Summary
Glaucoma is an optic neuropathy characterized by permanent visual field loss caused by the death of retinal ganglion cells (RGCs) and it is the leading cause of irreversible blindness worldwide. Consequently, there is an unmet need for the development of new strategies for its treatment. We investigated RGC replacement therapy as a treatment for ganglion cell loss. Human-induced pluripotent stem cells (hiPSCs) were differentiated into mature, functional RGCs in vitro, labeled with AAV2.7m8-SNCG-eGFP, and transplanted intravitreally in wild-type 4-month-old C57BL/6J mice. Survival of the transplanted hiPSC-RGCs was assessed by color fundus photography and histological studies confirmed the localization of the transplanted hiPSC-RGCs within the retina. Two-photon live imaging of retinal explants and electrophysiological studies confirmed that the morphology and function of the transplanted hiPSC-RGCs were similar to native RGCs. These experiments will provide key strategies to enhance the efficiency of stem cell replacement therapy for neurodegenerative diseases, including glaucoma.
Subject areas: Biological sciences, Bioengineering, Tissue engineering, Biotechnology, Cell biology, Stem cells research
Graphical abstract
Highlights
-
•
Intravitreal injections of hiPSC-RGCs in WT mice were successful at 94% (n = 15/16)
-
•
The average number of donor hiPSC-RGCs that survived was 672 (range 230 to 1535)
-
•
hiPSC-RGCs were detected in the GCL of retinas and expressed RGC-specific markers
-
•
hiPSC-RGCs showed similar morphology and functional activities as the murine RGCs
Biological sciences; Bioengineering; Tissue engineering; Biotechnology; Cell biology; Stem cells research.
Introduction
Glaucoma comprises a diverse group of optic neuropathies clinically characterized by progressive structural and functional deterioration of the optic nerve resulting in permanent visual field loss (Janssen et al., 2013; Weinreb et al., 2016). Primary open-angle glaucoma (POAG) is the most common form of glaucoma. In 2020, about 52.68 million individuals aged 40–80 were diagnosed with POAG. By 2040, that number is expected to increase to 79.76 million (Tham et al., 2014; Zhang et al., 2021c), making POAG the leading cause of irreversible blindness worldwide (Cook and Foster, 2012; Quigley and Broman, 2006). In the United States alone, 3 million Americans are projected to have glaucoma, which is estimated to double by the year 2050. Furthermore, glaucoma is responsible for 16.2 billion dollars of total direct medical costs (Rein et al., 2006). POAG is disproportionately more prevalent among senior individuals of African descent (>5%) compared to other groups of the population such as Hispanic or Latino (2.7%), Asian (∼2%), and Caucasian (1.5%) (Charlson et al., 2015; Salowe et al., 2015; Kapetanakis et al., 2016).
Despite the prevalence of POAG, its cause remains poorly understood. Disruption of the aqueous humor outflow pathway is known to increase intraocular pressure (IOP), which preferentially and progressively damages the retinal ganglion cells (RGCs) (Danford et al., 2017). RGCs are involved in transmitting visual signals from the photoreceptors to the brain (Heavner and Pevny, 2012). They are the first set of neuronal cells to be differentiated in the developing retina, and since RGCs do not regenerate, their death results in permanent vision loss. RGC death causes optic nerve atrophy and results in a severe reduction of visual function (Weinreb et al., 2016). Elevated IOP is a primary risk factor and the only treatable clinical characteristic of glaucoma (Liu and Allingham, 2017); however, increased IOP alone is neither sufficient nor necessary to cause RGC death leading to glaucoma (Sommer et al., 1991). According to the Baltimore Eye Survey, approximately 50% of all patients with glaucoma have normal-tension glaucoma with an eye pressure below 22 mm Hg (Sommer et al., 1991). Existing treatments for glaucoma target lowering the IOP and include medications such as anti-hypertensive drops, laser trabeculoplasty, and surgical interventions. Surgeries for glaucoma are plagued with complications that result in the need for additional surgery and frequent follow-up. Anti-hypertensive drops have very high rates of non-compliance stemming from the multiple daily drug regimens of eye drops, which can account for 35,000 doses throughout one’s lifetime. Importantly, anti-hypertensive drops should ideally be administered to patients before or during early vision loss. However, owing to the asymptomatic progression of glaucoma, patients often present with a late-stage disease that is irreversible, and no treatments are available once the RGC death has occurred (Cohen and Pasquale, 2014). Consequently, there is an unmet need for the development of new strategies for the treatment of glaucoma.
Cell replacement strategies are an innovative and promising alternative to treat glaucoma. One such method involves transferring healthy RGCs into the eyes of afflicted individuals to replace the function of the degenerated and dead ganglion cells. Prior RGC transplantation studies have produced variable outcomes and success (Hertz et al., 2014, Jagatha et al., 2009, Divya et al., 2017, Cho et al., 2012, Scruggs et al., 2019, Venugopalan et al., 2016, Miltner and La Torre, 2019, Suen et al., 2019, Oswald et al., 2021, Zhang et al., 2021a), but thus far have resulted in limited clinical use. The inconsistency in transplantation efficiency may be attributed to the availability and purity of a reliable donor source, differences in graft and host species, and route of delivery.
In this study, we transplanted mature human iPSC-derived retinal ganglion cells (hiPSCs-RGCs) intravitreally in wild-type C57BL/6J mice. Our protocol for generating hiPSC-RGCs represents one of the most versatile, robust, and highly reproducible methods for the large-scale production of pure RGC populations without any gene modification (Chavali et al., 2020). Following intravitreal injections, eGFP + hiPSC-RGCs were detected in 15 of the 16 wild-type mice, with an average successful transplantation rate of about 94%. Overall, an average of 672 donor hiPSC-RGCs were visible per explanted host retina. The transplanted RGCs integrated within the ganglion cell layer of host retinas and survived at least 5-months post-transplantation. Furthermore, the transplanted hiPSC-RGCs were functional and showed electrophysiologic profiles similar to native mouse RGCs. In conclusion, hiPSC-RGCs represent a promising source of donor cells that may aid in developing patient-tailored cell therapies designed to improve visual function in glaucoma.
Results
In vitro human induced pluripotent stem cells differentiation generates pure populations of retinal progenitor cells and retinal ganglion cells
Clinical translation of hiPSC-RGC transplantation in an in vivo model is highly dependent on the availability of a scalable differentiation protocol to efficiently generate mature and functional RGCs in an expedited and less labor-intensive manner. Our laboratory standardized the two-step/stage differentiation technique (Teotia et al., 2017), in which hiPSCs are initially differentiated to retinal progenitor cells (RPCs) via the step-wise addition of small molecules and peptide modulators in a chemically defined media. This is followed by a cross-hatching technique on day 24 that allows for the expansion and differentiation of RPCs into functionally mature iPSC-RGCs (Chavali et al., 2020).
The hiPSC lines obtained from de-identified patient-derived normal Caucasian and African American donors were differentiated into hiPSC-RPCs and hiPSC-RGCs (Pashos et al., 2017). Prior to transplantation, the purity of hiPSC-RPC and hiPSC-RGC populations were assessed using fluorescence-activated cell sorting (FACS) on day 15 and day 35, respectively. Ki67 and Chx10 were used to characterize hiPSC-RPCs and BRN3/ POU4F, gamma Synuclein (SNCG), CD90/ Thy1, and RNA-binding protein with multiple splicing (RBPMS) were used for the identification of hiPSC-RGCs, respectively.
We recently reported that hiPSC-RPCs generated using our method stained positive for Ki67 (95.5%) and Chx10 (82%) markers (Gudiseva et al., 2021). Similarly, hiPSC-RGCs stained positive for BRN3 (87%), SNCG (93%), CD90 (85.5%), and RBPMS (22.5%) (Gudiseva et al., 2021). Our differentiation protocol consistently generates RPCs and RGCs that are highly positive for the respective differentiation markers (Tables S1A and S1B). The hiPSC-RGCs can be maintained in culture for over 3 months with minimal cell loss and, therefore, serve as a readily available source of RGCs for transplantation studies.
Transplanted human-induced pluripotent stem cells -retinal ganglion cells were detected in the murine retina
For transplantation studies into the murine retina, we fluorescently labeled hiPSC-RGCs by transduction with AAV2.7m8-SNCG-eGFP, which expresses eGFP under the control of an RGC-specific gamma Synuclein (SNCG) promoter (Ross et al., 2021). Labeled hiPSC-RGCs expressed green fluorescence as early as 48 h post-transduction (Figure 1A).
Figure 1.
Images of hiPSC-RGCs expressing SNCG-eGFP after intravitreal injection
(A) Fluorescent images of the hiPSC-RGCs transduced with AAV2.7m8 SNCG-eGFP (4X (scale bar is 750 μm) and 10X (scale bar is 300 μm) magnification).
(B and C) hiPSC-RGCs transduced with SNCG-eGFP vector were injected into the vitreous space of wild-type C57BL/6J mice. Fundus photography of the transplanted hiPSC-RGCs was taken using BAF-cSLO (scale bar is 200 μm) and SD-OCT at 2-, 4-, and 6-weeks post-transplantation. See Table S2.
Owing to the high purity of transduced hiPSC-RGCs, 5 × 105 viable cells (∼Day 50) were intravitreally injected into the eye of 4-month-old wild-type C57BL/6J mice (n = 16). The contralateral eye was injected with 1X PBS as an experimental control. The mice were subsequently monitored for any signs of distress or complications. In vivo fundus images were taken using wide-field (WF) and ultra-widefield (UWF) blue autofluorescence- confocal scanning laser ophthalmology (BAF-cSLO) and spectral-domain optical coherence tomography (SD-OCT) to assess the presence of eGFP + cells at 2-, 4-, and 6-weeks post-injection (Figures 1B and 1C).
Following intravitreal delivery, eGFP + hiPSC-RGCs were detected within the ganglion cell layer of the murine retina as early as 2-weeks post-injection, seen as punctate hyperfluorescent foci in the BAF-cSLO and color fundus imaging (CFI) (Figure 1B). We did not observe any gross abnormalities in the fundus images of any saline- or hiPSC-RGCs injected eyes. SNCG-eGFP + donor cells were uniformly distributed across the pan-field of view of the mouse retina; however, they were predominantly localized adjacent to retinal veins than retinal arteries. The nearest neighbor index (NNI) (the ratio of mean nearest observed distance between eGFP + cells (Nnd = ∼3 μm) to mean random expected distance) was used to quantify the donor cell spatial distribution pattern. If the index is greater than 1, the cells are determined to be well dispersed, whereas if the index is less than 1, the cells are considered to be clustered. In our study, NNI was determined to be 0.511 (n = 3); hence the transplanted hiPSC-RGCs were partially clustered within the mouse retina (Clark and Evans, 1954; Keeley et al., 2020; Cressie, 2015).
On average, there were 672 (range 230 to 1535) hiPSC-RGCs detected following intravitreal injections (Table S2). Our average transplantation efficiency was determined to be 0.134%. Furthermore, recent studies indicate that transplantation is considered successful if 0.1% of the total transplanted donor RGCs survive in vivo (Oswald et al., 2021; Venugopalan et al., 2016). In that case, our transplantation experiments were successful in n = 9/16 mice.
