Abstract
Endotoxin (lipopolysaccharide [LPS]) is the major pathogenic factor of gram-negative septic shock, and endotoxin-induced death is associated with the host overproduction of tumor necrosis factor alpha (TNF-α). In the search for new antiendotoxin molecules, we studied the endotoxin-neutralizing capacity of a human lactoferrin-derived 33-mer synthetic peptide (GRRRRSVQWCAVSQPEATKCFQWQRNMRKVRGP; designated LF-33) representing the minimal sequence for lactoferrin binding to glycosaminoglycans. LF-33 inhibited the coagulation of the Limulus amebocyte lysate and the secretion of TNF-α by RAW 264.7 cells induced by lipid A and four different endotoxins with a potency comparable to that of polymyxin B. The first six residues at the N terminus of LF-33 were critical for its antiendotoxin activity. The endotoxin-neutralizing capacity of LF-33 and polymyxin B was attenuated by human serum. Coinjection of Escherichia coli LPS (125 ng) with LF-33 (2.5 μg) dramatically reduced the lethality of LPS in the galactosamine-sensitized mouse model. Significant protection of the mice against the lethal LPS challenge was also observed when LF-33 (100 μg) was given intravenously after intraperitoneal injection of LPS. Protection was correlated with a reduction in TNF-α levels in the mouse serum. These results demonstrate the endotoxin-neutralizing capability of LF-33 in vitro and in vivo and its potential use for the treatment of endotoxin-induced septic shock.
Endotoxin (lipopolysaccharide [LPS]) is a constitutive component of the outer membrane of gram-negative bacteria and is released when the bacteria die or multiply (29). It is estimated that every year in the United States, approximately 400,000 patients present with bacterial sepsis, of which 100,000 ultimately die of septic shock, and about half of these cases are caused by gram-negative bacteria (26). Gram-negative sepsis and septic shock primarily result from endotoxin-induced excessive production and release of inflammatory cytokines by cells of the immune system, particularly macrophages (3, 31). Tumor necrosis factor alpha (TNF-α) is the primary mediator of the systemic toxicity of endotoxin (3, 14). Consequently, neutralization of endotoxin represents an important aspect of a logical, multifaceted approach to treating this complex clinical syndrome (36). This approach is potentially specific since it does not interfere with the host defense.
Lipid A is the toxic portion of endotoxin (29). Monoclonal anti-lipid A antibodies have been tested for treating gram-negative sepsis and septic shock, but their clinical efficacy has not been demonstrated consistently (40), probably due to their poor ability to bind and neutralize endotoxin (41). Newer developments include identification of synthetic antiendotoxin peptides mimicking polymyxin B (33) and a number of cationic antiendotoxin peptides derived from host defense proteins. These include a recombinant 23-kDa fragment derived from bactericidal/permeability-increasing protein (10, 21), a 28-mer peptide derived from bee melittin (13), a 33-mer peptide derived from an 18-kDa cationic antibacterial protein (18), and synthetic peptides based on the crystal structure of Limulus anti-LPS factor (28).
Lactoferrin is an iron-binding glycoprotein that is synthesized by mucosal epithelium and neutrophils and released by these cells in response to inflammatory stimuli (16, 19). It has antimicrobial activities in vitro (19), and lactoferrin treatment in vivo has been reported to lower the incidence of gram-negative bacteremia (37). Structurally, it contains a strongly basic region close to the N terminus which binds to a variety of anionic biological molecules, including lipid A (1) and glycosaminoglycans that occur on the surface of most cells and in most extracellular matrices (20). Lactoferricin H (residues 1 to 47) and lactoferricin B (residues 17 to 41) are released by pepsinolysis of human and bovine lactoferrin, respectively, and may have more potent antibacterial activity than the native proteins (2). A region composed of residues 28 to 34 is reported to contribute to the high-affinity binding of lactoferrin and lactoferricin H to endotoxin (6, 39). Lactoferrin and lactoferricin B have been shown to inhibit the endotoxin-induced interleukin-6 response in human monocytic cells (23).
Previous studies have established that the N-terminal 33 residues of human lactoferrin represent the minimal sequence that mediates binding of the protein to glycosaminoglycans (20). This sequence contains a cationic head (residues 1 to 6) and tail (residues 28 to 33) which combine to form the glycosaminoglycan-binding site. In this study, we sought to assess the endotoxin-neutralizing capacity of a synthetic peptide, designated LF-33, corresponding to the first 33 residues of the secreted form of human lactoferrin. We measured the peptide-mediated inhibition of endotoxin-induced Limulus amebocyte lysate (LAL) coagulation with a sensitive LAL assay (43) and suppression of endotoxin-induced TNF-α secretion by the macrophage cell line RAW 264.7 (17). We also examined the ability of LF-33 to suppress endotoxin-induced TNF-α secretion in the presence of human serum. Finally, we evaluated the protection provided by LF-33 to galactosamine-sensitized mice against a lethal endotoxin challenge.
MATERIALS AND METHODS
Peptides.
