salisbury, England) to preserve cell viability and maximize cell recovery. The cells were stored in liquid nitrogen until used.
Preparation of STF.
STF were purified at the Center for Vaccine Development by a bulk shearing method from the rough S. typhi strain Ty2R (9, 23, 51). This preparation was further purified over a column of PMB followed by an ENDX-B15 lipopolysaccharide (LPS) removal column (Associates of Cape Cod, Inc., Woods Hole, Mass.) (2, 61). ENDX-B15 consists of endotoxin-neutralizing protein coupled to a bead matrix. Endotoxin-neutralizing protein has a high affinity for the LPSs of many gram-negative strains (2, 61). This STF preparation formed a single strong precipitate band with a rabbit anti-Ty2R flagellum antiserum in an Ouchterlony gel, confirming that the protein in the purified preparation was STF. The purity of the 10-mg/ml stock of STF was determined by sodium dodecyl sulfate (SDS)–10 to 15% polyacrylamide gel electrophoresis Tris acetate minigels. PhastGel, gradient media, buffers, and silver stains were purchased from Pharmacia Biotech, Piscataway, N.J., and used according to the manufacturer’s instructions. The gels were treated with periodic acid prior to silver staining to oxidize and detect any LPS present in the preparations (54). The STF preparations used in these studies consisted of a single flagellin band of ∼55 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (9). Less than 24 pg of LPS/ml were present in the purified STF preparation, as determined by using chromogenic Limulus amebocyte lysate kits (Associates of Cape Cod, Inc.) (level of sensitivity, 24 pg/ml).
T-cell proliferation assay.
A standard T-cell proliferation assay was used to examine the response to the mitogen phytohemagglutinin (PHA) and TT in the presence of whole-cell S. typhi and purified STF. Briefly, 1.5 × 105 PBMC were added in triplicate to the wells of 96-well plates in RPMI 1640 medium containing 10 mM HEPES, 50 μg of gentamicin/ml, and 10% heat-inactivated human serum (cRPMI). Antigens were added to the following final concentrations unless otherwise noted: STF, 4 μg/ml; TT, 2 μg/ml; BSA, 4 μg/ml; and the malarial circumsporozoite repeat antigen NANP50, 4 μg/ml. Whole-cell bacteria were added at 2 × 105 particles per well in a final volume of 200 μl. IM, 2-ME, and neutralizing monoclonal antibodies to human cytokines were added at the concentrations indicated in Results and the figure legends. The final volume was 200 μl/well. The cells were cultured for 2 days (for PHA) or 6 days (for TT) at 37°C and 5% CO2, and 1 μCi of tritiated thymidine/well was added. The plates were harvested 20 h later on a Wallac (Gaithersburg, Md.) cell harvester, and incorporated thymidine was measured on a Wallac Trilux Microbeta counter.
Measurement of NO− production and PGE2.
NO− was measured by using the Griess reaction (22). Culture supernatants (100 μl) from cells incubated with medium, STF, or TT were added to 100 μl of 1% sulfanilamide–0.1% naphthylethylene diamine dihydrochloride–2% H3PO4 and incubated for 10 min at room temperature. The absorbance at 562 nm was measured on an enzyme-linked immunosorbent assay (ELISA) plate reader (Titertech Instruments, Huntsville, Ala.). A serial dilution of NaNO2 was used as a standard. The limit of detection of this assay was 10 μM NO2. The production of prostaglandin E2 (PGE2) was determined with a commercial competitive ELISA kit (R&D Systems) on the same supernatants in which NO− was measured by the Griess reaction. The limit of detection of this ELISA was 50 pg of PGE2/ml.
Soluble antigen uptake.
The ability to take up soluble antigen was measured with fluorescein isothiocyanate (FITC)-conjugated dextran (molecular weight, 50,700; Sigma). hPBMC (106) were incubated in the presence of either TT (2 μg/ml), STF (4 μg/ml), or S. typhi LPS (Difco, Detroit, Mich.) (10 ng/ml) for 24 h preceding the assay in a 24-well plate in a final volume of 1 ml of cRPMI/well containing 10% heat-inactivated human serum. We have previously observed that a 10-ng/ml concentration of LPS induces strong macrophage activation (64). The cells were isolated by first removing the nonadherent cells and adding 1 ml of ice-cold phosphate-buffered saline (PBS) containing 10 mM EDTA. The plates were incubated for 10 to 20 min on ice, and the adherent cells were harvested and added to the corresponding nonadherent cells. After being washed, the cells were divided and placed in 2 ml of cRPMI containing 10% human serum, and 1 ml of each cell preparation was placed either at 37°C or on ice for 10 min. One hundred micrograms of FITC-dextran/ml was added to the cultures and allowed to incubate for 30 to 40 min at 37°C or on ice. The cells were then stained with an anti-CD14 allophycocyanin-labeled monoclonal antibody (Caltag, Burlingame, Calif.) for 20 min on ice, washed once with ice-cold PBS, and run immediately on an Epics Elite flow cytometer-cell sorter system (Coulter Corp., Miami, Fla.). Analysis was performed with the WinList software package (Verity Software House, Topham, Maine). The percentages of cells that incorporated FITC-dextran was obtained by subtracting the percentage of cells that incorporated FITC-dextran at 0°C (on ice) from the percentage of cells that incorporated FITC-dextran at 37°C.
Flow cytometry.
hPBMC were stained for surface markers after a 24-h incubation in the absence or presence of TT (2 μg/ml), STF (4 μg/ml), or LPS (10 ng/ml). Briefly, the cells were collected from the wells as described previously and washed once with PBS (pH 7.4). The cells were then incubated with murine anti-CD54–FITC monoclonal antibody or normal mouse immunoglobulin (Ig)-FITC (Pharmingen) for 30 min at 4°C. The cells were washed twice with PBS (pH 7.4) containing 0.1% sodium azide and fixed with 1% formaldehyde. The samples were run on a Coulter EPICS Elite flow cytometer-cell sorter system and analyzed with the WinList software package.
Statistical analysis.
Student’s t test was used to compare data among different groups.
