Abstract
Enteropathogenic yersiniae (Yersinia pseudotuberculosis and Yersinia enterocolitica) typically cause chronic disease as opposed to the closely related Yersinia pestis, the causative agent of bubonic plague. It is established that this difference reflects, in part, carriage by Y. pestis of a unique 9.6-kb pesticin or Pst plasmid (pPCP) encoding plasminogen activator (Pla) rather than distinctions between shared ∼70-kb low-calcium-response, or Lcr, plasmids (pCD in Y. pestis and pYV in enteropathogenic yersiniae) encoding cytotoxic Yops and anti-inflammatory V antigen. Pla is known to exist as a combination of 32.6-kDa (α-Pla) and slightly smaller (β-Pla) outer membrane proteins, of which at least one promotes bacterial dissemination in vivo and degradation of Yops in vitro. We show here that only α-Pla accumulates in Escherichia coli LE392/pPCP1 cultivated in enriched medium and that either autolysis or extraction of this isolate with 1.0 M NaCl results in release of soluble α and β forms possessing biological activity. This process also converted cell-bound α-Pla to β-Pla and smaller forms in Y. pestis KIM/pPCP1 and Y. pseudotuberculosis PB1/+/pPCP1 but did not promote solubilization. Pla-mediated posttranslational hydrolysis of pulse-labeled Yops in Y. pseudotuberculosis PB1/+/pPCP1 occurred more slowly than that in Y. pestis but was otherwise similar except for accumulation of stable degradation products of YadA, a pYV-mediated fibrillar adhesin not encoded in frame by pCD. Carriage of pPCP by Y. pseudotuberculosis did not significantly influence virulence in mice.
Bubonic plague caused by Yersinia pestis is among the most severe acute bacterial diseases of man. In contrast, closely related Yersinia pseudotuberculosis and the more distant Yersinia enterocolitica (44) typically cause chronic enteropathogenic infections in humans. Both syndromes depend upon the ability to express a low-calcium response (LCR) at 37°C but not 26°C, mediated by ∼70-kb shared Lcr plasmids (termed pCD in Y. pestis and pYV in enteropathogenic yersiniae). The LCR is characterized by either vegetative growth in vitro in the presence of ≥2.5 mM Ca2+ accompanied by downregulation of LcrF-regulated virulence factors (VirF in Y. enterocolitica) or else bacteriostasis associated with upregulation of these same determinants during cultivation in Ca2+-deficient (≤1.0 mM) medium (19, 47) (Lcr+). Lcr plasmids promoting this phenotype are functionally interchangeable (67) and essentially identical (49, 50) except that that from Y. pestis possesses frameshifts preventing expression of the fibrillar outer membrane adhesin YadA (31) and the lipoprotein YlpA (17) encoded by pYV (26, 54). Major effectors of virulence encoded by both pCD and pYV include anti-inflammatory V antigen and the secreted cytotoxins YopE, YopH, and YopO (YpkA) (19, 47).
The unique ability of Y. pestis to promote acute disease is caused in part by accessory virulence factors mediated by two plasmids not shared by the enteropathogenic yersiniae (4, 22, 51). One of these is a 9.6 kb-pesticin or Pst plasmid (pPCP) which encodes the 39.9-kDa bacteriocin pesticin (3, 10, 27, 48, 52), its 16-kDa immunity protein (48, 52), and a particulate plasminogen activator (2) termed Pla (Pst+). Pla facilitates dissemination of yersiniae in host tissues; thus, its presence is essential for expression of virulence via transmission by fleabite and other peripheral routes of infection (8). Invasion of tissue is accomplished by Pla-dependent adherence to host basement membrane and extracellular matrix, where plasminogen activation facilitates bacterial metastasis (35). A transient 34.6-kDa primary pla product is processed upon insertion into the outer membrane to yield 32.6-kDa α-Pla (58), which can then be hydrolyzed in ∼2 h (43) to yield a slightly smaller derivative, termed β-Pla (58); both forms, especially β-Pla, accumulate as major outer membrane proteins (43, 62). The apparent molecular mass of α-Pla (∼35 kDa) estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) exceeds the correct value determined by sequencing (59); this anomaly probably also occurs for β-Pla (∼33 kDa by SDS-PAGE).
Native Yops did not accumulate in wild-type cells of Y. pestis grown in a Ca2+-deficient environment that favored their net synthesis by Y. pseudotuberculosis (61), although analysis of unprocessed Lcr+ cells of Y. pestis grown in vitro revealed a large segment of YopE (13). Upon pulsing Ca2+-starved plague bacilli with [35S]methionine, radioactivity immediately appeared in a complete complement of native Yops but was promptly chased into small peptides (43, 56), except for a stable fragment of YopE probably identical to that noted above (13). This posttranslational degradation was attributed to the action of Pla (56, 57), a conclusion later verified by genetic analysis (60). These findings illustrate that Yops are cryptic in Lcr+ Pst+ isolates of Y. pestis grown in vitro but that they are obviously synthesized and delivered in an intact form in vivo because their structural genes are essential for the expression of disease (19, 47).
Essentially nothing is known about the mechanism whereby Pla mediates hydrolysis of Yops synthesized by Y. pestis or the significance, if any, of this phenomenon. In this report we demonstrate that soluble and biologically active α-Pla can be extracted from Escherichia coli LE392/pPCP1 by autolysis or with 1.0 M NaCl, α-Pla undergoes spontaneous conversion to β-Pla and smaller forms, and complete Pla-dependent posttranslational hydrolysis of Yops (but not YadA) also occurs in Y. pseudotuberculosis transformed with pPCP1. These findings are discussed within the context of current models defining the translocation of Yops into host cells by docked yersiniae (5, 19).
MATERIALS AND METHODS
Bacteria.
Salient properties of bacteria and plasmids used in this study are shown in Table 1. Transformation of E. coli LE392 with pPCP1 and derivatives of the latter containing Tn5 were previously described (58). E. coli LE392/pPCP1 was utilized in experiments concerned with extraction of soluble Pla and conversion of α-Pla to smaller derivatives. E. coli K802/pEK4 served as a source of Pla for generation of specific antiserum (6). DNA of pVK1 and pVK2 was isolated by the procedure of Clewell and Helinski (18) and transformed into Y. pseudotuberculosis strains 85 and 861 by freeze-thawing (20); transformants were selected on solid medium containing ampicillin. To minimize the potential of laboratory infection by Lcr+ cells of Y. pseudotuberculosis possessing pPCP1, experiments concerned with posttranslational hydrolysis of Pla and Yops in Y. pseudotuberculosis were undertaken with an avirulent gua isolate (intravenous 50% lethal dose > 107 bacteria) of strain PB1/+. The latter was induced and selected by methods previously described for Y. pestis (7) and then coconjugated with F+ to receive p2PCP::Tn1 containing a single copy of Tn1 in a silent region, as judged by mapping with restriction endonucleases (41). A methionine meiotroph (21) of nonpigmented (29) Lcr+ Pst+ Y. pestis KIM (substrain D39) and isogenic Lcr− and Pst− derivatives were used in comparative experiments. In mice Pgm− isolates are virulent (50% lethal dose ≈ 10 bacteria) when administered by intravenous injection but avirulent (50% lethal dose > 107 bacteria) when administered by peripheral routes (30, 63) due to the mutational loss of an ∼100-kb chromosomal sequence (23, 38) encoding the ability to absorb hemin, enzymes required for yersiniabactin synthesis, and the pesticin-yersiniabactin receptor (Δpgm) (47). Pst− derivatives of Y. pestis were obtained by elimination of pPCP by cold-curing (56).