Lactoferrin-derived peptides were synthesized by conventional Fmoc [N-(9-fluoreny)methoxycarbonyl] chemistry as described elsewhere (20). The 33-mer peptide (GRRRRSVQWCAVSQPEATKCFQWQRNMRKVRGP) corresponding to the first 33 residues at the N terminus of human lactoferrin is designated LF-33 (molecular weight [MW], 4,004). The 27-mer peptide, LF-27 (MW, 3,276), corresponds to LF-33 lacking its N-terminal six residues. Polymyxin B (MW, 1,066; Sigma, St. Louis, Mo.), an antiendotoxin peptide (4), is used as a reference for comparison throughout this study.
LPS.
Control standard endotoxins from Escherichia coli O113:H10 and Salmonella abortus equi (Associates of Cape Cod, Inc., Woods Hole, Mass.) had a potency of 10 endotoxin units (EU) per ng. LPS (purity >99%) from Neisseria meningitidis was prepared from the group B strain 6275 in our laboratory, and its potency was 25 EU/ng. Lipid A from E. coli K-12 (List Biological Laboratories, Inc., Campbell, Calif.) had a potency of 8.6 EU/ng. The potency of the LPS from Pseudomonas aeruginosa (Sigma) was 0.12 EU/ng. The potency of the endotoxin described above was determined with the Limulus enzyme-linked immunosorbent assay (ELISA) (43) in comparison with the U.S. Pharmacopeia reference standard endotoxin EC-5.
Limulus ELISA for determining the ENC50 of antiendotoxin agents.
The Limulus ELISA is an endotoxin assay based on activation of LAL coagulation by endotoxin and detection of the generated peptide C immunoreactivity with an ELISA using a monoclonal antibody to the peptide (43). Endotoxin was defined to be neutralized when it lost its ability to activate the LAL enzymes. The 50% endotoxin-neutralizing concentration (ENC50) reflects the potency of an antiendotoxin agent; a low ENC50 indicates high potency.
Briefly, 25 μl of endotoxin solution (200 EU/ml) was mixed with an equal volume of test materials in a series of twofold dilutions in 0.15 M NaCl in a sterile 96-well tissue culture plate (Nunc A/S, Roskilde, Denmark) and incubated at 37°C for 1 h in a dry-air incubator. The reaction mixtures were diluted 1,000-fold with endotoxin-free water. The endotoxin activity was then quantified with the use of the Limulus ELISA (43). In the Limulus ELISA, endotoxin activated the LAL coagulation at concentrations below 1 pg or 0.01 EU per ml (43). The high sensitivity of the assay allowed for very low levels of the endotoxin activity to be detected. Following incubation of endotoxin with test materials, a 1,000-fold dilution was introduced to eliminate any potential effects of the test materials on the LAL enzyme system. Neither serum nor any of these materials interfered with the enzymatic cascade of the LAL assay itself after a 1,000-fold dilution from their highest concentrations used in this study. For each assay, the LAL-endotoxin reaction was carried out under optimal conditions with a linear relationship between the concentration of endotoxin and the optical density at 490 nm. A sigmoid curve was usually obtained between optical density at 490 nm and the logarithmic concentration of an antiendotoxin agent. The concentration corresponding to the midpoint of the curve was designated ENC50.
Endotoxin-induced TNF-α secretion by RAW 264.7 cells.
The murine macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC, Rockville, Md.) and maintained as described previously (17). The concentration of endotoxin in all buffers and media was controlled to below 0.1 EU/ml. The following protocol was essentially described before and used here with minor modifications (17). Each well of a 96-well tissue culture plate was seeded with 150 μl of RAW 264.7 cells at 106 cells per ml of Dulbecco minimal essential medium (Life Technologies, Gaithersburg, Md.) supplemented with 10% heat-treated fetal bovine serum (Life Technologies), 25 mM HEPES (pH 7.3), penicillin (60 U/ml), and streptomycin (60 μg/ml). After overnight incubation at 37°C in a 6% CO2 incubator, the medium was aspirated and the cells were washed with three changes of endotoxin-free Hank’s balanced salt solution (HBSS; Life Technologies) supplemented with 25 mM HEPES (pH 7.3). Control endotoxin (10 ng/ml) and test materials were prepared in HBSS-HEPES. After incubation in a 37°C water bath for 1 h, 0.2 ml of these solutions was added in triplicate to each well and incubated at 37°C for 6 h. The supernatants were then collected and stored at −70°C before the measurement of TNF-α activity. Controls included HBSS-HEPES and test materials in the absence of endotoxin. TNF-α activity of the medium and test materials was below 160 pg/ml. The human serum used in some experiments was a pool from normal donors.
The cytotoxic assay.
TNF-α activity in the culture supernatant was determined on the basis of its cytotoxicity for the mouse fibrosarcoma cell line WEHI 164 (ATCC). We observed that this cell line was fourfold more sensitive to TNF-α than the commonly used L929 fibroblast cells, and the sensitivity was further increased fivefold by inclusion of actinomycin D (Life Technologies) in the medium (32). In this assay, the concentration of active TNF-α was correlated with cell death resulting from exposure to TNF-α. Cell death was measured colorimetrically with the viable dye MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] method (24). The specificity of the assay was verified by using a rabbit anti-mouse TNF-α antibody (Genzyme, Inc., Cambridge, Mass.). The antibody at a 1:100 dilution completely eliminated the cytotoxicity in the culture supernatant of the RAW 264.7 cells stimulated by endotoxin and in the mouse sera collected 1 h after intraperitoneal (i.p.) injection of endotoxin.