RESULTS
S. typhi decreases lymphoproliferative responses to PHA and TT.
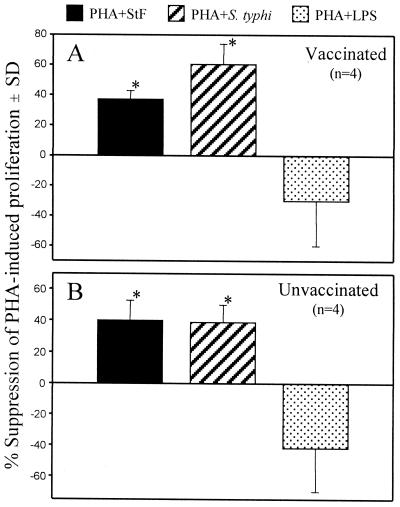
To determine whether whole-cell S. typhi and STF were able to alter T-cell proliferative responses, we investigated the effect of whole-cell S. typhi or STF on PHA-induced proliferation of hPBMC. The addition of whole-cell S. typhi decreased by ∼30 to 60% the ability of T cells to proliferate to PHA (Fig. 1). These suppressive effects were observed in both naive individuals and volunteers immunized orally with the S. typhi Ty21a typhoid vaccine (Fig. 1). Similar effects on PHA-induced proliferation were observed in vaccinated and unvaccinated volunteers when purified STF was added to the wells containing PHA (Fig. 1). In contrast, the addition of S. typhi LPS (10 ng/ml) exhibited a trend, albeit not statistically significant, to enhance PHA-induced lymphoproliferative responses (Fig. 1). Reductions in proliferative responses to PHA were not observed when BSA was added as a control protein (data not shown).
FIG. 1.
Suppression of PHA-induced proliferation by whole-cell S. typhi and STF. PBMC were cultured in the presence of PHA (2 μg/ml) alone or with STF (4 μg/ml), whole-cell S. typhi (2 × 105 particles per well), or LPS (10 ng/ml) for 3 days. Proliferation was determined by incorporation of tritiated thymidine. The data are expressed as mean percent suppression (standard deviation [SD]) of PHA-induced proliferation in four volunteers vaccinated with the S. typhi Ty21a typhoid vaccine (A) or four unvaccinated individuals (B) evaluated in two independent experiments with similar results. ∗, P < 0.05 compared to PHA-stimulated cells.
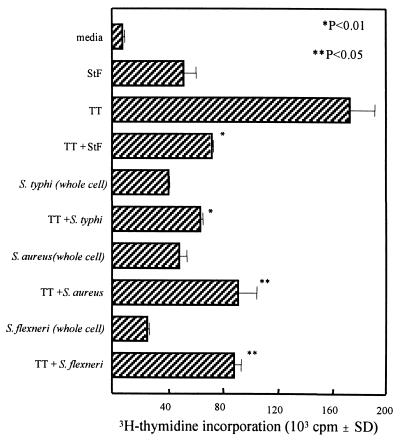
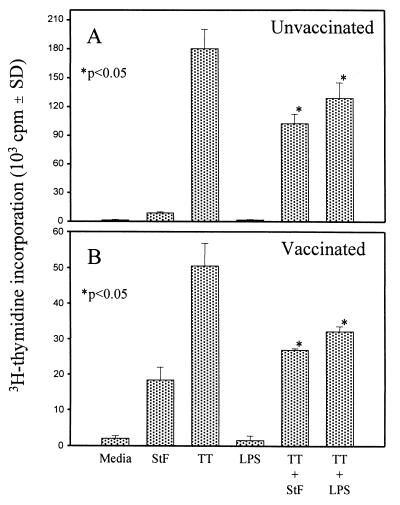
We next examined the ability of hPBMC to respond to the recall antigen TT in the presence of whole-cell bacteria or STF. Killed whole-cell S. typhi, S. aureus, and Shigella flexneri were all able to significantly reduce the proliferative responses of hPBMC to TT (Fig. 2). We also observed a significant reduction in proliferation to TT when LPS-free STF, as well as S. typhi LPS, was added to the cultures, indicating that both components are able to markedly suppress proliferative responses to TT (Fig. 2 and 3). These responses were observed in naive individuals as well as in volunteers immunized orally with the S. typhi Ty21a typhoid vaccine (Fig. 3). Addition of the control soluble protein BSA or the malarial circumsporozoite NANP50 repeat antigen had no effect on proliferation to TT (data not shown).
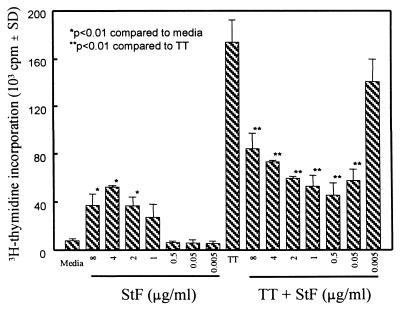
FIG. 2.
Suppression of PBMC proliferation to TT by STF and whole-cell killed bacteria. Whole-cell S. typhi, S. aureus, S. flexneri, and highly purified STF (4 μg/ml) were incubated with or without TT (2 μg/ml), and the ability to stimulate T-cell proliferation was determined. Shown are the results from an individual representative of two vaccinated volunteers evaluated in two independent experiments with similar results. The P values are as compared to cells treated with TT alone. SD, standard deviation.
FIG. 3.
STF and S. typhi LPS suppress PBMC proliferation to TT. PBMC were incubated in the absence or presence of soluble STF (4 μg/ml) or LPS (10 ng/ml) alone or with TT. Shown are results from an unvaccinated (A) and a vaccinated (B) volunteer from a total of seven individuals (three unvaccinated, four vaccinated) evaluated in five independent experiments with similar results. The P values are as compared to cells treated with TT alone. SD, standard deviation.
The observation that the suppressive effects of purified STF were similar in magnitude to those seen with whole-cell S. typhi suggested that STF is a major bacterial component mediating S. typhi-induced suppression of proliferation to TT. Therefore, we focused subsequent studies on the characterization of this STF-mediated phenomenon.
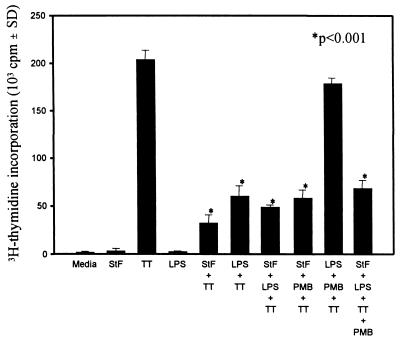
STF-induced suppression of PBMC proliferation to TT is not due to LPS contamination.
The ability of STF to reduce T-cell proliferation to TT was similar in magnitude to that observed with S. typhi LPS (10 ng/ml) (Fig. 3). The fact that our STF preparations had been rigorously purified and found to be LPS free by the Limulus amebocyte assay (sensitivity, 24 pg/ml) made it very unlikely that the observed decrease in T-cell proliferation to TT in the presence of STF was due to contaminating LPS. However, to explore this possibility further, we incubated hPBMC in the absence or presence of TT, LPS, or STF without or with PMB (4 μg/ml). PMB is known to bind LPS and inhibit its effects on a variety of cell types (48). As shown in Fig. 4, STF and LPS significantly decreased T-cell proliferation to TT. However, PMB was unable to reverse the suppression of proliferative responses induced by STF. On the contrary, PMB reversed the suppression of TT-induced proliferation mediated by S. typhi LPS. When STF and LPS were added together with TT, PMB was unable to reverse the suppression. These results confirm the fact that the suppression induced by STF on hPBMC proliferation to TT was not due to contaminating LPS in STF preparations.
FIG. 4.
