Abstract
Background
This real-life study aimed to assess omalizumab treatment patterns in adult and paediatric asthma patients, and to describe asthma control and healthcare resource use (HCRU) at omalizumab initiation and discontinuation.
Methods
The French healthcare database system (Système National des Données de Santé (SNDS)) was used to identify asthma patients aged ≥6 years who initiated omalizumab for at least 16 weeks from 2009 to 2019. We examined omalizumab treatment patterns using dispensation records.
Results
We identified 16 750 adults and 2453 children initiating omalizumab. Median treatment persistence before discontinuation (TSTOP) was 51.2 (95% CI 49.3–53.4) months in adults and 53.7 (95% CI 50.6–56.4) months in children. At 2 years of omalizumab exposure, rate of hospitalisation for asthma decreased by 75% and use of oral corticosteroids (OCS) by 30%, in adults and children. Among adults who discontinued omalizumab while asthma was controlled, 70%, 39% and 24% remained controlled and did not resume omalizumab at 1, 2 and 3 years after discontinuation, respectively. These proportions were higher in children (76%, 44% and 33%, respectively). Over 2 years of follow-up after discontinuation, HCRU remained stable in adults and children, notably rate of hospitalisations for asthma (none before TSTOP, 1.3% and 0.6% at 2 years) and use of OCS (in adults and children, respectively: 20.0% and 20.2% before TSTOP, 33.3% and 24.6% at 2 years).
Conclusion
This is the first large-scale study describing omalizumab real-life exposure patterns in adult and paediatric asthma patients in France with >10 years of follow-up. We showed the long-term maintenance of low HCRU in adults and children who discontinued omalizumab while asthma was controlled, notably for OCS use and hospitalisations for asthma.
Short abstract
This real-life study shows long-term maintenance of low healthcare resource use in adult and paediatric asthma patients who discontinued omalizumab therapy while asthma was controlled https://bit.ly/3kzWH2d
Introduction
Although real-life studies have provided valuable information regarding omalizumab therapy in severe asthmatic patients, questions remain about the optimal duration of omalizumab treatment [1–6]. While some studies have shown that most patients maintained asthma control after omalizumab discontinuation, others have shown a loss of asthma control [4, 7, 8]. It is to date unknown whether the beneficial effect of omalizumab in reducing asthma exacerbations and in healthcare resource use (HCRU) in real-life is maintained after treatment discontinuation, nor of what duration. Finally, available real-life data mainly concern adult patients; less data are available for children, and it is unclear whether omalizumab effectiveness and exposure patterns in real-life differ between the two populations.
Health administrative databases are valuable sources of data to examine drug exposure patterns and quantitatively assess HCRU in real-life [9, 10]. The first step to address the aforementioned questions is to describe treatment patterns in real-life over a long-term follow-up identifying long-term discontinuations, which can be considered as intentional, as opposed to temporary or short discontinuations currently described in the literature (gap ≥90 days between omalizumab administrations or discontinuation ≥60 days [11, 12]). In this context, using the French healthcare database system (Système National des Données de Santé (SNDS)), this study aimed to assess omalizumab treatment persistence, from initiation to long-term discontinuation, in adult and paediatric asthma patients in a real-world setting, and to describe asthma control at treatment initiation and discontinuation. We also provide a longitudinal dynamic analysis of HCRU around omalizumab initiation and discontinuation, including drugs, medical and paramedical visits, and hospitalisations.
Material and methods
Source of data
The SNDS gathers, at the individual level, information about reimbursed expenditure for outpatient and inpatient care, and reasons for hospital admissions (International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10)), with dates and related costs [13]. To date, the SNDS covers ∼99% of the French population [13]. It is an invaluable tool for exploring real-life HCRU in France [13–15], including asthma HRCU [16, 17].
The study was approved by the National Expertise Committee (CEREES; 1294482) and the French Personal Data Protection Agency (CNIL; AR-203-292). The data processing company (RCTs, Lyon, France) complies with the French Personal Data Protection Agency criteria for access to the SNDS (RERC181009).
Study population
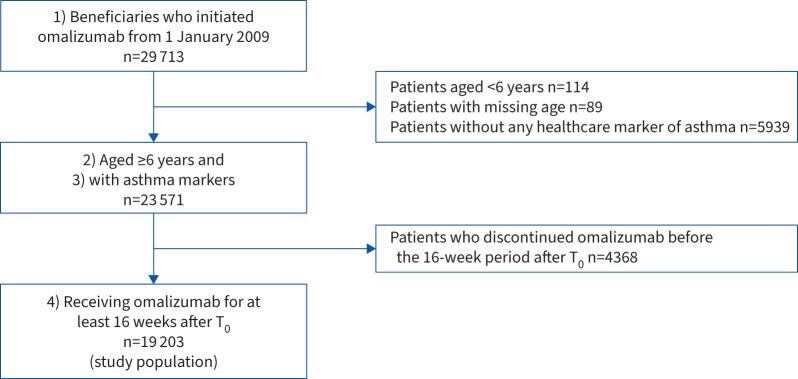
The study population included beneficiaries available in the SNDS 1) who initiated treatment with omalizumab from January 2009 to December 2019, 2) were aged ≥6 years at initiation, 3) with HCRU markers of asthma (to exclude omalizumab indication for chronic urticaria, another approved indication of omalizumab therapy during that time period; see supplementary appendix S2 [18–20]), and 4) who initiated omalizumab for a minimal cumulative exposure period of 16 weeks over 20 weeks from initiation (16 weeks after initiating omalizumab therapy corresponding to the time-point at which treatment effectiveness is assessed before further omalizumab injections are administered, as stated in the summary of product characteristics [21]). In this way, we aimed to include only those patients likely to benefit from omalizumab therapy and for whom evaluating the treatment patterns is relevant.
