Supplemental Digital Content is Available in the Text.
Increased levels of plasma oleoylethanolamide and stearoylethanolamide in patients with fibromyalgia and plasma palmitoylethanolamide and interstitial stearoylethanolamide in patients with chronic widespread pain suggest that the endocannabinoid system may be dysregulated in these conditions.
Keywords: Fibromyalgia, Chronic widespread pain, Endocannabinoid system, Endocannabinoids, N-acylethanolamines
Abstract
The endocannabinoid system (ECS) is an essential endogenous signaling system that may be involved in the pathophysiology of chronic widespread pain (CWP) and fibromyalgia syndrome (FMS). Further research is required to understand the role of ECS in the development and maintenance of CWP and FMS. We provided the first systematic review and meta-analysis exploring the clinical relevance of ECS alterations in patients with CWP and FMS by comparing plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients and healthy controls. A systematic search was conducted to identify studies that measured plasma and/or interstitial levels of endocannabinoids and N-acylethanolamines in patients with CWP or FMS and healthy controls. A total of 8 studies were included for qualitative review, and 7 studies were included for meta-analysis. The findings identified increased plasma levels of oleoylethanolamide and stearoylethanolamide in patients with FMS compared with those in controls (P = 0.005 and P < 0.0001, respectively) and increased plasma levels of palmitoylethanolamide and interstitial levels of stearoylethanolamide in patients with CWP compared with those in controls (P = 0.05 and P = 0.001, respectively). There were no significant differences in other ECS parameters. Most studies did not account for variables that may influence ECS function, including cannabis use, concomitant medication, comorbidities, physical activity, stress levels, circadian rhythm, sleep quality, and dietary factors, suggesting that future studies should explore the correlation between these variables and endocannabinoid activity. We highlight the importance of investigating endocannabinoid activity in CWP and FMS because it will underpin future translational research in the area.
1. Introduction
Fibromyalgia syndrome (FMS) is manifested by chronic widespread pain (CWP) as the cardinal symptom and other somatic and psychological impairments, including morning stiffness, severe fatigue, sleep disturbances, cognitive dysfunction, depression, and anxiety.7,51 Therefore, the terms fibromyalgia and CWP are often incorrectly used interchangeably. Chronic widespread pain may also occur as a symptom of other conditions, including musculoskeletal, metabolic, endocrine, psychological, neurological, and medication-related disorders.20 Failure to recognize other conditions that can masquerade as FMS could adversely affect patient outcomes.20 Chronic widespread pain and FMS are relatively common, affecting 2% to 5% of developed countries' populations.51 The most common criteria for diagnosis of FMS are the 1990 American College of Rheumatology (ACR) criteria, where CWP is defined as “pain lasting >3 months that affects both sides of the body both above and below the waist, including some part of the axial skeleton.”66 The ACR criteria were revised in 2010 and 2016 with the latest CWP definition standardized to pain in at least 4 of 5 pain regions.65 The heterogeneity of CWP and FMS risk factors and pathophysiological mechanisms requires individualized treatment strategies, with most having only moderately effective outcomes.40
The endocannabinoid system (ECS) is an essential endogenous signaling system involved in various physiological processes, including regulation of pain, inflammation, sleep, cognition, and energy metabolism.2,3,35,53,67 According to the classical definition, the ECS is composed of the type-1 and type-2 cannabinoid receptors (CB1 and CB2), endogenous cannabinoid receptor ligands (endocannabinoids), and enzymes responsible for endocannabinoid biosynthesis.12 A broader definition of the ECS also includes endocannabinoid-like lipid mediators, which belong to the same chemical class as the endocannabinoids (ie, amides, esters, and ethers of long-chain fatty acids) but bind to different receptors.12,61
Type-1 cannabinoid receptors are expressed mainly in the brain and, to a lesser extent, in the peripheral tissues, whereas CB2 receptors are primarily present in immune cells and tissues.38 The main endocannabinoids N-arachidonoylethanolamide (AEA or anandamide) and 2-arachidonoylglycerol (2-AG) bind to CB1 and CB2 receptors.3 Anandamide behaves as a partial agonist of CB1 but is virtually inactive at CB2, whereas 2-AG is a full agonist of CB1 and CB2.33 Therefore, AEA and 2-AG elicit various biological effects as cannabinoid receptor ligands, including the cannabinoid tetrad characterized by hypolocomotion, hypothermia, catalepsy, and analgesia.61 They also induce decreases in heart rate, blood, and intraocular pressure61 and show anti-inflammatory and immunomodulatory activity.62 Anandamide has also been shown to activate vanilloid receptors; however, its physiologic role as a vanilloid receptor agonist is not fully understood.11
Endocannabinoid-like lipid mediators such as N-acylethanolamines (NAEs), including palmitoylethanolamide (PEA), oleoylethanolamide (OEA), and stearoylethanolamide (SEA), exert their biological effects potentially through molecular targets other than cannabinoid receptors.61 Palmitoylethanolamide selectively activates peroxisome proliferator-activated receptor-alpha (PPAR-α) and G protein–coupled receptor 55 (GPR55)29,37 and demonstrates analgesic, anti-inflammatory, antiallergic, and neuroprotective properties.1 Oleoylethanolamide suppresses appetite, regulates food intake and body weight, and shows analgesic and anti-inflammatory properties using different mechanisms through activation of PPAR-α, GPR119, and vanilloid receptor 1 (TRPV1).29 Stearoylethanolamide demonstrates anti-inflammatory and neuroprotective properties,32 potentially through activation of PPAR-γ,5 although its molecular targets have yet to be fully elucidated.
In human studies, endocannabinoids and NAEs have been extracted from many biological fluids and tissues, including plasma and interstitial microdialysates, and quantified using various liquid chromatography methods coupled with mass spectrometry.68 Recently, research efforts have been devoted to understanding the relationship between ECS and chronic pain conditions, including FMS, suggesting that endocannabinoid deficiency could underpin the pathophysiology of such disorders.47–49 Therefore, further research is required to better understand the mechanisms of the ECS and implications for effectively treating CWP and FMS.
To date, it has not been established whether the endocannabinoid activity in patients with CWP and FMS correlates with the clinical status of the conditions. We explored this question with a systematic review and meta-analysis of the available evidence and investigated the clinical relevance of ECS alterations in patients with CWP and FMS by comparing the differences in plasma and interstitial levels of endocannabinoids and NAEs between patients and healthy controls.
2. Methods
The systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The protocol was registered in PROSPERO (CRD42021272567). The full search strategy can be found in the Supplementary Information—Research Protocol (available at http://links.lww.com/PR9/A177).
The systematic search was conducted by author I.K. from August 16, 2021, to August 18, 2021, using electronic databases MEDLINE (PubMed), CINAHL (Ebsco), PsycINFO (Ebsco), and Scopus (Elsevier) to identify publications reporting plasma and/or interstitial levels of endocannabinoids and NAEs in patients with CWP and FMS.
2.1. Inclusion criteria
The inclusion criteria were studies reporting plasma and/or interstitial levels of endocannabionoids (anandamide, 2-arachidonoylglycerol) and N-acylethanolamines (palmitoylethanolamide, oleoylethanolamide, stearoylethanolamide) in patients with clinically diagnosed CWP or FMS when compared against healthy controls.
