Abstract
The human granulocytic ehrlichiosis (HGE) agent resides and multiplies exclusively in cytoplasmic vacuoles of granulocytes. Double immunofluorescence labeling was used to characterize the nature of the HGE agent replicative inclusions and to compare them with inclusions containing the human monocytic ehrlichia, Ehrlichia chaffeensis, in HL-60 cells. Although both Ehrlichia spp. can coinfect HL-60 cells, they resided in separate inclusions. Inclusions of both Ehrlichia spp. were not labeled with either anti-lysosome-associated membrane protein 1 or anti-CD63. Accumulation of myeloperoxidase-positive granules were seen around HGE agent inclusions but not around E. chaffeensis inclusions. 3-(2,4-Dinitroanilino)-3′-amino-N-methyldipropylamine and acridine orange were not localized to either inclusion type. Vacuolar-type H+-ATPase was not colocalized with HGE agent inclusions but was weakly colocalized with E. chaffeensis inclusions. E. chaffeensis inclusions were labeled with the transferrin receptor, early endosomal antigen 1, and rab5, but HGE agent inclusions were not. Some HGE agent and E. chaffeensis inclusions colocalized with major histocompatibility complex class I and II antigens. These two inclusions were not labeled for annexins I, II, IV, and VI; α-adaptin; clathrin heavy chain; or β-coatomer protein. Vesicle-associated membrane protein 2 colocalized to both inclusions. The cation-independent mannose 6-phosphate receptor was not colocalized with either inclusion type. Endogenously synthesized sphingomyelin, from C6-NBD-ceramide, was not incorporated into either inclusion type. Brefeldin A did not affect the growth of either Ehrlichia sp. in HL-60 cells. These results suggest that the HGE agent resides in inclusions which are neither early nor late endosomes and does not fuse with lysosomes or Golgi-derived vesicles, while E. chaffeensis resides in an early endosomal compartment which accumulates the transferrin receptor.
Ehrlichia spp. belong to the family Rickettsiaceae and are obligatory intracellular bacteria of monocytes-macrophages or granulocytes but usually not both types of cells (43). The human granulocytic ehrlichiosis (HGE) agent has a granulocyte tropism and causes an emerging tick-borne zoonosis called HGE. HGE was first described in 1994 among 12 patients in Minnesota and Wisconsin (4, 12). More than 400 cases have since been reported in several additional states (22, 56). HGE has also been reported in Europe (3, 9, 20, 41). Patients with HGE show signs of illness characterized by fever, chills, headache, malaise, and/or myalgia (4, 22, 56). Severity may range from asymptomatic infection to severe morbidity and death in some cases. Laboratory tests show thrombocytopenia, leukopenia, elevation of C-reactive protein levels, and increased liver enzyme activities (4, 22, 56).
E. chaffeensis has a monocyte-macrophage tropism and was first isolated in Arkansas in 1990 from a patient suffering from another tick-borne disease called human monocytic ehrlichiosis (HME) (17). More than 460 cases of HME have been reported in 30 states since the disease was first reported in 1987 (40). The clinical signs of HME are similar to those of HGE.
Ehrlichia spp. survive and replicate in membrane-bound inclusions (parasitophorous vacuoles). Previous results obtained in our laboratory have shown that E. chaffeensis in human monocytic leukemia cell line THP-1 resides in early endosomes which do not fuse with lysosomes and selectively accumulate the transferrin receptor (TfR) (7). We have also shown that E. risticii, a monocytic ehrlichia infecting the horse, selectively prevents lysosomal fusion with ehrlichia-containing inclusions in murine macrophage-like cell line P388D1 (55). Recently, Mycobacterium tuberculosis (14), M. avium (50), Legionella pneumophila (13, 14), Coxiella burnetti (26), and Chlamydia trachomatis (26) have been shown to occupy unique cytoplasmic membrane-bound compartments which are distinct from endosomes or phagolysosomes, and each of these compartments is tailored to the needs of the particular organism. It is unknown whether all Ehrlichia spp. occupy similar or different cytoplasmic inclusion compartments. The 16S rRNA gene sequences of E. chaffeensis and the HGE agent differ by 7.5% (12), and their ultrastructures and antigenic compositions are distinct (42, 44, 45).
The purpose of this study was to examine the characteristics of the replicative inclusions of the HGE agent and E. chaffeensis independent of host cell differences by determining the localization of (i) various host cell membrane proteins specific to cytoplasmic compartments, (ii) cytoplasmic proteins which reversibly associate with membranes for forming vesicles for specific docking of cytoplasmic vacuoles (clathrin, adaptin, annexins, β-coatomer protein [β-COP], and rab), and/or (iii) membrane lipids characteristic of compartments in the exocytic pathway.
MATERIALS AND METHODS
Ehrlichia culture.
HGE agent strain HZ (45) and E. chaffeensis Arkansas (17) were propagated individually or dually infected in the HL-60 human promyelocytic leukemia cell line and incubated in RPMI 1640 medium (GIBCO-BRL, Grand Island, N.Y.) supplemented with 5% fetal bovine serum (Atlanta Biological, Norcross, Ga.), 2 mM l-glutamine (GIBCO-BRL), 1% 100× nonessential amino acids (GIBCO-BRL), and 1 mM sodium pyruvate (GIBCO-BRL) at 37°C in 5% CO2–95% air.
Double immunofluorescence labeling.
Cytocentrifuged (Cytospin2; Shandon, Inc., Pittsburgh, Pa.) preparations of HGE agent-infected, E. chaffeensis-infected, or dually infected HL-60 cells, all ≥70% infected as determined by Diff-Quik staining (Baxter Scientific Products, Obetz, Ohio), were fixed for 15 s in Diff-Quik fixative containing methanol. Infected cells were sequentially incubated with primary (Table 1) and secondary antibodies in phosphate-buffered saline (PBS; 0.009 M Na2HPO4, 0.005 M NaH2PO4, 0.15 M NaCl) for 1 h at 37°C. Anti-HGE agent and anti-E. chaffeensis sera were preabsorbed with uninfected HL-60 cells at 106/ml of serum for 1 h at 37°C. Lissamine rhodamine (LR)-conjugated anti-mouse, -rabbit, or -goat immunoglobulin G (IgG) at 7.5 μg/ml (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used to label the mouse, rabbit, or goat primary antibodies. Fluorescein isothiocyanate (FITC)-conjugated anti-horse or anti-dog IgG (Jackson) at 7.5 μg/ml was used to label horse anti-HGE agent or dog anti-E. chaffeensis antibodies. Labeled cells were mounted with a semipermanent mounting medium containing polyvinyl alcohol (Mowiol 4-88; Calbiochem, La Jolla, Calif.) and viewed by epifluorescence microscopy. As controls, uninfected cells were incubated with horse anti-HGE agent serum or dog anti-E. chaffeensis serum and FITC- or LR-conjugated anti-horse or anti-dog IgG. Uninfected cells incubated with the various mouse, rabbit, or goat antibodies also served as a control, and labeling of distinct structures in the cell served as a positive control. HGE agent- or E. chaffeensis-infected cells were incubated with anti-HGE agent or anti-E. chaffeensis serum, respectively, and FITC- or LR-conjugated anti-mouse, -rabbit, or -goat IgG to make sure there was no cross reactivity. Single labeling of infected HL-60 cells with each primary antibody and FITC- or LR-conjugated antibody was performed to ensure little cross-excitation through the LR or FITC filters, respectively.
