Highlights
-
•
Coronavirus disease 2019 (COVID-19) was highly associated with risk of severe maternal morbidity and mortality.
-
•
The risk of intensive care unit (ICU) admission or death was higher in the third trimester of pregnancy.
-
•
Serious adverse maternal events were more common following caesarean delivery.
-
•
Maternal death and morbidity was higher during the Gamma wave than the Delta wave.
-
•
COVID-19 vaccination reduced the risk of maternal death and ICU admission.
Keywords: Pregnant women, COVID-19, SARS-CoV-2, Maternal mortality, Adverse outcomes, Immunization
Abstract
Objectives
To identify factors associated with adverse maternal outcomes during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
This was a single-centre prospective cohort study at a maternity department in a public general hospital in Rio de Janeiro. All pregnant women evaluated for emergency care, labour and delivery, respiratory symptoms, obstetric reasons or medical reasons between May 2020 and March 2022 at the study institution were invited to enrol in this study. The endpoint was maternal mortality or intensive care unit (ICU) admission.
Results
In total, 1609 pregnant women were enrolled in this study. Of these, 25.5% (n=410) were infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) based on reverse transcription polymerase chain reaction or an antigen test. There were 21 deaths and 67 ICU admissions in 4% of the cohort. The incidence of severe maternal morbidity and mortality was higher during the Gamma wave than during the Delta wave (P=0.003). Vaccination conferred protection against the endpoint [relative risk (RR) 0.4, 95% confidence interval (CI) 0.1–0.9; P=0.0169]. Factors associated with severe morbidity and mortality included caesarean section (RR 3.7, 95% CI 1.7–7.9; P=0.0008), SARS-CoV-2 infection in the third trimester (RR 2.4, 95% CI 1.1–5.6; P=0.0006) and comorbidities (RR 3, 95% CI 1.8–5.2; P<0.0001).
Conclusions
COVID-19 was significantly associated with the risk of severe maternal morbidity and mortality. Immunization of pregnant women against COVID-19 was highly protective against adverse outcomes, and should be encouraged during pregnancy.
Introduction
Over 365,000 cases of coronavirus disease 2019 (COVID-19) and 3000 deaths have been reported among pregnant women in the Americas; many of these patients were already critically ill when hospitalized [25]. The study of severe maternal morbidity and mortality is an effective proxy measure of trends in maternal mortality, and is widely utilized in both developed and developing countries [11]. Pregnancy increases the frequency of severe maternal morbidity and mortality following viral infections [36]. Data from influenza A/H1N1 [13,30], severe acute respiratory syndrome [38], Middle East respiratory syndrome [1] and COVID-19 [32,37] outbreaks demonstrate that pregnant women are at greater risk of severe maternal outcomes following infection with these respiratory viruses. In this study, severe maternal morbidity and mortality was defined as a life-threatening condition requiring admission to an intensive care unit (ICU) or death.
When the COVID-19 pandemic reached Rio de Janeiro and community transmission began, health resources were redeployed to address the crisis. Like other at-risk populations, pregnant women were advised to remain at home to avoid potential exposure to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Despite these policies, maternal mortality in the state and throughout Brazil during the pandemic (2020–2021) increased compared with the pre-pandemic period (2018–2019) [10,31]. In response to the pandemic, healthcare institutions allocated staff and infrastructure to receive and treat pregnant women with COVID-19.
In March 2021, the Brazilian Ministry of Health authorized COVID-19 vaccination for pregnant women with comorbidities. At that time, CoronaVac (whole inactivated SARS-CoV-2, Sinovac Biotech/Butantan Institute, São Paulo, Brazil), the AstraZeneca adenovirus viral vector vaccine (Oxford/Biomanguinhos, Rio de Janeiro, Brazil) and the BNT162b2 mRNA vaccine (Pfizer-BioNTech, Kalamazoo, MI, USA) were authorized for use in Brazil. In mid-May 2021, the Ministry of Health halted administration of the AstraZeneca vaccine to pregnant women, and this suspension has remained in place ever since. In late June 2021, COVID-19 vaccination was made available to all pregnant women in Brazil, including those without comorbidities. In September 2021, the Ministry of Health issued guidance recommending that pregnant women should receive either BNT162b2 or CoronaVac. To date, few studies have investigated the effectiveness of inactivated vaccines for reducing severe maternal morbidity and mortality [15]. This article reports the results of a longitudinal prospective study in a maternity unit of a general hospital which recruited pregnant women evaluated for a clinical or obstetric reason during the course of their pregnancy. The objective of the study was to investigate factors associated with severe maternal morbidity and mortality during the COVID-19 pandemic. In addition, maternal mortality rates at the institution pre-pandemic and during the COVID-19 pandemic were compared.