2.2. Exclusion criteria
(1) In vitro and animal studies.
(2) Systematic reviews or meta-analyses.
(3) Studies lacking numerical data.
(4) Language other than English, German, or Russian.
2.3. Search terms
(1) “Chronic widespread pain” OR fibromyalgia.
(2) Endocannabinoid OR Anandamide OR N-arachidonoylethanolamide OR N-acylethanolamine OR Arachidonoylethanolamide OR 2-arachidonoylglycerol OR oleoylethanolamide OR palmitoylethanolamide OR stearoylethanolamide.
(3) 1 AND 2.
Search results were limited to human studies. Titles and abstracts of retrieved publications were imported into an EndNote Library. Duplicates were identified and removed. Studies were then analyzed by screening through titles and then abstracts by authors I.K. and J.S. Studies that clearly did not satisfy the inclusion criteria were excluded; full texts of the remaining studies were obtained. After reading the full texts, an agreement was reached between all review authors on a final selection of reports to be included. Author I.K. extracted data from all included studies into an electronic summary table, which was reviewed by author J.S.
Two reviewers (authors J.S. and I.K.) independently assessed the quality of the included studies using the modified Newcastle-Ottawa scale and through this process, achieved identical scores in every category, as well as overall (full consensus) (Table 1).
Table 1.
Quality assessment of included studies based on the Newcastle-Ottawa scale.
| Selection | Comparability | Outcome | Overall score (out of 8) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate case definition | Representativeness of the sample | Selection of controls | Definition of controls | Age | Sex | Assessment of the outcome | Statistical test | ||
| Baumeister et al.4 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 |
| Ghafouri et al.16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Hellström et al.21 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Kaufmann et al.34 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Stensson et al.56 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Stensson et al.55 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Stensson et al.57 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Stensson et al.54 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
Adapted version of the Newcastle-Ottawa quality assessment scale for case-control studies:
Selection (max 4 points):
(1) Is the case definition adequate? (1) yes, with independent validation (American College of Rheumatology 1990) (1 point); (2) yes, eg, record linkage or based on self-reports;and (3) No description.
(2) Representativeness of the sample: (1) consecutive or obviously representative series of cases (1 point); (2) potential for selection biases or not stated.
(3) Selection of controls: (1) community controls (1 point); (2) hospital controls; and (3) no description.
(4) Definition of controls: (1) no history of disease (1 point); (2) no description of source.
Comparability (max 2 points):
(1) Comparability of cases and controls based on the design or analysis: (1) study controls for age (1 point); (2) study controls for sex (1 point).
Outcome (max 2 points):
(1) Assessment of the outcome: (1) objective measure (1 point); (2) validated self-report measures (1 point); and (3) no information or nonvalidated measures.
(2) Statistical test: (1) The statistical test used to analyze the data is clearly described and appropriate, and the measurement of the association is presented, including confidence intervals and/or the probability level (P value) (1 point); (2) the statistical test is not appropriate, not described, or incomplete.
2.4. Outcomes and meta-analyses
We investigated the clinical relevance of ECS alterations in patients with CWP and FMS by comparing the differences in plasma and interstitial levels of endocannabinoids and NAEs between patients and healthy controls.
A quantitative analysis of the differences in ECS markers between patients and healthy controls was performed when 2 or more studies were available in each subgroup (FMS/CWP). Median values, interquartile ranges, standard errors, and confidence intervals (CIs) were converted to mean values and SDs following validated protocols.22,28
All meta-analyses were conducted in RevMan, version 5.4.59 The standardized mean difference (SMD) was used as summary statistics in meta-analyses. Because meta-analyses of observational studies are typically characterized by significant heterogeneity, we pooled data using random-effects models.42 Statistical significance between patients and controls was assessed with a z test, where P < 0.05 was considered statistically significant.
We assessed heterogeneity with the Cochrane Q test, where P < 0.10 represents statistically significant between-study heterogeneity, and the degree of heterogeneity was quantified with the I2 index, where I2 values above 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively.23,24 Sensitivity analyses were performed using the “leave-one-out” method by removing a single study each time and repeating the analysis to estimate the effect of any individual study on the overall SMD.
To identify factors affecting the levels of endocannabinoids and NAEs, qualitative data were collected, including the sampling, analytical methods and diagnostic criteria used, the number of participants, their age, sex, body mass index (BMI), medication use, cannabis use, comorbidities, the intensity of pain, and depression and anxiety levels.
3. Results
The initial database search identified 49 citations. After removing duplicates and screening titles, abstracts, and full texts, a total of 8 studies met the inclusion criteria for qualitative analysis.4,16,21,34,54–57 Of those 8 studies, 1 was excluded from meta-analysis because of lack of numerical data reported, leaving a total of 7 studies eligible for meta-analysis.4,16,21,54–57 See the PRISMA diagram in Figure 1.
Figure 1.
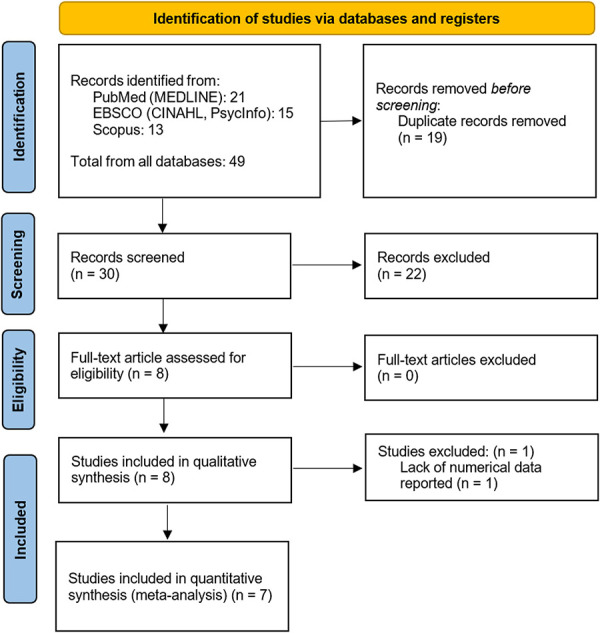
Preferred reporting items for systematic reviews and meta-analyses flow diagram of study selection process.
The characteristics of included studies are summarized in Table 2. Four articles investigated the ECS markers in patients with FMS4,34,54,57 and 4 studied CWP cohorts.16,21,55,56 One of the CWP studies reported morning and afternoon data21 with the data captured from separate sample groups (morning and afternoon); therefore, we included these study results as 2 individual reports. Two studies collected samples before and after exercise: one investigated plasma levels in patients with FMS54 and the other studied interstitial levels in patients with CWP.16 No other study reported data on the changes of ECS markers in response to exercise; therefore, only data collected before exercise were included in the meta-analyses.
Table 2.