SD-OCT images confirm the structural integrity of the mouse retina following intravitreal injections (Figure 1C). We evaluated the vitreous cavity for the presence of abnormal scattering that could be evidence of donor hiPSC-RGC remnants and/or the presence of immune cells at 2-, 4-, and 6-weeks following intravitreal injections.
Detection of the transplanted human-induced pluripotent stem cells-retinal ganglion cells within the murine retina
The eyes of wild-type mice injected with hiPSC-RGCs were enucleated 2- and 5-months post-transplantation. Immunohistochemistry (IHC) analysis of retinal sections (n = 3) was performed to determine the localization of the transplanted hiPSC-RGCs within the host retina. Retinal cryosections were co-stained with RGC-specific markers including BRN3, MAP2, and Synapsin I to confirm the identity of the transplanted cells.
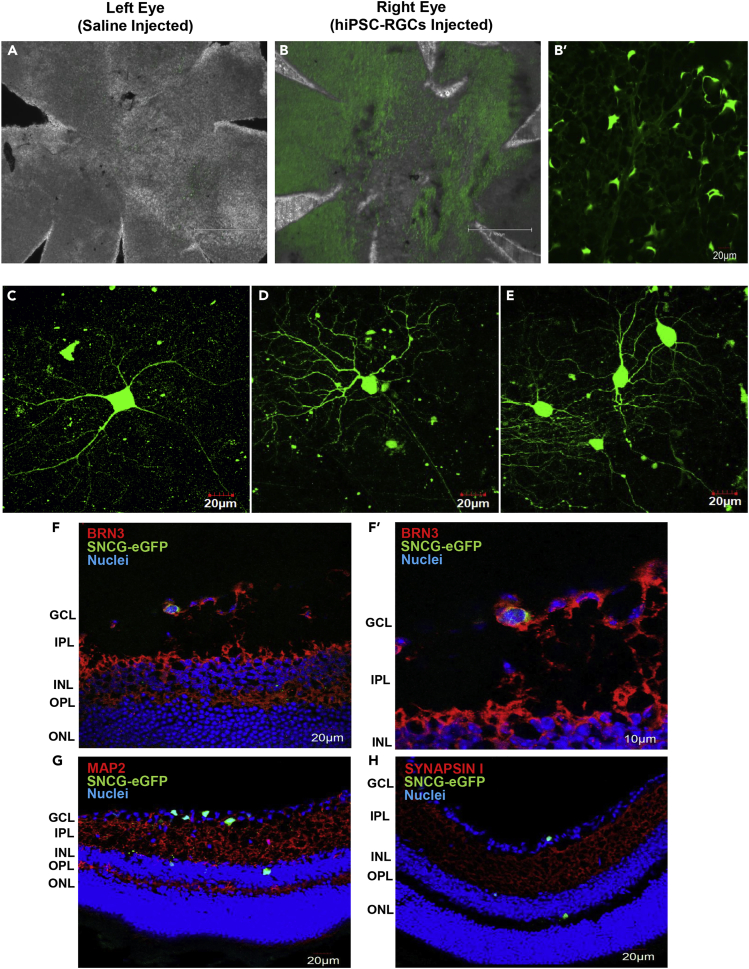
The majority of the transplanted hiPSC-RGCs were found within the ganglion cell layer (GCL) of the mouse retina interweaved with host ganglion cells (Figures 2B and 2B′), while a few displaced RGCs (dRGCs) migrated into the inner nuclear layer (Dräger and Olsen, 1981) (Figures 2F–2H). Transplanted hiPSC-RGCs exhibited morphological characteristics similar to endogenous RGCs with long, complex dendritic stratification and some with short-range neurite outgrowth (Figures 2C–2E). Furthermore, the donor cells had dendritic extensions into the inner plexiform layer, as seen in the side projection of two-photon Z-stack images acquired from the live murine retinal samples, thus suggesting that the hiPSC-RGCs are forming projections to the appropriate cell layer within the host retinal circuit (Videos S1 and S2).
Figure 2.
Images of transplanted hiPSC-RGCs in vivo
(A and B) Fluorescent images of the flattened whole-mount retina of saline-injected left eye and hiPSC-RGCs injected right eye (B = 4X (scale bar is 750 μm), B’ = 10X (scale bar is 20 μm)).
(C–E) Neurite outgrowth of transplanted hiPSC-RGCs taken as two-photon images acquired from live retinal samples. Cryosection images of transplanted hiPSC-RGCs (n = 3) were detected in the GCL and INL layer of the murine retina and co-stained for (F) Brn3, (G) MAP2, and (H) Synapsin I. The scale bar of images is 20 μm.
Characterization of the transplanted human-induced pluripotent stem cells-retinal ganglion cells within the murine retina
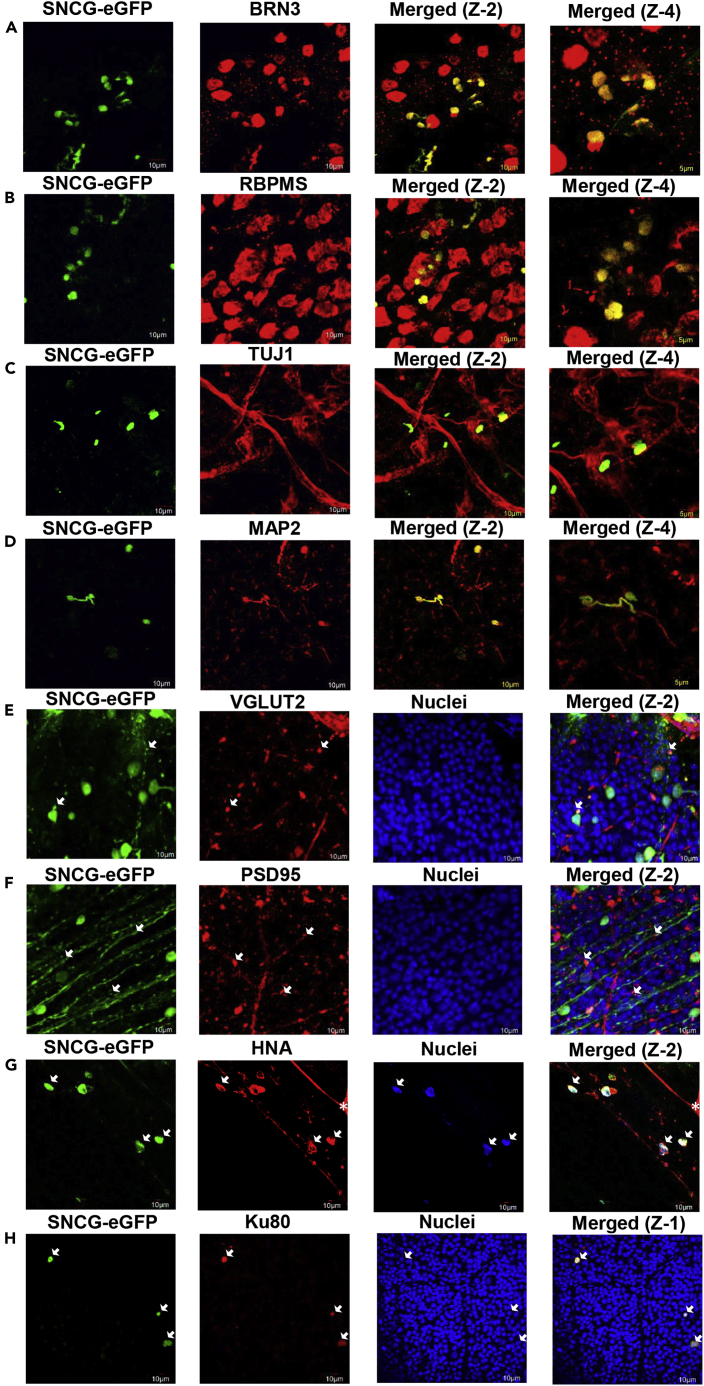
Whole-mount immunohistochemistry (n = 8) was performed on the retinas isolated from enucleated eyes 2- and 5- months after injection with hiPSC-RGCs. Flattened retinas were counter-stained for RGC-specific markers such as BRN3, RBPMS, MAP2, and TUJ1, including synaptic markers like VGLUT2 and PSD95.
All the SNCG-eGFP + hiPSC-RGCs detected in the mouse retinas expressed BRN3 (Figure 3A) and RBPMS (Figure 3B). Furthermore, TUJ1 (Figure 3C) and MAP2 (Figure 3D) antibodies were used to stain microtubule markers expressed primarily in neurons and they function to stabilize neuronal shape. To detect the presence of synapses of the transplanted hiPSC-RGCs, VGLUT2 and postsynaptic density protein 95 (PSD95) markers were used. VGLUT2 is one of the three vesicular glutamate transporters, involved in the accumulation of glutamate into synaptic vesicles before glutamate is released at the synaptic cleft for excitatory neurotransmission (Bellocchio et al., 2000). Co-staining mouse retina with VGLUT2 revealed that SNCG-eGFP + cells displayed the vesicular transporter (Figure 3E). PSD95 is a postsynaptic scaffolding protein that regulates the localization of neurotransmitter receptors and signaling molecules in excitatory neurons (Keith and El-Husseini, 2008). Transplanted SNCG-eGFP + cells are shown here to express PSD95 protein in the neurite terminal as the characteristic punctate pattern (Figure 3F). The transplanted hiPSC-RGCs expressed the assembling of synaptic protein markers in the host retina.
Figure 3.
Immuno-staining of the transplanted hiPSC-RGCs in mouse retina with RGC-specific markers
(A–H) Flattened whole-mount images of transplanted hiPSC-RGCs (n = 8) were co-stained with RGC-specific markers (A) BRN3, (B) RBPMS, (C) TUJ1, (D) MAP2, (E) VGLUT2, and (F) PSD95. Donor hiPSC-RGCs were also co-stained with (G) human nuclear antigen (HNA) and (H) Ku80 to confirm the donor origin of SNCG-eGFP + cells. ∗ Since the human nuclear antigen antibody was raised in mice, some cross-reactivity with the murine retina was observed. Z-1: Zoom-1 (scale bar is 10 μm), Z-2: Zoom-2 (scale bar is 10 μm), and Z-4: Zoom-4 (scale bar is 5 μm).
Human nuclear antigen (HNA) and Ku80 antibodies were used to label the donor hiPSC-RGCs to confirm the donor origin of the transplanted cells; all SNCG-eGFP + cells were either positive for human nuclear antigen or Ku80, thus suggesting no material exchange was observed between transplanted hiPSC-RGCs and the host RGCs (Figures 3G and 3H).