TABLE 1.
Plasmids and bacterial isolates used in this study
| Plasmid or bacterium | Description | Reference(s) or source |
|---|---|---|
| Plasmids | ||
| pPCP1 | 9.6-kb pesticin (Pst) plasmid encoding Pla | 26, 47 |
| pCD1 | 70.6-kb low calcium response (Lcr) plasmid | 26, 47 |
| pMT1 | ∼100-kb murine toxin/fraction 1 (Tox) plasmid | 26, 47 |
| pBR322 | Cloning vector | 41 |
| pEK4 | Vector portion of pBR322 ligated to the cloned 1.45-kb HindIII-SmaI fragment of pPCP from Y. pestis 358 encoding Pla under control of its own promoter | 6 |
| p2PCP::Tn1 | pPCP::Tn1 (insert in silent region) | This study |
| pVK1 | pPCP::Tn1 (insert in silent region) | 33 |
| pVK2 | pPCP::Tn1 (insert in pst) | 33 |
| E. coli | ||
| LE392 | F−hsdR5149(r− m−) supE44 supF58 lacY1 galK2 galT22 metB1 trpR55 λ− | 41 |
| K802 | F−hsdR2(r−K m+K) supE44 lacY1 galK2 galT22 metB1 λ− | American Type Culture Collection |
| Y. pseudotuberculosis | ||
| PB1/+ | pYV; serotype IB | 12 |
| PB1/0 | from PB1/+ | 12 |
| PB1/+ (B44) | trp bio gua, pYV; from PB1/+ | This study |
| PB1/0 (B45) | trp bio gua; from B44 | This study |
| PB1/+ (B53) | trp bio gua, pYV, pPCP; from B44 | This study |
| PB1/0 (B59) | trp bio gua, pPCP; from B53 | This study |
| 85 | pYV; serotype III | Culture collection of Institute “Microbe” |
| 85/1 | pYV, pPCP (pVK1) | This study |
| 85/2 | pYV, pPCP (pVK2) | This study |
| 861 | pYV; serotype II | H. Molleret |
| 861/1 | pYV, pPCP (pVK1) | This study |
| 861/2 | pYV, pPCP (pVK2) | This study |
| Y. pestis | ||
| KIM (D27) | Δpgm,a pPCP1, pCD1, pMT1 | 7 |
| KIM (D28) | Δpgm, pPCP1, pMT1 | From D27 |
| KIM (D45) | Δpgm, pCD1, pMT1 | 57 |
| KIM (D47) | Δpgm, pMT1 | From D45 |
| KIM (D39) | Δpgm, pPCP1, pCD1, pMT1, Met+b | 56 |
| KIM (D49) | Δpgm, pCD1, pMT1, Met+ | From D39 |
| KIM (D40) | Δpgm, pPCP1, pMT1, Met+ | From D39 |
| KIM (D50) | Δpgm, pMT1, Met+ | From D49 |
Retention of pPCP and its derivatives in yersiniae and E. coli was monitored in isolates recovered from experimental animals and in cultures used for experiments by determining the percentage of pesticinogenic colonies on pesticin agar, with Y. pseudotuberculosis PB1/0 used as an indicator (11). This value was always 100%, indicating that pPCP is retained tenaciously in Y. pseudotuberculosis and E. coli, as is known to be the case in Y. pestis (56).
Cultivation.
Bacteria were grown at 37°C in liquid media contained in Erlenmeyer flasks (10%, vol/vol) aerated at 200 rpm on a water bath shaker (model G76; New Brunswick Scientific Co., New Brunswick, N.J.). E. coli K802/pEK4 was cultivated until late logarithmic growth phase in Luria-Bertani medium (Difco Laboratories, Inc., Detroit, Mich.) supplemented with 50 μg of ampicillin (Sigma Chemical Co., St. Louis, Mo.) per ml. The culture then received 20 μg of chloramphenicol (Sigma) per ml and was incubated for an additional 12 h at the same temperature before preparation of supernatant fluids. A minor modification of pesticin medium (28) used here for preparation and characterization of Pla consisted of 2.5% Sheffield NZ Amine (Kraft Inc., Memphis, Tenn.), 0.5% yeast extract (Difco), the salt component of Higuchi’s defined medium (25) (final concentrations of 0.025 M K2HPO4, 0.01 M citric acid, 2.5 mM MgCl2, 0.1 mM FeSO4, and 0.01 mM MnCl2), 25 mM potassium d-gluconate, and 2.5 mM sodium thiosulfate (all adjusted to pH 7.0 with 10 N NaOH). Pulse-chase experiments were performed with the defined medium of Zahorchak and Brubaker (68) supplemented with 0.5 mM guanosine and 0.5 mM hypoxanthine but lacking added l-methionine.
Antisera.
To produce anti-Pla, cells of E. coli K802/pEK4 were grown as described above and removed by centrifugation (10,000 × g for 15 min), and the supernatant fluid was passed through a 0.2-μm-pore-size low-protein-binding membrane filter (Gelman Sciences, Ann Arbor, Mich.). Solid (NH4)2SO4 was then added to the resulting sterile filtrate, and the 30 to 70% saturated fraction was collected by centrifugation (12,000 × g for 60 min), suspended in 0.025 M Tris · Cl, pH 7.5, and dialyzed at 4°C against three changes of the same buffer. This material was applied in volumes of 2.0 ml containing 11.6 mg of protein to a column (1.6 by 60 cm) of Sephadex G-200 (Pharmacia, Uppsala, Sweden) equilibrated with 0.02 M Tris · Cl buffer, pH 7.5, containing 0.2 M NaCl and 0.02% NaN3, and then eluted at a flow rate of 0.4 ml/min in the same buffer. Eluted fractions (2.0 ml) were assayed for biological activity by use of fibrin plates as described previously (2). Positive samples were assayed by SDS-PAGE, pooled, and lyophilized. The resulting material was nearly homogeneous and, following immunization of rabbits, yielded antisera capable of recognizing Pla. To eliminate natural antibodies directed against Yersinia spp. and E. coli, the serum received disrupted and lyophilized cells of E. coli K-12, Y. pseudotuberculosis PB1, and Y. enterocolitica WA (20 mg apiece/ml) and was gently stirred for 30 min at 37°C and then overnight at 4°C followed by removal of particulate material by centrifugation (10,000 × g for 30 min). After this cycle was repeated three times, gamma globulin was isolated by chromatography on DEAE cellulose as described previously (64).