Main characteristics of studies included in qualitative analysis.
| First author (year) | No. of participants | Diagnostic criteria | ECS markers studied | Sampling/analytical method/s | Age, y | Male/female | BMI, kg/m2 | Cannabis use | Medication use | Comorbidities | Intensity of pain, depression and anxiety | Other blood markers | Main outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baumeister (2018) | n = 89 (FMS) n = 36 (HC) |
ACR 1990 | Plasma AEA, 2-AG, PEA | Venepuncture/liquid chromatography/multiple reaction monitoring (LC/MRM) | FMS: 55.4 (9.5) HC: 61.4 (12.4) |
FMS: 7/82 HC: 20/16 |
FMS: 29.2 (6.5) HC: 26.9 (4.0) |
Cannabis users were excluded from the study | Antidepressants: FMS: 41.6% (38) HC: 0% (0), P = 0.001 Opiates: FMS: 5.6% (6) HC: 0% (0), P = 0.1 NSAIDs: FMS: 58.4% (53) HC: 13.9% (5), P = 0.001 Channel blockers: FMS: 15.7% (14) HC: 0% (0), P = 0.01 |
Diabetes mellitus: FMS: 11.2% (10) HC: 2.8% (1), P = 0.1 |
Average pain/4 wk (NRS 0–10): FMS: 6.2 (1.6) HC: 0.0 (0.0), P = 0.001 Depression (HADS): FMS: 8.1 (4.4) HC: 3.0 (3.2), P = 0.001 Anxiety (HADS): FMS: 9.2 (4.2) HC: 3.6 (2.5), P = 0.001 |
Not reported | FMS (pmol/L): AEA: 0.56 (0.31) 2AG: 2.18 (1.20) PEA: 5.92 (2.14) |
HC (pmol/L): AEA: 0.55 (0.28) 2AG: 2.13 (1.20) PEA: 5.80 (2.53) |
BF10: AEA: 0.22 2AG: 0.21 PEA: 0.24 |
|
| Kaufmann (2008) | n = 22 (FMS) n = 22 (HC) |
ACR 1990 | Plasma AEA | Venepuncture/high performance liquid chromatography-tandem mass spectrometry (HPLC/MS–MS) | FMS: 53.1 (2.1) HC: 48.1 (5.1) |
FMS: 5/17 HC: 5/17 |
Not reported | Not reported | Not reported Participants had abstained from their medication for at least 24 h before blood sampling. Subjects taking immunosuppressive or acetylsalicylic acid medication were excluded |
Not reported Subjects with inflammatory conditions, diabetes mellitus, endocrinologic disorders including thyroid disease, infections, hypertension, muscle or joint diseases, acute injury, pregnancy, major depressive disorder, addiction, general anxiety disorder, psychosis, major personality disorder, or current compensation claims were excluded |
Pain (0–10 cm VAS): FMS: 6.4 (1.4) HC: 0.0 (0.0), P < 0.0001 Stress (PTSS-10): FMS: 46.7 (3.5) HC: 19.0 (2.3), P < 0.001 |
Plasma epinephrine and norepinephrine, serum cortisol, white blood cell count, neutrophil function (H2O2 production, adhesion and phagocytosis capabilities) | AEA (μg/L): FMS vs HC: P < 0.0001 |
|||
| Stensson (2018) | n = 104 (FMS) n = 116 (HC) |
ACR 1990 | Plasma AEA, OEA, PEA, SEA, 2-AG | Venepuncture/liquid chromatography-tandem mass spectrometry (LC-MS/MS) |
FMS: 50.8 (9.7) HC: 48.1 (5.1) |
FMS: 0/104 HC: 0/116 |
FMS: 27.7 (5.1) HC: 24.0 (3.7) |
Not reported | Not reported Participants had to refrain from analgesics, NSAIDS, or hypnotics for 48 h before examinations |
Not reported Patients with FMS with high blood pressure, osteoarthritis (hip/knee), other severe somatic or psychiatric disorders, and primary causes of pain other than FMS were excluded from the study |
Pain (100-mm VAS): FMS: 51.2 (21.6) HC: 2.7 (6.6) Depression (HAD-D): FMS: 7.3 (3.6) HC: 1.8 (2.4) Anxiety (HAD-A): FMS: 8.7 (4.1) HC: 3.4 (3.2) |
Not reported | FMS (nM): AEA: 0.31 (0.15) OEA: 6.2 (2.3) PEA: 9.5 (2.4) SEA: 2.6 (1.0) 2AG: 14.5 (9.2) |
HC (nM): AEA: 0.31 (0.15) OEA: 5.4 (1.8) PEA: 8.6 (2.5) SEA: 2.1 (0.9) 2AG: 11.1 (5.1) |
P: AEA: 0.99 OEA: 0.006 PEA: 0.010 SEA: 0.001 2AG: 0.001 |
|
| Stensson (2020) | Primary cohort: n = 37 (FMS) n = 33 (HC) |
ACR 1990 | Plasma AEA, OEA, PEA, SEA, 2-AG | Venepuncture/liquid chromatography-tandem mass spectrometry (LC-MS/MS) |
FMS: 50.1 (10.5) HC: 50.3 (12.8) |
FMS: 0/37 HC: 0/33 |
FMS: 26.5 (4.7) HC: 24.4 (4.4) |
Not reported | Not reported Participants had to refrain from analgesics, NSAIDS, or hypnotics for 48 h before examinations |
Not reported Patients with FMS with high blood pressure, osteoarthritis (hip/knee), other severe somatic or psychiatric disorders, and primary causes of pain other than FMS were excluded from the study |
Pain (100-mm VAS): FMS: 48.7 (22.9) HC: 1.9 (5.9), P = 0.001 Depression (HAD-D): FMS: 7.7 (4.2) HC: 3.1 (3.0), P = 0.001 Anxiety (HAD-A): FMS: 8.8 (4.7) HC: 1.5 (1.8), P = 0.001 |
Not reported | FMS (nM): Pre-exercise: AEA: 0.26 (0.13) OEA: 5.66 (1.92) PEA: 8.51 (2.03) SEA: 2.56 (1.18) 2AG: 12.8 (8.49) Post-exercise: AEA: 0.34 (0.18) OEA: 5.33 (2.11) PEA: 7.96 (2.69) SEA: 2.05 (1.10) 2AG: 11.5 (8.03) |
HC (nM): Pre-exercise: AEA: 0.31 (0.16) OEA: 5.39 (1.29) PEA: 8.72 (1.63) SEA: 2.05 (0.83) 2AG: 12.5 (3.97) Post-exercise: AEA: 0.25 (0.14) OEA: 6.06 (1.96) PEA: 9.18 (2.54) SEA: 2.57 (1.06) 2AG: 12.6 (6.17) |
P: Pre-exercise: AEA: 0.12 OEA: 0.50 PEA: 0.63 SEA: 0.04 2AG: 0.83 Post-exercise: AEA: 0.03 OEA: 0.14 PEA: 0.06 SEA: 0.05 2AG: 0.51 |
|
|
Hellström (2016) |
Morning sample: n = 5 (CWP) n = 15 (HC) Afternoon sample: n = 9 (CWP) n = 12 (HC) |