PMB reverses LPS-induced suppression of PBMC proliferation to TT but not that induced by STF. PBMC were cultured with TT in the presence of STF (4 μg/ml), LPS (10 ng/ml) or the combination of STF and LPS alone or with PMB (4 μg/ml) as indicated. Shown are results from an individual representative of a total of seven volunteers (five vaccinated, two unvaccinated) evaluated in two independent experiments with similar results. In wells containing TT, the P value is as compared to cells treated with TT alone. SD, standard deviation.
STF reduces proliferation to TT in a dose-dependent manner.
To determine the minimal concentration at which STF suppresses TT-induced proliferation, hPBMC were cultured in the absence or presence of serial dilutions of STF in the presence of TT. As shown in Fig. 5, maximum suppression occurs between 0.5 and 2 μg of STF/ml. Typically, at the highest concentration tested (8 μg of STF/ml) there was a significant decrease in TT proliferative responses, albeit at a lower level. The suppressive effect of STF was lost at concentrations below 0.05 μg/ml. These data demonstrate that very low concentrations of STF (50 to 500 ng/ml) are sufficient to significantly decrease the in vitro proliferative responses to TT.
FIG. 5.
Dose-response of STF-mediated suppression of TT-induced proliferation by PBMC. PBMC from a vaccinated volunteer were incubated with STF and/or TT at the indicated concentrations, and T-cell proliferation was determined. Similar results were obtained with PBMC from an additional vaccinated volunteer. SD, standard deviation.
Production of nitric oxide (NO−) by STF is not responsible for STF-induced suppression of proliferation to TT.
Al Ramadi et al. and Huang et al. demonstrated that the suppression of murine T-cell responses to Salmonella typhimurium antigens is due to Mφ production of NO− (3, 22). Although production of NO− by human macrophages is still controversial, consensus for a role for macrophage-derived NO− in human infection and other diseases is emerging (4, 6, 24, 34, 37, 41, 44, 56, 59).
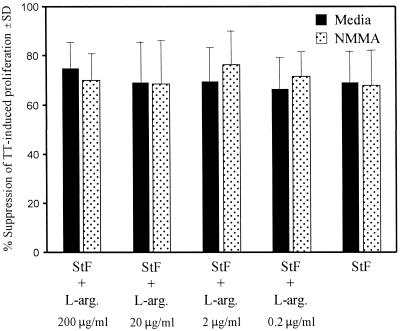
To determine whether the suppression observed with STF on TT-induced proliferation of hPBMC is due to the induction of the production of NO− by Mφ, hPBMC were cultured with TT alone or with STF or STF plus l-arginine in the absence or presence of 50 μM NMMA, a competitive inhibitor of NO− production (Fig. 6). Moreover, since the production of NO− is dependent upon the presence of l-arginine in the medium, we further evaluated the role of NO− production in STF-mediated suppression of TT-induced proliferation by determining whether the absence or presence of low l-arginine concentrations in the medium had any impact on STF-mediated suppression. To this end, we studied the effects of adding increasing concentrations of l-arginine to l-arginine-free RPMI 1640 on STF-mediated suppression of TT-induced proliferation.
FIG. 6.
Effects of NMMA on STF-mediated suppression of TT-induced proliferative responses. PBMC were incubated with TT alone (2 μg/ml) or with STF (4 μg/ml) or STF plus l-arginine (L-arg.) in the absence (Media) or presence of NMMA (50 μM), and T-cell proliferation was determined. The data are shown as the mean percentage of suppression (standard deviation [SD]) of proliferation to TT in the presence of STF alone or with NMMA, an inhibitor of nitric oxide synthesis, at decreasing concentrations of l-arginine induced by PBMC from three different vaccinated volunteers.
As shown in Fig. 6, the addition of NMMA had no significant effect on restoring the proliferative response to TT suppressed by the addition of STF. Furthermore, we observed that adding l-arginine to l-arginine-free RPMI 1640 up to the levels present in standard l-arginine-containing RPMI 1640 (∼200 μg/ml) (14), did not significantly alter STF-induced suppression of TT-induced proliferation (Fig. 6). Of note, all cultures contained low levels of l-arginine (∼6 to 23 μg/ml) from the 10% human serum-supplemented RPMI 1640 used in these studies (28). However, it is highly unlikely that Mφ were able to generate NO− from these very low concentrations of l-arginine in the presence of 50 μM NMMA. This concentration of NMMA has been shown to reverse the suppressive effects of NO− produced by splenocytes obtained from mice injected with S. typhimurium in a primary in vitro plaque-forming cell culture system that involved the use of media containing ∼130 μg of l-arginine/ml (minimal essential medium plus 10% fetal calf serum) (3).
To further explore the influence of NO− on STF-induced suppression of TT proliferation, the production of NO− was measured in cell culture supernatants generated in the absence or presence of either TT or STF. The production of nitric oxide was measured by the Griess reaction to detect the NO− conversion product NO2 (3). No production of NO− above the detection levels of our assay (10 μM) was observed in the presence of either of the antigens (data not shown).
Taken together, these results strongly suggest that STF-mediated suppression of TT-induced proliferation is not mediated by the production of NO−.
Production of prostaglandins and oxygen radicals by STF is not responsible for STF-induced suppression of proliferation to TT.
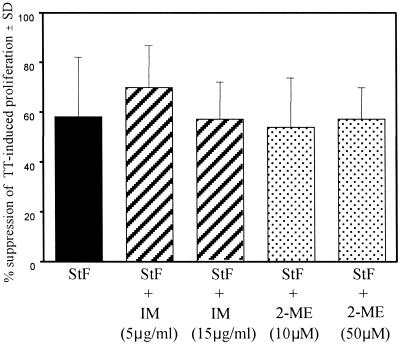
Prostaglandins and free oxygen radicals produced by Mφ have been shown to suppress T-cell responses (7, 36). To determine whether the suppression observed with STF of TT-induced proliferation of hPBMC is due to the production of these Mφ-derived products, hPBMC were cultured with TT alone or with STF alone or with IM, an inhibitor of prostaglandin synthesis, or the free oxygen radical scavenger 2-ME.
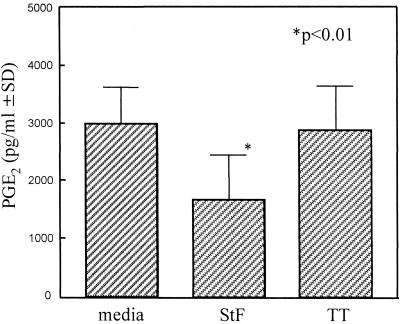
As shown in Fig. 7, nonsignificant reversals of S. typhi-mediated suppression of TT-induced proliferation by hPBMC were observed when IM (5 or 15 μg/ml) or 2-ME (10 or 50 μM) was added to the cultures. We further explored the possibility that PGE2 mediates STF-induced suppression of TT proliferation by measuring PGE2 secretion by hPBMC when exposed to STF or TT. PGE2 was measured with a commercial competitive ELISA kit. A background production of PGE2 was observed in the medium and TT wells, likely due to Mφ activation following adherence to plastic (30). A significant decrease in PGE2 production was observed in cultures containing STF compared to that in medium or TT (Fig. 8). However, no significant differences on PGE2 production were observed between cells cultured in the absence or presence of TT (Fig. 8). Taken together, these data indicate that the production of PGE2 or free oxygen radicals is unlikely to play a significant role in mediating the immunosuppressive effects of STF on TT-induced proliferation by hPBMC.