Function of the transplanted human-induced pluripotent stem cells-retinal ganglion cells
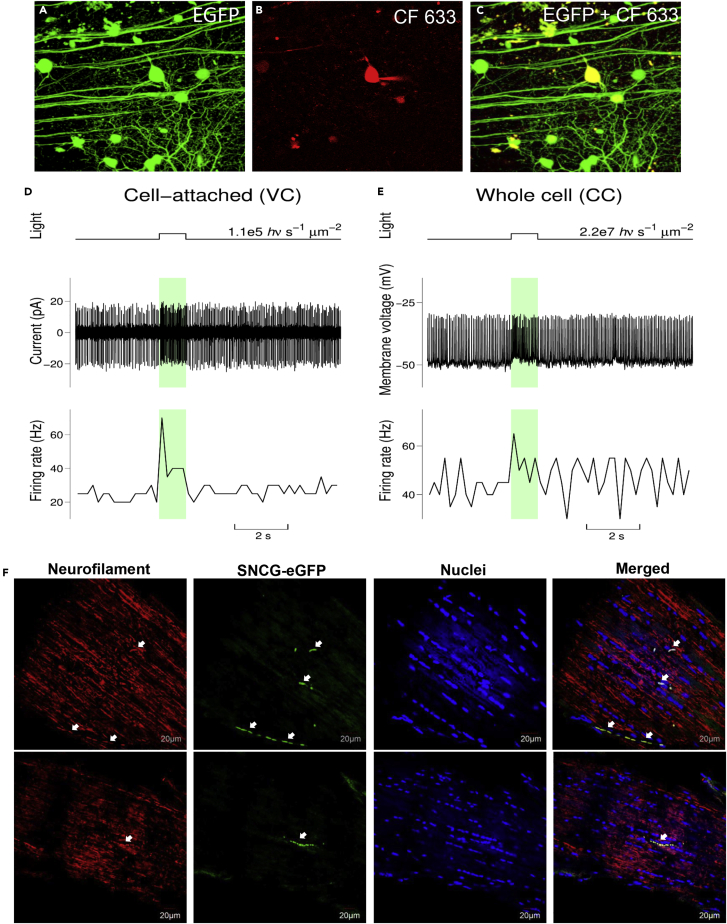
Transplanted retinas from mice aged 9 months (n = 4) were used for live two-photon imaging and electrophysiological studies. Two-photon live images revealed an elaborate dendritic arborization of transplanted hiPSC-RGCs in the host retina (Figures 4A–4C). Electrophysiologic responses to full-field photopic stimuli assessed functional integration. Light responses were first recorded in the cell-attached (loose patch) configuration in the voltage-clamp mode (Figure 4D) before rupturing the membrane and establishing the whole-cell configuration in the current-clamp mode (Figure 4E). Of the six recorded hiPSC-RGCs, two cells produced light responses with elevated baseline firing (Figure 4), two cells generated light responses with very low baseline firing (Figures S1D and S1E), and two cells produced increased firing over large, often spontaneous depolarizations. For one of the last two cells, the probability of synchronization between flash and spike firing was around 60%, well above 10%, which would be expected if spike firing was completely random. Depolarizations and spike firing observed for the other cell appear to be primarily spontaneous (Figures S1F and S2E).
Figure 4.
Injected hiPSCs integrate into the host retina and generate light responses
Two-photon images at the top row show targeted hiPSC-RGCs (centered) before, during, and after recording its light responses.
(A) Image shows eGFP fluorescence observed from hiPSC-RGCs in the retinal sample before any attempt to approach it with the recording pipettes. The image is a projection of the Z-stack consisting of 234 optical slices acquired with a 0.5 μm step.
(B) The red fluorescence from the CF 633 filled pipette and targeted cell is shown. This image comes from the single optical slice acquired at the end of the patch-clamp recording session. The patch pipette can be seen on the right side of the cell. A slight repositioning of the targeted cell was owing to its re-centering for the IR video system used to control pipette manipulations.
(C) The Z-stack projection (151 optical slices with 0.5 μm step) shows combined eGFP and CF 633 fluorescence after the completion of the pipette recording and withdrawal of the patch-pipette. The targeted cell has a bright yellow color. Note that eGFP + hiPSC-RGC processes observed before the patch-clamp recording (panel A) can still be readily identified after its completion (panel C). The scale bar is 50 μm.
(D and E) Light responses of the targeted cell recorded in the cell-attached configuration (voltage-clamp mode) and whole-cell configuration (current-clamp mode) are illustrated (n = 4). Here upper graphs give the time course of light stimuli and indicate flash intensities, the middle graphs plot current vs. time and membrane voltage vs. time traces (panels D and E respectively), and the bottom graphs plot firing rate vs time traces. The current vs. time trace from panel D has been high pass filtered, and the voltage vs. time trace from panel E has not been corrected for the LJP, the correction can be done by subtracting 15 mV from the reported values. The firing rate traces were calculated using a 200 ms time bin. The colored light bars indicate light stimulation events. See also Figures S1–S4.
(F) Detection of transplanted hiPSC-RGCs in the optic nerve of the mouse and its expression co-localized with the neurofilament marker (n = 2). The scale bar is 20 μm.
The hiPSC-RGCs firing rate increase in response to depolarizing stimuli appears to diminish upon repeated stimuli. It is unlikely that the dilution of the intracellular content caused such a decrease in responsivity by the pipette solution because it was detected in the cell-attached as well as in the whole-cell configurations. It was also observed that a brighter flash delivered later on could cause a larger membrane depolarization but a smaller increase in the firing rate compared to the preceding dim flash (Figures S3A and S3B). Moreover, a small depolarization in response to the first light flash could produce a larger increase in the firing rate than much larger depolarizations caused by current injections, when current injections were done after repeated light stimuli (Figures S2A–S2C). Figures 4 and S2 present data from the same cell, and a comparison of Z-stack projections before and after pipette recording (Figure 4C) does not show any broken/missing cellular processes, so the observed decrease in responsivity appears not to be owing to any physical damage caused to the cell by pipette manipulations. Progressively smaller increases in the firing rate in response to depolarizations caused by light exposures or current injections were observed for all recorded cells. The responsivity at least partially recovered when cells were rested without stimulation for 30 s or longer. Furthermore, the light responses produced by the transplanted SNCG-eGFP + hiPSC-RGCs were similar to those produced by the wild-type mouse RGCs (Figure S4).
To improve vision, axons of functionally integrated donor hiPSC-RGCs need to extend all the way to synapse with targets in the visual cortex of the brain. In this study, we processed optic nerves of eyes intravitreally injected with hiPSC-RGCs (n = 2) and detected graft axonal projections of a few eGFP + hiPSC-RGCs in the optic nerve head (ONH) and shown here to express neurofilament marker (Figure 4F). Neurofilament proteins are essential for the maintenance of the structure and shape of the neurons, and they are involved in the regulation of signal transduction and synaptic plasticity of the axons (Didonna and Opal, 2019). Furthermore, this suggests that the donor hiPSC-RGCs were aligned with and followed along with the host RGC axons.
Discussion
Transplantation experiments of immature retinal sheets into the eye can be traced as far back as 1964 (Tansley, 1964). However, the success of ganglion cell transplantation is highly dependent on the purity of the donor retinal cells. The efficiency of current retinal differentiation protocols across different cell lines such as mESC, hESC, miPSC, and hiPSC is highly variable and often results in low yield and/or low purity of retinal cell populations (Banin et al., 2006; Osakada et al., 2008; Lamba et al., 2006). Early attempts included co-culturing mESCs with extracted mouse retinal tissue to promote the differentiation towards retinal cell fate, but this resulted in few cells that expressed RGC-like markers (Ikeda et al., 2005; Meno et al., 1997; Del Barco Barrantes et al., 2003; Davis et al., 2000; Aoki et al., 2007). RGCs derived from cultured embryoid bodies that formed optic vesicle-appearing neurospheres were detected from days 20–50 and were reported to express BRN3 and Calretinin. This technique was reported in both hiPSCs and ESCs (Meyer et al., 2009, 2011). This method was then expanded to develop optic cup-like structures from mESCs and hESCs and has been reported to recapitulate in vivo retinal development (Nakano et al., 2012; Eiraku et al., 2011). RGCs have also been differentiated from miPSC- and mESC-derived 3D-retinal tissues but resulted in low cell purity (8% Brn3a+ and 5% RBPMS+) and thus required further enrichment with Thy1.2-based magnetic associated cell sorting (Oswald et al., 2021).
In recent years, the use of iPSCs for cell therapies has garnered much attention, as iPSCs are an excellent source of stem cells that can be directed to generate every key cell type in the eye and can be used to treat neurodegenerative diseases such as glaucoma (Singh et al., 2013). Furthermore, using human iPSCs to treat diseases can overcome some of the ethical concerns associated with using human embryonic stem cells (Chamling et al., 2016). Patient-derived iPSCs are an ideal source of donor cells as they circumvent host vs. donor transplant immunological rejection. Our differentiation protocol consistently produces large populations of hiPSC-derived neuronal cells expressing over 70% of RGC-specific markers such as BRN3, Thy1, and SNCG to date. However, it should be noted that although these markers are specific to RGCs within the retina, they are expressed by various non-retinal neuronal cells such as CNS neurons and motor neurons. Therefore, it is essential to employ different techniques to confirm in vitro cell identity by RNA sequencing analysis, DNA methylation patterns of RGC-specific genes, microarrays, or transcriptomic and proteomic approaches. For translational studies, it will be critical to determine the purity of donor RGCs to reduce the risk of teratoma formation from undifferentiated or heterogeneous cell populations. To achieve successful transplantation, it is also critical to identify different RGC subtypes, as each of the 20 different RGC subtypes identified to date has a unique pre- and post-synaptic function.
To provide a pre-clinical framework necessary to advance retinal ganglion cell transplantation as a therapy for glaucoma, we performed our preliminary in vivo studies in a wild-type adult C57BL/6J mouse model. Using murine models for transplantation studies can considerably reduce the risk, time, and costs of advancing the therapy into human clinical trials. For transplantation into the murine eye, we chose intravitreal injection among the different routes of hiPSC-RGCs administration, as it is the most targeted route of cell delivery to the retina without disrupting the blood-retinal barrier, and it bypasses systemic exposure (Varela-Fernández et al., 2020). However, since the vitreous space is a large cavity, it is difficult to control the dispersion of the injected cells to a specific area of the retina, and in some cases, the donor cells adhere to the posterior lens capsule and ciliary body (Johnson and Martin, 2008).
Our current transplantation methodology recovered about 50% of viable cells in the single-cell dissociation step. This step needs to be optimized to be less harsh on the cells and efficiently break up the cell clusters into single cells to perform injections with a higher number of live and healthy RGCs. Furthermore, cell sorting by FACS to remove dead cells after cell dissociation will improve hiPSC-RGC survival post-transplantation and their integration in the mouse retina. However, numerous cues influence the survival rate of the donor cells, such as the innate and adaptive immune system of the recipient animal, and factors in the ocular environment within the mouse, such as the blood flow, oxygen, and nutrient supply, which all have a direct and indirect role in the eventual homing and integration of the transplanted cells.