ACR 1990 | Plasma 2-AG, AEA, PEA, SEA, OEA, LEA | Venepuncture/Ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) |
CWP: 55.5 (6.0) HC: 47.5 (9.0) |
CWP: 0/14 HC: 0/27 |
FMS: 26.8 (4.3) HC: 24.0 (3.0) |
Not reported | Not reported All participants were asked not to use any pain medications except for paracetamol preparations 3 days before the blood sampling |
Not reported Subjects with rheumatoid arthritis, systemic lupus erythematosus, Bechterew disease, multiple sclerosis, epilepsy or Parkinson disease, type I diabetes, cardiovascular disease, or endocrine diseases were excluded |
Average pain/1 wk (NRS 0–10): CWP: 5.3 (1.3) HC: no pain Current pain (NRS 0–10): CWP: 3.8 (1.8) HC: no pain Depression/anxiety: Not reported |
9-HODE, 13-HODE, 9,10-DiHOME, 12,13-DiHOME, 9,10,13-TriHOME, 9,12,13-TriHOME, 13-oxo-ODE, 5-HETE, 8,9-DiHETrE |
CWP (nM) | HC (nM) | ||
|
AM: 2AG: 3.28 (2.12) AEA: 0.16 (0.16) PEA: 2.08 (1.12) SEA: 11.31 (8.34) OEA: 1.76 (1.01) LEA: 0.62 (0.60) |
PM: 8.30 (10.83) 0.22 (0.17) 2.96 (1.68) 10.69 (5.58) 1.29 (1.39) 0.71 (0.90) |
AM: 5.38 (3.22) 0.18 (0.05) 1.93 (0.53) 8.44 (3.74) 0.93 (1.25) 0.62 (0.14) |
PM: 6.39 (8.50) 0.19 (0.10) 2.73 (0.89) 11.22 (4.02) 1.77 (0.73) 0.95 (0.34) |
|||||||||||||
| Stensson (2017) | n = 17 (CWP) n = 21 (HC) |
ACR 1990 | Plasma OEA, PEA, SEA | Venepuncture/Liquid chromatography-tandem mass spectrometry (LC-MS/MS) |
CWP: 41.7 (10) HC: 47.9 (9.6) |
CWP: 0/17 HC: 0/21 |
FMS: 24.2 (2.1) HC: 26.8 (5.3) |
Not reported | Not reported. | Not reported. Subjects with bursitis, disorders of the spine, tendonitis, capsulitis, postoperative conditions (neck/shoulder), prior neck trauma, neurological disease, rheumatoid arthritis or any other systemic disease, metabolic disease, malignancy, severe psychiatric illness, pregnancy were excluded. |
Pain (NRS 0–10): CWP: 5.3 (2.1) HC: 0.0 (0.0), P < 0.001 Depression/Anxiety: Not reported |
IL-6, IL-8, IL-10, TNF-α, IL-1β | CWP (nM): OEA: 11.1 (3.0) PEA: 18.1 (9.7) SEA: 38.6 (28.7) |
HC (nM): OEA: 7.5 (3.7) PEA: 10.5 (6.2) SEA: 27.2 (20.7) |
P: OEA: 0.003 PEA: 0.006 SEA: 0.164 |
|
| Ghafouri (2013) | n = 18 (CWP) n = 24 (HC) |
ACR 1990 | Interstitial PEA and SEA (trapezius muscle) | Microdialysis/liquid chromatography-tandem mass spectrometry (LC–MS/MS) | CWP: 47.6 (8.3) HC: 42.8 (7.3) |
CWP: 0/18 HC: 0/24 |
FMS: 27.5 (4.0) HC: 24.3 (2.8) |
Not reported | Not reported Participants were asked not to take nonsteroidal anti-inflammatory drugs for 7 d and/or paracetamol medication for 12 h before the experiment |
Not reported Subjects with bursitis, disorders of the spine, tendonitis, capsulitis, postoperative conditions (neck/shoulder), prior neck trauma, neurological disease, rheumatoid arthritis or any other systemic disease, metabolic disease, malignancy, severe psychiatric illness, and pregnancy were excluded |
Pain (NRS 0–10): CWP vs HC: P = 0.001 Depression/anxiety: Not reported |
Not reported | CWP (nM): Pre-exercise: PEA: 1.23 (0.55) SEA: 1.48 (0.93) Post-exercise: PEA 0.95 (0.58) SEA: 0.95 (0.70) |
HC (nM): Pre-exercise: PEA: 1.17 (0.84) SEA: 0.98 (0.68) Post-exercise: PEA: 0.85 (0.55) SEA: 0.75 (0.43) |
P: Pre-exercise: PEA: NS SEA: NS Post-exercise: PEA: NS SEA: NS |
|
| Stensson (2016) | n = 17 (CWP) n = 19 (HC) |
ACR 1990 | Interstitial OEA, PEA, and SEA (trapezius muscle) | Microdialysis/liquid chromatography-tandem mass spectrometry (LC-MS/MS) |
CWP: 48.8 (10.0) HC: 41.8 (10.7) |
CWP: 0/17 HC: 0/19 |
FMS: 27.5 (5.7) HC: 24.5 (2.9) |
Not reported | Not reported. | Not reported Subjects with bursitis, disorders of the spine, tendonitis, capsulitis, postoperative conditions (neck/shoulder), prior neck trauma, neurological disease, rheumatoid arthritis or any other systemic disease, metabolic disease, malignancy, severe psychiatric illness, and pregnancy were excluded |
Pain (NRS 0–10): CWP vs HC: P < 0.001 Depression/anxiety: Not reported |
Not reported | CWP (nM) 120 min: OEA: 2.22 (1.78) PEA: 0.93 (0.50) SEA: 3.67 (3.54) |
HC (nM) 120 min: OEA: 0.34 (0.48) PEA: 0.55 (0.95) SEA: 0.74 (0.69) |
P: OEA: 0.000 PEA: 0.148 SEA: 0.001 |
|
2-AG, 2-arachidonoylglycycerol; ACR, American College of Rheumatology; AEA, N-arachidonoylethanolamide, or anandamide; AM, morning sample; CWP, chronic widespread pain; FMS, fibromyalgia syndrome; HCs, healthy controls; IL, interleukin; LEA, linoleoylethanolamide; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; PM, afternoon sample; SEA, stearoylethanolamide; TNF-α, tumor necrosis factor-α.
3.1. Meta-analyses
Seven individual meta-analyses were performed to compare the differences in plasma and interstitial levels of endocannabinoids and NAEs between people with CWP and FMS and healthy controls. Table 3 contains a summary of statistics on the meta-analyses.
Table 3.
Results of meta-analyses of plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with fibromyalgia syndrome and chronic widespread pain.