FIG. 7.
Effects of IM and 2-ME on STF-mediated suppression of TT-induced proliferative responses. PBMC were incubated with TT alone (2 μg/ml) or with STF (4 μg/ml) in the absence or presence of 2-ME or IM, and T-cell proliferation was determined. The data are shown as the mean percentage of suppression (standard deviation [SD]) of proliferation to TT in the presence of STF, alone or with the inhibitor IM or 2-ME, observed with PBMC from three different vaccinated volunteers.
FIG. 8.
Production of PGE2 by PBMC cultured with STF (4 μg/ml) or TT (2 μg/ml). PBMC were cultured in the absence (media) or presence of STF or TT, and PGE2 levels in the supernatants were measured by a competitive ELISA. Shown are the mean (standard deviation [SD]) PGE2 levels secreted by PBMC from four volunteers (three vaccinated, one unvaccinated). The P value is as compared to cells treated with medium or TT.
Failure of neutralizing antibodies to proinflammatory cytokines to restore proliferation to TT.
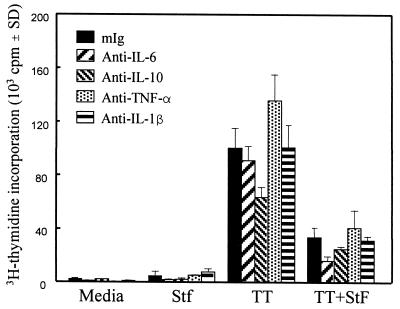
Recently, we demonstrated that incubation of hPBMC with STF causes the rapid production of high levels of the proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-10 (64). To explore the role of these potent immunomodulating cytokines in STF-induced suppression of proliferation to TT by hPBMC, neutralizing monoclonal antibodies were used to block the activities of these cytokines to determine whether their presence in the cultures caused the decrease in TT proliferative responses. As shown in Fig. 9, the addition of anti-proinflammatory cytokine antibodies (1 μg/ml) had no effect on restoring TT-induced proliferative responses. Similarly, the addition of combinations of neutralizing monoclonal antibodies to these cytokines in concentrations up to 5 μg/ml per antibody had no effect on reversing STF-induced suppression of TT-induced proliferation (data not shown). These results indicate that the production of high levels of the cytokines TNF-α, IL-1β, IL-6, and IL-10 was not directly responsible for the STF-induced suppression of hPBMC proliferative responses to TT.
FIG. 9.
Effects of neutralization of IL-1β, TNF-α, IL-6, or IL-10 on STF-induced suppression of the proliferation induced by TT. PBMC from a vaccinated individual were incubated with TT (2 μg/ml), STF (4 μg/ml), or a combination of TT and STF in the absence or presence of an optimal concentration (1 μg/ml) of neutralizing monoclonal antibodies to IL-1β, TNF-α, IL-6, or IL-10, and T-cell proliferation was determined. Similar results were obtained with PBMC from an additional vaccinated volunteer. mIg, normal mouse Ig. SD, standard deviation.
Soluble-antigen uptake is decreased by STF.
The ability of Mφ to take up antigen is critical to their role as antigen-presenting cells. Therefore, we investigated whether STF-mediated suppression of TT-induced proliferation by hPBMC might be the result, at least in part, of a reduced ability of Mφ to take up soluble antigen.
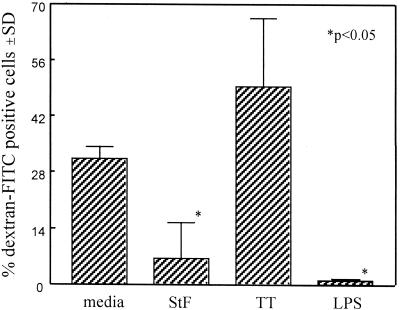
To this end, FITC-conjugated dextran (molecular weight, 50,700) was added to cultures which had previously been incubated with either TT or STF for 24 h. Antigen uptake was allowed to occur by incubating the culture at 37°C for 30 to 45 min. As a control, cultures were also incubated on ice, which inhibits the uptake of soluble antigen by Mφ. Compared to the cells incubated on ice (controls), when TT-stimulated hPBMC were incubated at 37°C with the FITC-conjugated dextran, a significant increase in the uptake of soluble antigen was observed (data not shown). Of the cells gated on the “Mφ region” (defined as cells with high forward light scatter versus high side light scatter during flow cytometric analysis) that had been preincubated with TT and exposed to FITC-dextran at 37°C, 49.4% ± 14% stained positive for FITC-dextran (Fig. 10). However, when the cells were preincubated with STF, only 6.5% ± 9% of the Mφ-gated cells became positive for the uptake of FITC-labeled dextran. LPS was also able to significantly decrease the ability of the Mφ-gated population to take up soluble antigen (<1% ± 0.2% of FITC-labeled cells). The latter observation is consistent with reports showing a decrease in the phagocytic ability of Mφ after exposure to LPS (49, 63). Throughout the various repeat experiments, we observed that the ability to take up FITC-labeled dextran was variable from individual to individual and among separate experiments. However, the majority of the individuals in the various repeat experiments yielded results similar to those of the representative experiment shown in Fig. 10. These results demonstrate that STF decreases the ability of Mφ to take up soluble antigen and may be responsible, at least in part, for the observed immunosuppressive effects of STF on the TT-induced proliferative responses of hPBMC.
FIG. 10.
Effects of STF on antigen uptake by Mφ. PBMC were incubated overnight in the absence (media) or presence of STF (4 μg/ml), TT (2 μg/ml), or LPS (10 ng/ml), and the uptake of FITC-labeled dextran was determined by flow cytometry, as described in Materials and Methods. The percentage of FITC-dextran-positive cells was calculated from cells gated on the Mφ region, characterized by high forward light scatter versus high side light scatter. Shown are the means (standard deviations [SD]) of the percentages of FITC-dextran-positive cells observed with PBMC from three volunteers (two vaccinated, one unvaccinated). The P value is as compared to cells treated with medium or TT.
Effects of STF on expression of the adhesion molecule CD54 (ICAM-1).