Extraction of Pla.
Bacteria were grown as noted above and harvested by centrifugation (10,000 × g for 30 min), and the original culture supernatant fluid was retained for analysis by SDS-PAGE and immunoblotting. The organisms were washed once in 0.033 M potassium phosphate buffer, pH 7.0 (phosphate buffer), and then suspended at an optical density (620 nm) of 50 in either phosphate buffer alone or 1.0 M NaCl in phosphate buffer. These preparations, contained in 500-ml centrifuge bottles (50%, vol/vol), were vigorously shaken (250 rpm) on an Orbit platform shaker (model 3520; Lab-Line Instruments, Inc., Melrose Park, Ill.) for 30 min at 26°C and then centrifuged as described above. The resulting bacterial pellet was suspended in phosphate buffer and prepared for SDS-PAGE; supernatant fluids were similarly analyzed either directly or after passage through a 0.2-μm-pore-size low-protein-binding membrane filter (Gelman).
Pulse-chase determinations.
The method of Sample et al. (57) was used to detect net synthesis and then degradation of Yops by Lcr+ Pst+ yersiniae. Briefly, the technique involved restriction of vegetative growth at 37°C in Ca2+-deficient medium lacking l-methionine and then pulsing the cultures for 15 s with carrier-free [35S]methionine (New England Nuclear, Boston, Mass.) to yield a final concentration of 10 μCi/ml. The organisms were then chased by addition of excess unlabeled l-methionine to yield a final concentration of 1.6 mM. Samples of whole culture were removed at intervals, precipitated with an equal volume of cold 10% trichloroacetic acid, centrifuged (5,000 × g for 10 min), and prepared for SDS-PAGE. After staining, the gels were dried and exposed to X-ray film.
Miscellaneous.
SDS-PAGE was performed by the method of Laemmli (34); separated proteins were visualized by silver staining (45). Alternatively, Coomassie blue was utilized for staining Pla; this procedure and that used for immunoblotting with the rabbit polyclonal anti-Pla described above have been described previously (9). SDS-PAGE of whole or processed bacteria was undertaken with ∼10 μg of protein/lane; samples of extracted bacteria contained ∼1 μg of protein. Protein quantity was estimated by the method of Lowry et al. (37). Outbred white mice (4 to 8 weeks) received bacteria by subcutaneous injection (10 mice per dose) to determine virulence. The 50% lethal dose was calculated by the method of Kerber as modified by Ashmarin and Vorob’iov (1). Appropriate measures were used for care and housing of laboratory animals.
RESULTS
Detection of Pla.
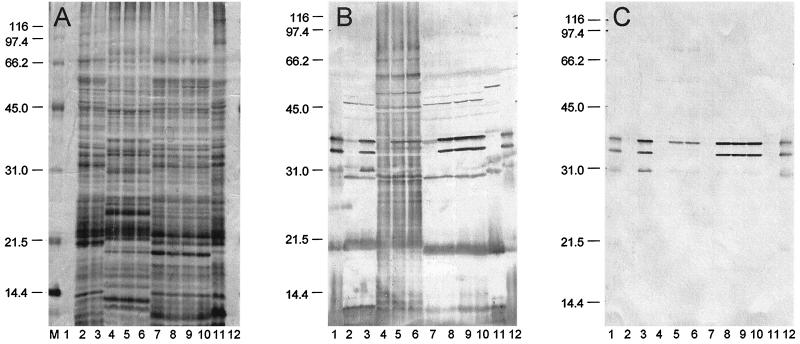
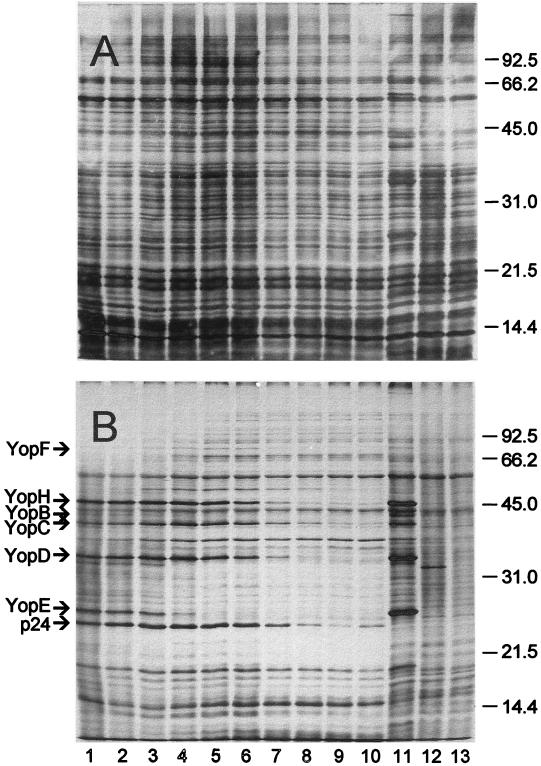
Cells of E. coli and various yersiniae carrying pPCP1 were subjected to SDS-PAGE and then silver stained. Pla was not visible in the resulting gel (Fig. 1A), although bands corresponding in molecular mass to α-Pla (35 kDa) and β-Pla (33 kDa) plus numerous cross-reacting antigens were observed upon immunoblotting with unabsorbed rabbit antiserum raised against purified Pla (Fig. 1B). Immunoblots specific for Pla were obtained by absorption of this antiserum followed by purification of remaining immunoglobulin G (Fig. 1C). Only α-Pla was detected in E. coli LE392/pPCP1, whereas both α- and β-Pla were produced by Y. pseudotuberculosis PB1/0/pPCP1. Cells of Y. pestis KIM/pPCP1 expressed both the α and β forms of Pla as well as a smaller variant of ∼31 kDa, termed γ-Pla (Fig. 1C).
FIG. 1.
Silver stain (A) and immunoblots of unabsorbed (B) and absorbed (C) rabbit polyclonal anti-Pla following SDS-PAGE of purified Pla (lanes 1 and 12), whole cells of Y. pestis KIM cured of pPCP1 (substrain D45) (lane 2), Y. pestis KIM/pPCP1 (substrain D27) (lane 3), E. coli LE392 (lane 4), E. coli LE392/pPCP1 (lane 5), E. coli LE392/p2PCP::Tn1 lane 6). Y. pseudotuberculosis PB1/0 (lane 7), Y. pseudotuberculosis PB1/0/pPCP1 (substrain B59) (lane 8), Y. pseudotuberculosis PB1/0/p2PCP:Tn1 (lane 9), Y. pseudotuberculosis PB1/0/pVK1 (lane 10), and Y. enterocolitica WA (lane 11). Molecular mass markers (in kilodaltons) are provided in the left margins. The procedure used to absorb the antiserum is described in Materials and Methods. Note that only α-Pla was expressed by E. coli/pPCP1, whereas Y. pseudotuberculosis PB1/0/pPCP1 produced β-Pla as did Y. pestis KIM/pPCP1 (which also generated a third, ∼31-kDa form, termed γ-Pla).