TABLE 1.
Primary antibodies used to label HGE agent- or E. chaffeensis-infected HL-60 cells
| Antigen | Source | Dilution or concn (μg/ml) | Source |
|---|---|---|---|
| LAMP-1 | Mouse | 1:10 (culture supernatant) | Developmental Studies; Hybridoma Bank, University of Iowa, Iowa City |
| Human CD63 | Mouse | 1:10 (culture supernatant) | Immunotech, Inc., Westbrook, Mass. |
| Human TfR | Mouse | 1:5 (culture supernatant) | Immunotech, Inc. |
| HLA-ABC (MHC class I) | Mouse | 6 | Immunotech, Inc. |
| HLA-DR (MHC class II) | Mouse | 6 | Immunotech, Inc. |
| Human annexins I, II, IV, and VI | Mouse | 20 | ICN Biomedicals, Inc., Costa Mesa, Calif. |
| Human VAMP2 | Mouse | 20 | StressGen Biotechnologies Corp., Victoria, British Columbia, Canada |
| Human vacuole-type H+-ATPase (73-kDa subunit) | Mouse | 1:50 (culture supernatant) | M. Forgac, Tufts University, Boston, Mass. |
| Human EEA1 | Mouse | 6 | Affinity Bioreagents, Inc., Golden, Colo. |
| Human clathrin heavy chain | Mouse | 10 | F. M. Brodsky, University of California, San Francisco |
| Human α-adaptin | Mouse | 1:50 (ascitic fluid in PBS) | Affinity Bioreagents, Inc. |
| Human β-COP | Rabbit | 1:100 (purified rabbit IgG in PBS) | Affinity Bioreagents, Inc. |
| Human rab5 | Rabbit | 1:100 (serum) | Mario Zerial, European Molecular Biology Laboratory, Heidelberg, Germany |
| Bovine CI-M-6-P Rc | Rabbit | 1:100 (serum) | W. J. Brown, Cornell University, Ithaca, N.Y. |
| HGE agent (BDS strain) | Horse | 1:100 (serum) | J. E. Madigan, University of California, Davis |
| E. chaffeensis Arkansas | Dog | 1:100 (serum) | Yasuko Rikihisa, Ohio State University, Columbus |
DAMP and acridine orange labeling.
An acidic granule kit (Oxford Biomedical Research, Inc., Oxford, Mich.) was used to label HL-60 cells with 3-(2,4-dinitroanilino)-3′-amino-N-methyldipropylamine (DAMP), which contains an antigenic dinitrophenol (DNP) group. Briefly, 2 × 105 uninfected, HGE agent-infected, or E. chaffeensis-infected HL-60 cells/ml were incubated with a 30 μM DAMP solution for 30 min at 37°C in 5% CO2–95% air. Cells were centrifuged and fixed in 3% (wt/vol) paraformaldehyde in buffer C (10 mM Na2HPO4, 150 mM NaCl, 2 mM MgCl2, pH 7.4) for 15 min at room temperature and subsequently washed once with 50 mM NH4Cl and twice with buffer C. The cells were then permeabilized by incubation for 15 min in buffer C containing 0.3% saponin. Permeabilized cells were sequentially labeled with primary mouse anti-DNP antibodies at a 1:10 dilution of stock solution, horse anti-HGE agent serum (1:100 dilution), or dog anti-E. chaffeensis serum (1:100 dilution) at 37°C for 1 h. The secondary antibody, LR-conjugated anti-mouse IgG, FITC-conjugated anti-horse IgG, or FITC-conjugated anti-dog IgG, was used as described earlier. Acridine orange (Sigma, St. Louis, Mo.) at a final concentration of 2 μg/ml was added to HGE agent-infected or E. chaffeensis-infected HL-60 cells, and the mixture was incubated at 37°C for 1 h before cytocentrifugation for 2 min at 450 × g to wash away the unincorporated dye. Cytospin preparations were mounted, and the cells were viewed by epifluorescence microscopy as described above.
Cytochemical localization of myeloperoxidase in infected cells.
HGE agent- or E. chaffeensis-infected HL-60 cells were fixed with 1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) containing 5% sucrose for 45 min. Cells were rinsed three times with 50 mM Tris (pH 7.4) containing 7.5% sucrose for 10 min and incubated in the substrate solution (73 mM sodium acetate, pH 6.2) containing 0.003% diaminobenzidine tetrachloride (Nacalai Tesque, Inc., Kyoto, Japan) for 5 min. The reaction was stopped by rinsing the cells three times with 50 mM Tris (pH 7.4) containing 7.5% sucrose. Cells were incubated at 4°C in reduced osmium tetroxide (1% OsO4 and 1% potassium ferrocyanide in 0.1 M sodium cacodylate buffer, pH 7.4) for 1 h, rinsed three times with cold 0.1 M sodium cacodylate buffer, and dehydrated with graded series of ethanol. Ultrathin (60-nm) sections were stained with lead citrate and observed under a Philips EM 300 transmission electron microscope.
C6-NBD-ceramide labeling.
Fluorescent C6-7-nitrobenzo-2-oxa-1,3-diazole (NBD)-ceramide (Molecular Probes, Eugene, Oreg.) was complexed with defatted bovine serum albumin (DFBSA) in RPMI medium to obtain final concentrations of C6-NBD-ceramide and DFBSA of ∼5 μM as described previously (26). Infected or uninfected HL-60 cells (106) were incubated in the presence of the C6-NBD-ceramide–DFBSA complex in RPMI medium for 30 min at 4°C in a multiwell plate and then washed with 10 mM HEPES-buffered calcium and magnesium-free PBS (HCMF). Cells were incubated in RPMI medium containing 0.03% DFBSA for either 0.5, 1, 2, or 4 h to allow back exchange of excess fluorescent probe from the plasma membrane and washed gently with HCMF before collection by centrifugation. Cells were resuspended in serum-free RPMI medium, and 50 μl of the cell suspension was cytocentrifuged for 2 min at 450 × g. Cytocentrifuged cells on slides were mounted with Mowiol mounting medium and examined by epifluorescence microscopy for NBD and by Nomarski differential interference microscopy for ehrlichial inclusions.
BFA treatment of HGE agent- or E. chaffeensis-infected cells.
HGE agent- or E. chaffeensis-infected HL-60 cells (∼90%) were placed in RPMI medium supplemented with 10% fetal bovine serum. Brefeldin A (BFA; Sigma) was added to the culture at 20 μg/ml. Cells were incubated for 24 h at 37°C in 5% CO2–95% air and examined by Diff-Quik staining or fixed and processed for electron microscopy as described previously (55).
RESULTS
Double immunofluorescence labeling and electron microscopy of dually infected cells.