Briefly, 96-well tissue culture plates were seeded with 100 μl of WEHI 164 cells (5 × 104 cells) in RPMI 1640 medium (Life Technologies) containing 10% heat-treated fetal bovine serum, 25 mM HEPES (pH 7.3), penicillin (60 U/ml), streptomycin (60 μg/ml), and actinomycin D (4 μg/ml). After a 2-h incubation at 37°C in a 6% CO2 incubator, 10 μl of twofold serially diluted samples (culture supernatants) or standards (murine recombinant TNF-α; Genzyme) was added to each well and the wells were incubated for 20 h. Cell viability was then determined by the addition of 10 μl of MTT (thiazolyl blue; Sigma) stock solution (5 mg/ml in saline) to each well, and the incubation was allowed to continue for 6 h. One hundred eighty microliters of acid-isopropanol (containing 40 mM HCl) was added to dissolve the generated dark blue crystals. The plate was read at 570 nm with a reference of 630 nm in a microplate reader. The amount of TNF-α that led to 50% killing of the seeded cells was defined as 1 U, equivalent to approximately 15 pg of recombinant TNF-α under the present condition. A standard curve was obtained by incubating known amounts of the recombinant TNF-α with the WEHI 164 cells.
To exclude any potential cytotoxicity of LF-33, the procedure described above was followed except that WEHI 164 cells were replaced by RAW 264.7 cells and the concentration of cells seeded in each well was 1.5 × 105 per 150 μl of medium to mimic the conditions in the stimulation experiment. At the highest concentration of LF-33 (10 μM) used in this study, no cytotoxicity to RAW 264.7 cells was detected.
Galactosamine-sensitized mouse model.
Mice are typically resistant to endotoxin. However, the sensitivity of mice to endotoxin can be enhanced more than 1,000-fold by coinjection with a liver-specific inhibitor, galactosamine (11, 12). An essential feature of this in vivo model is that systemically released TNF-α causes liver damage due to TNF-α-mediated liver cell death, which can be scored by measuring lethality. In our study, i.p. injection of 125 ng of E. coli LPS together with 15 mg of galactosamine hydrochloride (Sigma) in 0.5 ml of 0.15 M NaCl induced nearly 100% lethality in 8- to 10-week-old female NIH/Swiss mice (body weight, 20 to 25 g/mouse). LF-33 was either injected intravenously (i.v.) through tail veins 10 min after the i.p. injection of the LPS-galactosamine mixture or coinjected i.p. with LPS and galactosamine. Lethality was observed for 72 h after injection. In experiments involving measurement of the TNF-α level in serum, blood samples were collected in serum separator tubes (Becton Dickinson, Rutherford, N.J.) 60 to 90 min postinjection, and sera were obtained after centrifugation. The TNF-α level in serum was measured by the cytotoxic assay described above. The peak TNF-α level in serum was found between 60 and 90 min after i.p. injection of LPS.
Statistics.
We performed all endotoxin and TNF-α measurements in triplicate in each experiment. At least two independent experiments were performed for all data. Values are given as the mean ± standard deviation (SD) and were compared by using the unpaired Student t test. Lethality is compared by use of Fisher’s exact test.
RESULTS
Inhibition of endotoxin-induced LAL coagulation.
ENC50 values of each antiendotoxin agent against lipid A and four different types of LPS are listed in Table 1. A low ENC50 indicates high potency of endotoxin neutralization. The potency of each antiendotoxin agent varied depending on the type of endotoxin. LF-33 was more potent than polymyxin B, on a molar basis, at neutralizing all forms of endotoxin tested. In contrast, LF-27 was approximately 10-fold-less potent than LF-33 at neutralizing lipid A and E. coli LPS and had no detectable activity against the other three LPSs. Human serum showed various degrees of inhibition of endotoxin-induced LAL coagulation but had no effect on lipid A (Table 1).
TABLE 1.
ENC50 of antiendotoxin agents determined by the Limulus ELISA
| Agent | ENC50 (mean ± SD) against endotoxina
|
||||
|---|---|---|---|---|---|
| Lipid A | E. coli LPS | S. abortus equi LPS | P. aeruginosa LPS | N. meningitidis LPS | |
| LF-33 | 0.10 ± 0.017 μM | 0.52 ± 0.078 μM | 0.39 ± 0.054 μM | 2.08 ± 0.37 μM | 3.08 ± 0.44 μM |
| LF-27 | 0.63 ± 0.093 μM | 7.45 ± 1.12 μM | >300 μM | >300 μM | >300 μM |
| Polymyxin B | 0.19 ± 0.021 μM | 1.95 ± 0.26 μM | 18.2 ± 2.85 μM | 3.91 ± 0.73 μM | >100 μM |
| Human serum | >50% | 0.03% ± 0.005% | 2.1% ± 0.29% | 0.63% ± 0.11% | 2.52% ± 0.41% |
The ENC50 against 200 EU of endotoxin per ml was measured.