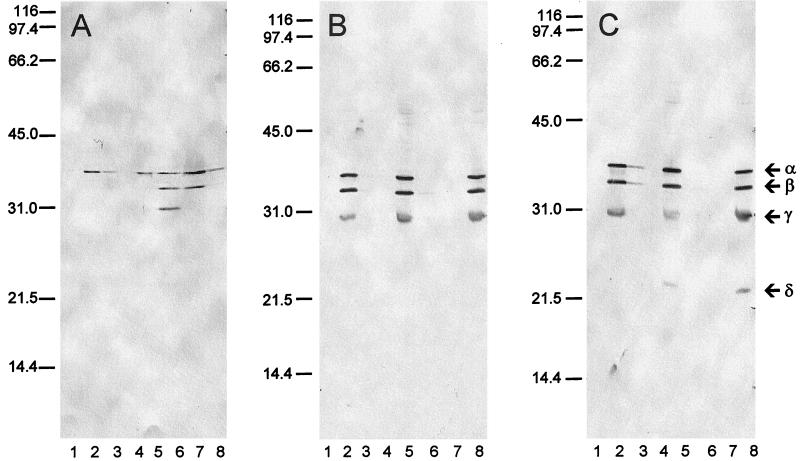
Solubilization of α-Pla.
In no case was Pla expressed in culture supernatant fluids (Fig. 2, lanes 1). We attempted its controlled solubilization and defined its conversion to degradation products by extraction of Pst+ bacteria with phosphate buffer alone or with 1.0 M NaCl in phosphate buffer. Only the α form was detected at significant levels in whole cells of E. coli LE392/pPCP1 after washing and suspension in phosphate buffer (Fig. 2A, lane 2). In contrast, similarly washed and suspended Lcr− cells of Y. pseudotuberculosis PB1/0 (Fig. 2B, lane 2) and Y. pestis KIM harboring pPCP1 (Fig. 2C, lane 2) expressed α-Pla as well as the β and γ forms. Attempts to extract significant soluble Pla from the three organisms with phosphate buffer alone were not successful. In contrast, extraction with 1.0 M NaCl in phosphate buffer resulted in recovery from E. coli LE392/pPCP1 of α-, β-, and γ-Pla (Fig. 2A, lane 6). The same process did not release significant Pla in any form from Pst+ yersiniae (Fig. 2B, lane 6; Fig. 2C, lane 6), although it generated a small derivative in Y. pestis, termed δ-Pla, which remained associated with the bacteria (Fig. 2C, lane 8).
FIG. 2.
Preparations obtained from E. coli LE392/pPCP1 (A), Y. pseudotuberculosis PB1/0/pPCP1 (substrain B59) (B), and Y. pestis KIM/pPCP1 (substrain D28) (C). Shown are culture supernatant fluid (lane 1); centrifuged whole cells suspended at an optical density (620 nm) of 50 in 0.033 M potassium phosphate buffer, pH 7.0 (phosphate buffer) (lane 2); supernatant recovered upon extraction (30 min) and then centrifugation of whole cells suspended in phosphate buffer (lane 3); supernatant recovered upon extraction (30 min) and then centrifugation of whole cells suspended in phosphate buffer after passage through a low-protein-binding membrane filter (lane 4); whole cells after extraction with phosphate buffer (lane 5); supernatant recovered upon extraction (30 min) and centrifugation of whole cells suspended in phosphate buffer plus 1.0 M NaCl (lane 6); supernatant recovered upon extraction (30 min) and centrifugation of whole cells suspended in phosphate buffer plus 1.0 M NaCl after passage through a low-protein-binding membrane filter (lane 7); and whole cells after extraction with phosphate buffer plus 1.0 M NaCl (lane 8). The positions of the α, β, γ, and δ forms of Pla are identified in the right margin.
The α and β derivatives extracted from the surface of E. coli LE392/pPCP1 with 1.0 M NaCl in phosphate buffer were soluble, as demonstrated by their ability to pass through a low-protein-binding filter, whereas smaller γ-Pla was lost during this procedure, indicating that it was denatured (Fig. 2A, lane 7). This observation illustrates that soluble Pla can be removed from E. coli LE392/pPCP1 (but not Pst+ yersiniae) by extraction with 1.0 NaCl in phosphate buffer. The filtered material obtained from E. coli LE392/pPCP1 with 1.0 M NaCl possessed potent biological activity (>105 U/10 μl) as judged by direct assay on fibrin plates.
Pst+ phenotype of Y. pseudotuberculosis.
A Pst+ isolate of a gua auxotroph of Y. pseudotuberculosis PB1/+ was selected by coconjugation of plasmid p2PCP::Tn1 accompanied by loss of F+. Comparison of this isolate with wild-type Y. pestis showed that both organisms expressed similar levels of pesticin, coagulase, and plasminogen activator activities. Subsequent mutational loss of pPCP1 in both species resulted in the disappearance of these activities (Table 2).
TABLE 2.
Specific activities of various products in whole cells of Y. pseudotuberculosis PB1/+ and Y. pestis KIM
| Organism | Sp act (U/mg of protein)a of:
|
|||
|---|---|---|---|---|
| pPCP | Pesticin | Coagulase | Plasminogen activator | |
| Y. pestis | +b | 46,000 | 2.0 | 840 |
| Y. pestis | 0 | 0 | 0 | 0 |
| Y. pseudotuberculosis | + | 51,500 | 2.4 | 925 |
| Y. pseudotuberculosis | 0 | 0 | 0 | 0 |
Units are defined according to Straley and Brubaker (64).
+, carriage of pPCP.