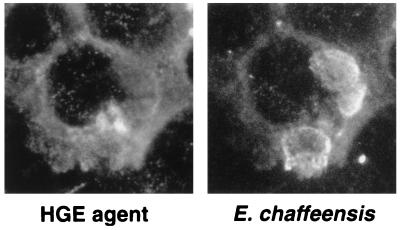
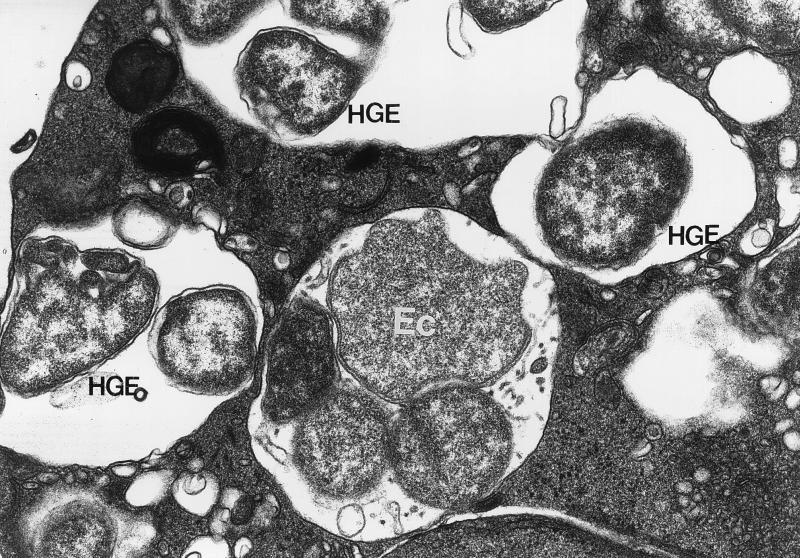
HL-60 cells were infected with the host cell-free HGE agent and E. chaffeensis concurrently to determine if they shared the same replicative inclusions following entrance into the host cell. At 3 days postinfection (allowing stable and logarithmic growth), double immunofluorescence labeling revealed the presence of separate HGE agent and E. chaffeensis inclusions in the cytoplasm of the same HL-60 cell (Fig. 1). No HL-60 cells revealed ehrlichial inclusions containing both organisms together. Electron microscopic (EM) analysis of dually infected HL-60 cells confirmed the presence of the HGE agent and E. chaffeensis in separate compartments within the same host cell (Fig. 2). The HGE agent was distinguished from E. chaffeensis by the presence of denser ribosomal clumping, loosely packed morulae, and the presence of a ruffled outer membrane, while E. chaffeensis could be distinguished by tighter packaging of organisms into the inclusions, the presence of tubular appendicular structures in the lumen of the inclusion, a less ruffled outer membrane, and less dense ribosomal clumps. These results obtained with dually infected cells suggest that two Ehrlichia spp. occupy distinct cytoplasmic compartments which do not fuse with each other.
FIG. 1.
Double infection of HL-60 cells with the HGE agent and E. chaffeensis. The host cell-free HGE agent and E. chaffeensis were used simultaneously to infect HL-60 cells. The paired photomicrographs show the LR-labeled HGE agent and FITC-labeled E. chaffeensis in the same cell. These results are representative of three independent labeling experiments. Magnification, ×750.
FIG. 2.
EM analysis of dually HGE agent- and E. chaffeensis-infected HL-60 cells. Shown are the HGE agent and E. chaffeensis in inclusions in an HL-60 cell. The tightly packed E. chaffeensis inclusion (Ec, center) is seen surrounded by three separate HGE agent (HGE) inclusions characterized by loose packing of the organisms into the inclusions. Magnification, ×33,000.
Lysosomal markers.
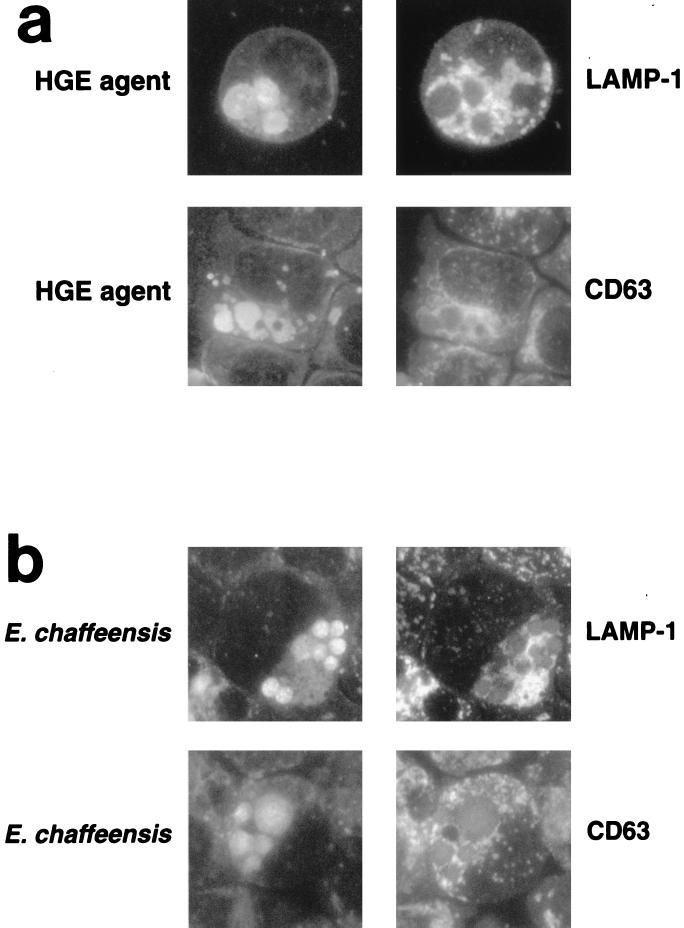
CD63 and human lysosome-associated membrane protein 1 (LAMP-1) are unique membrane glycoproteins found in late endosomes and lysosomes (11). Neither HGE agent nor E. chaffeensis inclusions colocalized with CD63 or LAMP-1, although lysosome-like structures were strongly labeled throughout the cytoplasm of the cell, with a higher population residing around the HGE agent inclusions (Fig. 3).
FIG. 3.
Double immunofluorescence labeling of the HGE agent (a) or E. chaffeensis (b) and late endosomal or lysosomal glycoproteins. Paired photomicrographs showing the FITC-labeled HGE agent or E. chaffeensis and LR-labeled late endosome and lysosome markers. The markers are LAMP-1 and CD63. The markers used were LAMP-1 and CD63. These results are representative of three independent labeling experiments. Magnification, ×750.
Myeloperoxidase localization.
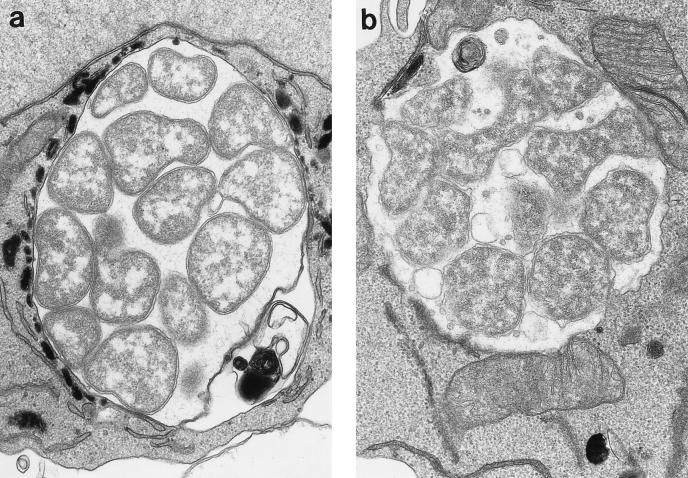
EM cytochemical analysis of myeloperoxidase localization in HGE agent-infected HL-60 cells revealed a large population of lysosomes which surrounded the ehrlichial inclusions (Fig. 4a); however, the myeloperoxidase activity was not detected inside inclusions, indicating the absence of lysosomal fusion. In contrast, not only were E. chaffeensis inclusions negative for myeloperoxidase, but no accumulation of peroxidase-positive granules around the inclusions was seen; instead, more mitochondria were seen adjacent to the inclusion (Fig. 4b), as previously noted in infected DH82 cells (44).