Suppression of endotoxin-induced TNF-α secretion by LF-33.
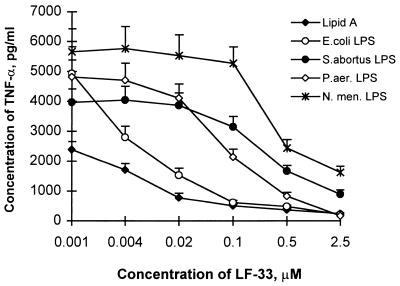
RAW 264.7 cells secrete TNF-α upon exposure to endotoxin (32). A linear relationship between TNF-α secretion and endotoxin concentration was observed at endotoxin concentrations below 20 ng/ml for lipid A and the various LPSs used in this study, and a concentration of 10 ng/ml of endotoxin was selected for the TNF-α-inducing experiments. Mixing endotoxin with increasing concentrations of LF-33 resulted in a dose-dependent suppression of endotoxin-induced TNF-α secretion (Fig. 1). Similar to the results of the LAL assay, the potency of LF-33 varied depending on the type of endotoxin. The LF-33 concentrations needed to suppress TNF-α secretion induced by endotoxin (10 ng/ml) by 50% were approximately 0.01 μM for E. coli LPS and lipid A, 0.1 μM for LPS from P. aeruginosa, and 0.5 μM for LPS from S. abortus equi and N. meningitidis. The effects of LF-27, polymyxin B, and human serum on endotoxin-induced TNF-α secretion are shown in Table 2 for a comparison. LF-33 exhibited a slightly higher potency than polymyxin B in suppressing TNF-α secretion induced by different types of endotoxin, whereas an equimolar concentration of LF-27 or 10% human serum had no effect on endotoxin-induced TNF-α secretion.
FIG. 1.
Dose-dependent suppression by LF-33 of endotoxin-induced TNF-α secretion by RAW 264.7 cells. Endotoxin at 10 ng/ml was incubated at 37°C for 1 h with LF-33 at the concentrations indicated before being exposed to RAW 264.7 cells. All data were the means of triplicates in representative experiments. S. abortus, S. abortus equi; P. aer, P. aeruginosa; N. men, N. meningitidis.
TABLE 2.
Suppression by antiendotoxin agents of endotoxin-induced TNF-α secretion by RAW 264.7 cellsa
| Agent (concn) | TNF-α secretion (pg/ml [mean ± SD]) induced by endotoxinb
|
||||
|---|---|---|---|---|---|
| Lipid A | E. coli LPS | S. abortus equi LPS | P. aeruginosa LPS | N. meningitidis LPS | |
| Endotoxin control | 2,386 ± 269 | 4,928 ± 896 | 3,969 ± 443 | 4,813 ± 571 | 5,658 ± 770 |
| LF-33 (2.5 μM) | 239* ± 29.6 | 211* ± 31.4 | 886* ± 149 | 164* ± 23.3 | 1,624* ± 203 |
| LF-27 (3 μM) | 3,182 ± 412 | 4,912 ± 588 | 3,985 ± 497 | 4,633 ± 603 | 7,306 ± 1,008 |
| Polymyxin B (2.5 μM) | 537* ± 77.9 | 314* ± 42.6 | 1,115* ± 181 | 246* ± 32.5 | 2,829* ± 455 |
| Human serum (10%) | 2,663 ± 337 | 4,817 ± 964 | 5,726 ± 1,126 | 4,428 ± 677 | 8,817 ± 1,711 |
Endotoxin was incubated with test agents at the concentrations indicated at 37°C for 1 h before being exposed to RAW 264.7 cells.
TNF-α secretion induced by endotoxin at 10 ng/ml. *, significantly different from control value at a one-tailed P value of <0.05 in the unpaired t test.
Effect of human serum on the LF-33 suppression of endotoxin-induced TNF-α secretion.
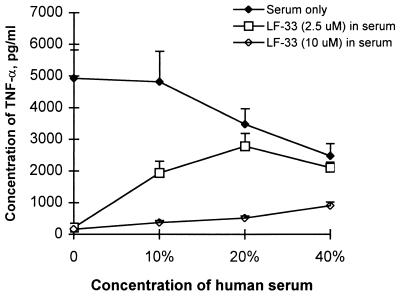
To test the suppression of endotoxin-induced TNF-α secretion under more physiological conditions, LF-33 or polymyxin B was added to human serum (final concentration, 10%) before the addition of endotoxin. As shown in Table 3, the suppressive effect of the peptides was attenuated substantially in the presence of 10% human serum, although the serum effect could be overcome by increasing the concentration of LF-33 (Table 3 and Fig. 2). However, if the peptide was mixed with endotoxin 5 min before the addition of serum, the effect of the serum on the neutralization of endotoxin by the peptides was greatly reduced (Table 4).
TABLE 3.