The inner limiting membrane/neural fiber layer (ILM/NFL) of the eye acts as a physical barrier to the successful engraftment of donor RGCs into the GCL layer of the retina (Zhang et al., 2021b). Previously, the use of collagenase to digest ILM (Dalkara et al., 2009) or mechanical peeling of the ILM (Takahashi et al., 2017; Teo et al., 2018) has been shown to be deleterious to the retinal structure. In our study, we performed intravitreal injections of 0.0001% Pronase E (n = 5) 4-weeks prior to cell transplantation to digest the ILM and enhance cell engraftment. However, using Pronase E induced cataract formation and inflammation as observed by cSLO imaging (Figure S5) and did not increase the efficiency of transplantation. We observed that C57BL/6J mice that received hiPSC-RGCs without Pronase E had a significant number of successfully transplanted cells. Furthermore, it is reported that there is a greater probability that transplanted RGCs will extend their dendrites into the IPL and towards the optic nerve when the ILM layer of the host is intact (Venugopalan et al., 2016; Zhang et al., 2021a), perhaps owing to the presence of laminin in ILM that functions as a neuritogenic signal and growth substrate (Randlett et al., 2011; Riccomagno et al., 2014).
Another factor that may influence the success of the transplantation is the immunological response mediated by the host. In terms of transplantation sites, the vitreous cavity of the eye is considered to be relatively immune-privileged. However, since we did not immune suppress our mice before injection, and the hiPSC-RGCs are xenografts in mice, the eventual number of donor cells that survive the transplantation may depend on the response facilitated by the resident innate neuroinflammatory cells such as retinal macroglia (astrocytes and Müller glia) and microglia. To this end, we stained for the microglia-specific marker (Iba1) in the retinal sections transplanted with hiPSC-RGCs and did not detect the presence of microglia (Data not shown). However, we plan to conduct an exhaustive study to determine the immune response enacted by the mice against the injected hiPSCs-RGCs. Furthermore, if this study were translated into a human clinical trial, the chances of immunologic rejection would be considerably reduced if autologous patient-derived iPSC-RGCs were used for transplantation.
In recent years, intercellular material transfer between donor and host cells has confounded the interpretation of transplantation studies (Nickerson et al., 2018; Pearson et al., 2016; Waldron et al., 2018; Singh et al., 2016; Santos-Ferreira et al., 2016). To address the concern of material transfer and/or leakage of eGFP from AAV2.7m8 virus to host mouse RGCs, two human-specific markers (human nuclear antigen and Ku80) were used to identify the lineage of the transplanted eGFP + cells specifically. Our data from the immunohistochemistry studies revealed that all eGFP + cells in the mouse retina were positive for either human nuclear antigen or Ku80 markers. Hence, no intercellular material transfer was observed between donor hiPSC-RGCs and mouse RGCs. However, an extensive parallel study must be conducted to prove this case conclusively.
Transplanted hiPSCs successfully integrated, produced axonal and elaborate dendritic connections in the host retinas, and were capable of generating light responses. Responses could be detected at moderate intensities (1e5 photon s−1 μm−2). However, most responses were observed at 10–100 times brighter intensities. One of the reasons for the lower light sensitivity appears to be the reduced ability of these cells to fire upon repeated stimuli. To some degree, this can be compensated by increasing flash intensity and light-dependent membrane depolarization. The observed decrease in light sensitivity cannot be explained by photoreceptor bleaching alone; brighter flashes delivered later during the experiment produced larger depolarizations (suggesting a more powerful signal originating from photoreceptors) but a smaller increase in the firing rate compared to earlier, dimmer flashes. Accordingly, large depolarizations caused by current injections through patch pipettes resulted in smaller than expected increases in the firing rate when performed after repeated light stimuli. It appears that hiPSCs may require a longer resting time to recover their ability to increase their firing rate in response to depolarizing stimuli. Partial restoration of this ability was observed when cells were rested in the dark for at least 30 s.
The RGC light responses are known to be either driven by photoreceptors or originate in melanopsin-expressing RGCs themselves. However, the kinetics of photoreceptor-driven and melanopsin-driven responses are markedly different, with the latter being much slower in development and especially recovery (Schmidt et al., 2011). In the case of hiPSC-RGCs, exposure to light induces a sharp increase in their firing rate, which goes back to the baseline level in less than a second after the light offset. Such kinetics are not consistent with the known kinetics of melanopsin-driven responses, and thus hiPSC-RGC responses should be photoreceptor driven.
When designing a cell replacement strategy, the unique microenvironment present within aged glaucomatous host retinas must also be considered. We are currently conducting hiPSC-RGC injections in mouse models of N-Methyl-D-Aspartate (NMDA) induced RGC loss and optic nerve crush model of optic neuropathy by following published protocols that recapitulate critical aspects of the glaucomatous disease model (Lam et al., 1999; Honda et al., 2019; Khan et al., 2013; Chen et al., 2013). Thus far, our intravitreal injection technique resulted in the successful detection of eGFP + hiPSC-RGCs in NMDA (n = 10/10) and optic nerve crush (n = 7/8) models of glaucoma (Data not shown). Studies are underway to determine the long-term survival of donor cells within a damaged neuro-retinal circuitry. Glaucomatous retinas may exhibit an increased ability to readily accept donor hiPSC-RGCs compared to wild-type adult retinas. Furthermore, we plan to investigate axonal growth and rewiring of hiPSC-RGCs within the existing inner retinal neural circuit and ultimately to targets within the brain such as the suprachiasmatic nucleus, lateral geniculate nucleus, olivary pretectal nucleus, and superior colliculus.
In summary, we have shown that our two-step hiPSC differentiation protocol consistently generates a large population of pure RGCs reported to date. Furthermore, our method does not employ any additional purification step or gene modification as commonly required by other differentiation protocols. Intravitreal injections of hiPSC-RGCs into the vitreous cavity of adult wild-type C57BL/6J mice resulted in a successful transplantation rate of about 94%, and an average number of donor hiPSC-RGCs that survived was calculated to be 672 (range 230 to 1535). In relation to other groups that are also trying to regenerate RGCs via transplantation, their successful transplantation rate was reported as 10% (Venugopalan et al., 2016; Hertz et al., 2014) and >65% (Oswald et al., 2021). The transplantation efficiency in these studies ranged from 50 to >2,000 GFP + RGCs (Venugopalan et al., 2016) and 0.5–5% (Oswald et al., 2021), respectively. In our study, the transplanted cells were localized within the ganglion cell layer and expressed RGC-specific markers within the host retinas. Furthermore, the transplanted hiPSC-RGCs exhibited similar morphology and functional activities as the endogenous murine RGCs. The long-term goal of our study is to develop a disease-modifying RGC therapy to treat ganglion cell loss in glaucoma. Even though our study is in the early stages of development, it has the potential to provide the necessary framework required for extending induced pluripotent stem cell studies beyond the pre-clinical stages of development and into human clinical trials. This novel strategy will help alleviate a significant emotional and financial burden endured by patients with glaucoma and society.
Limitations of the study
In our current study, on average, about 0.134% of 500,000 intravitreally injected hiPSC-RGCs survived in wild-type mice after 5-months of transplantation. For the development of successful cell therapy, it is essential to improve the survivability of transplanted hiPSC-RGCs. Furthermore, the experiments were only done in wild-type mice and not in a model of glaucoma or retinal ganglion cell death. Additionally, we did not investigate whether the RGCs extend axons into the brain.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Ki67/MK167 | Novus Biologicals | Cat#: NB500-170SS |
| Chx10 | Millipore | Cat#: AB9016; RRID:AB_2216009 |
| CD90 | Novus Biologicals | Cat#: AF2067 |
| anti-sheep Alexa Fluor 405 | Abcam | Cat#: ab175676 |
| anti-BRN3 Alexa Fluor 594 | Santa Cruz | Cat#: sc-390780 |
| anti-SNCG Alexa Fluor 488 | Santa Cruz | Cat#: sc-65979; RRID:AB_1120824 |
| anti-RBPMS Alexa Fluor 647 | Novus Biologicals | Cat#: NBP273835AF647 |
| BRN3 | Santa Cruz | Cat#: sc-6026; RRID:AB_673441 |
| RBPMS | Sigma-Aldrich | Cat#: ABN1362 |
| Tubulin β 3 | BioLegend | Cat#: 801201; RRID:AB_2313773 |
| GFP | Novus Biologicals | Cat#: NB600-308 |
| PSD95 | Cell Signaling Technology | Cat#: 3409S |
| VGLUT2 | Cell Signaling Technology | Cat#: 14487S |
| Ku80 | Cell Signaling Technology | Cat#: 2180S |
| Human Nuclear Antigen | Novus Biologicals | Cat#: NBP2-34342 |
| anti-goat Alexa Fluor-594 | ThermoFisher Scientific | Cat#: A11080 |
| anti-mouse Alexa Fluor-594 | ThermoFisher Scientific | Cat#: A11020 |
| anti-rabbit Alexa Fluor-594 | ThermoFisher Scientific | Cat#: A21442 |
| anti-rabbit Alexa Fluor-488 | ThermoFisher Scientific | Cat#: A11034 |
| MAP2 | Santa Cruz | Cat#: sc-74421; RRID:AB_1126215 |
| Synapsin I | Sigma-Aldrich | Cat#: 574777 |
| Neurofilament antibody | Sigma-Aldrich | Cat#: MAB5266 |
| DAPI (4′,6-diamidino-2-phenylindole) | Invitrogen | Cat#: P36935 |
| DAPI (1 mg/ ml) | ThermoFisher Scientific | Cat#: 62248 |
| CF 633, Hydrazide | Sigma-Aldrich | Cat#: SCJ4600037 |
| Bacterial and virus strains | ||
| AAV2.7m8-SNCG.eGFP | Center for Advanced Retinal and Ocular Therapeutics (CAROT) and F.M. Kirby Center for Molecular Ophthalmology, University of Pennsylvania | N/A |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| basic fibroblast growth factor (bFGF) | R&D Systems | Cat#: 233-FB |
| Matrigel Growth Factor (GFR) | Corning | Cat#: 354230 |
| XAV939 (X) (Wnt inhibitor) | R&D Systems | Cat#: 3748 |
| SB431542 (SB) (TGF-β inhibitor) | R&D Systems | Cat#: 1614 |
| LDN193189 (L) (BMP inhibitor) | R&D Systems | Cat#: 6053 |
| Nicotinamide | Sigma-Aldrich | Cat#: N0636 |
| IGF1 | R&D Systems | Cat#: 291-G1 |
| Follistatin 300 | R&D Systems | Cat#: 669-FO |
| Cyclopamine | R&D Systems | Cat#: 1623/1 |
| DAPT | Stemgent | Cat#: 04-0041 |
| Y-27632 dihydrochloride (Rock inhibitor) | R&D Systems | Cat#: 1254/1 |
| Forskolin | Selleckchem | Cat#: S2449 |
| N6,2′-O-Dibutyryladenosine 3′,5′- cyclic monophosphate sodium salt (cAMP) | Sigma-Aldrich | Cat#: D0627 |
| BDNF | R&D Systems | Cat#: 248-BDB |
| NT4 | R&D systems | Cat#: 268-N4 |
| CNTF | R&D systems | Cat#: 257-NT |
| Critical commercial assays | ||
| Deposited data | ||
| Single-cell RNA sequencing data | Harini et al., 2021 | https://doi.org/10.3390/genes12122015 |
| Experimental models: Cell lines | ||
| Human: Undifferentiated induced pluripotent stem cells (hiPSCs) | UPenn iPSC Core | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: Wild type C57BL/6J | The Jackson Laboratory | RRID:IMSR_JAX:000664 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| ImageJ | Schneider et al., 2012 | https://doi.org/10.1038/nmeth.2089 |
| Ilastik cell density counting software | Berg et al., 2019 | https://www.ilastik.org/ |
| Other | ||
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Venkata Ramana Murthy Chavali (vchavali@pennmedicine.upenn.edu; murthy.chavali@gmail.com).