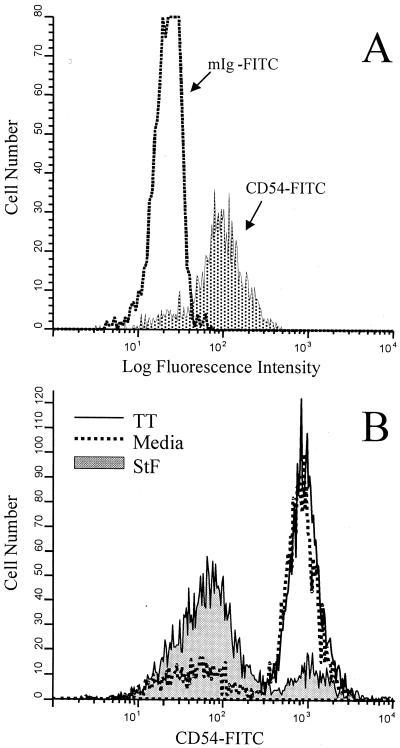
Pryjma et al. (39) and Tsuyuguchi et al. (55) have demonstrated that exposure to whole-cell bacteria results in decreased expression of cell surface adhesion molecules, including CD54 (intracellular adhesion molecule-1 [ICAM-1]). Since CD54 is a cell adhesion molecule on APC that plays a critical role in specific T-cell activation by enhancing the interactions between APC and T cells through binding to the CD11a-CD18 complex on T cells (47), we examined the expression of CD54 on human Mφ after exposure to STF. As shown in Fig. 11, there was a significant reduction in the expression of CD54 on human Mφ (e.g., cells gated on the “monocyte region”) as compared to cells incubated in the absence (media) or presence of TT. The monocyte region was defined based on the forward scatter versus side scatter characteristics of monocytes, as previously described (45, 64). In agreement with previous observations (47), marked increases in the expression of CD54 (compared to cells stained immediately after isolation) were observed as a result of the Mφ activation that takes place during the overnight incubation in medium (Fig. 11). The observed decreases in CD54 expression by Mφ following incubation with STF suggest that STF-mediated suppression of T-cell proliferative responses to antigens and mitogens is mediated, at least in part, through downregulation of CD54 expression and/or abrogation of the upregulation that follows Mφ activation.
FIG. 11.
Effects of STF on Mφ expression of CD54. PBMC from a vaccinated volunteer were stained immediately after isolation (A) or cultured overnight in the absence (Media) or presence of TT (2 μg/ml) or STF (2 μg/ml) (B). The cells were labeled with control antibodies (FITC-labeled mouse IgG [mIg-FITC]) or CD54-FITC, as described in Materials and Methods. The histograms shown correspond to cells gated on the Mφ region, characterized by high forward light scatter versus high side light scatter. (A) Dotted area represents cells expressing CD54 immediately after isolation. (B) Shaded area represents cells expressing CD54 after incubation with STF. Shown are results from an individual representative of five volunteers (four vaccinated, one unvaccinated) evaluated in two independent experiments with similar results.
DISCUSSION
In this manuscript we report that STF, as well as whole-cell S. typhi, markedly suppress lymphocyte proliferation by hPBMC in response to mitogens (e.g., PHA) and antigens (e.g., TT). These suppressive effects were observed in unvaccinated volunteers as well as in individuals previously immunized orally with the S. typhi Ty21a typhoid vaccine, indicating that STF-mediated effects on TT-induced proliferation are not dependent on the presence of acquired anti-S. typhi-specific immune responses. Moreover, we provide evidence that supports the hypothesis that these phenomena are the result, at least in part, of suppression of antigen uptake and decreased Mφ expression of CD54, leading to diminished antigen presentation.
The results showing that whole-cell S. typhi markedly suppresses proliferative responses to mitogens and antigens are consistent with those of Pryjma et al. showing that whole-cell bacteria (e.g., S. aureus, Escherichia coli, Pseudomonas aeruginosa, and S. enteritidis) suppress the in vitro responses to TT (39). We have now extended these observations by demonstrating that at least one of the major components on the surface of S. typhi, i.e., STF, at concentrations as low as 50 ng/ml, appears to be responsible for the observed immunomodulatory effects.
It is important to note that the STF-mediated suppressive effects observed in these studies were not due to LPS present in STF preparations. This assertion in based on four independent observations: (i) the levels of LPS added to the cultures at the final concentrations of STF used in these experiments could not have exceeded ∼1 fg/ml; (ii) unlike the LPS-mediated suppression of TT-induced proliferation, the suppression of TT-induced proliferation mediated by STF was not abrogated by PMB (Fig. 4); (iii) as opposed to STF, LPS did not suppress PHA-induced proliferation (Fig. 1); and (iv) in contrast to the strong suppression of CD54 expression in activated Mφ mediated by STF (Fig. 11), LPS is known to markedly increase CD54 expression in Mφ (19, 42).
To investigate the mechanisms involved in STF-mediated suppression of lymphoproliferative responses, we studied the effects of STF on several of the key phenomena associated with Mφ function. We concluded that production of NO−, PGE2, and free oxygen radicals was not a significant contributor to the STF-mediated suppression of TT-induced proliferation, since we did not observe increased levels of NO− or PGE2 in the supernatants of Mφ exposed to STF nor were inhibitors of NO−, prostaglandins, or free oxygen radical scavengers able to revert the observed suppression. Interestingly, we observed a decrease in the baseline release of PGE2 in the presence of STF (Fig. 8). Furthermore, we were unable to show that STF-mediated suppression was a result of the production of proinflammatory cytokines, since neutralizing antibodies to TNF-α, IL-1β, IL-6, and IL-10 had no effect on the ability of STF to suppress hPBMC proliferation to TT.
Results from our experiments appear to indicate that decreases in T-cell proliferation to TT after incubation with STF are the result of at least two independent mechanisms: (i) reduced antigen uptake and (ii) downregulation of adhesion and costimulatory molecules. Concerning reduced antigen uptake, we speculate that the decreased ability of human Mφ preincubated with STF to take up soluble antigen, as shown by the FITC-dextran experiments, would directly affect the amount of antigen available for presentation. It has been demonstrated that in the presence of high levels of TNF-α, Mφ and dendritic cells (DC) lose the in vivo ability to present soluble antigen by decreasing their ability to take up soluble antigen (29, 40, 46, 52). These effects are mediated, at least in part, through downregulation of mannose receptors, which play an important role in antigen capture (40). Since we previously demonstrated that incubation of hPBMC with STF induces high levels of the proinflammatory cytokines TNF-α and IL-1β (64), it was therefore conceivable that the high levels of these cytokines induced by STF are partly responsible for the observed effects. However, the fact that neutralizing antibodies to these cytokines did not block STF-mediated effects suggests that STF might mediate their effects through other, yet-undetermined mechanisms that, acting independently of these cytokines, lead to the immunoregulatory responses described above. Interestingly, using S. typhimurium phoP mutants, Wick et al. recently demonstrated that PhoP-regulated gene products have the ability to decrease the processing and presentation of S. typhimurium antigens by macrophages, suggesting a role for this virulence locus in the suppression of the induction of specific immunity (62).