Methods
Setting
This prospective cohort study was performed at Adão Pereira Nunes General Hospital, an emergency clinical care unit that provides specialized care for medium- and high-risk pregnancies in a low-income region of the state of Rio de Janeiro. Approximately 15,000 consultations occur each year, with 4200 admissions and 3400 deliveries. The hospital had 51 beds including 17 ICU beds prior to the pandemic, and this was increased by 90 additional ICU beds during the pandemic period.
All pregnant women evaluated at the maternity department for clinical emergency care, labour and delivery, any respiratory symptoms, any obstetric reasons or any clinical reasons between May 2020 and March 2022 were invited to participate in this study, irrespective of whether or not they presented with clinical signs and symptoms of COVID-19.
Study procedures included collection of a nasopharyngeal specimen for testing for SARS-CoV-2 via real-time reverse transcription polymerase chain reaction or a rapid antigen detection test provided by the Brazilian Single Unified Health System. Screened participants were enrolled in the study after their test results became available. Participants who were enrolled before delivery and tested negative were retested upon admission for labour and delivery. If the second test near delivery was positive, the participant was reclassified as positive. Participants with both positive and negative results were enrolled and followed through delivery, hospital discharge and post partum for 42 days.
Exposures and outcomes
The exposure variables were trimester of pregnancy at study enrolment, race/ethnicity, number of prenatal visits, caesarean delivery, maternal comorbidities, and history of SARS-CoV-2 vaccination. These variables were selected because they are known causes of maternal mortality in low-income countries, and because they have been investigated in previous studies of risk factors for severe morbidity and mortality among women with SARS-CoV-2 during pregnancy [20]. The number of prenatal visits was converted into a binary variable representing whether or not the participant completed the minimum number of prenatal visits (i.e. four) based on national guidelines [4]. The exposure variables were stratified by SARS-CoV-2 infection status.
The outcome was maternal mortality or severe morbidity, defined as ICU admission as a proxy for a life-threatening condition. A COVID-19-associated death after SARS-CoV-2 infection during pregnancy was defined as the death of a person with a positive test during pregnancy who subsequently died in pregnancy or within 42 post partum. Additionally, women identified with SARS-CoV-2 infection were categorized according to the COVID-19 disease classification of the National Institutes of Health [24] as asymptomatic or with mild, moderate, severe or critical disease.
Statistical analysis
The study was divided into periods corresponding to the specific local circulation of SARS-CoV-2 variants based on the most abundant circulating lineage according to regional genomic surveillance [5]. The proxy periods were 15 May–4 October 2020 for the B.1.1.33 variant, 5 October 2020–18 January 2021 for the Zeta variant, 19 January 19–21 June 2021 for the Gamma variant, 22 June–10 December 2021 for the Delta variant, and 11 December 2021–6 March 2022 for the Omicron variant. The number of primary outcomes was tallied per proxy period, and the incidence of severe maternal morbidity and mortality events per 100,000 was compared by wave. In addition, a Cochran–Mantel–Hansel test was performed to assess if there was a significant association between each exposure variable and severe maternal morbidity and mortality, stratified for SARS-CoV-2 infection. The incidence of maternal mortality was calculated for the site during the pre-pandemic (2018–2019) and pandemic (2020–2021) periods. The incidence of maternal mortality was calculated as the number of pregnant women who died (numerator) divided by the total number of live births (denominator) at the study institution. The maternal mortality rates in 2018–2019 and 2020–2021 were compared using a Farrington–Manning test. Statistical analysis was performed using SAS 9.4.
Ethical approval
The study was reviewed and approved by the Brazilian National Ethics Committee on 5 May 2020 (CAAE: 30639420.0.0000.5262), and conducted in accordance with principles of the Code of Ethics of the World Medical Association (Declaration of Helsinki) and Good Clinical Practice guidelines of the International Conference on Harmonization, with general principles of protection of humans participating in research. With respect to risk level, taking a swab for the diagnosis of SARS-CoV-2 infection was a study procedure, but was not the standard of care upon admission to the maternity department. The informed study consent form described this risk of the study procedure, and written consent was provided by the participant (or their legal representative when participants were unable to provide informed consent for themselves).