| Outcome | No. of reports | No. of subjects | SMD (95% CI) | P (overall effect) | Heterogeneity I2, χ2, P | |
|---|---|---|---|---|---|---|
| Case | Control | |||||
| Plasma AEA | ||||||
| FMS vs HC | 3 | 230 | 185 | −0.05 (−0.25, 0.15) | 0.61 | 0%, 1.77, 0.41 |
| Plasma 2-AG | ||||||
| FMS vs HC | 3 | 230 | 185 | 0.22 (−0.09, 0.53) | 0.16 | 52%, 4.20, 0.12 |
| Plasma PEA | ||||||
| FMS vs HC | 3 | 230 | 185 | 0.15 (−0.14, 0.44) | 0.31 | 46%, 3.73, 0.15 |
| CWP vs HC | 3 | 31 | 48 | 0.53 (0.01, 1.06) | 0.05 | 17%, 2.40, 0.30 |
| Total | 6 | 261 | 233 | 0.24 (−0.02, 0.51) | 0.07 | 37%, 7.98, 0.16 |
| Plasma OEA | ||||||
| FMS vs HC | 2 | 141 | 149 | 0.33 (0.10, 0.57) | 0.005 | 0%, 0.68, 0.41 |
| CWP vs HC | 3 | 31 | 48 | 0.44 (−0.47, 1.35) | 0.34 | 70%, 6.77, 0.03 |
| Total | 5 | 172 | 197 | 0.37 (0.00, 0.73) | 0.05 | 49%, 7.91, 0.09 |
| Plasma SEA | ||||||
| FMS vs HC | 2 | 141 | 149 | 0.52 (0.28, 0.75) | <0.0001 | 0%, 0.02, 0.90 |
| CWP vs HC | 3 | 31 | 48 | 0.31 (−0.15, 0.77) | 0.19 | 0%, 1.27, 0.53 |
| Total | 5 | 172 | 197 | 0.47 (0.27, 0.68) | <0.00001 | 0%, 1.90, 0.75 |
| Interstitial PEA | ||||||
| CWP vs HC | 2 | 35 | 43 | 0.26 (−0.19, 0.71) | 0.25 | 0%, 0.76, 0.38 |
| Interstitial SEA | ||||||
| CWP vs HC | 2 | 35 | 43 | 0.86 (0.33, 1.38) | 0.001 | 19%, 1.24, 0.27 |
Bold font indicates statistical significance (P ≤ 0.05).2-AG, 2-arachidonoylglycycerol; AEA, N-arachidonoylethanolamide or anandamide; CWP, chronic widespread pain; FMS, fibromyalgia syndrome; HCs, healthy controls; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; SEA, stearoylethanolamide.
3.1.1. Meta-analyses (x3) on plasma oleoylethanolamide, stearoylethanolamide, and palmitoylethanolamide in chronic widespread pain and fibromyalgia syndrome subgroups
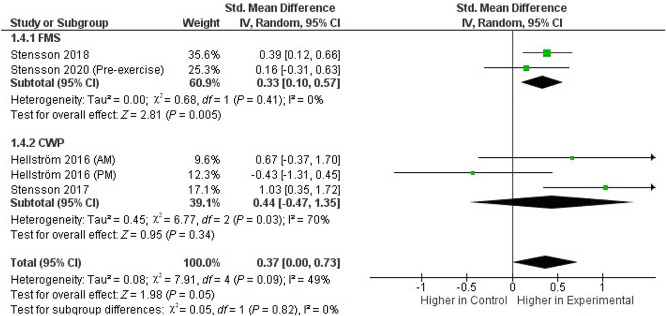
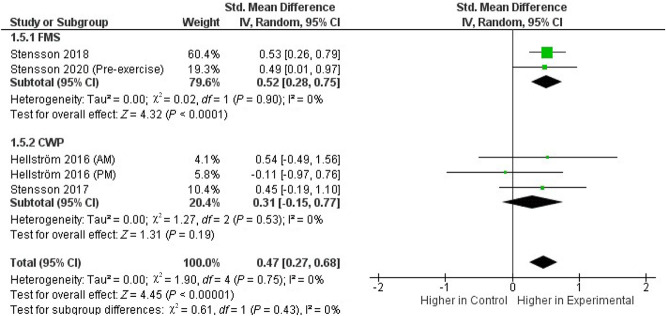
We pooled the data from 2 FMS54,57 and 2 CWP studies21,55 that investigated OEA and SEA plasma concentrations. Across these 4 studies, which included in total 172 patients and 197 controls, the whole-group analysis demonstrated significantly higher concentrations of both OEA (Fig. 2) and SEA (Fig. 3) in the experimental group compared with those in healthy controls (SMD, 0.37; 95% CI, 0.00–0.73; P = 0.05 and SMD, 0.47; 95% CI, 0.27–0.68; P < 0.0001, respectively), with evidence of low heterogeneity (I2 = 49% and I2 = 0%, respectively). Increased plasma levels of both OEA and SEA were found in patients in the FMS subgroup (P = 0.005 and P < 0.0001, respectively), but not in the CWP subgroup (P = 0.34 and P = 0.19, respectively).
Figure 2.
Forest plot of standardized mean difference (SMD) in plasma OEA levels in patients (FMS and CWP) and healthy controls. Weights obtained from random-effects analysis. Horizontal lines represent 95% CIs. The arrow indicates that the upper limit of the CI for that study is equal or superior to the upper limit of the SMD indicated at the bottom of the graph. The diamond shows the overall pooled SMD. The vertical line is the line of no effect, representing no difference between patients and healthy controls. CI, confidence interval; CWP, chronic widespread pain; FMS, fibromyalgia syndrome; OEA, oleoylethanolamide.
Figure 3.
Forest plot of Standardized Mean Difference (SMD) in plasma SEA levels in patients (FMS and CWP) and healthy controls. Weights obtained from random-effects analysis. Horizontal lines represent 95% CIs. The arrow indicates that the upper limit of the CI for that study is equal or superior to the upper limit of the SMD indicated at the bottom of the graph. The diamond shows the overall pooled SMD. The vertical line is the line of no effect, representing no difference between patients and healthy controls. CI, confidence interval; CWP, chronic widespread pain; FMS, fibromyalgia syndrome; SEA, stearoylethanolamide.
Three FMS and 2 CWP studies investigated plasma concentrations of PEA4,21,54,55,57 in a total of 261 patients and 233 controls. In the whole-group analysis, no significant difference was found in plasma PEA concentrations in the experimental group compared with those in healthy controls (P = 0.07), with evidence of low heterogeneity (I2 = 37%). Increased plasma PEA levels were found in patients in the CWP subgroup (P = 0.05), but not in the FMS subgroup (P = 0.31).
3.1.2. Meta-analyses (x2) on plasma N-arachidonoylethanolamide and 2-arachidonoylglycerol in fibromyalgia syndrome
Three FMS studies reported data on plasma AEA levels and 2-AG levels, totaling 230 patients and 285 controls.4,54,57 No significant difference was found in plasma AEA concentrations in patients with FMS compared with those in healthy controls (SMD, −0.05; 95% CI, −0.25 to 0.15; P = 0.61; I2 = 0%). Similarly, no significant difference was found in plasma 2-AG concentrations in patients with FMS compared with those in healthy controls (P = 0.16), with evidence of moderate heterogeneity (I2 = 52%).
3.1.3. Meta-analyses (x2) on interstitial palmitoylethanolamide and stearoylethanolamide in chronic widespread pain
Two CWP studies investigated interstitial levels of PEA and SEA in the trapezius muscle.16,56 One of these studies collected data before exercise (140 minutes after the insertion of the microdialysis catheter into the muscle) and after 20 minutes of standardized repetitive low-force exercise.16 Another study conducted the measurements at 20, 40, 60, 80, and 120 minutes after the insertion of the microdialysis catheter into the muscle.56 To ensure consistency between studies, we included the data before exercise from the first study16 and the data collected at 120 minutes from the second study.56 Across these 2 studies, which included 35 patients and 43 controls in total, significantly higher concentrations of SEA were reported in patients with CWP compared with those in healthy controls (SMD, 0.86; 95% CI, 0.33–1.38; P = 0.001), with evidence of low heterogeneity (I2 = 19%). No significant difference was found in interstitial PEA concentrations in patients with CWP compared with those in healthy controls (P = 0.25, I2 = 0%).