The second mechanism leading to suppression, i.e., downregulation of important accessory and costimulatory molecules, is supported by our results showing a significant reduction in the expression of CD54 by human Mφ after exposure to STF (Fig. 11). These observations with highly purified STF support the hypothesis proposed by Pryjma (39) and Tsuyuguchi (55) based on studies with whole-cell bacteria, that the downregulation of adhesion and costimulatory molecules might lead to decreased cell-cell interactions between Mφ and T cells and diminished proliferative responses. Moreover, although not presented here, this hypothesis is also supported by previous research in our laboratory showing a substantial decrease in the expression of CD14 on human Mφ exposed to STF (64). Although the decreases in soluble antigen uptake would be unlikely to lead to the reduction in PHA responses induced by STF, the downregulation of adhesion molecules, such as CD54, may explain these observations. In fact, it is well known that T-cell responses to mitogens, such as PHA, is dependent on expression of adhesion molecules on APC (12, 26, 35). Current studies are directed toward further exploring the effects of STF on Mφ adhesion and accessory molecule expression. Taken together, the results presented in this report suggest that exposure to STF decreases the ability of Mφ to effectively present antigen to T cells.
S. typhi is an intracellular human pathogen known to have profound effects on the functions of the cells it infects. In macrophages, S. typhimurium inhibits the fusion of the phagosome with the lysosome and upon contact with the surface of the cell causes membrane ruffling in both Mφ and epithelial cells (8, 13). The results presented here showing that purified LPS-free STF decreases PGE2 production and reduces the ability to take up soluble antigen and the expression of CD54, as well as our previous work showing that STF is a potent inducer of proinflammatory cytokines by human Mφ and that it downregulates CD14 expression (64), clearly indicate that STF profoundly affects Mφ activation, differentiation, and function.
Current research on DC has shown that when human Mφ are cultured in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 followed by TNF-α, they differentiate into CD14(−) CD83(+) DC (27, 52, 65). Work by Sallusto et al. (40) demonstrated that DC incubated with TNF-α have a 10-fold decrease in their ability to present soluble TT. Furthermore, the Mycobacterium tuberculosis Calmette-Guérin bacillus has been shown to induce the release of TNF-α from cultured DC with a concurrent decrease in the uptake of soluble FITC-dextran (53). These observations suggest that high levels of TNF-α, such as those induced by STF, can cause changes in Mφ and/or DC differentiation and function which decrease the abilities of these cells to take up and present antigen or that these are concurrent, independent phenomena. Based on our observations that culturing hPBMC with STF causes the rapid and high-level production of TNF-α, as well as IL-1β, IL-10, and IL-6 (64), with a concurrent decrease in the expression of CD14 and decreased antigen uptake and CD54 expression, it is conceivable that exposure to STF leads Mφ to differentiate towards DC-like cells.
Recent evidence supports the hypothesis that there are several stages of DC maturation (5). The first stage, called the immature DC, consists of cells in peripheral tissues that have the ability to actively capture and process antigen (5). In the second stage, the cells migrate through blood or lymph to secondary lymphoid organs. These cells have a decreased ability to capture and present antigen, with concurrent alterations in the expression of adhesion molecules. The changes in expression of adhesion molecules are believed to allow these migratory cells to reach lymph nodes and other immune sites. Evidence supporting this hypothesis has been reported with murine and human DC. In mice, Hill et al. have shown that DC isolated from blood express decreased levels of CD54, class II MHC, and B7 in comparison to DC isolated from lymph nodes (20). In humans, while CD54 is present on mature lymphoid DC, it is not detectable on immature blood-derived DC or Langerhans cells (5, 65). In the final stage of maturation, “mature DC,” the cells reacquire the ability to present antigen very efficiently. The changes observed in the final stage of maturation are thought to be induced by either physical interactions or cytokine signals in the lymph nodes (5).
In support of this model, Jonuleit et al. have recently demonstrated that the addition of TNF-α–IL-1β–IL-6 to cultures of hPBMC-DC derived from granulocyte-macrophage colony-stimulating factor–IL-4 induces DC maturation (25). DC derived in this manner stimulate CD4+ T cells to produce IFN-γ but not IL-4. Similarly, we have been able to detect the production of IFN-γ but not IL-4 in STF-stimulated hPBMC (64). Moreover, Palucka et al. have shown that monocytes readily convert from monocytes to macrophages or DC depending upon the cytokines present (38). The presence of TNF-α and IL-1β generated DC with a less stable phenotype, which required a further signal to fully convert to mature DC. While removal of cytokines caused the DC to revert to macrophages, additional cytokines, Mφ-conditioned medium, or the ligation of CD40 fully maturated the cells to efficient antigen-presenting DC (38). This is supportive of a model in which the environment determines the functional phenotype of the cell. These observations may also explain the variability we observed in our FITC-dextran experiments. Varying cytokine concentrations and/or the kinetics of production of these cytokines or other, still-undetermined phenomena, could alter the degree of maturation of Mφ into DC, leading to variability in the suppression of FITC-dextran uptake in the experiments.
Based on the above discussion, we hypothesize that bacteria and their components, such as STF, might cause human Mφ to differentiate into a stage like that of the migratory stage of DC. This shift towards a migratory cell phenotype might facilitate the migration of these cells into secondary lymphoid tissues. Once the cells reach the lymph nodes and other lymphoid tissues, they might interact with T cells and/or stromal cells and, in the presence of cytokines and other factors in the local microenvironment, differentiate into mature, highly efficient APC. This process would lead to an increased probability of Mφ that had captured antigen in peripheral tissues coming into contact with specific T cells reactive to the antigens being presented.
S. typhi, as well as other intracellular pathogens, may have capitalized upon the process described above. By surviving within Mφ, which subsequently differentiate into migratory cells, S. typhi are carried systematically to the spleen and lymph nodes and throughout the reticuloendothelial system. We speculate that vaccine development can capitalize upon these mechanisms used by S. typhi to spread systemically as well. Attenuated S. typhi is being extensively studied as a carrier of foreign antigens in multicomponent vaccines (1, 10, 18, 32, 33, 43). These attenuated strains of S. typhi, alone or carrying foreign antigens, have a limited intracellular survival and are not likely to replicate within the host cells for more than a few replication cycles. However, it is likely that by the time that S. typhi organisms stop replicating intracellularly and die, the cells carrying the bacteria would have already delivered S. typhi antigens, as well as the expressed foreign antigens, to secondary lymphoid organs. In this way, vaccines based on attenuated S. typhi would be targeting the foreign antigens to lymphoid sites, where they will be more likely to be presented to specific T cells and other highly efficient APC, thus augmenting the efficacy of vaccination. Further research into Mφ maturation, differentiation, and trafficking induced by bacteria and bacterial components, such as STF, is ongoing to test this hypothesis.