Suppression by antiendotoxin peptides of endotoxin-induced TNF-α secretion by RAW 264.7 cells in culture medium containing 10% human seruma
| Peptide (concn) | TNF-α secretion (pg/ml [mean ± SD]) induced by endotoxinb
|
||||
|---|---|---|---|---|---|
| Lipid A | E. coli LPS | S. abortus equi LPS | P. aeruginosa LPS | N. meningitidis LPS | |
| Endotoxin control | 2,663 ± 337 | 4,817 ± 964 | 5,726 ± 1126 | 4,428 ± 677 | 8,817 ± 1,711 |
| Polymyxin B (2.5 μM) | 1,209* ± 199 | 1,259* ± 321 | 4,090 ± 806 | 4,206 ± 956 | 8,181 ± 1,032 |
| LF-33 (2.5 μM) | 1,518* ± 311 | 1,938* ± 376 | 5,627 ± 1,037 | 2,776* ± 478 | 7,998 ± 976 |
| LF-33 (10 μM) | 185* ± 25.6 | 369* ± 67.1 | 1,191* ± 264 | 833* ± 138 | 6,473 ± 787 |
Endotoxin was incubated at 37°C for 1 h with peptides at the concentrations indicated in culture medium containing 10% human serum before being exposed to RAW 264.7 cells.
TNF-α secretion induced by endotoxin at 10 ng/ml. *, significantly different from control value at a one-tailed P value of <0.05 in the unpaired t test.
FIG. 2.
Dose-dependent suppression by LF-33 of endotoxin-induced TNF-α secretion by RAW 264.7 cells in human serum. E. coli LPS at 10 ng/ml was incubated at 37°C for 1 h with human serum and LF-33 at the concentrations indicated before being exposed to RAW 264.7 cells. All data were the means of triplicates in representative experiments.
TABLE 4.
Suppression by antiendotoxin peptides of endotoxin-induced TNF-α secretion by RAW 264.7 cells in culture medium containing 10% human serum: effect of the mixing sequence with serum
| Peptide (concn) | TNF-α secretion (pg/ml [mean ± SD]) induced by endotoxina
|
|||
|---|---|---|---|---|
|
E. coli LPS mixing with:
|
P. aeruginosa LPS mixing with:
|
|||
| Peptide first | Serum first | Peptide first | Serum first | |
| LPS control | 4,817 ± 964 | 4,817 ± 964 | 4,428 ± 677 | 4,428 ± 677 |
| LF-33 (2.5 μM) | 575** ± 115 | 1,938* ± 376 | 610** ± 160 | 2,776* ± 478 |
| Polymyxin B (2.5 μM) | 525** ± 130 | 1,259* ± 321 | 883** ± 261 | 4,206 ± 956 |
TNF-α secretion induced by endotoxin at 10 ng/ml. *, significantly different from control value at a one-tailed P value of <0.05 in the unpaired t test; **, significantly different from the serum-first value for the same peptide at a one-tailed P value of <0.01 in the unpaired t test.
Effect of LF-33 on endotoxin-induced lethality and the TNF-α level in serum in the galactosamine-sensitized mouse model.
Injection of 125 ng of E. coli LPS per animal by the i.p. route induced nearly 100% lethality in the galactosamine-sensitized mice. As shown in Table 5, the endotoxin-induced lethality was dramatically reduced by injecting LF-33. Small amounts of LF-33 (2.5 μg per animal), when injected simultaneously with endotoxin, reduced the lethality from 93% (14 of 15 animals dead) to 6% (1 of 15 animals dead). In addition, LF-33 also significantly reduced the lethality when injected i.v. 10 min subsequent to the i.p. injection of endotoxin (Table 5), although a 40-fold-greater amount of LF-33 was required. The protection was correlated with the reduction of the TNF-α level in mouse serum (Table 5).
TABLE 5.
Protection of animals against the lethality of LPS provided by LF-33 in the galactosamine-sensitized mouse model
| Mouse group and i.p. injection dose/mousea | Lethality (no. dead/ total no.)b | Serum TNF-α level (pg/ml [mean ± SD])c |
|---|---|---|
| (i) 5 ng of LPS | 1/5 | NDd |
| (ii) 25 ng of LPS | 3/5 | ND |
| (iii) 125 ng of LPS | 14/15 | 5,801 ± 3,120 |
| (iv) 125 ng of LPS + 2.5 μg of LF-33 | 1/15* | 885** ± 657 |
| (v) 125 ng of LPS + 20 μg of LF-33 (i.v.) | 5/5 | 4,611 ± 1,897 |
| (vi) 125 ng of LPS + 100 μg of LF-33 (i.v.) | 3/15* | 1,125** ± 1,166 |
In groups (i) to (iv), all materials were injected i.p., whereas in groups (v) and (vi), LPS and galactosamine (15 mg per mouse) were injected i.p. but LF-33 was injected i.v. 10 min after LPS injection. The mouse strain used was NIH/Swiss, and the mice weighed between 20 and 22 g each. The total volumes for i.p. and i.v. injection per mouse were 0.5 and 0.2 ml, respectively.