Results
In total, 1787 pregnant women were screened for study participation, with 1609 evaluable singleton pregnancies available for final analysis (hereafter ‘the cohort’) (Figure 1). The frequency of SARS-CoV-2 infection at enrolment was 27.6% (n=494). This included some participants with missing data on covariates, who were subsequently excluded, as discussed below. The median maternal age was 26 years (interquartile range 22–31) (Table 1). Most participants self-reported being of black or mixed race. In total, 557/1609 (34.6%) patients were vaccinated [282 (18%) with inactivated virus vaccine; 81 (5.2%) with adenovirus vector vaccines, 270 (17.2%) with the mRNA vaccine and 20 (1.3%) with heterologous vaccination doses]. A subset of 260/1609 women (16.2%) were enrolled before SARS-CoV-2 vaccines became available in April 2021.
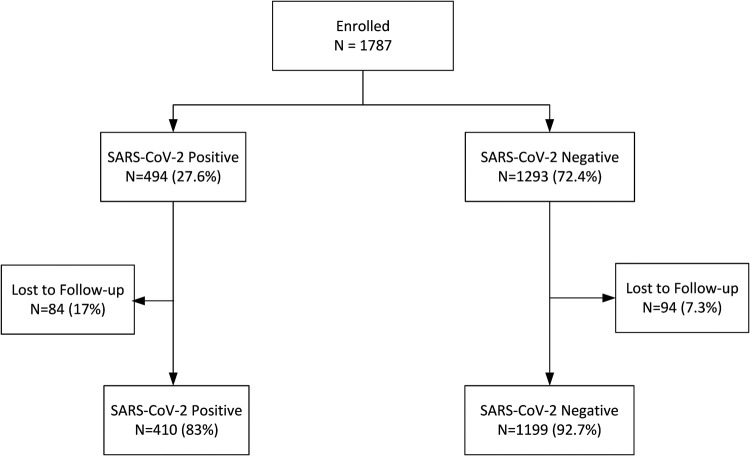
Figure 1.
Flow chart of participants enrolled in the analysis (n=1609 final participants; of these, 410 were severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) positive and 1199 were SARS-CoV-2 negative). Loss to follow-up indicates that no further data were available beyond the enrolment visit. Positive denotes results following screening by reverse transcription polymerase chain reaction or a rapid antigen test at enrolment, or near labour and delivery.
Table 1.
Demographics, and clinical and obstetric characteristics of the study participants.
| Median maternal age (IQR) (n=1609), years | 26 (22–31) |
| Race (n=1546), n (%) | |
| White | 219 (14.2%) |
| Non-white | 1327 (85.5%) |
| COVID-19 morbimortality (n=410), n (%) | |
| Asymptomatic | 121/410 (29.5%) |
| Mild/moderate | 247/410 (60.2%) |
| Severe/critical | 42/410 (10.2%) |
| Vaccinated (among 1609 women)a, n (%) | 557 (34.6%) |
| Type of vaccine (n=557), n (%) | |
| Ad26.COV2.S | 3 (0.5%) |
| BNT162b2 | 233 (41.8%) |
| ChAdOx1 nCov-19 | 60 (10.8%) |
| Coronavac | 245 (44%) |
| Heterologous | 16 (2.9%) |
| Median parity (IQR) (n=1609), n | 2 (1–3) |
| Trimester at enrolment (n=1609), n (%) | |
| First | 108 (6.7%) |
| Second | 261 (16.2%) |
| Third | 1240 (77.1%) |
| Medical comorbidities (n=1609), n (%) | |
| Anaemia | 117 (7.3%) |
| Asthma | 85 (5.3%) |
| Chronic hypertension | 160 (9.9%) |
| Pre-pregnancy BMI >30 kg/m2 | 77 (4.8%) |
| Obstetric complications (n=1609), n (%) | |
| Pre-eclampsia | 328 (20.4%) |
| HELLP syndrome | 4 (0.2%) |
| Gestational diabetes | 105 (6.5%) |
| Birth outcomes (n=1609), n (%) | |
| Vaginal delivery | 841 (52.3%) |
| Caesarean section | 668 (41.5%) |
| Stillbirth or miscarriage | 100 (6.2%) |
IQR, interquartile range; COVID-19, coronavirus disease 2019; BMI, body mass index; HELLP, haemolysis, elevated liver enzymes, low platelets.