FIG. 4.
Localization of myeloperoxidase in HGE agent- and E. chaffeensis-infected HL-60 cells. The EM images show the localization of myeloperoxidase in granules surrounding HGE agent inclusions (a), suggesting that docking of lysosomes with HGE agent inclusions occurs without fusion, and in contrast, lack of localization of myeloperoxidase-positive granules around E. chaffeensis inclusions with, instead, more frequent presence of mitochondria adjacent to the inclusions (b). Magnifications, ×19,500 (a) and ×22,100 (b).
Acidic markers.
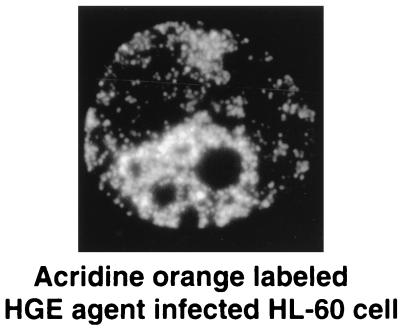
Vacuole-type H+-ATPase is located in early and late endosomes, lysosomes, and the Golgi apparatus and is required for acidification of these compartments (31). It differs from mitochondrial and bacterial H+-ATPase both antigenically and biochemically. HGE agent inclusions were not labeled for vacuole-type H+-ATPase (data not shown), although endosome- or lysosome-like structures scattered throughout the cytoplasm of infected and uninfected HL-60 cells were positively labeled for vacuole-type H+-ATPase. E. chaffeensis in HL-60 cells, as in THP-1 cells (7), showed weak colocalization with vacuole-type H+-ATPase (data not shown). The weak base DAMP, which accumulates in acidic compartments in direct proportion to compartment acidity (2), did not accumulate in HGE agent inclusions, although infected and uninfected HL-60 cells showed strong accumulation of DAMP in endosome- or lysosome-like compartments, which were scattered throughout the cytoplasm of the cells. Infected HL-60 cells exposed to DAMP only without exposure to anti-Ehrlichia sp. antibody also showed no labeling of ehrlichial inclusions. Unlike that in THP-1 cells (7), E. chaffeensis inclusions in HL-60 cells did not accumulate DAMP; however, a population of endosome- or lysosome-like vesicles accumulated DAMP. The HGE agent or E. chaffeensis inclusions did not accumulate the acridine orange dye after 24 h of incubation, but accumulation of labeled granules was seen around HGE agent inclusions (Fig. 5).
FIG. 5.
Acridine orange labeling of HGE agent-infected HL-60 cells. The photomicrograph shows lysosomes and late endosomes populating the area of an HGE agent inclusion. Magnification, ×800.
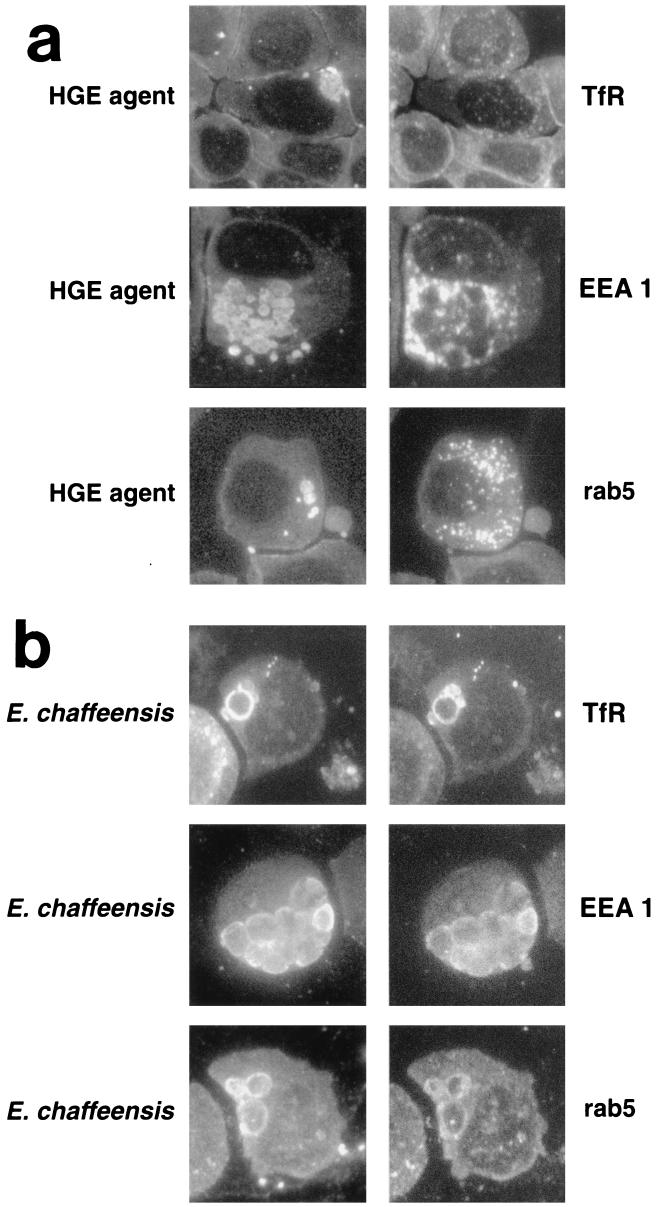
Early endosomal markers.
The TfR (24), the cytoplasmic reversibly associating protein rab5 (a small GTPase) (53), and the hydrophilic peripheral membrane protein early endosomal antigen 1 (EEA1) (37) are found in early endosomes. The association of EEA1 with the endosomal membrane requires rab5-GTP (49). HGE agent inclusions did not colocalize with the TfR, rab5, or EEA1 (Fig. 6a); however, strongly labeled endosome-like vesicles were found scattered throughout the cytoplasm of infected and uninfected HL-60 cells. The TfR was more abundant than rab5 or EEA1. As in THP-1 cells (7), E. chaffeensis inclusions colocalized with the TfR (Fig. 6b). In addition, both EEA1 and rab5 also colocalized with E. chaffeensis inclusions (Fig. 6b). Based on the localization of these unique early endosomal membrane proteins, the data suggests that the HGE agent does not reside in a TfR endosome or an early endosome while E. chaffeensis does.
FIG. 6.
Double immunofluorescence labeling of the HGE agent (a) or E. chaffeensis (b) and early endosomal markers. Paired photomicrographs showing the FITC- or LR-labeled HGE agent or E. chaffeensis and LR- or FITC-labeled early endosomal markers TfR, EEA1, and rab5. These results are representative of three independent labeling experiments. Magnification, ×750.