*, Significantly different from group (iii) value at a one-tailed P value of <0.01 in Fisher’s exact test.
**, Significantly different from group (iii) value at a one-tailed P value of <0.01 in the unpaired t test.
ND, not done.
DISCUSSION
Current treatments for gram-negative sepsis and septic shock rely on antibiotics to control the infection and intensive-care support to correct the dysfunction of cardiovascular, respiratory, and other organ systems. Research into the pathogenesis of this fatal clinical syndrome points to endotoxin as a principal pathogenic factor and TNF-α as a primary mediator of endotoxicity. Although a number of antiendotoxin proteins and peptides have been reported (10, 13, 18, 28, 33), there is still no antiendotoxin agent licensed for clinical use to supplement the current therapy. Our study establishes that the human lactoferrin-derived peptide LF-33 possesses a potent endotoxin-neutralizing capacity in vitro and in vivo as shown by its ability to suppress endotoxin-induced LAL coagulation and TNF-α secretion by RAW 264.7 cells and to protect animals from a lethal endotoxin challenge.
Because the lipid A portion of endotoxin is responsible for the activation of LAL (15), stimulation of TNF-α secretion (29), and lethality in mice (29), LF-33 likely exerts its antiendotoxin actions by binding to the lipid A portion and consequently blocking the biological effects of endotoxin. This is supported by its direct neutralization of lipid A in addition to four different types of endotoxin (Table 1). The only difference between the sequence of LF-33 and LF-27 is that LF-27 lacks the first six residues (GRRRRS) at the N terminus of LF-33. Remarkably, this deletion led to a dramatic loss of the endotoxin-neutralizing capacity of the peptide in the LAL assay and complete loss in the TNF-α bioassay (Tables 1 and 2), indicating the importance of the cationic head of LF-33 in neutralizing endotoxin. This cluster of basic residues has previously been shown to be required for the binding of lactoferrin to other anionic molecules, including glycosaminoglycans (20). Because most known antiendotoxin peptides are cationic in nature and both the lipid A and oligosaccharide core portion of LPS are anionic, electrostatic forces may contribute to the binding of endotoxin and the neutralizing cationic peptides. Indeed, the presence of additional ethanolamine groups (positively charged) in the lipid A portion of LPS from N. meningitidis and S. abortus equi, but their absence in LPS from E. coli and P. aeruginosa (27, 30), is consistent with the observed greater potency of LF-33 and polymyxin B in suppressing TNF-α secretion induced by endotoxin from the latter types of bacteria compared to the former types (Table 2 and Fig. 1).
LF-27 showed some detectable antiendotoxin activity against lipid A and E. coli LPS but was essentially inactive against the other three types of LPS examined in the LAL assay (Table 1) and completely inactive in inhibiting the TNF-α production induced by all five of the endotoxins tested (Table 2). These apparent discrepancies likely reflect the difference in affinities of LF-27 for the different types of endotoxins and the difference in sensitivities and complexities of the two assay systems. The LAL assay is more than 100 times more sensitive than the TNF-α bioassay for detecting endotoxin and therefore would allow the low-affinity binding between LF-27 and endotoxin to be detected.
The endotoxin-neutralizing activity of human serum has been observed previously in the Limulus assay (8, 42) and confirmed by our study, as shown in Table 1 and Fig. 2. Noticeably, much lower concentrations (<2.5% compared to >10%) of human serum are needed to neutralize the endotoxin activity measured in the Limulus assay (Table 1) than in the cell assay (endotoxin-induced TNF-α secretion by RAW 264.7 cells) (Fig. 2 and Table 2). Binding of serum proteins to endotoxin neutralizes the endotoxin activity in the Limulus assay by preventing endotoxin from activating LAL (8). However, this is not necessarily true in the cell assay. It is now known that the initial step in the endotoxin-induced cellular response is the binding of endotoxin to serum LPS-binding protein (LBP). Endotoxin in this complexed form is much more effective than free endotoxin in binding to CD14, a glycosylphosphatidylinositol-anchored membrane protein on myeloid cells (22, 34), and subsequently triggering and enhancing the production and release of inflammatory mediators including TNF-α (38). Thus, in the cell assay, LBP in the serum can counteract the endotoxin-neutralizing activity of other serum LPS-binding molecules such as lipoproteins. In addition, since EDTA potentiates and divalent cations (Mg2+ and Ca2+) reduce the endotoxin-neutralizing capacity of human serum (25, 42), the presence of divalent cations in the incubation medium in the cell assay should further decrease the endotoxin-neutralizing capacity of the serum.