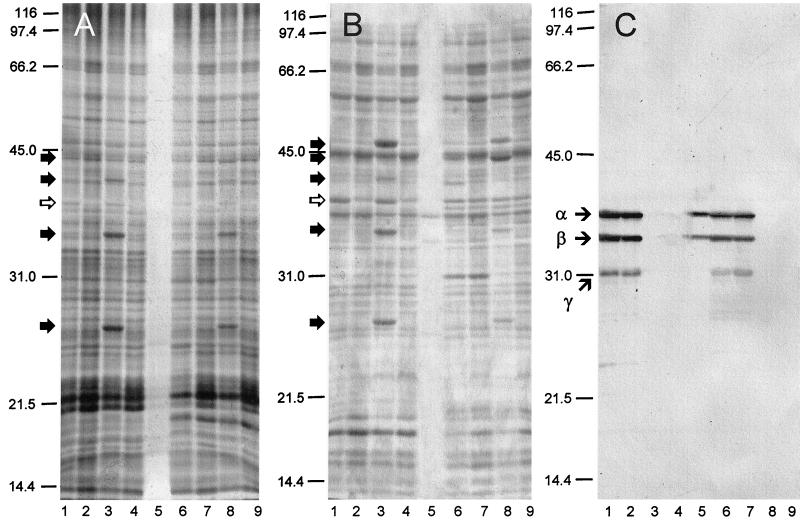
Following induction of the LCR, yersiniae were harvested, washed, and subjected to analysis by SDS-PAGE. After staining with silver (Fig. 3A) or Coomassie blue (Fig. 3B) or immunoblotting with anti-Pla (Fig. 3C), major Yops were recognized by comparison with isogenic mutants lacking pCD (pYV), pPCP, or both. Major discernible Yops in Lcr+ Pst− cells of Y. pseudotuberculosis (Fig. 3A and B, lanes 3) and Y. pestis (Fig. 3A and B, lanes 8) possessed molecular weights corresponding to values previously reported for YopH, YopC, YopD, and YopE (47). Those known to be expressed at lower concentrations were not detected by this procedure, nor were any Yops observed in Lcr+ Pst+ cells of Y. pseudotuberculosis (Fig. 3, lanes 1) or Y. pestis (Fig. 3, lanes 6). An Lcr+-specific peptide of ∼37 kDa, established as V antigen, was observed in both Lcr+ yersiniae (Fig. 3A and B, lanes 1, 3, 6, and 8). No form of Pla was detectable via silver staining (Fig. 3A, lane 5), but both α- and β-Pla were revealed by staining with Coomassie blue (Fig. 3B, lane 5) and by immunoblotting (Fig. 3C).
FIG. 3.
Effect of pPCP1 on expression of Yops in whole bacteria subjected to silver staining (A), Coomassie blue staining (B), and immunoblotting with rabbit polyclonal anti-Pla (C). Shown are the Y. pseudotuberculosis PB1/pPCP1, pYV (substrain B53) (lane 1); Y. pseudotuberculosis PB1/pPCP1 (substrain B59) (lane 2); Y. pseudotuberculosis PB1/pYV (substrain B44) (lane 3); Y. pseudotuberculosis PB1 (substrain B45) (lane 4); soluble (filtered) Pla extracted from E. coli LE392/pPCP1 in 1.0 M NaCl in 0.033 M potassium phosphate buffer, pH 7.0 (lane 5); Y. pestis KIM/pPCP1, pCD1 (substrain D27) (lane 6); Y. pestis KIM/pPCP1 (substrain D28) (lane 7); Y. pestis KIM/pCD1 (substrain D45) (lane 8); and Y. pestis KIM (substrain D47) (lane 9). (A and B) The solid arrows to the left of the gels indicate individual Yops, and the open arrows designate V antigen. (C) Arrows to the left of the immunoblot indicate the α, β, and γ forms of Pla.
Pla-mediated degradation of Yops.
A 15 s pulse with [35S]methionine followed by a 1-h chase with excess unlabeled methionine was undertaken to demonstrate synthesis and concomitant degradation of Yops in Lcr+ Pst+ cells of Y. pseudotuberculosis PB1 (substrain B53) maintained in the LCR. Radioactive proteins in samples removed at intervals throughout this period were separated by SDS-PAGE and subjected to silver staining (Fig. 4A) and radiography (Fig. 4B). Lcr+-specific peptides of ∼76 (YopF), 46 (YopH), 44 (YopB), 42 (YopC), 34 (YopD), and 25 (YopE) kDa underwent prompt posttranslational degradation and were not detected after 1 h. The stained gel (Fig. 4A, lanes 1 to 10) also showed the presence of YadA (∼200 kDa) and three similarly high molecular weight putative degradation products of this adhesin; native YadA was not well labeled within the short pulse used in these experiments (Fig. 4B, lanes 1 and 11).
FIG. 4.
Silver-stained gel (A) and corresponding autoradiogram (B) of trichloroacetic acid-precipitated material from cultures of Y. pseudotuberculosis PB1 cultivated at 37°C in Ca2+-deficient medium. Lcr+ Pst+ cells (substrain B53) were pulsed for 15 s with [35S]methionine and then chased with unlabeled methionine for 0 s (lane 1), 15 s (lane 2), 30 s (lane 3), 1 min (lane 4), 2 min (lane 5), 4 min (lane 6), 8 min (lane 7), 15 min (lane 8), 30 min (lane 9), and 1 h (lane 10). Control lanes (pulsed for 15 s and then chased for 1 h) contained isogenic Lcr+ Pst− (substrain B44) (lane 11), Lcr− Pst+ (substrain B59) (lane 12), and Lcr− Pst− (substrain B45) (lane 13) derivatives. Molecular mass markers (in kilodaltons) are provided in the right margin, and the positions of individual Yops are shown in the left margin; Bla and p24 indicate β-lactamase and the stable YopE degradation product (11), respectively. Note the presence of YadA and its derivatives in the silver stain.
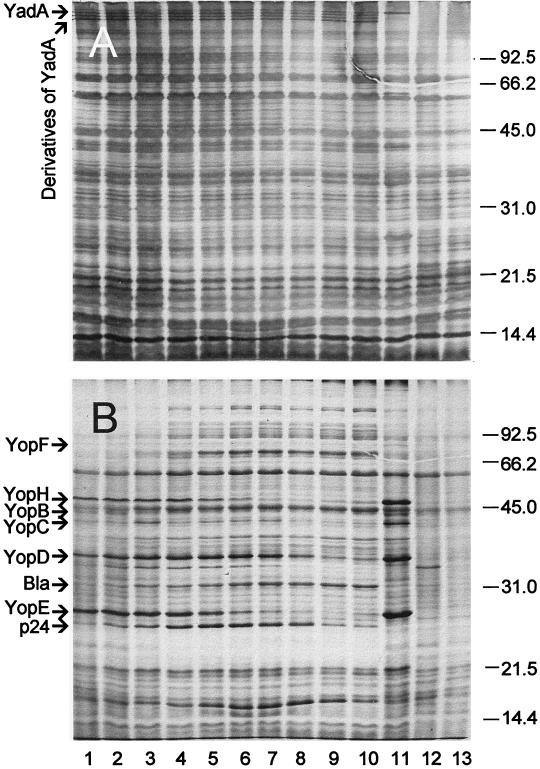
A parallel determination with Y. pestis KIM (substrain D39) was performed for purposes of comparison (Fig. 5). One major difference was the anticipated absence of YadA and its presumed degradation products. The kinetics of hydrolysis of YopF, YopH, YopB, and YopC were similar between the two species, whereas degradation of YopD occurred more slowly in Pst+ cells of Y. pseudotuberculosis (where its destruction required 1 h, as opposed to 8 min in Y. pestis). Both yersiniae converted YopE into its stabilized ∼24-kDa degradation product. This process, however, was accomplished in 1 min by Y. pestis, as opposed to 15 min for Y. pseudotuberculosis.