In total, 1349 participants were recruited after vaccines became available to pregnant women.
A total of 70 participants (4.4%) reached the endpoint of ICU admission (n=67) or death (n=21). Of these, 55/70 (79%) participants had confirmed COVID-19. The most common reasons for ICU admission were respiratory failure (n=59, 84%), pre-eclampsia (n=7, 10%), sepsis (n=6, 9%), haemorrhage requiring transfusion (n=3, 4%), pulmonary thromboembolism (n=1, 1%) and pulmonary oedema (n=1, 1%). Among the 21 deceased participants, 18 (86%) were admitted to the ICU (median duration of hospitalization 8 days) and 17 (81%) required invasive mechanical ventilation. Among the participants who died, 86% (18/21) had a diagnosis of COVID-19. In total, six participants died during pregnancy (29%) and 15 (71%) died after a live birth (median of 13 days post partum); 12 of the latter were caesarean deliveries (80%). Six women who died (28.5%) had underlying medical conditions, including chronic hypertension (n=1), obesity (n=3), diabetes (n=1) and pre-existing pulmonary disease (n=1). The maternal mortality rate during the pandemic (2020 and 2021) at the study institution was 415 per 100,000 live births. In contrast, in the pre-pandemic years (2018 and 2019), the maternal mortality rate at the study institution was 225 per 100,000 live births (P=0.035).
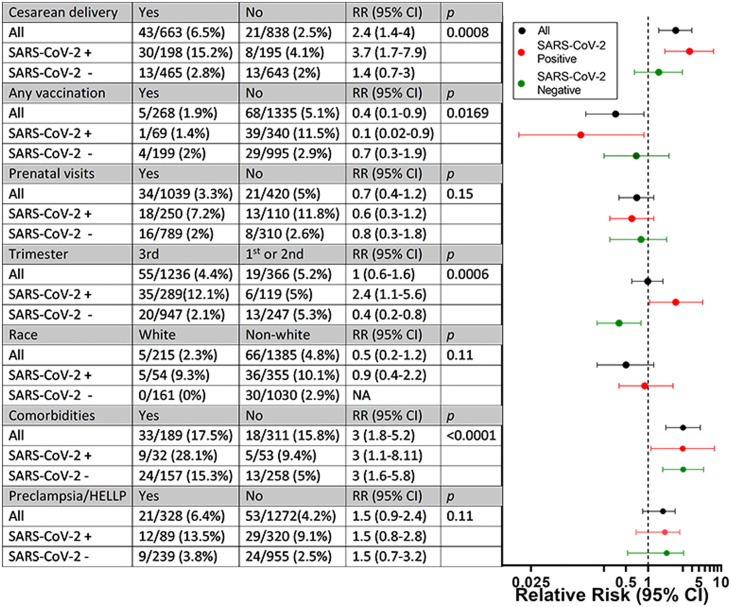
The median interval from symptom onset to severe maternal morbidity and mortality was 6 days. Five (1.9%) women with severe maternal morbidity and mortality were vaccinated and 68 (5.1%) were unvaccinated. Vaccination significantly reduced the risk of severe maternal morbidity and mortality (P=0.0169). The reduction in relative risk (RR) was greater for pregnant women infected with SARS-CoV-2 compared with uninfected pregnant women (Figure 2). Among 70 participants with severe maternal morbidity and mortality, 38 (54.3%) were recruited before vaccination was made available to pregnant women, and 32 (45.7%) were recruited afterwards. Among 32 participants with severe maternal morbidity and mortality who enrolled in the study after vaccines became available, 29 were unvaccinated (90.6%) and three (9.4%) were partially vaccinated with one dose of the BNT162b2 vaccine. In total, 410 of 494 participants (83%) infected with SARS-CoV-2 at study entry had complete data on vaccination and other covariates, and thus were included in the final analysis (Table 1). With respect to clinical characteristics, 121/410 (29.5%) participants were asymptomatic and 289/410 (70.5%) were symptomatic (critical/severe n=42, mild/moderate n=247). Of the symptomatic participants, 83/289 (28.7%) were hospitalized. In total, 22/83 (26.5%) patients required oxygen therapy and 9/83 (10.8%) required invasive ventilation; extracorporeal membrane oxygenation was not available. None of the infected asymptomatic pregnant women died or required admission.