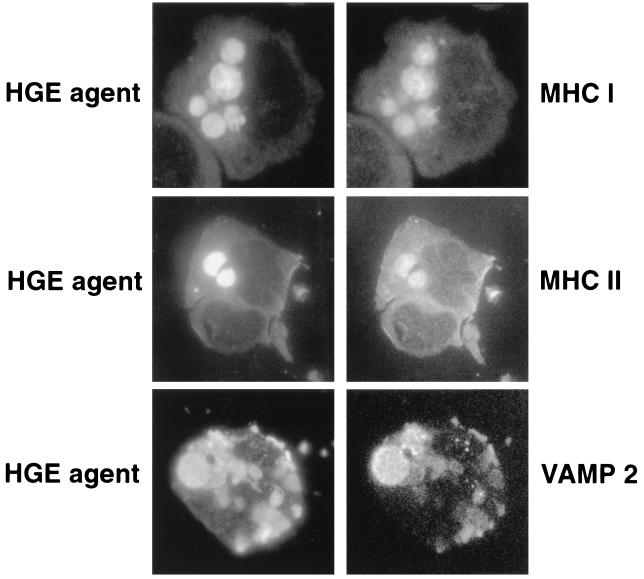
MHC class I and II molecules.
Major histocompatibility complex (MHC) class I and II molecules are membrane glycoproteins which are constitutively present on the surface of monocytes and neutrophils and in the cytoplasmic membrane compartment but are distributed differently in the endosomal-lysosomal pathway. MHC class I molecules are trafficked from the Golgi apparatus to the plasma membrane. Class I molecules are excluded during endocytosis or are rapidly redirected back to the plasma membrane following endocytosis (39). In contrast, MHC class II molecules are trafficked from the Golgi apparatus to specialized MHC class II-containing endosomes, which are subsequently directed to the plasma membrane (1). Both MHC class I and II molecules are located on a distinct class of endosomes separate from the TfR-containing endosomes during endocytosis. Approximately 20 to 25% (100 infected cells each were scored for three independent experiments) of HGE agent inclusions contained the MHC class I heavy chain associated with β2 microglobulin and the MHC class II molecules (Fig. 7). A small percentage of E. chaffeensis inclusions in HL-60 cells colocalized with MHC class I and II molecules (∼10 and 5%, respectively). Uninfected and infected cells both contained other small endosome-like structures in the peripheral cytoplasm which were labeled for MHC class I and II molecules.
FIG. 7.
Double immunofluorescence labeling of the HGE agent, MHC class I and II molecules, and VAMP2. The paired photomicrographs show the FITC- or LR-labeled HGE agent and LR-labeled MHC class I or II molecules or FITC-labeled VAMP2. These results are representative of three independent labeling experiments. Magnification, ×750.
Endocytic markers.
The clathrin heavy chain is attached to membrane proteins via the adapter protein α-adaptin (51), which aids vesicle transport to endosomes from the plasma membrane. HGE agent and E. chaffeensis replicative inclusions did not colocalize with either the clathrin heavy chain or α-adaptin, but strongly labeled endosome-like inclusions were present throughout the cytoplasm of both infected and uninfected cells. Vesicles labeled for α-adaptin often localized near the plasma membrane, while vesicles labeled for the clathrin heavy chain were generally more randomly located throughout the cytoplasm (data not shown).
Cytoplasmic reversibly associating proteins.
The annexins are a family of structurally related Ca2+-regulated, phospholipid-binding proteins which are suggested to have roles in many cellular processes such as exocytosis, ion permeation, lipid metabolism, and membrane fusion (36). Since E. risticii is sensitive to Ca2+-calmodulin inhibitors (47), we examined the localization of annexins in ehrlichia-infected cells. For both the HGE agent and E. chaffeensis, neither annexin I, II, IV, nor VI translocated from the cytoplasm to the ehrlichial inclusions themselves or to areas surrounding the inclusions. Annexins IV and VI and smaller populations of annexins I and II showed strong labeling of small granules which were diffusely and evenly distributed throughout the cytoplasm of both infected and uninfected cells.
VAMP2.
Vesicle-associated membrane protein (VAMP2), also known as synaptobrevin-2, is an integral membrane protein which plays a role in vesicular transport and neurotransmission (10). In human blood neutrophils, VAMP2 has been found in high concentrations in secretory vesicles and tertiary granules but only in low concentrations in primary and secondary granules (10). Approximately 40% of HGE agent inclusions and 15 to 20% of E. chaffeensis inclusions were labeled with VAMP2 in a punctate pattern (Fig. 7). Both VAMP2-labeled and unlabeled ehrlichial inclusions were found within the same host cell. Both uninfected and infected HL-60 cells had a small population of VAMP2-positive vesicles primarily in the peripheral cytoplasm.
Exocytic pathway markers.
β-COP is involved in the regulation of intracellular protein transport between the intermediate compartment of the endoplasmic reticulum and the Golgi complex (18). HGE agent and E. chaffeensis inclusions showed no colocalization of β-COP (data not shown). Anti-β-COP was found to colocalize in the Golgi apparatus and in a large population of vesicles in the region of the Golgi apparatus in both infected and uninfected HL-60 cells.
Ceramide is cleaved to sphingolipid in the Golgi apparatus. C6-NBD-ceramide, a fluorescently labeled ceramide, has been used to study trafficking of sphingolipid in viable cells (30). We utilized C6-NBD-ceramide to determine whether endogenously synthesized sphingomyelin is incorporated into HGE agent inclusions. C6-NBD-ceramide labeling of HGE agent-infected and uninfected cells revealed accumulation of the fluorescent sphingolipid in the Golgi apparatus and in a small population of vesicles concentrated around the Golgi apparatus. HGE agent inclusions visualized by Nomarski interference microscopy did not accumulate C6-NBD-ceramide. Plasma membranes of uninfected and infected cells did not contain fluorescence following back exchange with DFBSA.
CI-M-6-P Rc.
Prelysosomal vesicles, late endosomes, and trans-Golgi vesicles contain the cation-independent mannose-6-phosphate receptor (CI-M-6-P Rc), which delivers newly synthesized, soluble lysosomal enzymes to the prelysosomes from the trans-Golgi network (25). The CI-M-6-P Rc releases its ligands to the acidic lumen of prelysosomes, and the Rc is rapidly cycled back to the trans-Golgi network to initiate another cycle (25). The CI-M-6-P Rc was not detected on the membranes of either HGE agent or E. chaffeensis inclusions (data not shown), although CI-M-6-P Rc-positive vesicles were detected throughout the cytoplasm of the cell.
BFA treatment.
HGE agent- or E. chaffeensis-infected HL-60 cells were treated with BFA to assess the requirement of vesicular transport from the endoplasmic reticulum and Golgi complex for ehrlichial survival. BFA is a fungal metabolite which inhibits antegrade (but not retrograde) transport by causing redistribution and loss of the stacked cisternae of the Golgi complex (23). Following 24 h of BFA treatment, light microscopy with Diff-Quik staining showed the presence of many juxtanuclear vacuoles and the presence of the viable HGE agent or E. chaffeensis in morphologically normal-appearing inclusions. EM analysis of HGE agent- or E. chaffeensis-infected HL-60 cells revealed morphologically normal-appearing ehrlichial inclusions containing viable organisms. HL-60 cells showed complete loss of the Golgi complex but retention of an enlarged, swollen endoplasmic reticulum. Many juxtanuclear vacuoles were also present.
All of the double immunofluorescence labeling results obtained are summarized in Table 2.
TABLE 2.