Materials availability
There are restrictions to the availability of hiPSC-RGCs due to patent pending on the differentiation protocol.
Experimental model and subject details
Human iPSC culture
Undifferentiated human-induced pluripotent stem cells (hiPSCs) were derived and characterized by DNA fingerprinting, karyotype analysis, qRT-PCR, and FACS, showing complete analysis of iPSC characteristics (Duong et al., 2018; Sullivan et al., 2014; Maguire et al., 2016). All human sample collection protocols were approved by the University of Pennsylvania and the Children’s Hospital of Philadelphia Human Subjects Research Institutional Review Board following the Declaration of Helsinki. All methods were performed per the relevant research guidelines and regulations of the University of Pennsylvania. Written informed consent was obtained from all human cell donors. The iPSCs were maintained in an iPSC medium: StemMACs iPSC-Brew XF (Miltenyi Biotec, catalog #: 130-107-086, North Rhine-Westphalia, Germany) containing 50X supplement (Miltenyi Biotec, catalog #: 130-107-087, North Rhine-Westphalia, Germany), and 5 ng/mL of basic fibroblast growth factor (bFGF; R&D Systems, catalog #: 233-FB, Minneapolis, MN, USA) on 0.1% gelatin-coated dishes with irradiated mouse embryonic fibroblast (iMEFs).
Retinal progenitor cell (RPC) generation and conditions
hiPSCs were cultured on 0.1% gelatinized plates containing iMEFs in 37°C, 5% O2, and 5% CO2 conditions. Cells were maintained until approximately 75% confluence, then feeder cells were depleted, and approximately 1.5 × 106 iPSCs were seeded in one well of a 6-well tissue culture dish (Corning, catalog # 229106, Corning, NY, USA) coated with 1:100 diluted growth factor reduced Matrigel Growth Factor (GFR; Corning, catalog #: 354230, Corning, NY, USA). hiPSCs were maintained in an iPSC: MEF-conditioned medium (80:20) + 20 ng/mL of bFGF and 5 ng/mL of stable bFGF. The MEF-conditioned media was prepared by plating iMEFs onto 0.1% gelatin at a density of 20,000 cells/cm2 in iPSC media. Two days post-plating, media was collected, filtered, and either used directly or cryopreserved for later use. hiPSCs were maintained at 37°C at 5% O2 and 5% CO2 until reaching 100% confluence then transferred to 37°C, 5% CO2 overnight prior to induction. On day 0, iPSC: MEF-conditioned media was changed to RPC induction media: DMEM/F12 (50:50; Corning, catalog #: 10-092-cm, Corning, NY, USA), 1% Penn/Strep (Life Technologies, catalog #: 10378–016, Carlsbad, CA, USA), 1% Glutamine MAX (Life Technologies, catalog #: 35050–061, Carlsbad, CA, USA), 1% NEAA (Life Technologies, catalog #: 11140–050, Carlsbad, CA, USA), 0.1 mM 2-ME (Life Technologies, catalog #: 21985–023, Carlsbad, CA, USA), 2% B27 supplement (w/o vitamin A; Life Technologies, catalog #: 12587–010, Carlsbad, CA, USA), 1% N2 supplement (Life Technologies, catalog #: 17502–048, Carlsbad, CA, USA), containing 2 μM XAV939 (X) (Wnt inhibitor; R&D Systems, catalog #: 3748, Minneapolis, MN, USA), 10 μM SB431542 (SB) (TGF-β inhibitor; R&D Systems, catalog #: 1614, Minneapolis, MN, USA), 100 nM LDN193189 (L) (BMP inhibitor; R&D Systems, catalog #: 6053, Minneapolis, MN, USA), 10 mM nicotinamide (Sigma-Aldrich, catalog #: N0636, St. Louis, MO, USA), and 10 ng/mL IGF1 (R&D Systems, catalog #: 291-G1, Minneapolis, MN, USA). Cultures were fed daily for 4 days. On day 4, culture media was exchanged with RPC induction media containing: 2 μM XAV939, 10 μM SB431542, 100 nM LDN193189, 10 ng/mL IGF1, and 10 ng/mL bFGF. Media was changed daily for 21 days (Chavali et al., 2020).
Retinal ganglion cell (RGC) differentiation
Before RGC differentiation, the medium was changed daily using RGC induction media containing 250 ng/mL Shh (R&D systems, catalog #: 8908-SH, Minneapolis, MN, USA), 100 ng/mL FGF8 (R&D Systems, catalog #: 423-F8, Minneapolis, MN, USA) for 2 days. For RGC differentiation on day 24, cells were manually crossed into small clusters with Leibovitz’s medium containing 34 μM D-Glucose (Research Products International, catalog #: G32045-500, Mt. Prospect, IL, USA) using the crosshatching technique as previously described (Espuny-Camacho et al., 2013), then re-plated at a density of 1.0 × 105 cells/well of 6-well plate coated with 1:100 diluted growth factor reduced Matrigel Growth Factor in RGCs induction media containing: 100 ng/mL Follistatin 300 (R&D Systems, catalog #: 669-FO, Minneapolis, MN, USA), 0.5 μM Cyclopamine (R&D Systems, catalog #: 1623/1, Minneapolis, MN, USA), 3 μM DAPT (Stemgent, catalog #: 04–0041, Cambridge, MA, USA), and 4.2 μM Y-27632 dihydrochloride (Rock inhibitor; R&D Systems, catalog #: 1254/1, Minneapolis, MN, USA). Media was changed 24 h post-plating with RGC induction media containing: 100 ng/mL Follistatin 300 and 3 μM DAPT daily for 2 days. From day 27, media was changed to RGC induction media containing: 3 μM DAPT, 10 μM Rock inhibitor (Y27632), 5 μM Forskolin (Selleckchem, catalog #: S2449, Houston, TX, USA), 400 μM N6,2′-O-Dibutyryladenosine 3′,5′- cyclic monophosphate sodium salt (cAMP; Sigma-Aldrich, catalog #: D0627, St. Louis, MO, USA), 40 ng/mL BDNF (R&D Systems, catalog #: 248-BDB, Minneapolis, MN, USA), 5 ng/mL NT4 (R&D systems, catalog #: 268-N4, Minneapolis, MN, USA), and 10 ng/mL CNTF (R&D systems, catalog #: 257-NT, Minneapolis, MN, USA). Media was changed every 2–3 days until day 36. After maturation (D36), the medium was exchanged every 3–4 days with RGC induction media containing: 3 μM DAPT and 10 μM Rock inhibitor (Y27632) (Chavali et al., 2020).
AAV vector design and production
AAV expression cassettes were flanked by the AAV2 inverted terminal repeats. A pro-viral plasmid driving expression of enhanced green fluorescent protein (eGFP) (McDougald et al., 2018) was driven by codon optimized SNCG (gamma-synuclein promoter) (Chaffiol et al., 2017) and contains identical cis regulatory elements. AAV2.7m8-SNCG.eGFP vector was generated and purified with CsCl gradient by the CAROT research vector core at the University of Pennsylvania (Costello et al., 2006).
hiPSC-RGCs (Day 40) were transduced with AAV2.7m8 virus-containing SNCG-eGFP vector at a concentration of 1.92 × 1010 vg/mL. hiPSC-RGCs expressed green fluorescence as early as 48 h post-transduction, the cells were washed with 1X PBS three days after the virus was first applied and media was changed at least two times before hiPSC-RGCs were intravitreally injected (∼Day 50) into the eye of C57BL/6J mice.
Animal husbandry
Wild type C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and raised in a 12-h light/dark cycle. Animals were housed at the University of Pennsylvania in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research as well as with institutional and federal regulations.
Method details
Flow cytometry analysis of hiPSC-RPCs and hiPSC-RGCs
hiPSC-RPCs and hiPSC-RGCs cultures were lifted using TrypLE Express (Invitrogen, catalog #: 12605–010, Waltham, MA, USA) and collected by centrifugation at 1,600 rpm for 5 min at 4°C. The pelleted cells were resuspended in 1X PBS supplemented with 0.5% bovine serum albumin and 0.1% Na-Azide (FACS buffer). Cells were fixed in 4% paraformaldehyde (PFA) (v/v) for 15 min at room temperature (RT) followed by permeabilization using 0.5% Tween 20 (v/v) for 10 min at RT. Cells were incubated with various antibodies: anti-Ki67/MK167 (Novus Biologicals, catalog #: NB500-170SS, Littleton, CO, USA), anti-Chx10 (Millipore, catalog #: AB9016, Burlington, MA, USA), anti-CD90 (Thy1; Novus Biologicals, catalog #: AF2067, Littleton, CO, USA), anti-sheep Alexa Fluor 405 (Abcam, catalog #: ab175676, Cambridge, UK), anti-BRN3 Alexa Fluor 594 (Santa Cruz, catalog #: sc-390780, Dallas, TX, USA), anti-SNCG Alexa Fluor 488 (Santa Cruz, catalog #: sc-65979, Dallas, TX, USA), and anti-RBPMS Alexa Fluor 647 (Novus Biologicals, catalog #: NBP273835AF647, Littleton, CO, USA). Stained cells were analyzed using LSR B and LSRFortessa B at Penn Cytomics and Cell Sorting Resource Laboratory. Data were further analyzed using the FCS Express software.
Intravitreal injections of hiPSC-RGC
4-month-old wild type C57BL/6J mice were anesthetized by isoflurane inhalation to effect (2–2.5%). For our injections, a 30- gauge needle was used to create a small incision at the limbus of the eye. Followed by 2 μL of 5 × 105 live hiPSC-RGCs transduced with AAV2.7m8 virus-containing SNCG-eGFP vector was injected into the vitreous space of C57BL/6 mouse’s right eye (n = 16) using a 33-gauge 0.5″ blunt needle attached to a 5 μL Hamilton syringe (Hamilton Company, catalog #: 7803–05, Reno, NV, USA). The contralateral eye injected with 2 μL of 1X PBS served as the experimental control. Following the injections, Prednisolone 1%/Gentamicin 0.3% ophthalmic ointment was applied topically on the eyes. The animals were placed on a heating pad until awake and monitored periodically for any signs of distress or complications.