FIG. 5.
Silver-stained gel (A) and corresponding autoradiogram (B) of trichloroacetic acid-precipitated material from cultures of Y. pestis KIM (substrain D39) and corresponding derivatives. See the legend to Fig. 4 for details.
Virulence of Pst+ derivatives of Y. pseudotuberculosis.
In order to determine if expression of Pla by Y. pseudotuberculosis influences virulence in mice, we compared the subcutaneous 50% lethal doses of wild-type isolates transformed with Pst plasmids (pVK1 and pVK2). As shown in Table 3, carriage of these plasmids did not significantly enhance invasion of tissues as judged by only modest decreases in virulence.
TABLE 3.
The influence of pPCP on the subcutaneous 50% lethal dose of Lcr+ cells of Y. pseudotuberculosis in mice
| Strain | pPCP | 50% lethal dose | Ra |
|---|---|---|---|
| 85 | 0 | 6.7 × 101 | |
| 85/1 | + (pVK1)b | 8.2 × 102 | 12.2 |
| 85/2 | + (pVK2) | 2.1 × 103 | 31.3 |
| 861 | 0 | 7.5 × 105 | |
| 861/1 | + (pVK1) | 6.2 × 106 | 8.3 |
| 861/2 | + (pVK2) | 4.8 × 106 | 6.4 |
R, ratio of 50% lethal dose of the parent to that of the isogenic derivative transformed with pPCP.
+, carriage of pPCP.
DISCUSSION
Pla possesses significant homology to the outer membrane protease OmpT of E. coli (59) now known to be active under extreme denaturing conditions (66). OmpT from certain clinical isolates of E. coli also activated plasminogen, although additional features of surface architecture unique to pathogenic strains may have contributed to this capability (39). It is therefore of interest that the N terminus of native OmpP (a similar outer membrane protease possessing 87% identity to OmpT) was vulnerable to proteolytic digestion whereas its C-terminal region was protected. This finding indicated that the catalytic center of OmpP is located at or near the N terminus and that its C-terminal end promotes attachment to the outer membrane (32). Similarly, introduction of hexahistidine at the N terminus of Pla prevented biological activity whereas its addition at the C terminus prevented anchoring (unpublished observations). In view of these findings, we abandoned the prospect of engineering a soluble form of Pla and considered alternatives known to facilitate physical removal of native membrane proteins, including autolysis and extraction with monovalent cations (14, 36, 53).
These processes failed to release Pla in active form from Y. pestis or Y. pseudotuberculosis carrying pPCP, although particulate samples of high specific activity were sometimes obtained. However, their application to certain isolates of E. coli transformed with pPCP, including strains K802 and LE392, resulted in extraction of active enzyme. The reason for this difference is unknown, but a likely possibility is that Pla is processed and inserted into the outer membrane of E. coli LE392 exactly like OmpT or OmpP but that differences in primary structure result in faulty anchoring and thus permit solubilization upon autolysis or increased ionic strength. We emphasize that this phenomenon was observed only for the noted E. coli K-12 derivatives and does not apply to all strains of the species.
Although the site cleaved by plasminogen activators to generate plasmin is established (55), essentially nothing is known about the posttranslational conversion of Pla, the mechanism whereby Pla degrades Yops, or the biological significance of this reaction. Inability to define these processes reflects the fact that heretofore Pla could not be prepared in a form sufficiently homogeneous to rigorously determine its mechanisms of conversion and catalysis. These issues can now be addressed by using the rabbit polyclonal antiserum raised against Pla from E. coli LE392/pPCP1.
Of initial interest was the finding that cells of this isolate grown in an enriched medium (28) expressed only cell-bound α-Pla as opposed to those of Y. pseudotuberculosis PB1/0/pPCP1 or Y. pestis KIM/pPCP1, which also formed β-Pla. These results are in contrast to those of Sodeinde and Goguen (58), who detected cell-bound α- and β-Pla in E. coli cultivated in a minimal medium. We have repeated this observation with E. coli LE392/pPCP1 but have not yet identified variables that account for the appearance of β-Pla under austere conditions. However, we noted that the process of extracting E. coli LE392/pPCP1 with NaCl after growth in the enriched medium generated both β- and γ-Pla, suggesting that the smaller forms arise as sheared derivatives of α-Pla. Extraction failed to remove soluble forms of Pla from Pst+ cells of Y. pestis or Y. pseudotuberculosis (but did generate cell-bound γ- and δ-Pla), thus emphasizing that Pla is tenaciously bound to the outer membrane of yersiniae.
The anti-Pla serum enabled precise localization of α-Pla and its derivatives in stained gels. These determinations prompted the unexpected observation that Pla cannot be visualized by silver staining. Fortunately, Pla stained readily with Coomassie blue, which we recommend for determining its presence by SDS-PAGE. These experiments provided assurance that Y. pseudotuberculosis PB1/+/pPCP1 does indeed express levels of Pla similar to those in wild-type Y. pestis, thereby permitting a direct comparison of Yop degradation by the two isolates.
Yops of Lcr+ Pst+ cells of Y. pseudotuberculosis underwent hydrolysis in a manner similar to that previously reported for Y. pestis. Two distinctions, however, were noted between the two species. First, at least portions of the process occurred more rapidly in Y. pestis, even though the specific activities of Pla in the two isolates were equivalent as determined by fibrinolytic assay. This phenomenon is illustrated by the prompt disappearance of YopE in Pst+ cells of Y. pestis KIM with immediate conversion to its stable derivative (13), as opposed to more leisurely degradation in Lcr+ Pst+ cells of Y. pseudotuberculosis PB1. Second, YadA of the latter underwent evident Pla-mediated alteration, resulting in the appearance of four distinct structural elements, with the molecular mass of the largest equal to that of native YadA in the Lcr+ Pst− control. The significance of this observation is not fully understood, but these components probably represent native YadA and the YadA heteropolymeric subunits described by Gripenberg-Lerche et al. (24).
No major differences in virulence were found between wild-type Y. pseudotuberculosis and its Pst+ derivatives. Accordingly, expression of Pla did not significantly increase the virulence of these strains of Y. pseudotuberculosis by subcutaneous injection as is known to occur in Y. pestis (8). This difference emphasizes that avirulence caused by mutational loss of a native plasmid does not guarantee that acquisition of that plasmid by a naive bacterium will necessarily assure enhanced virulence. Many possibilities could account for this distinction, including pPCP-mediated disruption of pYV partitioning, interference with iron assimilation by pesticin, and hydrolysis of some unique Y. pseudotuberculosis-specific virulence factor by Pla. At present, we favor the notion that YadA, known to reduce the virulence of Y. pestis (54), remains at least partially intact in Y. pseudotuberculosis harboring pPCP (and thus may still retard dissemination of the bacteria from peripheral sites of injection). Resolution of this inconsistency is beyond the scope of the present study.