Colocalization of cellular compartment markers with HGE agent or E. chaffeensis inclusions
| Host cell membrane, cytoplasmic protein, or lipid (molecular mass [kDa]) | Degree of colocalization witha:
|
Cellular compartment(s) labeled (reference) | |
|---|---|---|---|
| HGE agent | E. chaffeensis | ||
| Clathrin heavy chain (180) | − | − | Plasma membrane, endosomes (38) |
| α-Adaptin (100) | − | − | Plasma membrane, endosomes (51) |
| Early endosomal antigen 1 (180) | − | ++++ | Early endosomes (37) |
| rab5 (25.5) | − | ++++ | Plasma membrane, early endosomes (53) |
| TfR (90–95) | − | ++++ | Plasma membrane, early endosomes (24) |
| MHC class I molecules (44) | ++ | + | Plasma membrane, endoplasmic reticulum, endosomes (39) |
| MHC class II molecules (30) | ++ | + | Plasma membrane, Golgi apparatus, endosomes (1) |
| CI-M-6-P Rc (215) | − | − | Plasma membrane, trans-Golgi network, prelysomal compartment (25) |
| Vacuole-type H+-ATPase (73) | − | ± | Endosomes, lysosomes (33) |
| LAMP-1 (120) | − | − | Late endosomes, lysosomes (11) |
| CD63 (∼60) | − | − | Late endosomes, lysosomes (11) |
| Annexins I, II, IV, and VI (38, 36, 36, and 70, respectively) | − | − | Chromaffin granules, early endosomes (36) |
| VAMP2 (12) | +++ | ++ | Synaptic and secretory vesicles, 2° and 3° granules (10) |
| β-COP (110) | − | − | Golgi apparatus, Golgi-derived vesicles (18) |
| C6-NBD-ceramide (0.576) | − | ND | Golgi apparatus, Golgi-derived vesicles (30) |
++++, >90%; +++, ∼40%; ++, ∼20; +, ∼10%; −, none; ±, most colocalized but weak labeling; ND, not done.
DISCUSSION
Ehrlichia spp. survive and replicate exclusively within inclusions in monocytes-macrophages or granulocytes, which are the primary effector cells of antimicrobial defense. Therefore, ehrlichiae apparently create an inclusion environment which is conducive to survival and replication. The present study revealed that the HGE agent resides in a unique cytoplasmic compartment, which is distinct from that occupied by E. chaffeensis, within the same cell, and distinct from those occupied by any other intracellular organisms studied thus far. Our parallel study (6) showed that E. chaffeensis infection of THP-1 cells is inhibited by monodansylcadaverine, as is E. risticii infection (34, 46), suggesting that both organisms enter by receptor-mediated endocytosis (16). Previous results obtained with E. risticii have shown that entrance is dependent upon surface antigenic proteins expressed on the host cell and the ehrlichial organism (34, 35). The replication of these two Ehrlichia spp. in separate inclusions suggests that they have evolved distinct mechanisms to survive in their particular intracellular niches. For example, the receptor and signaling for internalization may be distinct for these two ehrlichial organisms. These differences may influence ehrlichial metabolism, e.g., iron acquisition, as suggested in our parallel work (6), and pathogenesis.
The absence of LAMP-1 and CD63 in the HGE agent and E. chaffeensis inclusions in HL-60 cells indicates that fusion between late endosomes and lysosomes and ehrlichial inclusions does not occur. These results are in agreement with our previous observations of E. chaffeensis inclusions in THP-1 cells (7) and with ultrastructural studies of E. risticii inclusions in P388D1 cells (55). This evasion of late endosomal and lysosomal fusion by ehrlichial inclusions must be critical for survival and replication and conserved universally among different Ehrlichia spp. Lysosomal distribution, as examined by the presence of LAMP-1 and CD63, myeloperoxidase localization, and acridine orange vital staining, clearly demonstrated accumulation of lysosomes adjacent to the HGE agent inclusions, which appeared to be docking but not fusing with the inclusion membranes. For synaptic vesicles, vesicle fusion but not docking is GTP dependent (53). These results allow speculation that the HGE agent may block a GTPase activity present on the inclusion membrane. This may also explain the relative instability of the HGE agent in culture. LAMP-1 was also not found at any detectable level with C. trachomatis (23) and L. pneumophila (14) inclusions, but inclusions of M. avium (50), M. marinum (5), and C. burnetti (23) did contain LAMP-1.
Previous results obtained with E. risticii have suggested a role for clathrin-mediated endocytosis because its internalization is inhibited by monodansylcadaverine (34, 46). Both HGE agent and E. chaffeensis inclusions, however, lacked colocalization with both the clathrin heavy chain and α-adaptin. Our previous study with E. chaffeensis in THP-1 cells also did not show the clathrin heavy chain with ehrlichial inclusions (7). Clathrin-mediated endocytosis is a pathway utilized by many receptors (e.g., the TfR) and virus entry, which are sensitive to monodansylcadaverine (16, 38). The association of clathrin heavy and light chains with the receptor allows formation of basket-like structures on the cytoplasmic side of the plasma membrane which are pinched off to form clathrin-coated vesicles (38). Clathrin is attached to the plasma membrane receptor via a distinct adapter complex including α-adaptin (51). This clathrin-associated complex is released from the endocytic vesicle immediately preceding fusion to a target membrane in an ATP-dependent manner (8). It is unknown whether the α-adaptin is fully or partially removed prior to fusion with the target membrane. The absence of clathrin and α-adaptin from ehrlichial inclusions suggests that the inclusion is distinct from budding TfR endosomes (33), and their association with a newly formed ehrlichial inclusion during entry into the host cell and replication may have an extremely short half-life, if the association occurs at all, because TfR-ligand complex internalization is initiated by clathrin-mediated endocytosis (24). Ehrlichial organisms partially internalized in a coated pit were occasionally observed (42); therefore, the absence may not rule out the use of clathrin-mediated endocytosis as a mechanism for entrance into the host cell. E. chaffeensis shows similarity to E. sennetsu (6)- and M. tuberculosis (14)-containing phagosomes in that they both fuse with early endosomes, allowing acquisition of the TfR. L. pneumophila or C. trachomatis, in contrast, was not found to acquire the TfR (14, 26), similar to the HGE agent. The inclusion membrane of E. chaffeensis may contain specific recognition molecules which allow fusion of TfR endosomes or accumulation of newly synthesized TfR directly trafficked from the Golgi apparatus.
We have determined that, similar to E. chaffeensis inclusions in THP-1 cells (7) and M. tuberculosis (14), a fraction of E. chaffeensis and HGE agent inclusions in HL-60 cells acquire or are slow to remove MHC class I and II molecules. Although the MHC class I and II molecules on a small percentage of the ehrlichial inclusions may be derived directly from the plasma membrane or newly synthesized molecules derived from Golgi vesicles that have been directly trafficked to the inclusions, our results suggest that the persistence of MHC class I and II molecules on ehrlichial inclusions is due to inhibition of normal membrane molecule sorting following phagocytosis and inhibition of normal maturation of the ehrlichial phagosome along the endosomal-lysosomal pathway. Taken together with results obtained with early endosomal markers, these findings suggest that persistence of MHC class I and II molecules is independent from the presence of early endosomal markers.