In-vivo ocular fundus imaging
Non-invasive retinal imaging was performed using Confocal Scanning Laser Ophthalmoscopy (“cSLO”, model Spectralis HRA, Heidelberg Engineering, Inc., Heidelberg, Germany), Color Fundus Imaging (“CFI”, model Micron III, Phoenix Instruments, Inc., Naperville, IL) and Spectral-Domain Optical Coherence Tomography (“SD-OCT”, model Envisu R2200 UHR, Bioptigen, Inc., Morrisville, NC, USA) at 2-, 4-, and 6-weeks post-injection. Mice were anesthetized using Ketamine (100 mg/kg)/Xylazine (8 mg/kg), pupils dilated with a combination of 2.5% Phenylephrine/1% Tropicamide, followed by topical anesthesia to the cornea using 0.5% Proparacaine. Ocular protection against evaporative corneal desiccation during the imaging session was accomplished by using various combinations of artificial tears (Refresh, Genteal, and Balanced Salt Solution) and ocular eye shields. First, Wide-field (WF-55° FOV) and Ultra-wide field (UWF-105° FOV) cSLO images were collected in each eye with the plane of focus trained on the retina ganglion cell layer/nerve fiber layer region. BluePeak Autofluorescence (BAF-cSLO, 486nm excitation/500–680 nm emission) images were collected with the optic nerve centrally positioned within the image field of view (FOV) frame. This procedure was repeated with the Micron III system using the blue excitation and green auto-fluorescence emission channel. SD-OCT was performed last to assess the posterior pole retina and vitreous body structure. Posterior pole images included the posterior lens, vitreous body, retina, and choroid from a 50° FOV with the optic nerve central positioned. Following the imaging assessment, mouse eyes were covered with unmedicated ophthalmic ointment (Puralube Vet Ointment) and placed on a warmed heating pad (model 50-7220-F, Harvard Apparatus, Inc.) until recovered.
Retinal histology and RGC quantification
Flattened whole mount
Eyes were harvested, fixed in 4% PFA at RT for 1 h, and washed with 1X PBS, followed by isolation and dissection of the retina. Retinas were prepared as flattened whole mounts (n = 8), washed with 1X PBS three times, and permeabilized in 0.5% Triton X-100 in PBS by freezing at −80°C for 15 min. Retinas were incubated overnight at 4°C in a humidified chamber with either BRN3 (Santa Cruz, catalog #: sc-6026, Dallas, TX, USA), RBPMS (Sigma-Aldrich, catalog #: ABN1362, St. Louis, MO, USA), Tubulin β 3 (TUJ1; BioLegend, catalog #: 801201, San Diego, CA, USA), GFP (Novus Biologicals, catalog #: NB600-308, Littleton, CO, USA), PSD95 (Cell Signaling Technology, catalog #: 3409S, Danvers, MA, USA), VGLUT2 (Cell Signaling Technology, catalog #: 14487S, Danvers, MA, USA), Ku80 (Cell Signaling Technology, catalog #: 2180S, Danvers, MA, USA), or Human Nuclear Antigen (Novus Biologicals, catalog #: NBP2-34342, Littleton, CO, USA). The next day, the primary antibody was removed, and the retinas were washed with 1X PBS five times for 5 min each, followed by incubation with appropriate secondary antibodies: anti-goat Alexa Fluor-594 (ThermoFisher Scientific, catalog #: A11080, Waltham, MA, USA), anti-mouse Alexa Fluor 594 (ThermoFisher Scientific, catalog #: A11020, Waltham, MA, USA), anti-rabbit Alexa Fluor-594 (ThermoFisher Scientific, catalog #: A21442, Waltham, MA, USA), and anti-rabbit Alexa Fluor-488 (ThermoFisher Scientific, catalog #: A11034, Waltham, MA, USA) for 1 h at RT. The secondary antibody was discarded, and the retinas were washed with 1X PBS seven times for 5 min each. The coverslips were then mounted on slides using proLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen, catalog #: P36935, Waltham, MA, USA). In the case of the retinal sample stained against Ku80, in the last step of IHC staining, the retina was stained with DAPI (1 mg/mL) (ThermoFisher Scientific, catalog #: 62248, Waltham, MA, USA) for 10 min at RT. Following incubation, the retina was washed with 1X PBS three times for 5 min each, and coverslips were then mounted on the slide using fluorescent mounting media (KPL, catalog #: 95059–434). Slides were observed under an Invitrogen EVOS M5000 fluorescent microscope and Olympus FV1000 Confocal laser scanning microscope and images were captured using 4X, 10X, and 40X objectives with the use of appropriate filters and lasers. Images were analyzed using ImageJ software.
Cryosections
Enucleated eyes were fixed in 4% PFA overnight at 4°C. For cryo-sectioning, eyecups were dissected, and the lens was extracted (n = 3). The retinal cups were first transferred to 15% sucrose solution for 1 h at 4°C and were then again transferred to 30% sucrose solution and incubated overnight at 4°C. Eyes were embedded in optimal cutting temperature (OCT) compound, frozen on dry ice blocks, and stored at −20°C for long-term storage. Using a cryostat, 14 μm sections of the retinal cups were generated. For immunohistochemistry, the cryo-sections were washed with 1X PBS three times for 5 min each. The slides were incubated with blocking buffer (1% BSA, 0.1% Triton X-100, and 1% normal donkey serum in 1 X PBS) in a humidified chamber for 1 h at RT. The slides were then incubated overnight at 4°C using the following antibodies: BRN3 (Santa Cruz, catalog #: sc-6026, Dallas, TX, USA), MAP2 (Santa Cruz, catalog #: sc-74421, Dallas, TX, USA), and Synapsin I (Sigma-Aldrich, catalog #: 574777, St. Louis, MO, USA). The next day, samples were washed with 1X PBS three times for 5 min each, followed by incubation with appropriate secondary antibodies: anti-goat Alexa Fluor 594 (ThermoFisher Scientific, catalog #: A11080, Waltham, MA, USA), anti-mouse Alexa Fluor 594 (ThermoFisher Scientific, catalog #: A11020, Waltham, MA, USA), and anti-rabbit Alexa Fluor-594 (ThermoFisher Scientific, catalog #: A21442, Waltham, MA, USA) for 1 h at RT. The coverslips were then mounted on slides using proLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen, catalog #: P36935, Waltham, MA, USA). Slides were observed under an Olympus FV1000 Confocal laser scanning microscope, and images were captured using 40X objective with the use of appropriate filters and lasers. Images were analyzed using ImageJ software.
Voltage reading of transplanted hiPSC-RGCs
All animal procedures conformed to National Institutes of Health guidelines for animals in research and of the University of Pennsylvania Committee for the Care and Use of Animals. Mice were sacrificed by the injection of a lethal dose of ketamine/xylazine mixture (100 μg/g ketamine and 10 μg/g xylazine) followed by cervical dislocation, and eyes were enucleated and dissected in a Petri dish filled with Ames solution (Sigma-Aldrich Inc., Burlington, MA, USA) maintained at room temperature and continuously oxygenated with a mixture of 95% O2 and 5% CO2. Retinas were flat-mounted ganglion cells side up in the recording chamber perfused with oxygenated Ames solution maintained at 35–37°C using TC-344C two-channel temperature controller (Warner Instruments, Holliston, MA, USA). All procedures were done under dim red-light illumination. Two-photon imaging using Olympus FV1000 MPE Multiphoton Laser Scanning Microscope (Olympus Corporation of the Americas, Center Valley, PA, USA) was employed to identify, image, and target eGFP-expressing hiPSCs in a mouse retina. The wavelength for two-photon excitation was set at 920 nm. All pipette manipulations were done using a different IR viewing system providing regular video frame rates. It included a Dage-MTI CCD 72 IR camera and controller (Dage-MTI Inc., Michigan City, IN, USA), a Sony Trinitron TV monitor (Sony Corporation of America, New York, NY, USA), and a #87 IR filter (Lee Filters, Andover, England) installed in the microscope’s light path for a regularly transmitted light illumination. If needed, imaging could be briefly switched back to two-photon mode right before touching the cell membrane with the pipette tip to confirm that the red dye CF 633 filled pipette is indeed positioned next to the targeted EGFP expressing hiPSC. At the end of the electrophysiological recording two-photon imaging was used again to confirm that the targeted cell was filled with the CF 633 dye. Images were processed using Olympus Fluoview software and ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018).
The hiPSCs were recorded in the cell-attached and whole-cell modes (n = 4). First, an Ames-filled pipette was used to break through the inner limiting membrane and clean a small area around a cell membrane region to be patched. After that, it was replaced with another Ames-filled pipette used to record spiking activity from the targeted cell in the cell-attached (also known as the loose patch) configuration in the voltage-clamp mode. In this configuration, a seal is formed between the pipette and cell membrane, although it is not required to be as tight as a seal used in patch-clamp recording, and a cell membrane is not ruptured. Finally, a pipette filled with intracellular solution (112 mM K-Gluconate, 12 mM NaCl, 2 mM MgCl2, 1 mM EGTA, 10 mM HEPES, Ph adjusted to 7.4 using 26 mM of KOH) was used to achieve a tight seal (typically above 2–3 GΩ) with the cellular membrane, a whole-cell configuration established by rupturing a piece of the membrane inside the pipette with a brief pulse of suction (in the voltage-clamp mode). Cellar responses to depolarizing inputs caused by light stimulation or current injection were recorded in the current-clamp mode. All pipettes were fabricated using a Sutter P-87 puller (Sutter Instrument Company, Novato, CA, USA). Resistance of the Ames-filled pipettes was around 5–7 MΩ, and the resistance of patch-pipettes was around 7–9 MΩ. Light stimulation included calibrated full-field flashes of 500 nm light delivered from below the stage using a custom LED-based system. Cellular responses were amplified using Warner Instruments PC-505B patch-clamp amplifier, Axon Digidata 1400 digitizer, and Clampex software (Molecular Devices, San Jose, CA, USA) was used to record data on the computer hard disk and to control light and current stimulation. Custom Matlab-based code was used for data analysis (Math Works, Natick, MA, USA). Because of fast action potential spike kinetics (spike duration typically is less than 3 ms) and a real-world limitation of the current-clamp system, spikes can be reliably detected from current vs. time traces recorded both in voltage and current clamp modes. To detect spikes, these traces were high pass filtered (4 pole Butterworth filter, cutoff frequency 250 Hz), and a 4 STD detection threshold was used. Reported voltage traces recorded in the current-clamp mode were not corrected for the liquid junction potential (LJP), estimated to be around 15 mV (to correct for LJP 15 mV should be subtracted from the reported values).
Axon analysis
Optic nerves were isolated, processed, and embedded in paraffin (n = 2). 5 μm longitudinal paraffin sections of the optic nerve were deparaffinized and rehydrated, followed by incubation with blocking buffer (1% BSA, 0.1% Triton X-100, and 1% normal donkey serum in 1 X PBS) in a humidified chamber for 1 h at RT. The slides were then incubated overnight at 4°C with a Neurofilament antibody (Sigma-Aldrich, catalog #: MAB5266, St. Louis, MO, USA). The next day, samples were washed with 1X PBS three times for 5 min each, followed by incubation with anti-mouse Alexa Fluor 594 (ThermoFisher Scientific, catalog #: A11020, Waltham, MA, USA), staining was performed for 1 h at RT. The coverslips were then mounted on slides using proLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen, catalog #: P36935, Waltham, MA, USA). Slides were observed under an Olympus FV1000 Confocal laser scanning microscope, and images were captured using 60X objective with the use of appropriate filters and lasers.