Major pCD (pYV)-encoded virulence factors include the cytotoxic Yops noted previously. Despite the essential nature of these proteins, they are promptly degraded by Pla during expression in vitro. Recent work on the nature of the LCR and results reported here may provide a basis for understanding this enigma. Classical studies on the regulation of macromolecular synthesis in prokaryotes have established the existence of global regulatory mechanisms that assure constant ratios of protein to DNA, thereby preventing lethal unbalanced growth (40). Bacteriostasis of yersiniae in vitro resulting from Ca2+privation at 37°C reflects termination of ongoing rounds of DNA synthesis but continued synthesis of mRNA (15, 68, 69) known to largely encode pCD encoded functions, especially Yops (42, 43). The ratio of DNA to protein would thus become skewed if Yops were permitted to accumulate within the cytoplasm of static Ca2+-starved bacteria; therefore, a process is required to assure their elimination, either by turnover or export into culture supernatant fluid. Both mechanisms are utilized as judged by the occurrence of type III secretion and concomitant Pla-mediated degradation. It is not yet clear if an identical secretion process also accounts for translocation of Yops into host cell cytoplasm by docked yersiniae. In this context, it may be significant that YopE can be secreted by two distinct systems (16); perhaps only one such mechanism is linked to subsequent Pla-mediated hydrolysis.
In any event, it is now evident that cytotoxic Yops are innocuous to the host unless translocated during bacterium-host cell contact (19). Accordingly, the ability of Pla to hydrolyze excreted but not translocated Yops is unlikely to interfere with expression of virulence. Indeed, this function might even favor the ability of Y. pestis to cause acute disease by eliminating soluble Yops from Ca2+-deficient necrotic lesions in visceral organs where they would otherwise be recognized as foreign proteins. It is significant in this regard that visceral lesions in mice formed by Lcr+ cells of Y. pseudotuberculosis arose as abscesses surrounded by professional phagocytes, whereas those generated by Y. pestis appeared as pure necrotic foci entirely lacking in signs of inflammation (46, 65).
ACKNOWLEDGMENTS
We are most grateful to Elena G. Boolgakova and Michael N. Kireev from the Institute “Microbe,” Saratov, Russia, for excellent technical assistance. In addition, the help provided by Janet M. Fowler of the Department of Microbiology, Michigan State University, was invaluable.
REFERENCES
- 1.Ashmarin I P, Vorob’iov A A. Statistical methods in microbiological studies. Leningrad, Russia: Medgiz; 1962. pp. 85–93. . (In Russian.) [Google Scholar]
- 2.Beesley E D, Brubaker R R, Janssen W A, Surgalla M J. Pesticins. III. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967;94:19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Gurion R, Hertman I. Bacteriocin-like material produced by Pasteurella pestis. J Gen Microbiol. 1958;19:289–297. doi: 10.1099/00221287-19-2-289. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981;5:183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- 5.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N, delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Boolgakova E G, Filippov A A, Kutyrev V V, Protsenko O A. Development of the technology of obtaining components of chemical anti-plague and anti-cholera vaccines on the basis of recombinant strains. Mol Genet Mikrobiol Virusol. 1994;4:31. . (In Russian.) [Google Scholar]
- 7.Brubaker R R. Interconversion of purine mononucleotides in Pasteurella pestis. Infect Immun. 1970;1:446–454. doi: 10.1128/iai.1.5.446-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker R R, Beesley E D, Surgalla M J. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science. 1965;149:422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- 9.Brubaker R R, Sample A K, Yu D-Z, Zahorchak R J, Hu P C, Fowler J M. Proteolysis of V antigen from Yersinia pestis. Microb Pathog. 1987;2:49–62. doi: 10.1016/0882-4010(87)90114-8. [DOI] [PubMed] [Google Scholar]
- 10.Brubaker R R, Surgalla M J. Pesticins. I. Pesticin-bacterium interrelationships, and environmental factors influencing activity. J Bacteriol. 1961;82:940–949. doi: 10.1128/jb.82.6.940-949.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brubaker R R, Surgalla M J. Pesticins. II. Production of pesticin I and II. J Bacteriol. 1962;84:539–545. doi: 10.1128/jb.84.3.539-545.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows T W, Bacon G W. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- 13.Chalvignac M A, Carniel E, Tram C, Joseph-Francois A, Mollaret H H. In vitro expression of a 22-kilodalton Yersinia pestis polypeptide immunologically related to the 25-kilodalton plasmid-encoded protein of the three pathogenic Yersinia species. Infect Immun. 1988;56:2576–2580. doi: 10.1128/iai.56.10.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang H W, Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977;16:4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- 15.Charnetzky W T, Brubaker R R. RNA synthesis in Yersinia pestis during growth restriction in calcium-deficient medium. J Bacteriol. 1982;149:1089–1095. doi: 10.1128/jb.149.3.1089-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 17.China B, Michiels T, Cornelis G R. The pYV plasmid of Yersinia encodes a lipoprotein, YlpA, related to TraT. Mol Microbiol. 1990;4:1585–1593. doi: 10.1111/j.1365-2958.1990.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 18.Clewell D B, Helinski D R. Supercoiled circular DNA complex in Escherichia coli: purification and induced conversion to an open circular form. Proc Natl Acad Sci USA. 1969;8:1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 20.Dityatkin S A, Iliyashenko B N, Lisovskaya K V. Transfection of frozen and thawed bacteria by isolated DNA of phage. Genetika. 1972;8:107–112. . (In Russian.) [Google Scholar]
- 21.Englesberg E, Ingraham L. Meiotrophic mutants of Pasteurella pestis and their use in elucidation of nutritional requirements. Proc Natl Acad Sci USA. 1957;43:369–372. doi: 10.1073/pnas.43.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferber D M, Brubaker R R. Plasmids in Yersinia pestis. Infect Immun. 1981;31:839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fetherston J D, Schuetze P, Perry R D. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol Microbiol. 1992;6:2693–2704. doi: 10.1111/j.1365-2958.1992.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 24.Gripenberg-Lerche C, Skurnik M, Toivanen P. Role of YadA-mediated collagen binding in arthritogenecity of Yersinia enterocolitica serotype O:8: experimental studies with rats. Infect Immun. 1995;63:3222–3226. doi: 10.1128/iai.63.8.3222-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higuchi K, Kupferberg L L, Smith J L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959;77:317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu P, Elliott J, McCready P, Skowronski E, Garnes J, Kobayashi A, Carrano A V, Brubaker R R, Garcia E. Structural organization of virulence-associated plasmids of Yersinia pestis. J Bacteriol. 1998;180:5192–5202. doi: 10.1128/jb.180.19.5192-5202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu P C, Brubaker R R. Characterization of pesticin: separation of antibacterial activities. J Biol Chem. 1974;249:4749–4753. [PubMed] [Google Scholar]