Our results showed that HGE agent inclusions did not colocalize with the 73-kDa subunit of the vacuole-type H+-ATPase and that the weak base DAMP did not accumulate in ehrlichial inclusions. This suggests that HGE agent inclusions do not contain a functional vacuole-type H+ ATPase at any detectable level. A lack of acridine orange labeling of HGE agent inclusions confirmed the neutrality and lack of vacuole-type H+ ATPase on the HGE agent inclusions. These findings are distinct from those on E. chaffeensis inclusions in THP-1 and HL-60 cells, which have low levels of vacuole-type H+-ATPase (7). E. chaffeensis inclusions in HL-60 cells are less acidic than those in THP-1 cells, since the weak base DAMP did not accumulate in ehrlichial inclusions in HL-60 cells to any detectable level. Metabolic activities of host cell-free E. risticii and E. sennetsu organisms have been shown to be very sensitive to an acidic pH (54). Lack of or weak acidification of ehrlichial inclusions, therefore, may be another requirement conserved among different Ehrlichia spp. Like E. chaffeensis inclusions, C. burnetti inclusions contained vacuole-type H+-ATPase (26); however, vacuole-type H+-ATPase was not found in M. avium (50), M. marinum (5), or C. trachomatis (26) inclusions. The presence of a vacuole-type H+-ATPase may give E. chaffeensis inclusions a weak acidic nature to cause prolonged retention of the TfR in the inclusion, since acidification of endosomes was reported to delay recycling of the TfR back to the plasma membrane (27).
Our results show that some HGE agent- and E. chaffeensis-infected HL-60 cells colocalized with VAMP2. In neutrophils, VAMP2 is believed to play a role in controlling vesicular targeting, docking, and fusion through interactions with other proteins, such as the soluble protein NSF (N-ethylmaleimide sensitivity factor) and the connecting cytoplasmic protein known as SNAP (soluble NSF attachment protein) (48). Due to the highly conserved and ubiquitous nature of both NSF and SNAP, it is thought that the specificity for docking and fusion results from the existence of a unique SNAP receptor residing on the vesicles (i.e., VAMP2) (48). VAMP2 on ehrlichial inclusions may act as a mechanism to acquire lipid membrane or essential nutrients for the growing ehrlichial organism through regulated vesicle trafficking.
Through double immunofluorescence labeling, we found that neither HGE agent or E. chaffeensis inclusions colocalized with annexin I, II, IV, or VI. Results obtained with C. trachomatis in HeLa cells suggest a role for annexins III, IV, and V in the redistribution of chlamydial inclusions (32). It remains to be seen if other annexin family members occur or if transient association of annexins with ehrlichial inclusions occurs.
The failure to incorporate fluorescent sphingolipid into the inclusions indicates that the HGE agent does not intercept vesicular antegrade traffic to the plasma membrane from the trans-Golgi network. C. burnetti-containing vacuoles also did not incorporate fluorescent sphingolipids (26); however, specific trafficking of fluorescent sphingolipids to C. trachomatis inclusions occurs (26). Through the addition of BFA to HGE agent- or E. chaffeensis-infected cells, we have determined that these Ehrlichia spp. do not rely on secretory vesicles or their contents trafficked from the endoplasmic reticulum or the Golgi apparatus. Since vesicular traffic between the trans-Golgi network endosomes and the cell surface does proceed in the presence of BFA (29), proteins of the trans-Golgi network and endosomes are still able to intermix, which may be sufficient to allow ehrlichial growth or survival for at least 24 h. These results do support the finding obtained by β-COP and C6-NBD-ceramide labeling that neither the HGE agent nor E. chaffeensis intercepts regulated protein transport between the endoplasmic reticulum and the Golgi complex, intra-Golgi transport, or the trafficking of Golgi-derived vesicles (exocytic pathway) being transported to the plasma membrane or another cytoplasmic destination.
CI-M-6-P Rc data, along with the results obtained for LAMP-1 and CD63, suggests that neither the HGE agent nor E. chaffeensis resides in late endosomes or in transport vesicles between the Golgi and lysosomes. The exclusion of the CI-M-6-P Rc has also been shown for inclusions of M. marinum (5) and C. burnetti (26). Heinzen et al. have reported an absence of CI-M-6-P Rc in C. trachomatis inclusions (26); however, Van Ooij et al. more recently reported the presence of the CI-M-6-P Rc around the C. trachomatis inclusions from as early as 4 h postinfection to as late as 20 h postinfection (52).
The HL-60 cell line was derived from a patient with acute promyelocytic leukemia and can be induced by various agents to differentiate into granulocytes, monocytes, or macrophages (15). We also found that another myelocytic leukemia cell line, THP-1, supports the growth of both the HGE agent and E. chaffeensis (6). The ability to support the growth of both the HGE agent and E. chaffeensis indicates that these cell lines have the receptor and cellular physiology necessary to accommodate both types of ehrlichiae, unlike mature peripheral blood granulocytes or monocytes. Our observation coincides with the previous reports of the presence of E. chaffeensis in bone marrow mononuclear cells (19, 21, 40). Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the HGE agent (28). Infection of bone marrow progenitors may be partially responsible for the hematologic abnormality and pathogenesis seen in human ehrlichioses.
ACKNOWLEDGMENTS
This work was supported by grants RO1 AI30010 and F32 AI09968 from the National Institutes of Health. The H4A3 monoclonal antibody developed by James E. K. Hilfreth and J. Thomas August was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, Md., and the Department of Biological Sciences, University of Iowa, Iowa City, under contract N01-HD-2-3144 from the NICHD.
We appreciate Hyung-Yong Kim’s assistance with myeloperoxidase cytochemical analysis, HGE agent cultivation, and the Diff-Quik fixation method; and Evelyn Handly’s assistance with EM sample preparation.
ADDENDUM IN PROOF
The lack of TfR and vacuolar H+-ATPase in HGE agent inclusions has recently been shown (P. J. Webster, W. Ijdo, L. M. Chicoine, and E. Fikrig, J. Clin. Investig. 101:1932–1941, 1998).
REFERENCES
- 1.Amigorena S, Drake J R, Webster P, Mellman I. Transient accumulation of new class II MHC in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R G W, Falck J R, Goldstein J L, Brown M S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci USA. 1984;81:4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakken J S, Krueth J, Tilden R L, Dumler J S, Kristansen R F. Serologic evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1997;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- 4.Bakken J S, Dumler J S, Chen S M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest United States. a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 5.Barker L P, George K M, Falkow S, Small P L C. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect Immun. 1997;65:1497–1504. doi: 10.1128/iai.65.4.1497-1504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnewall, R. E., N. Ohashi, and Y. Rikihisa. Ehrlichia chaffeensis and E. sennetsu reside in transferrin endosomes and up-regulate transferrin receptor mRNA by activating IRP1; however, the HGE agent does not. Submitted for publication.