LF-33 appears to be more potent than polymyxin B at suppressing endotoxin-induced LAL coagulation and TNF-α secretion by RAW 264.7 cells under serum-free conditions (Table 1 and 2). This suggests that LF-33 may have a greater intrinsic capacity to neutralize endotoxin than polymyxin B does. In the presence of human serum, however, their antiendotoxin potencies become more similar since, although human serum significantly attenuates the endotoxin-neutralizing capacity of both peptides, it has a greater blocking effect on LF-33 (compare Tables 2 and 3). A similar effect of human serum has also been observed with other cationic antiendotoxin peptides such as Limulus anti-LPS factor-derived peptides (28) and a synthetic antiendotoxin peptide (5) in different assay systems. This appears to be due to the interaction of these peptides with serum proteins that effectively reduce the availability of the peptides for binding to endotoxin. Consistent with this explanation is our observation that mixing LF-33 with serum before endotoxin dramatically reduces the ability of the peptide to suppress endotoxin-induced TNF-α secretion (Table 4). For example, serum LBP may compete with LF-33 in binding to endotoxin, since lactoferrin has recently been shown to inhibit the endotoxin interaction with CD14 by competing for the binding of endotoxin to LBP (7). The blocking effect of human serum may partly explain the inability of a low dose of LF-33 to protect mice against the lethality of endotoxin when the peptide was injected into blood after i.p. injection of endotoxin, whereas almost complete protection was observed when they were mixed before i.p. injection (Table 5). Thus, increasing the dose of LF-33 could overcome the serum blocking effect (Table 3 and Fig. 2) and protect the mice against the lethality of endotoxin even when the peptide was injected separately from endotoxin (Table 5).
In conclusion, we have shown that a novel synthetic peptide representing the minimal sequence that mediates binding of human lactoferrin to glycosaminoglycans, LF-33, has potent endotoxin-neutralizing properties in vitro and in vivo against lipid A and different types of LPS. The endotoxin-neutralizing capacity of LF-33 is greater than that of polymyxin B when tested in serum-free media and comparable to that of polymyxin B in the presence of human serum. In a separate study, LF-33 has also been found to be bactericidal to various gram-negative bacteria (20a). The dual properties of LF-33 in neutralizing endotoxin and killing bacteria may present potential advantages over the conventional antibiotics such as β-lactams and quinolones, since these antibiotics are known to promote endotoxin release but have no endotoxin-neutralizing activity and can thus cause endotoxemia during antimicrobial therapy (9, 35). Considering that cationic peptides generally have a short half-life in blood while clinical endotoxemia can be intermittent and recurrent, we are currently investigating agents that can overcome the serum attenuation of the anti-LPS potency of these peptides and are designing analogues with enhanced half-life and efficacy in blood.
ACKNOWLEDGMENTS
We thank Claus Koch of the Statens Seruminstitut, Copenhagen, Denmark, for providing us with the essential regents for the Limulus ELISA and Carl E. Frasch, Che-Hung Lee, and Karin Elkins from the U.S. Food and Drug Administration for their valuable suggestions.
Gui-Hang Zhang was supported by a Visiting Fellowship from the Fogarty International Center, National Institutes of Health, and David M. Mann was supported by a grant (award AI39691) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Appelmelk B J, An Y-Q, Geerts M, Thijs B G, DeBoer H A, MacLaren D M, DeGraaff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Cooperstock M S. Inactivation of endotoxin by polymyxin B. Antimicrob Agents Chemother. 1974;6:422–425. doi: 10.1128/aac.6.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demistri M T, Velucchi M, Bracci L, Rustici A, Porro M, Villa P, Ghezzi P. Inhibition of LPS-induced systemic and local TNF production by a synthetic anti-endotoxin peptide (SAEP-2) J Endotoxin Res. 1996;3:445–454. [Google Scholar]
- 6.Elass-Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli O55:B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias P S, Mazurier J, Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emancipator K, Csako G, Elin R J. In vivo inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun. 1992;60:596–601. doi: 10.1128/iai.60.2.596-601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans M E, Pollack M. Effect of antibiotic class and concentration on the release of lipopolysaccharide from Escherichia coli. J Infect Dis. 1993;167:1336–1343. doi: 10.1093/infdis/167.6.1336. [DOI] [PubMed] [Google Scholar]
- 10.Fisher C J, Marra M N, Palardy J E, Marchbanks C R, Scott R W, Opal S M. Human neutrophil bactericidal/permeability-increasing protein reduces mortality rate from endotoxin challenge: a placebo-controlled study. Crit Care Med. 1994;22:553–558. doi: 10.1097/00003246-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Freudenberg M A, Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough M, Hancock R E W, Kelly N M. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun. 1996;64:4922–4927. doi: 10.1128/iai.64.12.4922-4927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heumann D, Le Roy D, Glauser M P. Contribution of TNF and of LPS in endotoxemic shock in mice: a reappraisal. J Endotoxin Res. 1996;3:87–92. [Google Scholar]
- 15.Iwanaga S, Miyata T, Tokunaga F, Muta T. Molecular mechanism of hemolymph clotting system in Limulus. Thromb Res. 1992;68:1–32. doi: 10.1016/0049-3848(92)90124-s. [DOI] [PubMed] [Google Scholar]
- 16.Jeremy B. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16:417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 17.Kelly N M, Young L, Cross A. Differential induction of tumor necrosis factor by bacteria expressing rough and smooth lipopolysaccharide phenotypes. Infect Immun. 1991;59:4491–4496. doi: 10.1128/iai.59.12.4491-4496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larrick J W, Hirata M, Balint R F, Lee J, Zhong J, Wright S C. Human CAP18: a novel antimicrobiol lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 20.Mann D M, Romm E, Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 20a.Mann, D. M., et al. Submitted for publication.