- 28.Hu P-C, Yang G C H, Brubaker R R. Specificity, induction, and absorption of pesticin. J Bacteriol. 1972;112:212–219. doi: 10.1128/jb.112.1.212-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson S, Burrows T W. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956;37:570–576. [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson S, Burrows T W. The virulence enhancing effect of iron on non-pigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 31.Kapperud G, Namork E, Skurnik M, Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987;55:2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann A, Stierhof Y D, Henning U. New outer membrane-associated protease of Escherichia coli K-12. J Bacteriol. 1994;176:359–367. doi: 10.1128/jb.176.2.359-367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutyrev V V, Popov Y A, Protsenko O A. Pathogenicity plasmids of the plague microbe (Yersinia pestis) Mol Genet Mikrobiol Virusol. 1986;6:3–11. [PubMed] [Google Scholar]
- 34.Laemmli U K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lähteenmäki K, Virkola R, Sarén A, Emödy L, Korhonen T K. Expression of the plasminogen activator Pla of Yersinia pestis enhances bacterial attachment to the mammalian extracellular matrix. Infect Immun. 1998;66:5755–5762. doi: 10.1128/iai.66.12.5755-5762.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leite D S, Yanno T, Pestana de Castro A F. Production, purification and partial characterization of a new adhesive factor (F42) produced by enterotoxigenic Escherichia coli isolated from pigs. Ann Inst Pasteur Microbiol. 1988;139:295–306. doi: 10.1016/0769-2609(88)90021-x. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Lucier T S, Brubaker R R. Determination of genome size, macrorestriction pattern polymorphism, and nonpigmentation-specific deletion in Yersinia pestis by pulsed-field gel electrophoresis. J Bacteriol. 1992;174:2078–2086. doi: 10.1128/jb.174.7.2078-2086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundrigan M D, Webb R M. Prevalence of ompT among Escherichia coli isolates of human origin. FEMS Microbiol Lett. 1992;76:51–56. doi: 10.1016/0378-1097(92)90362-r. [DOI] [PubMed] [Google Scholar]
- 40.Maaløe O, Kjeldgaard N O. Control of macromolecular synthesis. W. A. New York, N.Y: Benjamin; 1966. [Google Scholar]
- 41.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 42.Mehigh R J, Brubaker R R. Major stable peptides of Yersinia pestis synthesized during the low-calcium response. Infect Immun. 1993;61:13–22. doi: 10.1128/iai.61.1.13-22.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehigh R J, Sample A K, Brubaker R R. Expression of the low-calcium response in Yersinia pestis. Microb Pathog. 1989;6:203–217. doi: 10.1016/0882-4010(89)90070-3. [DOI] [PubMed] [Google Scholar]
- 44.Moore R L, Brubaker R R. Hybridization of deoxyribonucleotide sequences of Yersinia enterocolitica and other selected members of Enterobacteriaceae. Int J Syst Bacteriol. 1975;25:336–339. [Google Scholar]
- 45.Morrisey J H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilsl H, Killmann H, Hantke K, Braun V. Periplasmic location of the pesticin immunity protein suggests inactivation of pesticin in the periplasm. J Bacteriol. 1996;178:2431–2435. doi: 10.1128/jb.178.8.2431-2435.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portnoy D A, Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981;148:877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Portnoy D A, Wolf-Watz H, Bölin I, Beeder A B, Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984;43:108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Protsenko O A, Anisimov P I, Mosarov O T, Donnov N P, Popov Y A, Kokushkin A M. Detection and characterization of Yersinia pestis plasmids determining pesticin I, fraction 1 antigen and mouse toxin synthesis. Genetika. 1983;19:1081–1090. [PubMed] [Google Scholar]
- 52.Rakin A, Boogakona J, Heesemann J. Structural and functional organization of the Yersinia pestis bacteriocin pesticin gene cluster. Microbiology. 1996;142:3415–3424. doi: 10.1099/13500872-142-12-3415. [DOI] [PubMed] [Google Scholar]
- 53.Rivas S, Bolland S, Cabezon E, Goni F M, de la Cruz F. TrwD, a protein encoded by the IncW plasmid R388, displays an ATP hydrolase activity essential for bacterial conjugation. J Biol Chem. 1997;272:25583–25590. doi: 10.1074/jbc.272.41.25583. [DOI] [PubMed] [Google Scholar]
- 54.Rosqvist R, Skurnik M, Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988;334:522–525. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- 55.Saksela O, Rifkin D B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- 56.Sample A K, Brubaker R R. Post-translational regulation of Lcr plasmid-mediated peptides in pesticinogenic Yersinia pestis. Microb Pathog. 1987;3:239–248. doi: 10.1016/0882-4010(87)90057-x. [DOI] [PubMed] [Google Scholar]
- 57.Sample A K, Fowler J M, Brubaker R R. Modulation of the low calcium response in Yersinia pestis by plasmid-plasmid interaction. Microb Pathog. 1987;2:443–453. doi: 10.1016/0882-4010(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 58.Sodeinde O A, Goguen J D. Genetic analysis of the 9.5-kilobase virulence plasmid of Yersinia pestis. Infect Immun. 1988;56:2743–2748. doi: 10.1128/iai.56.10.2743-2748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sodeinde O A, Goguen J D. Nucleotide sequence of the plasminogen activator gene of Yersinia pestis: relationship to ompT of Escherichia coli and gene E of Salmonella typhimurium. Infect Immun. 1989;57:1517–1523. doi: 10.1128/iai.57.5.1517-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sodeinde O A, Sample A K, Brubaker R R, Goguen J D. Plasminogen activator/coagulase gene of Yersinia pestis is responsible for degradation of plasmid-encoded outer membrane proteins. Infect Immun. 1988;56:2749–2752. doi: 10.1128/iai.56.10.2749-2752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straley S C, Brubaker R R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci USA. 1981;78:1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straley S C, Brubaker R R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982;36:129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Une T, Brubaker R R. In vivo comparison of avirulent Vwa− and Pgm− or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Une T, Brubaker R R. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984;133:2226–2230. [PubMed] [Google Scholar]
- 65.Une T, Nakajima R, Brubaker R R. Roles of V antigen in promoting virulence in Yersinia. Contrib Microbiol Immunol. 1986;9:179–185. [PubMed] [Google Scholar]
- 66.White C B, Chen Q, Kenyon G L, Babbitt P C. A novel activity of ompT. Proteolysis under extreme denaturing conditions. J Biol Chem. 1995;270:12990–12994. doi: 10.1074/jbc.270.22.12990. [DOI] [PubMed] [Google Scholar]
- 67.Wolf-Watz H, Portnoy D A, Bölin I, Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985;48:241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zahorchak R J, Brubaker R R. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect Immun. 1982;38:935–959. doi: 10.1128/iai.38.3.953-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zahorchak R J, Charnetzky W T, Little R V, Brubaker R R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979;39:792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]