- 7.Barnewall R E, Rikihisa Y, Lee E H. Ehrlichia chaffeensis inclusions are early endosomes which selectively accumulate transferrin receptor. Infect Immun. 1997;65:1455–1461. doi: 10.1128/iai.65.4.1455-1461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braell W A, Schlossman D M, Schmid S L, Rothman J E. Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol. 1984;99:734–741. doi: 10.1083/jcb.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouqui P, Dumler J S, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–783. doi: 10.1016/s0140-6736(95)91544-3. [DOI] [PubMed] [Google Scholar]
- 10.Brummel J H, Volchuk A, Sengelov H, Borregaard N, Cieutat A-M, Bainton D F, Grinstein S, Klip A. Subcellular distribution of docking/fusion proteins in neutrophils, secretory cells with multiple exocytic compartments. J Immunol. 1995;155:5750–5759. [PubMed] [Google Scholar]
- 11.Chen J W, Chen G L, D’Souza M P, Murphy T L, August J T. Lysosomal membrane glycoproteins: properties of LAMP-1 and LAMP-2. Biochem Soc Symp. 1986;51:97–112. [PubMed] [Google Scholar]
- 12.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens D L, Horowitz M A. Membrane sorting during phagocytosis: selective exclusion of major histocompatibility complex molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J Exp Med. 1992;175:1317–1326. doi: 10.1084/jem.175.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemens D L, Horowitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins S J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 16.Davies P J A, Davies D R, Levitzki A, Maxfield F R, Milhaud P, Willingham M C, Pastan I H. Transglutaminase is essential in receptor-mediated endocytosis of α2-microglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- 17.Dawson J E, Anderson B E, Fishbein D, Sanchez J, Goldsmith C, Wilson K, Duntly C. Isolation and characterization of an Ehrlichia sp. from a patient with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duden R, Allan V, Kreis T. Involvement of β-COP in membrane traffic through the Golgi complex. Trends Cell Biol. 1991;1:14–19. doi: 10.1016/0962-8924(91)90064-g. [DOI] [PubMed] [Google Scholar]
- 19.Dumler J E, Dawson J E, Walker D H. Human ehrlichiosis: hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993;24:391–396. doi: 10.1016/0046-8177(93)90087-w. [DOI] [PubMed] [Google Scholar]
- 20.Dumler J E, Dotevall L, Gustafson R, Granstrom M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J Infect Dis. 1997;175:720–722. doi: 10.1093/infdis/175.3.720. [DOI] [PubMed] [Google Scholar]
- 21.Dumler J E, Sutker W L, Walker D H. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 22.Dumler J E, Bakken J S. Human granulocytic ehrlichiosis in Wisconsin and Minnesota: a frequent infection with the potential for persistence. J Infect Dis. 1996;173:1027–1030. doi: 10.1093/infdis/173.4.1027. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara T, Oda K C, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 24.Geuze H J, Slot J W, Strous G J A M, Peppard J, Von Figura K, Hasilik A, Schwarts A L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984;37:195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- 25.Geuze H J, Stoorvogel W, Strous G J, Slot J W, Bleekemolen J E, Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988;107:2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetti and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson L S, Dunn K W, Pytowski B, McGraw T E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor’s internalization motif. Mol Biol Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein M B, Miller J E, Nelson C M, Goodman J L. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J Infect Dis. 1997;176:1405–1409. doi: 10.1086/517332. [DOI] [PubMed] [Google Scholar]
- 29.Lippincott-Schwartz J, Yuan L C, Tipper C, Amherdt M, Orci L, Klausner R D. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 30.Lipsky N G, Pagano R E. Sphingolipid metabolism in cultured fibroblasts: microscopic and biochemical studies employing a fluorescent ceramide analogue. Proc Natl Acad Sci USA. 1983;80:2608–2612. doi: 10.1073/pnas.80.9.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacs G L, Rotstein O D, Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H+-ATPase in murine macrophages. J Biol Chem. 1990;265:21099–21107. [PubMed] [Google Scholar]
- 32.Majeed M, Ernst J D, Magnusson K, Kihlstrom E, Stendahl O. Selective translocation of annexins during intracellular redistribution of Chlamydia trachomatis in HeLa and McCoy cells. Infect Immun. 1994;62:126–134. doi: 10.1128/iai.62.1.126-134.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquez-Sterling N I, Herman I M, Pesacreta T, Arai H, Terres G, Forgac M. Immunolocalization of the vacuolar-type (H+)-ATPase from clathrin-coated vesicles. Eur J Cell Biol. 1991;56:19–33. [PubMed] [Google Scholar]
- 34.Messick J B, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and growth in host cells by flow cytometry. Infect Immun. 1993;61:3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messick J B, Rikihisa Y. Inhibition of binding, entry, or intracellular proliferation of Ehrlichia risticii in P388D1 cells by anti-E. risticii serum, immunoglobulin G, or Fab fragment. Infect Immun. 1994;62:3156–3161. doi: 10.1128/iai.62.8.3156-3161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss S E. Annexins. Trends Cell Biol. 1997;7:87–89. doi: 10.1016/S0962-8924(96)10049-0. [DOI] [PubMed] [Google Scholar]
- 37.Mu F, Callaghan J M, Steele-Mortimer O, Stenmark H, Parton R G, Campbell P L, McCluskey J, Yeo J, Tock E P C, Toh B. EEA1, an early endosome-associated protein. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee S, Ghosh R N, Maxfield F R. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 39.Neefjes J, Stollorz V, Peters P, Geuze H, Ploegh H. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 40.Paddock C D, Sumner J W, Merrill Shore G, Bartley D C, Elie R C, McQuade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrovec M, Lotric-Furlan S, Zupanc T A, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popov V L, Han V C, Chen S-M, Dumler J E, Feng H-M, Andreadis T G, Tesh R B, Walker D H. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. 1998;47:235–251. doi: 10.1099/00222615-47-3-235. [DOI] [PubMed] [Google Scholar]
- 43.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rikihisa Y. Proceedings of the 5th International Symposium on Rickettsiae and Rickettsial Diseases. Bratislava, Slovak Republic: International Society for Rickettsiae and Rickettsial Diseases, Slovak Academy of Sciences; 1996. Ehrlichiae; pp. 272–286. [Google Scholar]
- 45.Rikihisa Y, Zhi N, Wormser G P, Wen B, Horowitz H M, Hechemy K E. Ultrastructural and antigenic characterization of granulocytic ehrlichia directly isolated and cultivated from a patient in New York State. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 46.Rikihisa Y, Zhang Y, Park J. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect Immun. 1994;62:5126–5132. doi: 10.1128/iai.62.11.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rikihisa Y, Zhang Y, Park J. Role of Ca2+ and calmodulin on ehrlichial survival in macrophages. Infect Immun. 1995;63:2310–2316. doi: 10.1128/iai.63.6.2310-2316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothman J E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 49.Simonsen A, Lippe R, Christoforidis S, Gaullier J-M, Brech A, Callaghaan J, Toh B-H, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 50.Sturgill-Koszycki S, Schlesinger P H, Chakrborty P L, Haddix P L, Collons H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russel D G. Lack of acidification in mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 51.Traub L M. Clathrin-associated adaptor proteins—putting it all together. Trends Cell Biol. 1997;7:43–46. doi: 10.1016/S0962-8924(96)20042-X. [DOI] [PubMed] [Google Scholar]
- 52.van Ooij C, Apodaca G, Engel J. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect Immun. 1997;65:758–766. doi: 10.1128/iai.65.2.758-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitale G, Alexandrov K, Ullrich O, Horiuchi H, Giner A, Dobson C, Baykova O, Gournier H, Stenmark H, Zerial M. The GDP/GTP cycle of Rab5 in the regulation of endocytotic membrane traffic. Cold Spring Harbor Symp Quant Biol. 1995;LX:211–220. doi: 10.1101/sqb.1995.060.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Weiss E, Dasch G A, Kang Y-H, Westfall H N. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by Renografin gradient centrifugation. J Bacteriol. 1988;170:5012–5017. doi: 10.1128/jb.170.11.5012-5017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells M Y, Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988;56:3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wormser G, Mckenna D, Aguero-Rosenfeld M, et al. Human granulocytic ehrlichiosis—New York. Morbid Mortal Weekly Rep. 1995;44:593–595. [PubMed] [Google Scholar]