ACKNOWLEDGMENT
This work was supported by Grant RO1 AI36525 from the National Institute of Allergy and Infectious Diseases, NIH.
REFERENCES
- 1.Aggarwal A, Kumar S, Jaffe R, Hone D, Gross M, Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990;172:1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert G, Baldwin G, Thompson C, Wainwright N, Novitsky T J, Gillis Z, Parsonnet J, Fleisher G R, Siber G R. Limulus antilipopolysaccharide factor protects rabbits from meningococcal endotoxin shock. J Infect Dis. 1992;165:494–500. doi: 10.1093/infdis/165.3.494. [DOI] [PubMed] [Google Scholar]
- 3.al Ramadi B K, Meissler J J, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 4.Arias M, Zabaleta J, Rodriguez J I, Rojas M, Paris S C, Garcia L F. Failure to induce nitric oxide production by human monocyte-derived macrophages. Manipulation of biochemical pathways. Allergol Immunopathol. 1997;25:280–288. [PubMed] [Google Scholar]
- 5.Austyn J M. Antigen uptake and presentation by dendritic leukocytes. Semin Immunol. 1992;4:227–236. [PubMed] [Google Scholar]
- 6.Bonecini-Almeida M G, Chitale S, Boutsikakis I, Geng J, Doo H, He S, Ho J L. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 7.Boraschi D, Ghezzi P, Pasqualetto E, Salmona M, Nencioni L, Soldateschi D, Villa L, Tagliabue A. Interferon decreases production of hydrogen peroxide by macrophages: correlation with reduction of suppressive capacity and of anti-microbial activity. Immunology. 1983;50:359–368. [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier N A, Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991;59:2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsiotis M, Weinstein D L, Karch H, Holder I A, O’Brien A D. Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun. 1984;46:814–818. doi: 10.1128/iai.46.3.814-818.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatfield S N, Dougan G. Attenuated Salmonella as a live vector for expression of foreign antigens. Part i. Expressing bacterial antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 331–341. [Google Scholar]
- 11.da Silva R. Endocytosis and vacuolar function. In: Herzenberg L A, Weir D M, Blackwell C, editors. Weir’s handbook of experimental immunology. Cambridge, Mass: Blackwell Science; 1996. pp. 168.1–168.12. [Google Scholar]
- 12.Dixon J F, Uyemura K. Highly purified human T-lymphocytes do not respond to mitogens—including calcium ionophore and phorbol ester. Immunol Investig. 1987;16:189–200. doi: 10.3109/08820138709030575. [DOI] [PubMed] [Google Scholar]
- 13.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 14.Freshney R I. The culture environment: substrate, gas phase, medium and temperature. In: Freshney R I, editor. Culture of animal cells. New York, N.Y: Wiley-Liss; 1994. pp. 71–103. [Google Scholar]
- 15.Geppert T D, Davis L S, Gur H, Wacholtz M C, Lipsky P E. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990;117:5–66. doi: 10.1111/j.1600-065x.1990.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 16.Gercken J, Pryjma J, Ernst M, Flad H D. Defective antigen presentation by Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:3472–3478. doi: 10.1128/iai.62.8.3472-3478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germain R N. Antigen processing and presentation. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1993. pp. 629–676. [Google Scholar]
- 18.Gonzalez C, Hone D, Noriega F R, Tacket C O, Davis J R, Losonsky G A, Nataro J P, Hoffman S, Malik A, Nardin E, Sztein M B, Heppner D G, Fouts T R, Isibasi A, Levine M M. Salmonella typhi vaccine strain CVD 908 expressing the circumsporozoite protein of Plasmodium falciparum: strain construction and safety and immunogenicity in humans. J Infect Dis. 1994;169:927–931. doi: 10.1093/infdis/169.4.927. [DOI] [PubMed] [Google Scholar]
- 19.Heinzelmann M, Mercer-Jones M A, Gardner S A, Wilson M A, Polk H C. Bacterial cell wall products increase monocyte HLA-DR and ICAM-1 without affecting lymphocyte CD18 expression. Cell Immunol. 1997;176:127–134. doi: 10.1006/cimm.1997.1089. [DOI] [PubMed] [Google Scholar]
- 20.Hill S, Coates J P, Kimber I, Knight S C. Differential function of dendritic cells isolated from blood and lymph nodes. Immunology. 1994;83:295–301. [PMC free article] [PubMed] [Google Scholar]
- 21.Hirose K, Ezaki T, Miyake M, Li T, Khan A Q, Kawamura Y, Yokoyama H, Takami T. Survival of Vi-capsulated and Vi-deleted Salmonella typhi strains in cultured macrophage expressing different levels of CD14 antigen. FEMS Microbiol Lett. 1997;147:259–265. doi: 10.1111/j.1574-6968.1997.tb10251.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Schwacha M G, Eisenstein T K. Attenuated Salmonella vaccine-induced suppression of murine spleen cell responses to mitogen is mediated by macrophage nitric oxide: quantitative aspects. Infect Immun. 1996;64:3786–3792. doi: 10.1128/iai.64.9.3786-3792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda T, Kamiya R, Yamaguchi S. Excretion of flagellin by a short-flagella mutant of Salmonella typhimurium. J Bacteriol. 1983;153:506–510. doi: 10.1128/jb.153.1.506-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jesch N K, Dorper M, Enders G, Rieder G, Vogelmeier C, Messmer K, Krombach F. Expression of inducible nitric oxide synthase and formation of nitric oxide by alveolar macrophages: an interspecies comparison. Environ Health Perspect. 1997;105S:1297–1300. doi: 10.1289/ehp.97105s51297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk A H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 26.Kern J A, Reed J C, Daniele R P, Nowell P C. The role of the accessory cell in mitogen-stimulated human T cell gene expression. J Immunol. 1986;137:764–769. [PubMed] [Google Scholar]
- 27.Kiertscher S M, Roth M D. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59:208–218. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 28.Konings C H. A kinetic procedure for the estimation of arginine in serum using arginine kinase. Clin Chim Acta. 1988;176:185–193. doi: 10.1016/0009-8981(88)90206-9. [DOI] [PubMed] [Google Scholar]
- 29.Kowalczyk D, Mytar B, Jasinski M, Pryjma J, Zembala M. Modulation of monocyte antigen-presenting capacity by tumour necrosis factor-alpha (TNF): opposing effects of exogenous TNF before and after an antigen pulse and the role of TNF gene activation in monocytes. Immunol Lett. 1995;44:51–57. doi: 10.1016/0165-2478(94)00184-s. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel S L, Duque R E. The macrophage adherence phenomenon: its relationship to prostaglandin E2 and superoxide anion production and changes in transmembrane potential. Prostaglandins. 1983;26:893–904. doi: 10.1016/0090-6980(83)90152-1. [DOI] [PubMed] [Google Scholar]