Figure 2.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) stratified analysis of exposures during pregnancy and risk of severe maternal morbidity and mortality. SARS-CoV-2 positivity was based on reverse transcription polymerase chain reaction or SARS-CoV-2 rapid antigen test. Red lines, SARS-CoV-2-positive patients; green lines, SARS-CoV-2-negative patients; black lines, all participants. The relative risks (RRs) in the table correspond to the x-coordinate of the horizontal line in the figure. RR was calculated based on the proportion of participants with severe maternal morbidity and mortality relative to those without such outcomes. HELLP, haemolysis, elevated liver enzymes, low platelets; CI, confidence interval.
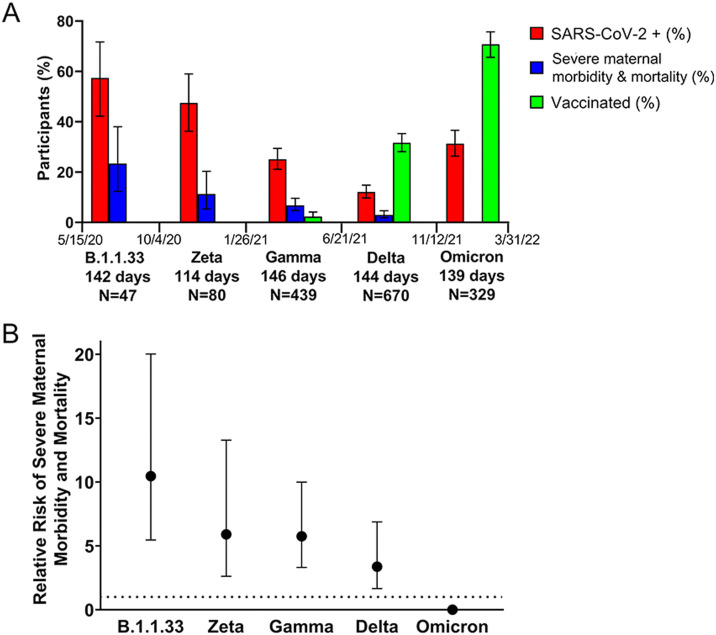
In total, there were 11 severe maternal morbidity and mortality events among 47 participants enrolled during the B1.1.1.33 wave (23%), nine events among 80 women recruited during the Zeta wave (11.3%), 30 events among 439 women recruited during the Gamma wave (6.8%), 20 events among 670 women recruited during the Delta wave (3%), and no events among 329 participants recruited during the Omicron wave (Figure 3). The RR of a severe maternal morbidity and mortality event was 10.5 [95% confidence interval (CI) 5.5–20] during the B.1.1.33 wave, 5.9 (95% CI 2.6–13.3) during the Zeta wave, 5.8 (95% CI 3.3–10) during the Gamma wave, 3.4 (95% CI 1.7-6.9) during the Delta wave, and zero during the Omicron wave (Figure 3). This is equivalent to 6833 severe maternal morbidity and mortality events per 100,000 during the Gamma wave and 2895 during the Delta wave (Fisher's exact test P=0.003). Comparing waves, the risk of severe maternal morbidity and mortality was 2.4 times higher during the Gamma wave compared with the Delta wave.
Figure 3.
Circulating severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants over time and severe maternal morbidity and mortality. (A) Percentage of participants positive for SARS-CoV-2, percentage of cases of maternal morbidity and mortality, and percentage of vaccinated women according to the period in which the predominant variants circulated [±95% confidence interval (CI)]. (B) Effect of infection with different SARS-CoV-2 variants of concern (VOCs) on the risk of maternal morbidity and mortality (±95% CI).
There was an overall association between caesarean delivery and severe maternal morbidity and mortality after adjusting for SARS-CoV-2 infection (P=0.004) (Figure 2). The RR of severe maternal morbidity and mortality was greater for pregnant women infected with SARS-CoV-2 who underwent caesarean delivery compared with pregnant women who were not infected with SARS-CoV-2 who underwent caesarean delivery. The RR was also greater among participants who enrolled during the third trimester of pregnancy compared with the first or second trimesters (P=0.011). Comorbidities increased the RR of severe maternal morbidity and mortality approximately three-fold regardless of whether or not women were infected with SARS-CoV-2 (P<0.0001). Finally, there was a trend towards increased risk of severe maternal morbidity and mortality associated with pre-eclampsia and HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome (P=0.11).