Quantification and statistical analysis
The total number of eGFP + hiPSC-RGCs detected in mouse retinas was quantified using Ilastik cell density counting software (Heidelberg, Germany) (Berg et al., 2019) and validated by manual counting in ImageJ software (NIH, Bethesda, MD, USA). Data are represented as mean +/- SEM.
Acknowledgments
Supported in part by the National Eye Institute of the National Institutes of Health under Award Number R21EY028273-01A1 (V.R.M.C). BrightFocus Foundation grant (V.R.M.C), Research to Prevent Blindness Unrestricted Grant Funds to Scheie Eye Institute (V.R.M.C), F.M. Kirby Foundation, and the Paul and Evanina Bell Mackall Foundation Trust, the Center for Advanced Retinal and Ocular Therapeutics (CAROT), the Foundation Fighting Blindness (FBB), Research to Prevent Blindness (RPB), Lisa Dean Moseley Foundation, Shared Instrumentation Grant NIH S10OD026860, the National Institute of Health vision core grant P30EY001583-26 [Vision Research Core Module E: Imaging and Electrophysiology], National Institutes of Health, Department of Health and Human Services, under eyeGENETM and contract Nos. HHSN260220700001C and HHSN263201200001C. We thank the Brody Family Medical Trust Fellowship, administered by the Philadelphia Foundation, with the College of Physicians of Philadelphia (V.V). 3D printed object courtesy of the University of Pennsylvania Libraries’ Biotech Commons.
Author contributions
Conceptualization, V.R.M.C and V.V.; Methodology, V.R.M.C and V.V.; Investigation, V.V, S.N, B.A.B, and K.E.U.; Formal analysis, V.V and S.N.; Writing- Original Draft, V.V.; Writing- Review & Editing, V.V, V.R.M.C, S.N, B.A.B, K.E.U, J.H, and Y.B.; Visualization, V.V.; Funding Acquisition, V.R.M.C.; Resources, V.R.M.C.; Supervision, V.R.M.C. All authors read and approved the final article.
Declaration of interests
All affiliations are listed on the title page of the article.
All funding sources for this study are listed in the “acknowledgments” section of the article.
We, the authors and our immediate family members have no financial interests to declare.
We, the authors and our immediate family members, have no positions to declare and are not members of the journal’s advisory board.
We, the authors, have a patent related to this work, which is noted in the “declaration of interests” section of the article and on this form below, and we have noted the patents of immediate family members. “There are restrictions to the availability of the hiPSC-RGCs owing to patent pending on the differentiation protocol.”
Inclusion and diversity
A) Relating to the scientific content of the paper: We worked to ensure sex balance in the selection of non-human subjects, diversity in experimental samples through the selection of the cell lines, and diversity in experimental samples through the selection of the genomic datasets.
B) Relating to authorship and attribution: One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research.
Published: November 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105308.
Supplemental information
Data and code availability
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Aoki H., Hara A., Niwa M., Motohashi T., Suzuki T., Kunisada T. An in vitro mouse model for retinal ganglion cell replacement therapy using eye-like structures differentiated from ES cells. Exp. Eye Res. 2007;84:868–875. doi: 10.1016/j.exer.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Banin E., Obolensky A., Idelson M., Hemo I., Reinhardtz E., Pikarsky E., Ben-Hur T., Reubinoff B. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells (Dayton, Ohio) 2006;24:246–257. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- Bellocchio E.E., Reimer R.J., Fremeau R.T., Jr., Edwards R.H. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science (New York, N.Y.) 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Berg S., Kutra D., Kroeger T., Straehle C.N., Kausler B.X., Haubold C., Schiegg M., Ales J., Beier T., Rudy M., Eren K., Cervantes J.I., Xu B., Beuttenmueller F., Wolny A., Zhang C., Koethe U., Hamprecht F.A., Kreshuk A. Ilastik: interactive machine learning for (Bio)image analysis. Nat. Methods. 2019;16:1226–1232. doi: 10.1038/s41592-019-0582-9. [DOI] [PubMed] [Google Scholar]
- Chaffiol A., Caplette R., Jaillard C., Brazhnikova E., Desrosiers M., Dubus E., Duhamel L., Macé E., Marre O., Benoit P., Hantraye P., Bemelmans A.P., Bamberg E., Duebel J., Sahel J.A., Picaud S., Dalkara D. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 2017;25:2546–2560. doi: 10.1016/j.ymthe.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamling X., Sluch V.M., Zack D.J. The potential of human stem cells for the study and treatment of glaucoma. Invest. Ophthalmol. Vis. Sci. 2016;57:ORSFi1–ORSFi6. doi: 10.1167/iovs.15-18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson E.S., Sankar P.S., Miller-Ellis E., Regina M., Fertig R., Salinas J., Pistilli M., Salowe R.J., Rhodes A.L., Merritt W.T., Chua M., Trachtman B.T., Gudiseva H.V., Collins D.W., Chavali V.R.M., Nichols C., Henderer J., Ying G.S., Varma R., Jorgenson E., O'brien J.M. The primary open-angle African American glaucoma genetics study: baseline demographics. Ophthalmology. 2015;122:711–720. doi: 10.1016/j.ophtha.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavali V.R.M., Haider N., Rathi S., Vrathasha V., Alapati T., He J., Gill K., Nikonov R., Duong T.T., Mcdougald D.S., Nikonov S., O’brien J., Mills J.A. Dual SMAD inhibition and Wnt inhibition enable efficient and reproducible differentiations of induced pluripotent stem cells into retinal ganglion cells. Sci. Rep. 2020;10:11828. doi: 10.1038/s41598-020-68811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Khan R.S., Cwanger A., Song Y., Steenstra C., Bang S., Cheah J.H., Dunaief J., Shindler K.S., Snyder S.H., Kim S.F. Dexras1, a small GTPase, is required for glutamate-NMDA neurotoxicity. J. Neurosci. 2013;33:3582–3587. doi: 10.1523/JNEUROSCI.1497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H., Mao C.A., Klein W.H. Adult mice transplanted with embryonic retinal progenitor cells: new approach for repairing damaged optic nerves. Mol. Vis. 2012;18:2658–2672. [PMC free article] [PubMed] [Google Scholar]
- Clark P.J., Evans F.C. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology. 1954;35:445–453. [Google Scholar]
- Cohen L.P., Pasquale L.R. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb. Perspect. Med. 2014;4:a017236. doi: 10.1101/cshperspect.a017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C., Foster P. Epidemiology of glaucoma: what's new? Can. J. Ophthalmol. 2012;47:223–226. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Costello F., Coupland S., Hodge W., Lorello G.R., Koroluk J., Pan Y.I., Freedman M.S., Zackon D.H., Kardon R.H. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 2006;59:963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Cressie N. John Wiley & Sons; 2015. Statistics for Spatial Data. [Google Scholar]
- Dalkara D., Kolstad K.D., Caporale N., Visel M., Klimczak R.R., Schaffer D.V., Flannery J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danford I.D., Verkuil L.D., Choi D.J., Collins D.W., Gudiseva H.V., Uyhazi K.E., Lau M.K., Kanu L.N., Grant G.R., Chavali V.R.M., O'brien J.M. Characterizing the “POAGome”: a bioinformatics-driven approach to primary open-angle glaucoma. Prog. Retin. Eye Res. 2017;58:89–114. doi: 10.1016/j.preteyeres.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.A., Matzuk M.M., Reh T.A. Activin A promotes progenitor differentiation into photoreceptors in rodent retina. Mol. Cell. Neurosci. 2000;15:11–21. doi: 10.1006/mcne.1999.0806. [DOI] [PubMed] [Google Scholar]
- Del Barco Barrantes I., Davidson G., Gröne H.J., Westphal H., Niehrs C. Dkk1 and noggin cooperate in mammalian head induction. Genes Dev. 2003;17:2239–2244. doi: 10.1101/gad.269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didonna A., Opal P. The role of neurofilament aggregation in neurodegeneration: lessons from rare inherited neurological disorders. Mol. Neurodegener. 2019;14:19. doi: 10.1186/s13024-019-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya M.S., Rasheed V.A., Schmidt T., Lalitha S., Hattar S., James J. Intraocular injection of ES cell-derived neural progenitors improve visual function in retinal ganglion cell-depleted mouse models. Front. Cell. Neurosci. 2017;11:295. doi: 10.3389/fncel.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger U.C., Olsen J.F. Ganglion cell distribution in the retina of the mouse. Invest. Ophthalmol. Vis. Sci. 1981;20:285–293. [PubMed] [Google Scholar]
- Duong T.T., Vasireddy V., Ramachandran P., Herrera P.S., Leo L., Merkel C., Bennett J., Mills J.A. Use of induced pluripotent stem cell models to probe the pathogenesis of Choroideremia and to develop a potential treatment. Stem Cell Res. 2018;27:140–150. doi: 10.1016/j.scr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I., Michelsen K.A., Gall D., Linaro D., Hasche A., Bonnefont J., Bali C., Orduz D., Bilheu A., Herpoel A., et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Gudiseva H.V., Vrathasha V., He J., Bungatavula D., O’brien J.M., Chavali V.R.M. 2021. Single Cell Sequencing of Induced Pluripotent Stem Cell Derived Retinal Ganglion Cells (iPSC-RGC) Reveals Distinct Molecular Signatures and RGC Subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavner W., Pevny L. Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008391. doi: 10.1101/cshperspect.a008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz J., Qu B., Hu Y., Patel R.D., Valenzuela D.A., Goldberg J.L. Survival and integration of developing and progenitor-derived retinal ganglion cells following transplantation. Cell Transplant. 2014;23:855–872. doi: 10.3727/096368913X667024. [DOI] [PubMed] [Google Scholar]
- Honda S., Namekata K., Kimura A., Guo X., Harada C., Murakami A., Matsuda A., Harada T. Survival of alpha and intrinsically photosensitive retinal ganglion cells in NMDA-induced neurotoxicity and a mouse model of normal tension glaucoma. Invest. Ophthalmol. Vis. Sci. 2019;60:3696–3707. doi: 10.1167/iovs.19-27145. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Osakada F., Watanabe K., Mizuseki K., Haraguchi T., Miyoshi H., Kamiya D., Honda Y., Sasai N., Yoshimura N., Takahashi M., Sasai Y. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatha B., Divya M.S., Sanalkumar R., Indulekha C.L., Vidyanand S., Divya T.S., Das A.V., James J. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochem. Biophys. Res. Commun. 2009;380:230–235. doi: 10.1016/j.bbrc.2009.01.038. [DOI] [PubMed] [Google Scholar]
- Janssen S.F., Gorgels T.G.M.F., Ramdas W.D., Klaver C.C.W., Van Duijn C.M., Jansonius N.M., Bergen A.A.B. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog. Retin. Eye Res. 2013;37:31–67. doi: 10.1016/j.preteyeres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Johnson T.V., Martin K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Invest. Ophthalmol. Vis. Sci. 2008;49:3503–3512. doi: 10.1167/iovs.07-1601. [DOI] [PubMed] [Google Scholar]