- 31.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 32.Levine M M, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol. 1996;44:193–196. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 33.Levine M M, Galen J E, Sztein M B, Beier M, Noriega F R. Attenuated Salmonella as a live vector for expression of foreign antigens. Part iii. Salmonella expressing protozoal antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 351–361. [Google Scholar]
- 34.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Maio M, Tessitori G, Pinto A, Temponi M, Colombatti A, Ferrone S. Differential role of distinct determinants of intercellular adhesion molecule-1 in immunologic phenomena. J Immunol. 1989;143:181–188. [PubMed] [Google Scholar]
- 36.Metzger Z, Hoffeld J T, Oppenheim J J. Macrophage-mediated suppression. I. Evidence for participation of both hydrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980;124:983–988. [PubMed] [Google Scholar]
- 37.Muhl H, Dinarello C A. Macrophage inflammatory protein-1 alpha production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor N(G)-monomethyl-l-arginine. J Immunol. 1997;159:5063–5069. [PubMed] [Google Scholar]
- 38.Palucka K A, Taquet N, Sanchez-Chapuis F, Gluckman J C. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–4595. [PubMed] [Google Scholar]
- 39.Pryjma J, Baran J, Ernst M, Woloszyn M, Flad H D. Altered antigen-presenting capacity of human monocytes after phagocytosis of bacteria. Infect Immun. 1994;62:1961–1967. doi: 10.1128/iai.62.5.1961-1967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarchielli P, Orlacchio A, Vicinanza F, Pelliccioli G P, Tognoloni M, Saccardi C, Gallai V. Cytokine secretion and nitric oxide production by mononuclear cells of patients with multiple sclerosis. J Neuroimmunol. 1997;80:76–86. doi: 10.1016/s0165-5728(97)00136-7. [DOI] [PubMed] [Google Scholar]
- 42.Schmittel A, Scheibenbogen C, Keilholz U. Lipopolysaccharide effectively up-regulates B7-1 (CD80) expression and costimulatory function of human monocytes. Scand J Immunol. 1995;42:701–704. doi: 10.1111/j.1365-3083.1995.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 43.Schodel F. Attenuated Salmonella as a live vector for expression of foreign antigens. Part ii. Carrying viral antigens. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 343–349. [Google Scholar]
- 44.Schoedon G, Schneemann M, Walter R, Blau N, Hofer S, Schaffner A. Nitric oxide and infection: another view. Clin Infect Dis. 1995;21(Suppl. 2):S152–S157. doi: 10.1093/clinids/21.supplement_2.s152. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro H M. Data analysis. In: Shapiro H M, editor. Practical flow cytometry. New York, N.Y: Wiley-Liss; 1995. pp. 179–216. [Google Scholar]
- 46.Shepherd V L, Lane K B, Abdolrasulnia R. Ingestion of Candida albicans down-regulates mannose receptor expression on rat macrophages. Arch Biochem Biophys. 1997;344:350–356. doi: 10.1006/abbi.1997.0219. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu Y, van Seventer G A, Horgan K J, Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 48.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J. 1996;315:679–686. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundaram R, O’Connor M, Cicero M, Ghaffar A, Gangemi J D, Mayer E P. Lipopolysaccharide-induced suppression of erythrocyte binding and phagocytosis via Fc gamma RI, Fc gamma RII, Fc gamma RIII, and CR3 receptors in murine macrophages. J Leukoc Biol. 1993;54:81–88. doi: 10.1002/jlb.54.1.81. [DOI] [PubMed] [Google Scholar]
- 50.Sztein M B, Mitchell G. Recent advances in immunology: impact on vaccine development. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 99–125. [Google Scholar]
- 51.Sztein M B, Wasserman S S, Tacket C O, Edelman R, Hone D, Lindberg A A, Levine M M. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 52.Thurnher M, Papesh C, Ramoner R, Gastl G, Bock G, Radmayr C, Klocker H, Bartsch G. In vitro generation of CD83+ human blood dendritic cells for active tumor immunotherapy. Exp Hematol. 1997;25:232–237. [PubMed] [Google Scholar]
- 53.Thurnher M, Ramoner R, Gastl G, Radmayr C, Bock G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–134. doi: 10.1002/(sici)1097-0215(19970106)70:1<128::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 54.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 55.Tsuyuguchi I, Kawasumi H, Takashima T, Tsuyuguchi T, Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990;58:1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tunctan B, Okur H, Calisir C H, Abacioglu H, Cakici I, Kanzik I, Abacioglu N. Comparison of nitric oxide production by monocyte/macrophages in healthy subjects and patients with active pulmonary tuberculosis. Pharmacol Res. 1998;37:219–226. doi: 10.1006/phrs.1997.0284. [DOI] [PubMed] [Google Scholar]
- 57.Unanue E R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 58.Van Furth R, Van Den Berg B. Phagocytosis and intracellular killing of microorganisms by polymorphonuclear and mononuclear phagocytes. In: Herzenberg L A, Weir D M, Blackwell C, editors. Weir’s handbook of experimental immunology. Cambridge, Mass: Blackwell Science; 1996. pp. 167.1–167.17. [Google Scholar]
- 59.Vitek M P, Snell J, Dawson H, Colton C A. Modulation of nitric oxide production in human macrophages by apolipoprotein-E and amyloid-beta peptide. Biochem Biophys Res Commun. 1997;240:391–394. doi: 10.1006/bbrc.1997.7408. [DOI] [PubMed] [Google Scholar]
- 60.Vladoianu I R, Chang H R, Pechere J C. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog. 1990;8:83–90. doi: 10.1016/0882-4010(90)90072-x. [DOI] [PubMed] [Google Scholar]
- 61.Wainwright N R, Miller R J, Paus E, Novitsky T J, Fletcher M A, McKenna T M, Williams T. Endotoxin binding and neutralizing activity by a protein from Limulus polyphemus. In: Nowotny A, Spitzer J J, Ziegler E J, editors. Cellular and molecular aspects of endotoxin reactions. Amsterdam, The Netherlands: Elsevier Science Publishers; 1990. pp. 315–325. [Google Scholar]
- 62.Wick M J, Harding C V, Twesten N J, Normark S J, Pfeifer J D. The phoP locus influences processing and presentation of Salmonella typhimurium antigens by activated macrophages. Mol Microbiol. 1995;16:465–476. doi: 10.1111/j.1365-2958.1995.tb02411.x. [DOI] [PubMed] [Google Scholar]
- 63.Wonderling R S, Ghaffar A, Mayer E P. Lipopolysaccharide-induced suppression of phagocytosis: effects on the phagocytic machinery. Immunopharmacol Immunotoxicol. 1996;18:267–289. doi: 10.3109/08923979609052736. [DOI] [PubMed] [Google Scholar]
- 64.Wyant, T., M. K. Tanner, and M. B. Sztein. Salmonella typhi flagella are potent inducers of pro-inflammatory cytokine secretion by human monocytes in vitro. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 65.Zhou L J, Tedder T F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]