Discussion
In the study cohort, the risk of severe maternal morbidity and mortality was lower among pregnant women who were vaccinated against SARS-CoV-2, and increased in women who underwent a caesarean section; had SARS-CoV-2 infection in the third trimester of pregnancy; and had comorbidities including asthma, chronic hypertension and obesity. Compared with pregnancy cohorts in higher-income countries, the present study population had a dramatically higher overall frequency of severe maternal morbidity and mortality, but better outcomes during the Delta wave compared with populations in other geographic locations [7]. In addition, the overall incidence of maternal mortality at this maternity unit was higher during the COVID-19 pandemic than in previous years.
A novel contribution of this study is that it reports the effects of the Gamma variant on pregnant women, which were more prevalent in Brazil than in other countries [29]. The results indicate that the risk of severe maternal morbidity and mortality during the Gamma wave was higher compared with the Delta wave. The present findings differ from those in other countries where the Delta variant was the most virulent variant of concern [8], including a pregnancy cohort in the USA [23]. The Delta variant may have been less virulent in the study cohort because it was immediately preceded by the Gamma variant, and Gamma exposure conferred partial immunity to the Delta variant. There may also have been fewer cases of severe maternal morbidity and mortality during the Delta wave because vaccination ramped up during the Delta wave.
In a multi-centric study of COVID-19 in pregnancy that included some low-income countries, the maternal mortality rate was 1.6% [35]. This is similar to the 1.3% maternal mortality rate at the present study site, which is a resource-limited setting. The present study site also had a higher rate of morbidity and mortality than what has been reported in Spain, the USA and the UK [18,22]. In addition, in this study, there were no severe maternal morbidity and mortality events during the Omicron wave. This is consistent with a cohort study of pregnant women in other geographic regions during the Omicron BA1 wave, which found that it was less virulent than prior variants of concern [2]. As in previous studies [39], most of the COVID-19-related deaths in the present study (85%) were due to acute respiratory distress syndrome (ARDS). In addition to the systemic immunological changes of pregnancy that have the potential to impact lung function, anatomical changes also occur in the respiratory system. Along with the reduction in total lung capacity and inability to clear secretions, the hypercoagulable state of pregnancy with increased thrombin production and an increase in intravascular inflammation also renders pregnant women more susceptible to severe respiratory infections and COVID-19 complications [36]. Furthermore, the endothelial cell dysfunction associated with COVID-19 must play an important role in the onset and progression of ARDS [33,34].
Early in the COVID-19 pandemic, it was hypothesized that caesarean sections might improve pregnancy outcomes [26]. However, the present study found that the risk of severe maternal morbidity and mortality was approximately four times higher among SARS-CoV-2-positive women who had a caesarean section compared with those who did not. With respect to the risk of maternal death, 71% of deaths in the study cohort occurred in the postpartum period, 80% of which were after caesarean delivery. It is possible that pregnancy outcomes were worse following caesarean section because SARS-CoV-2 infection dysregulates cytokine expression at delivery and increases the risk of thromboembolism [3,9,12]. On the other hand, caesarean section could potentially be a marker of disease morbidity and mortality risk, and be performed preferentially in women who are severely ill to improve health outcomes in situations such as pre-eclampsia, heart failure or respiratory failure, or even to salvage the fetus [14]. However, further studies are needed to elucidate the mechanisms through which SARS-CoV-2 and mode of delivery are associated with severe maternal morbidity and mortality, and whether association implies causation in this scenario.
In this study, women with SARS-CoV-2 infection in the third trimester were more likely to experience adverse outcomes compared with women with SARS-CoV-2 infection in the first or second trimesters. In addition to caesarean section in the third trimester, the association between third trimester diagnosis and severe maternal morbidity could be due to increased cardiovascular load, haemodilution and elevated production of inflammatory cytokines near delivery [21,28]. Moreover, the decrease in functional pulmonary residual capacity and the elevation of pre-thrombotic profiles could increase the risk of severe maternal morbidity during the third trimester [16]. The present findings are consistent with a multi-centre study in Latin America which found that the risk of dying from COVID-19 was highest among pregnant women infected during the third trimester [19].