4. Discussion
Fibromyalgia syndrome is a syndrome of unknown etiology; although several theories have been proposed, the exact pathophysiology is still poorly understood.30 An extensive search for causality has led to the proposal of an endocannabinoid deficiency in the pathophysiology of FMS, suggesting that reduced ECS tone with decreased endocannabinoids and upregulated cannabinoid receptor activity might be involved in FMS.47 However, this hypothesis has not yet been supported by objective clinical data.
To our knowledge, this is the first systematic review and meta-analysis exploring the clinical relevance of ECS alterations in patients with CWP and FMS by comparing the differences in plasma and interstitial levels of endocannabinoids and NAEs between patients and healthy controls. Contrary to the endocannabinoid deficiency theory, the main findings identified increased plasma levels of OEA and SEA in people with FMS compared with those in controls and increased plasma levels of PEA and interstitial levels of SEA in people with CWP compared with those in controls. There were no significant differences in other ECS parameters, including plasma AEA and 2-AG levels, between patients with CWP or FMS and healthy controls.
The one major impediment to investigating the function of the endocannabinoids is their short lifetime.31,64 They are produced on demand and are rapidly metabolized by their degrading enzymes,27 making it difficult to study their effects in vivo and limiting their use as a therapeutic intervention. Recent advances have focused on developing compounds that inhibit the endocannabinoid degrading enzymes, thereby increasing their availability in pain-regulatory circuits.27
In addition, there are several peripheral factors that may affect endocannabinoid and NAE levels, including exogenous cannabinoids,12 inflammation,2 hypothalamic-pituitary-adrenal (HPO) axis activation,26 alterations of the gut microbiome and metabolic function,8 and medications.60 These peripheral factors may be associated with increased SEA, OEA, and PEA levels seen in patients with CWP and FMS in this review.
Nonopioid analgesics, such as NSAIDs, paracetamol, and dipyrone, may potentially elevate endocannabinoid levels through several mechanisms, including inhibition of endocannabinoid cellular uptake, stimulation of endocannabinoid biosynthesis and release, and inhibition of the activity of degradative enzymes (FAAH and COX-2).60 Because prolonged washout periods of analgesics and antidepressants in patients with chronic pain may be unethical and challenging to perform, most of the studies in this review instructed their participants to abstain from their medication only for 12 to 48 hours before the investigations, which could affect the results by affecting the levels of endocannabinoid and NAEs in the participants. Furthermore, the endogenous activity of the ECS is affected by exogenous cannabinoids, such as delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which directly or indirectly modulate levels of endocannabinoids by acting as either agonists or antagonists to the cannabinoid receptors and modifying endocannabinoid cellular reuptake and endocannabinoid enzyme activity.12 One publication included in this review excluded cannabis users from their study,4 whereas others did not provide any information on current or prior cannabis use among their participants. Future studies should use standardized measures to assess medication and cannabis use to clarify their association with levels of endocannabinoids and NAEs in people with CWP and FMS.
The ECS plays an important role in energy metabolism, with evidence suggesting a correlation between circulation endocannabinoids and body weight and adiposity. Higher plasma and saliva levels of endocannabinoids and NAEs have previously been reported in obese individuals than those of normal weight.10,13,41 In most studies included in this review, the BMI in participants in the experimental groups was higher than that in participants in the control groups, providing another potential confounder influencing the results. One study identified BMI as a covariate for plasma AEA and PEA levels in patients with FMS.4 Similarly, a study conducted by Stensson et al.57 found a significant positive correlation between plasma 2-AG levels and BMI in patients with FMS, while an earlier study by the same authors found no correlation between BMI and interstitial levels of OEA, PEA, and SEA in patients with CWP.
Endocannabinoid system activity is altered in many inflammatory, metabolic, psychological, and psychiatric disorders.43,52,62,67 Thus, significantly increased endocannabinoid levels have been found in several health conditions associated with systemic inflammation, including chronic hepatitis C,45 cirrhosis,9 pancreatitis,46 atherosclerosis,39 myocardial infarction,63 endometriosis,50 and repeated exposure to sunlight.14 Furthermore, acute or chronic exposure to physical or psychological stress may also increase endocannabinoid levels.26 Most of the studies in this review excluded individuals with conditions that would interfere with the outcomes, including diabetes, osteoarthritis, hypertension, malignancies, and other inflammatory, metabolic, psychiatric, and neurological conditions.16,21,34,54–57 One study identified diabetes mellitus as a covariate for plasma 2-AG levels in patients with FMS,4 while a study by Stensson et al.57 reported a significant positive correlation between plasma AEA levels and depression scores in patients with FMS. A study by Stensson et al.56 found no correlation between plasma levels of OEA, PEA, and SEA and inflammatory cytokines in patients with CWP.
In addition, endocannabinoids levels may change significantly throughout the day, depending on many factors, including satiety levels, distress, physical activity, and sleep quality.25,58 Recent studies have shown that circulating endocannabinoids exhibit significant circadian rhythmicity, where levels of 2-AG increased continuously across the morning, peaking in the early to mid-afternoon and were significantly enhanced by sleep restriction,18 whereas AEA, PEA, and OEA demonstrated a biphasic pattern with lesser amplitude peaking during early sleep and mid-afternoon and were not affected by sleep restriction.17 One study in this review attempted to correlate hours of sleep the previous night with plasma AEA, 2-AG, and PEA levels in patients with FMS; however, no such correlation was reported.4
Endocannabinoid levels are sensitive to several aspects of food intake. Research showed that circulating levels of AEA significantly increased before meal consumption and then decreased postprandially, whereas no meal-related changes were observed for 2-AG.15 The response of 2-AG to food seems to be affected by its perceived hedonic (pleasure-driven) value. Thus, circulating levels of 2AG increased before, during, and after consumption of favorite foods, but not after nonhedonic eating.44 In addition, prolonged consumption of high-fat diets may decrease endogenous levels of NAEs (ie, OEA and PEA) and encourage food overconsumption.19 Most studies in this review did not control or report dietary factors. One study reported time since last food intake as a covariate for plasma AEA and PEA levels in patients with FMS.4
Several studies have examined the effects of physical exercise on levels of endocannabinoids and NAEs, including 2 studies in this review, one of which explored plasma levels of AEA, OEA, PEA, SEA, and 2-AG before and after a 15-week resistance exercise program in patients with FMS,54 whereas the other studied interstitial levels of PEA and SEA in the trapezius muscle before and after 20 minutes of repetitive low-force exercise in patients with CWP.16 Owing to the limited number of studies, it was not possible to perform the quantitative synthesis of this data as part of this review. Nonetheless, the first study reported significantly increased AEA plasma levels in both female patients with FMS and healthy controls and significantly decreased SEA levels in patients with FMS after the 15-week exercise program.54 The second study demonstrated significantly lower interstitial levels of PEA and SEA in the CWP cohort postexercise.16
Many factors can affect ECS activity making it difficult to achieve reliable results without tight control measures during experiments to understand and mitigate effects from such factors on those results. Future studies should explore the correlation between endocannabinoid levels and comorbidities, stress levels, physical activity, sleep quality, circadian rhythm, and dietary factors in people with CWP and FMS.
Moreover, further research is required to investigate other factors that may influence ECS tone in people with CWP and FMS, including changes in the expression of CB1 and CB2 receptors and the activity of endocannabinoid synthesizing and degrading enzymes.