- Kapetanakis V.V., Chan M.P.Y., Foster P.J., Cook D.G., Owen C.G., Rudnicka A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br. J. Ophthalmol. 2016;100:86–93. doi: 10.1136/bjophthalmol-2015-307223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley P.W., Eglen S.J., Reese B.E. From random to regular: variation in the patterning of retinal mosaics. J. Comp. Neurol. 2020;528:2135–2160. doi: 10.1002/cne.24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D., El-Husseini A. Excitation control: balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 2008;1:4. doi: 10.3389/neuro.02.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R., Chen Y., Cwanger A., Song Y., Dunaief J., Kim S., Shindler K. Dexras1 mediates retinal ganglion cell loss induced by NMDA excitotoxicity. Invest. Ophthalmol. Vis. Sci. 2013;54:1414. [Google Scholar]
- Lam T.T., Abler A.S., Kwong J.M., Tso M.O. N-methyl-D-aspartate (NMDA)–induced apoptosis in rat retina. Invest. Ophthalmol. Vis. Sci. 1999;40:2391–2397. [PubMed] [Google Scholar]
- Lamba D.A., Karl M.O., Ware C.B., Reh T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Allingham R.R. Major review: molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017;160:62–84. doi: 10.1016/j.exer.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J.A., Gagne A., Mills J.A., Gadue P., French D.L. Generation of human control iPS cell line CHOPWT9 from healthy adult peripheral blood mononuclear cells. Stem Cell Res. 2016;16:14–16. doi: 10.1016/j.scr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Mcdougald D.S., Dine K.E., Zezulin A.U., Bennett J., Shindler K.S. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Invest. Ophthalmol. Vis. Sci. 2018;59:1212–1220. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno C., Ito Y., Saijoh Y., Matsuda Y., Tashiro K., Kuhara S., Hamada H. Two closely-related left-right asymmetrically expressed genes, lefty-1 and lefty-2: their distinct expression domains, chromosomal linkage and direct neuralizing activity in Xenopus embryos. Gene Cell. 1997;2:513–524. doi: 10.1046/j.1365-2443.1997.1400338.x. [DOI] [PubMed] [Google Scholar]
- Meyer J.S., Howden S.E., Wallace K.A., Verhoeven A.D., Wright L.S., Capowski E.E., Pinilla I., Martin J.M., Tian S., Stewart R., et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.S., Shearer R.L., Capowski E.E., Wright L.S., Wallace K.A., Mcmillan E.L., Zhang S.C., Gamm D.M. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner A.M., La Torre A. Retinal ganglion cell replacement: current status and challenges ahead. Dev. Dyn. 2019;248:118–128. doi: 10.1002/dvdy.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nickerson P.E.B., Ortin-Martinez A., Wallace V.A. Material exchange in photoreceptor transplantation: updating our understanding of donor/host communication and the future of cell engraftment science. Front. Neural Circ. 2018;12:17. doi: 10.3389/fncir.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Akaike A., Sasai Y., Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Oswald J., Kegeles E., Minelli T., Volchkov P., Baranov P. Transplantation of miPSC/mESC-derived retinal ganglion cells into healthy and glaucomatous retinas. Mol. Ther. Methods Clin. Dev. 2021;21:180–198. doi: 10.1016/j.omtm.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashos E.E., Park Y., Wang X., Raghavan A., Yang W., Abbey D., Peters D.T., Arbelaez J., Hernandez M., Kuperwasser N., Li W., Lian Z., Liu Y., Lv W., Lytle-Gabbin S.L., Marchadier D.H., Rogov P., Shi J., Slovik K.J., Stylianou I.M., Wang L., Yan R., Zhang X., Kathiresan S., Duncan S.A., Mikkelsen T.S., Morrisey E.E., Rader D.J., Brown C.D., Musunuru K. Large, diverse population cohorts of hiPSCs and derived hepatocyte-like cells reveal functional genetic variation at blood lipid-associated loci. Cell Stem Cell. 2017;20:558–570.e10. doi: 10.1016/j.stem.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R.A., Gonzalez-Cordero A., West E.L., Ribeiro J.R., Aghaizu N., Goh D., Sampson R.D., Georgiadis A., Waldron P.V., Duran Y., Naeem A., Kloc M., Cristante E., Kruczek K., Warre-Cornish K., Sowden J.C., Smith A.J., Ali R.R. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat. Commun. 2016;7:13029. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O., Poggi L., Zolessi F.R., Harris W.A. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70:266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein D.B., Zhang P., Wirth K.E., Lee P.P., Hoerger T.J., Mccall N., Klein R., Tielsch J.M., Vijan S., Saaddine J. The economic burden of major adult visual disorders in the United States. Arch. Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- Riccomagno M.M., Sun L.O., Brady C.M., Alexandropoulos K., Seo S., Kurokawa M., Kolodkin A.L. Cas adaptor proteins organize the retinal ganglion cell layer downstream of integrin signaling. Neuron. 2014;81:779–786. doi: 10.1016/j.neuron.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A.G., Mcdougald D.S., Khan R.S., Duong T.T., Dine K.E., Aravand P., Bennett J., Chavali V.R.M., Shindler K.S. Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1. Gene Ther. 2021;28:256–264. doi: 10.1038/s41434-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salowe R., Salinas J., Farbman N.H., Mohammed A., Warren J.Z., Rhodes A., Brucker A., Regina M., Miller-Ellis E., Sankar P.S., Lehman A., O'brien J.M. Primary open-angle glaucoma in individuals of african descent: a review of risk factors. J. Clin. Exp. Ophthalmol. 2015;6:450. doi: 10.4172/2155-9570.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J., Ader M. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat. Commun. 2016;7:13028. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.M., Do M.T.H., Dacey D., Lucas R., Hattar S., Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J. Neurosci. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs B.A., Jiao C., Cranston C.M., Kaalberg E., Wang K., Russell S.R., Wiley L.A., Mullins R.F., Stone E.M., Tucker B.A. Optimizing donor cellular dissociation and subretinal injection parameters for stem cell-based treatments. Stem Cell. Transl. Med. 2019;8:797–809. doi: 10.1002/sctm.18-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Balmer J., Barnard A.R., Aslam S.A., Moralli D., Green C.M., Barnea-Cramer A., Duncan I., Maclaren R.E. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat. Commun. 2016;7:13537. doi: 10.1038/ncomms13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Shen W., Kuai D., Martin J.M., Guo X., Smith M.A., Perez E.T., Phillips M.J., Simonett J.M., Wallace K.A., et al. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum. Mol. Genet. 2013;22:593–607. doi: 10.1093/hmg/dds469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A., Tielsch J.M., Katz J., Quigley H.A., Gottsch J.D., Javitt J., Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: the Baltimore Eye Survey. Arch. Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- Suen H.C., Qian Y., Liao J., Luk C.S., Lee W.T., Ng J.K.W., Chan T.T.H., Hou H.W., Li I., Li K., Chan W.Y., Feng B., Gao L., Jiang X., Liu Y.H., Rudd J.A., Hobbs R., Qi H., Ng T.K., Mak H.K., Leung K.S., Lee T.L. Transplantation of retinal ganglion cells derived from male germline stem cell as a potential treatment to glaucoma. Stem Cells Dev. 2019;28:1365–1375. doi: 10.1089/scd.2019.0060. [DOI] [PubMed] [Google Scholar]
- Sullivan S.K., Mills J.A., Koukouritaki S.B., Vo K.K., Lyde R.B., Paluru P., Zhao G., Zhai L., Sullivan L.M., Wang Y., et al. High-level transgene expression in induced pluripotent stem cell–derived megakaryocytes: correction of Glanzmann thrombasthenia. Blood. 2014;123:753–757. doi: 10.1182/blood-2013-10-530725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Igarashi T., Miyake K., Kobayashi M., Yaguchi C., Iijima O., Yamazaki Y., Katakai Y., Miyake N., Kameya S., Shimada T., Takahashi H., Okada T. Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Mol. Ther. 2017;25:296–302. doi: 10.1016/j.ymthe.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansley K. The gecko retina. Vis. Res. 1964;4:33–37. doi: 10.1016/0042-6989(64)90029-x. [DOI] [PubMed] [Google Scholar]
- Teo K.Y.C., Lee S.Y., Barathi A.V., Tun S.B.B., Tan L., Constable I.J. Surgical removal of internal limiting membrane and layering of AAV vector on the retina under air enhances gene transfection in a nonhuman primate. Invest. Ophthalmol. Vis. Sci. 2018;59:3574–3583. doi: 10.1167/iovs.18-24333. [DOI] [PubMed] [Google Scholar]
- Teotia P., Chopra D.A., Dravid S.M., Van Hook M.J., Qiu F., Morrison J., Rizzino A., Ahmad I. Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically defined recapitulation of developmental mechanism. Stem Cells. 2017;35:572–585. doi: 10.1002/stem.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Varela-Fernández R., Díaz-Tomé V., Luaces-Rodríguez A., Conde-Penedo A., García-Otero X., Luzardo-Álvarez A., Fernández-Ferreiro A., Otero-Espinar F. Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12:269. doi: 10.3390/pharmaceutics12030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan P., Wang Y., Nguyen T., Huang A., Muller K.J., Goldberg J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016;7:10472. doi: 10.1038/ncomms10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron P.V., Di Marco F., Kruczek K., Ribeiro J., Graca A.B., Hippert C., Aghaizu N.D., Kalargyrou A.A., Barber A.C., Grimaldi G., Duran Y., Blackford S.J.I., Kloc M., Goh D., Zabala Aldunate E., Sampson R.D., Bainbridge J.W.B., Smith A.J., Gonzalez-Cordero A., Sowden J.C., Ali R.R., Pearson R.A. Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Rep. 2018;10:406–421. doi: 10.1016/j.stemcr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb R.N., Leung C.K.S., Crowston J.G., Medeiros F.A., Friedman D.S., Wiggs J.L., Martin K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Prim. 2016;2:16067. doi: 10.1038/nrdp.2016.67. [DOI] [PubMed] [Google Scholar]
- Zhang K.Y., Aguzzi E.A., Johnson T.V. Retinal ganglion cell transplantation: approaches for overcoming challenges to functional integration. Cells. 2021;10:1426. doi: 10.3390/cells10061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.Y., Tuffy C., Mertz J.L., Quillen S., Wechsler L., Quigley H.A., Zack D.J., Johnson T.V. Role of the internal limiting membrane in structural engraftment and topographic spacing of transplanted human stem cell-derived retinal ganglion cells. Stem Cell Rep. 2021;16:149–167. doi: 10.1016/j.stemcr.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Wang J., Li Y., Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci. Rep. 2021;11:13762. doi: 10.1038/s41598-021-92971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.