In the study cohort, there were no severe maternal morbidity and mortality events among fully vaccinated study participants. Most pregnant women in this study were immunized with BNT162b2 or an inactivated virus vaccine. BNT162b2 appeared to be effective in the present study population, and in large pregnancy cohorts in Sweden and Israel [6,17]. Regarding inactivated vaccines, to the authors’ knowledge, there is little real-world data on their effectiveness in pregnant women, but this is provided in the present analysis. Some pregnant women are hesitant to be vaccinated due to concerns about adverse effects on the fetus. The present findings show that pregnant women should not postpone vaccination until after delivery.
A large international study of COVID-19 during pregnancy found that pre-eclampsia increased the risk of adverse maternal outcomes significantly [27]. However, the present study only found a tendency towards higher risk of severe maternal morbidity and mortality with pre-eclampsia (P>0.05). The effects of comorbidities and pre-eclampsia on the RR of maternal adverse outcomes were similar in participants who were SARS-CoV-2 positive and negative. It was hypothesized that as the study site is a reference centre for high-risk pregnancies, the frequency of pre-eclampsia and underlying comorbidities was relatively high in the women who presented for care at this maternity unit.
The strengths of this study include its longitudinal design and the fact that patients were invited to participate irrespective of respiratory symptoms. As a result, this analysis provides an unbiased estimate of the prevalence of laboratory-confirmed SARS-CoV-2 infection in 1609 pregnant women. The limitations of this study include the loss to follow-up inherent to observational cohorts (Figure 1), including a higher rate of loss to follow-up among women infected with SARS-CoV-2 (17%) compared with those who were not infected (7.3%).
In conclusion, COVID-19 was significantly associated with the risk of severe maternal morbidity and mortality. Vaccination was associated with a four-fold reduction in ICU admission and maternal death, providing support for the immunization of pregnant women against COVID-19, which should be encouraged during prenatal care. This underscores the importance of expanding the participation of pregnant women in clinical trials in order to accelerate the regulatory approval of vaccines and other novel therapeutics in this vulnerable population.
Conflict of interest statement
None declared.
Funding
Thís study was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro - Brazil (Grants E-26/210.596/2019; E-26/200.935/2022; E-26/211.565/2019; E-26/210.146/2020), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (Finance Code 001), the Brazilian National Council for Scientific and Technological Development (311562/2021-3), and the Simons Foundation Autism Research Initiative (866410). The funding sources did not play a role in this study.
References
- 1.Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52:501–503. doi: 10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birol Ilter P, Prasad S, Berkkan M, Mutlu MA, Tekin AB, Celik E, et al. Clinical severity of SARS-CoV-2 infection among vaccinated and unvaccinated pregnancies during the Omicron wave. Ultrasound Obstet Gynecol. 2022;59:560–562. doi: 10.1002/uog.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest. 2016;150:572–596. doi: 10.1016/j.chest.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Brazilian Ministry of Health . Ministry of Health; Brazil: 2012. Assistência pré-natal: manual técnico. [Google Scholar]
- 5.Carvalho MS, Bastos LS, Fuller T, Cruz OG, Damasceno L, Calvet G, et al. Incidence of SARS-CoV-2 over four epidemic waves in a low-resource community in Rio de Janeiro, Brazil: a prospective cohort study. Lancet Region Health Americas. 2022;12 doi: 10.1016/j.lana.2022.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chodick G, Tene L, Rotem RS, Patalon T, Gazit S, Ben-Tov A, et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eid J, Abdelwahab M, Caplan M, Bilbe C, Hajmurad S, Costantine MM, et al. Increasing oxygen requirements and disease severity in pregnant individuals with the SARS-CoV-2 Delta variant. Am J Obstet Gynecol. 2022;4 doi: 10.1016/j.ajogmf.2022.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisman DN, Tuite AR. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. Can Med Assoc J. 2021;193:E1619–E1625. doi: 10.1503/cmaj.211248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foo SS, Cambou MC, Mok T, Fajardo VM, Jung KL, Fuller T, et al. The systemic inflammatory landscape of COVID-19 in pregnancy: extensive serum proteomic profiling of mother–infant dyads with in utero SARS-CoV-2. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco RPV, Lacerda L, Rodrigues AS. Obstetric Observatory BRAZIL – COVID-19: 1031 maternal deaths because of COVID-19 and the unequal access to health care services. Clinics. 2021;76:e3120. doi: 10.6061/clinics/2021/e3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geller SE, Rosenberg D, Cox SM, Brown ML, Simonson L, Driscoll CA, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191:939–944. doi: 10.1016/j.ajog.2004.05.099. [DOI] [PubMed] [Google Scholar]