- 21.Marra M N, Thornton M B, Snable J L, Wilde C G, Scott R W. Endotoxin-binding and -neutralizing properties of recombinant bactericidal/permeability-increasing protein and monoclonal antibodies HA-1A and E5. Crit Care Med. 1994;22:559–565. doi: 10.1097/00003246-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mathison J C, Tobias P S, Wolfson E, Ulevitch R J. Plasma LPS-binding protein, a key component in macrophage recognition of gram-negative LPS. J Immunol. 1992;194:200–206. [PubMed] [Google Scholar]
- 23.Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I, Hanson L A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Ogata M, Fletcher M F, Kloczewiak M, Loiselle P M, Zanzot E M, Vermeulen M W, Warren H S. Effect of anticoagulants on binding and neutralization of lipopolysaccharide by the peptide immunoglobulin conjugate CAP18106–138-immunoglobulin G in whole blood. Infect Immun. 1997;65:2160–2167. doi: 10.1128/iai.65.6.2160-2167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrillo J E. Shock syndromes related to sepsis. In: Bennett J C, Plum F, editors. Cecil textbook of medicine. 20th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1996. pp. 496–501. [Google Scholar]
- 27.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 28.Reid C, Wahl C, Miethke T, Wellnhofer G, Landgraft C, Schneider-Mergener J, Hoess A. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J Biol Chem. 1996;271:28120–28127. doi: 10.1074/jbc.271.45.28120. [DOI] [PubMed] [Google Scholar]
- 29.Rietschel E T, Kirikae T, Schade F U, Ulmer A J, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke H-D, Kusumoto S, Zaahringer U. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 30.Rietschel E T, Wollenweber H-W, Brade H, Zaahringer U, Lindner B, Seydel U, Bradaczek H, Barnickel G, Labischinski H, Giesbrecht P. Structure and conformation of the lipid A component of lipopolysaccharides. In: Rietschel E T, editor. Handbook of endotoxin. 1. Chemistry of endotoxin. New York, N.Y: Elsevier Science Publishers B. V.; 1984. pp. 187–220. [Google Scholar]
- 31.Rosenstreich D L, Vogel S N. Central role of macrophages in the host response to endotoxin. In: Schlessinger D, editor. Microbiology–1980. Washington, D.C: American Society for Microbiology; 1980. pp. 11–15. [Google Scholar]
- 32.Ruff M R, Gifford G E. Tumor necrosis factor. Lymphokines. 1981;2:235–272. [Google Scholar]
- 33.Rustici A, Velucchi M, Faggioni R, Sironi M, Ghezzi P, Quataert S, Green B, Porro M. Molecular mapping and detoxification of the lipid A binding site by synthetic peptides. Science. 1993;259:361–365. doi: 10.1126/science.8420003. [DOI] [PubMed] [Google Scholar]
- 34.Schumann R R. Function of LPS-binding protein (LBP) and CD14, the receptor for LPS/LBP complex: a short review. Res Immunol. 1992;143:11–15. doi: 10.1016/0923-2494(92)80074-u. [DOI] [PubMed] [Google Scholar]
- 35.Shenep J L, Barton R P, Mogan K A. Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental gram-negative bacterial sepsis. J Infect Dis. 1985;151:1012–1018. doi: 10.1093/infdis/151.6.1012. [DOI] [PubMed] [Google Scholar]
- 36.Tanamoto K-I. Novel strategies to attenuate the effect of lipopolysaccharide on the immune response. Curr Opin Infect Dis. 1997;10:177–182. [Google Scholar]
- 37.Trumpler U, Straub P W, Rosemund A. Antibacterial prophylaxis with lactoferrin in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1989;8:310–313. doi: 10.1007/BF01963459. [DOI] [PubMed] [Google Scholar]
- 38.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 39.van Berkel P H C, Geerts M E J, van Veen H A, Mericskay M, de Boer H A, Nuijens J H. N-terminal stretch Arg2, Arg3, Arg4, Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhoef J, Hustinx W M N, Frasa H, Hoepelman A I M. Issues in the adjunct therapy of severe sepsis. J Antimicrob Chemother. 1996;38:167–182. doi: 10.1093/jac/38.2.167. [DOI] [PubMed] [Google Scholar]
- 41.Warren H S, Amato S F, Fitting C, Black K M, Loiselle P M, Pasternack M S, Cavaillon J-M. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993;177:89–97. doi: 10.1084/jem.177.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang G H, Baek L, Bertelsen T, Koch C. Quantification of the endotoxin-neutralizing capacity of serum and plasma. APMIS. 1995;103:721–730. doi: 10.1111/j.1699-0463.1995.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G H, Baek L, Nielsen P E, Buchardt O, Koch C. Sensitive quantitation of endotoxin by enzyme-linked immunosorbent assay with a monoclonal antibody against Limulus peptide C. J Clin Microbiol. 1994;32:416–422. doi: 10.1128/jcm.32.2.416-422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]