Investigating endocannabinoid activity in these conditions is particularly relevant because it provides the scientific basis for future translational research, ie, clinical trials that explore exogenous cannabinoids as novel therapeutics in FMS. Evidence of enhancing ECS tone by using exogenous cannabinoids (ie, CBD or THC) in individuals with FMS is limited but promising and, if initial results are confirmed, could represent a new therapeutic approach.36
4.1. Limitations
This systematic review and meta-analysis have several limitations. Firstly, all quantitative analyses of plasma and interstitial levels of endocannabinoids and NAEs were based on a small number of studies with relatively small samples sizes.
Secondly, most studies provided limited data on cannabis use, concomitant medication, comorbidities, physical activity, stress levels, circadian rhythm, sleep quality, and dietary factors, variables that may influence ECS function. Future studies should use standardized measures to assess these variables to clarify their association with levels of endocannabinoids and NAEs.
Thirdly, most of the studies investigated levels of endocannabinoids and NAEs in female-only cohorts. The number of diagnosed cases of FMS in men is relatively small; therefore, most research on the condition has been performed on women, often overlooking the study of FMS in men. Evidence suggests that sex differences exist in the function and expression of ECS components, potentially due to interactions between the ECS and hormones and differences in metabolism and cannabinoid receptor expression.6 Therefore, the role of sex in ECS function in CWP and FMS should be further explored in future studies.
Fourthly, most studies in this review were performed by a single research group in one geographic area, which likely limits the generalizability of the findings. Therefore, it is important to expand this research to a broader population.
5. Conclusions
This study investigated the prognostic and diagnostic potential of measuring plasma and interstitial levels of endocannabinoids and NAEs in patients with CWP and FMS. However, most of the studies did not account for important variables that may influence ECS function, including cannabis use, concomitant medication, comorbidities, physical activity, stress levels, circadian rhythm, sleep quality, and dietary factors. Owing to these limitations, the review was not conclusive as to whether plasma and interstitial levels of endocannabinoids and NAEs are clinically relevant in patients with CWP and FMS. Understanding endocannabinoid activity in CWP and FMS will provide the scientific basis for the use of exogenous cannabinoids as potential therapeutics in these disease states and underpin further translational research in the area.
Disclosures
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: L. N. Warne holds the position of Head of Research & Innovation at Little Green Pharma Ltd; I. Kurlyandchik holds a PhD scholarship, which is partially funded by Little Green Pharma Ltd. The other authors have no competing interests to declare.
Acknowledgements
The authors would like to thank Southern Cross University and Little Green Pharma Ltd for providing the PhD scholarship for I. Kurlyandchik.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A177.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Romy Lauche, Email: romy.lauche@scu.edu.au.
Evelin Tiralongo, Email: E.Tiralongo@griffith.edu.au.
Leon N. Warne, Email: l.Warne@lgp.global.
Janet Schloss, Email: janet.schloss@scu.edu.au.
References
- [1].Alhouayek M, Muccioli GG. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov Today 2014;19:1632–9. [DOI] [PubMed] [Google Scholar]
- [2].Barrie N, Manolios N. The endocannabinoid system in pain and inflammation: its relevance to rheumatic disease. Eur J Rheumatol 2017;4:210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci 2012;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baumeister D, Eich W, Lerner R, Lutz B, Bindila L, Tesarz J. Plasma parameters of the endocannabinoid system are unaltered in fibromyalgia. Psychother Psychosom 2018;87:377–9. [DOI] [PubMed] [Google Scholar]
- [5].Berdyshev AG, Kosiakova HV, Onopchenko OV, Panchuk RR, Stoika RS, Hula NM. N-Stearoylethanolamine suppresses the pro-inflammatory cytokines production by inhibition of NF-κB translocation. Prostaglandins Other Lipid Mediat 2015;121:91–6. [DOI] [PubMed] [Google Scholar]
- [6].Blanton HL, Barnes RC, Mchann MC, Bilbrey JA, Wilkerson JL, Guindon J. Sex differences and the endocannabinoid system in pain. Pharmacol Biochem Behav 2021;202:173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borchers AT, Gershwin ME. Fibromyalgia: a critical and comprehensive review. Clin Rev Allergy Immunol 2015;49:100–51. [DOI] [PubMed] [Google Scholar]
- [8].Cani PD, Plovier H, Van Hul M, Geurts L, Delzenne NM, Druart C, Everard A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 2016;12:133–43. [DOI] [PubMed] [Google Scholar]
- [9].Caraceni P, Viola A, Piscitelli F, Giannone F, Berzigotti A, Cescon M, Domenicali M, Petrosino S, Giampalma E, Riili A, Grazi G, Golfieri R, Zoli M, Bernardi M, Di Marzo V. Circulating and hepatic endocannabinoids and endocannabinoid-related molecules in patients with cirrhosis. Liver Int 2010;30:816–25. [DOI] [PubMed] [Google Scholar]
- [10].Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–9. [DOI] [PubMed] [Google Scholar]
- [11].De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem 2001;276:12856–63. [DOI] [PubMed] [Google Scholar]
- [12].Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 2015;12:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005;54:2838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Felton SJ, Kendall AC, Almaedani AFM, Urquhart P, Webb AR, Kift R, Vail A, Nicolaou A, Rhodes LE. Serum endocannabinoids and N-acyl ethanolamines and the influence of simulated solar UVR exposure in humans in vivo. Photochem Photobiol Sci 2017;16:564–74. [DOI] [PubMed] [Google Scholar]
- [15].Gatta-Cherifi B, Matias I, Vallée M, Tabarin A, Marsicano G, Piazza PV, Cota D. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes (Lond) 2012;36:880–5. [DOI] [PubMed] [Google Scholar]
- [16].Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, Gerdle B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. PAIN 2013;154:1649–58. [DOI] [PubMed] [Google Scholar]
- [17].Hanlon EC. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 2020;111:104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, Hillard CJ, Van Cauter E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016;39:653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hansen HS, Diep TA. N-acylethanolamines, anandamide and food intake. Biochem Pharmacol 2009;78:553–60. [DOI] [PubMed] [Google Scholar]
- [20].Häuser W, Perrot S, Sommer C, Shir Y, Fitzcharles M-A. Diagnostic confounders of chronic widespread pain: not always fibromyalgia. Pain Rep 2017;2:e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hellström F, Gouveia-Figueira S, Nording ML, Björklund M, Fowler CJ. Association between plasma concentrations of linoleic acid-derived oxylipins and the perceived pain scores in an exploratory study in women with chronic neck pain. BMC Musculoskelet Disord 2016;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handb Syst Rev Interv 2019:143–76. doi: 10.1002/9781119536604.CH6. [DOI] [Google Scholar]