- 12.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 14.Katz D, Bateman BT, Kjaer K, Turner DP, Spence NZ, Habib AS, et al. The Society for Obstetric Anesthesia and Perinatology Coronavirus Disease 2019 Registry: an analysis of outcomes among pregnant women delivering during the initial severe acute respiratory syndrome coronavirus-2 outbreak in the United States. Anesth Analges. 2021;133:462–473. doi: 10.1213/ANE.0000000000005592. [DOI] [PubMed] [Google Scholar]
- 15.Kharbanda EO, Vazquez-Benitez G. COVID-19 mRNA vaccines during pregnancy: new evidence to help address vaccine hesitancy. JAMA. 2022;327:1451–1453. doi: 10.1001/jama.2022.2459. [DOI] [PubMed] [Google Scholar]
- 16.Lapinsky SE. Acute respiratory failure in pregnancy. Obstet Med. 2015;8:126–132. doi: 10.1177/1753495X15589223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnus MC, Ortqvist AK, Dahlqwist E, Ljung R, Skar F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327:1469–1477. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marín Gabriel MA, Reyne Vergeli M, Caserío Carbonero S, Sole L, Carrizosa Molina T, Rivero Calle I, et al. Maternal, perinatal and neonatal outcomes with COVID-19: a multicenter study of 242 pregnancies and their 248 infant newborns during their first month of life. Pediatr Infect Dis J. 2020;39:e393–e397. doi: 10.1097/INF.0000000000002902. [DOI] [PubMed] [Google Scholar]
- 19.Maza-Arnedo F, Paternina-Caicedo A, Sosa CG, de Mucio B, Rojas-Suarez J, Say L, et al. Maternal mortality linked to COVID-19 in Latin America: results from a multi-country collaborative database of 447 deaths. Lancet Region Health Americas. 2022;12 doi: 10.1016/j.lana.2022.100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 22.Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mupanomunda M, Fakih MG, Miller C, Ottenbacher A, Winegar AL, Roberts P, et al. Comparison of severe maternal morbidities associated with delivery during periods of circulation of specific SARS-CoV-2 variants. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health . NIH; Bethesda, MD: 2020. Clinical presentation of people with SARS-CoV-2 infection. Available at. [Google Scholar]; https://www.covid19treatmentguidelines.nih.gov/overview/clinical-presentation/#:~:text=Patients%20with%20COVID%2D19%20are,may%20experience%20rapid%20clinical%20deterioration
- 25.Pan-American Health Organization . PAHO; Washington, DC: 2022. Weekly press briefing on COVID-19 Director's remarks. [Google Scholar]
- 26.Patel P, Kulkarni S, Guerrero M, Persaud C, Zuberi J, Rebein B. Emergency cesarean section at 38 weeks of gestation with COVID-19 pneumonia: a case report. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.926591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García-May PK, Mhatre M, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289. doi: 10.1016/j.ajog.2021.05.014. e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieper PG. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol. 2015;12:718–729. doi: 10.1038/nrcardio.2015.172. [DOI] [PubMed] [Google Scholar]
- 29.Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case–control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vital Statistics - Births and Deaths [in Portuguese]. State of Rio de Janeiro Health Department, Rio de Janeiro, Brazil. 2020. https://www.saude.rj.gov.br/informacao-sus/dados-sus/2020/11/estatisticas-vitais-obitos-e-nascimentos.
- 32.Takemoto MLS, Menezes MO, Andreucci CB, Nakamura-Pereira M, Amorim MMR, Katz L, et al. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int J Gynaecol Obstet. 2020;151:154–156. doi: 10.1002/ijgo.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wastnedge EAN, Reynolds RM, Boeckel SRV, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101:303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westgren M, Pettersson K, Hagberg H, Acharya G. Severe maternal morbidity and mortality associated with COVID-19: the risk should not be downplayed. Acta Obstet Gynecol Scand. 2020;99:815–816. doi: 10.1111/aogs.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H, Zhu HM, Yuan CH, Yao C, Luo W, Shen X, Wang J, Shao JB, Xiang Y. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:10. doi: 10.1001/jamanetworkopen.2020.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]