- [23].Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018;43:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hillard CJ, Beatka M, Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr Physiol 2016;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hossain MZ, Ando H, Unno S, Kitagawa J. Targeting peripherally restricted cannabinoid receptor 1, cannabinoid receptor 2, and endocannabinoid-degrading enzymes for the treatment of neuropathic pain including neuropathic orofacial pain. Int J Mol Sci 2020;21:1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Im DS. GPR119 and GPR55 as receptors for fatty acid ethanolamides, oleoylethanolamide and palmitoylethanolamide. Int J Mol Sci 2021;22:1034–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jahan F, Nanji K, Qidwai W, Qasim R. Fibromyalgia syndrome: an overview of pathophysiology, diagnosis and management. Oman Med J 2012;27:192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Járai Z, Wagner JA, Goparaju SK, Wang L, Razdan RK, Sugiura T, Zimmer AM, Bonner TI, Zimmer A, Kunos G. Cardiovascular effects of 2-arachidonoyl glycerol in anesthetized mice. Hypertension 2000;35:679–84. [DOI] [PubMed] [Google Scholar]
- [32].Kasatkina LA, Heinemann A, Hudz YA, Thomas D, Sturm EM. Stearoylethanolamide interferes with retrograde endocannabinoid signalling and supports the blood-brain barrier integrity under acute systemic inflammation. Biochem Pharmacol 2020;174:113783. [DOI] [PubMed] [Google Scholar]
- [33].Kasatkina LA, Rittchen S, Sturm EM. Neuroprotective and immunomodulatory action of the endocannabinoid system under neuroinflammation. Int J Mol Sci 2021;22:5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaufmann I, Schelling G, Eisner C, Richter HP, Krauseneck T, Vogeser M, Hauer D, Campolongo P, Chouker A, Beyer A, Thiel M. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology 2008;33:676–85. [DOI] [PubMed] [Google Scholar]
- [35].Kesner AJ, Lovinger DM. Cannabinoids, endocannabinoids and sleep. Front Mol Neurosci 2020;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kurlyandchik I, Tiralongo E, Schloss J. Safety and efficacy of medicinal cannabis in the treatment of fibromyalgia: a systematic review. J Altern Complement Med 2021;27:198–213. [DOI] [PubMed] [Google Scholar]
- [37].Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 2005;67:15–19. [DOI] [PubMed] [Google Scholar]
- [38].Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocr 2008;20(suppl 1)10–14. [DOI] [PubMed] [Google Scholar]
- [39].Maeda N, Osanai T, Kushibiki M, Fujiwara T, Tamura Y, Oowada S, Higuma T, Sasaki S, Yokoyama J, Yoshimachi F, Matsunaga T, Hanada H, Okumura K. Increased serum anandamide level at ruptured plaque site in patients with acute myocardial infarction. Fundam Clin Pharmacol 2009;23:351–7. [DOI] [PubMed] [Google Scholar]
- [40].Mascarenhas RO, Souza MB, Oliveira MX, Lacerda AC, Mendonça VA, Henschke N, Oliveira VC. Association of therapies with reduced pain and improved quality of life in patients with fibromyalgia: a systematic review and meta-analysis. JAMA Intern Med 2021;181:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, Piazza PV, Cota D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One 2012;7:e42399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Metelli S, Chaimani A. Challenges in meta-analyses with observational studies. Evid Based Ment Health 2020;23:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Minichino A, Senior M, Brondino N, Zhang SH, Godwlewska BR, Burnet PWJ, Cipriani A, Lennox BR. Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiatry 2019;76:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, Maj M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab 2012;97:E917–24. [DOI] [PubMed] [Google Scholar]
- [45].Patsenker E, Sachse P, Chicca A, Gachet MS, Schneider V, Mattsson J, Lanz C, Worni M, de Gottardi A, Semmo M, Hampe J, Schafmayer C, Brenneisen R, Gertsch J, Stickel F, Semmo N. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. Int J Mol Sci 2015;16:7057–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pezzilli R, Ciuffreda P, Ottria R, Ravelli A, D'Eril GM, Barassi A. Serum endocannabinoids in assessing pain in patients with chronic pancreatitis and in those with pancreatic ductal adenocarcinoma. Scand J Gastroenterol 2017;52:1133–9. [DOI] [PubMed] [Google Scholar]
- [47].Russo EB. Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res 2016;1:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett 2004;25:31–9. [PubMed] [Google Scholar]
- [49].Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett 2008;29:192–200. [PubMed] [Google Scholar]
- [50].Sanchez AM, Cioffi R, Viganò P, Candiani M, Verde R, Piscitelli F, Di Marzo V, Garavaglia E, Panina-Bordignon P. Elevated systemic levels of endocannabinoids and related mediators across the menstrual cycle in women with endometriosis. Reprod Sci 2016;23:1071–9. [DOI] [PubMed] [Google Scholar]
- [51].Shipley M. Chronic widespread pain and fibromyalgia syndrome. Medicine (Baltimore) 2018;46:252–5. [Google Scholar]
- [52].Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 2013;17:475–90. [DOI] [PubMed] [Google Scholar]
- [53].Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 2013;17:475–90. [DOI] [PubMed] [Google Scholar]
- [54].Stensson N, Gerdle B, Ernberg M, Mannerkorpi K, Kosek EVA, Ghafouri B. Increased anandamide and decreased pain and depression after exercise in fibromyalgia. Med Sci Sports Exerc 2020;52:1617–28. [DOI] [PubMed] [Google Scholar]
- [55].Stensson N, Ghafouri B, Gerdle B, Ghafouri N. Alterations of anti-inflammatory lipids in plasma from women with chronic widespread pain—a case control study. Lipids Health Dis 2017;16:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Stensson N, Ghafouri B, Ghafouri N, Gerdle B. High levels of endogenous lipid mediators (N-acylethanolamines) in women with chronic widespread pain during acute tissue trauma. Mol Pain 2016;12:1744806916662886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stensson N, Ghafouri N, Ernberg M, Mannerkorpi K, Kosek E, Gerdle B, Ghafouri B. The relationship of endocannabinoidome lipid mediators with pain and psychological stress in women with fibromyalgia: a case-control study. J Pain 2018;19:1318–28. [DOI] [PubMed] [Google Scholar]
- [58].Stone NL, Millar SA, Herrod PJJ, Barrett DA, Ortori CA, Mellon VA, O'Sullivan SE, Morena M. An analysis of endocannabinoid concentrations and mood following singing and exercise in healthy volunteers. Front Behav Neurosci 2018;12:269–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].The Cochrane Collaboration. Review manager (RevMan), [Computer program]. Version 5.4, The Cochrane Collaboration, 2020. [Google Scholar]
- [60].Topuz RD, Gündüz Ö, Karadağ ÇH, Ulugöl A. Non-opioid analgesics and the endocannabinoid system. Balkan Med J 2020;37:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tsuboi K, Uyama T, Okamoto Y, Ueda N. Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflamm Regen 2018;38:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 2015;97:1049–70. [DOI] [PubMed] [Google Scholar]
- [63].Weis F, Beiras-Fernandez A, Sodian R, Kaczmarek I, Reichart B, Beiras A, Schelling G, Kreth S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J Mol Cell Cardiol 2010;48:1187–93. [DOI] [PubMed] [Google Scholar]
- [64].Willoughby KA, Moore SF, Martin BR, Ellis EF. The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther 1997;282:243–7. [PubMed] [Google Scholar]
- [65].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [66].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Michael Franklin C, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, John Reynolds W, Romano TJ, Jon Russell I, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- [67].Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci 2011;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zoerner AA, Gutzki FM, Batkai S, May M, Rakers C, Engeli S, Jordan J, Tsikas D. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim Biophys Acta 2011;1811:706–23. [DOI] [PubMed] [Google Scholar]