Study design and settings
This was a nationwide drug utilisation study based on secondary data. The index date T0 corresponded to the first dispensation of omalizumab (supplementary appendix S1). Exposure patterns were described from T0 to 31 December 2019 or the patient's death.
Sociodemographic characteristics were described at T0. Treatment history was assessed over the year before T0. Comorbid conditions were determined using mapping algorithms based on the 3-year history of reimbursed healthcare expenditure before T0 (supplementary appendix S2). Asthma control (i.e. absence of asthma exacerbation markers) and the use of Global Initiative for Asthma (GINA) Step 4–5 treatments, as a proxy for asthma severity, were assessed based on reimbursed expenditure for the year preceding T0 (supplementary appendix S2).
HCRU around omalizumab initiation was assessed in patients who initiated omalizumab from 2012 to ensure exhaustivity for inpatient visits. They were examined from the year preceding T0 to 2 years after T0, per 6-month sequence, among which only patients who had an available follow-up and were treated by omalizumab for the overall sequence were included. HCRU around omalizumab discontinuation (TSTOP) was assessed similarly, from the year preceding TSTOP to 2 years after TSTOP.
Exposure to omalizumab
Exposure patterns were modelled individually based on the dynamics of the dispensing dates and the number and nature of the box(es) [22]. The following assumptions were made: 1) the period covered by one omalizumab dispensation was 30 days and 2) the minimal period without any omalizumab dispensation to define a long-term discontinuation was 1 year (supplementary appendix S3). Indicators to describe omalizumab exposure patterns were treatment persistence and the medication possession ratio (MPR) as a proxy for adherence. Omalizumab treatment persistence was defined as the duration from T0 to the first long-term discontinuation, thus corresponding to the first continuous therapy sequence of omalizumab [23]. The MPR was defined as the ratio of number of days covered with quantity of omalizumab dispensed by the duration of time from T0 to long-term discontinuation; a MPR >80% indicated adherence.
Statistical analysis
A Kaplan–Meier method was used to estimate omalizumab treatment persistence; censored patients corresponded to patients whose follow-up period ended, or who died, before any long-term discontinuation. The probability to pursue omalizumab, i.e. to be exposed within the first continuous therapy sequence before any long-term discontinuation, was calculated, for each year, with its 95% confidence intervals using the log-log transformation. No replacement of missing values was performed; no codes indicate that data is missing in the SNDS. Analyses were performed using SAS version 9.4.6.0 (SAS Institute, Cary, NC, USA).
Results
Study population
In total, 19 203 patients met all the selection criteria, including 16 750 adults and 2453 children (figure 1). The sociodemographic characteristics, comorbid conditions and treatment history are presented in table 1.
FIGURE 1.
Patient selection flowchart. T0: omalizumab initiation.
TABLE 1.
Patient’s sociodemographic characteristics at omalizumab initiation (T0), medical history (3-year period before T0) and treatment history (year before T0)
| Adults | Children | |
| Patients | 16 750 | 2453 |
| Age (years) | 52.1±15.6 | 11.9±3.2 |
| Age group (years) | ||
| [6–12[ | 1072 (43.7) | |
| [12–18[ | 1381 (56.3) | |
| [18–65[ | 12 861 (76.8) | |
| ≥65 | 3889 (23.2) | |
| Female | 10 331 (61.7) | 1004 (40.9) |
| CMUc status | 1597 (9.5) | 473 (19.3) |
| Adjusted Charlson Comorbidity Index# | ||
| 1 or 2 | 9235 (55.1) | 2395 (97.6) |
| 3 or 4 | 4338 (25.9) | 41 (1.7) |
| ≥5 | 3177 (19.0) | 17 (0.7) |
| Comorbid conditions of interest | ||
| Disease of the digestive system | 7678 (45.8) | 286 (11.7) |
| Hypertension | 4987 (29.8) | 13 (0.5) |
| Depressive disorders | 2633 (15.7) | 38 (1.5) |
| Dyslipidaemia | 2423 (14.5) | 0 (0.0) |
| Diabetes | 1552 (9.3) | 16 (0.7) |
| Osteoporosis | 923 (5.5) | 0 (0.0) |
| Asthma characteristics | ||
| GINA Step 4–5 treatments to control asthma¶ | 11 145 (66.5) | 1141 (46.5) |
| High-dose ICS with (LABA or LAMA or LTRA or theophylline) | 7025 (41.9) | 693 (28.3) |
| ≥6 dispensations of OCS ≥5 mg daily with ≥1 ATC R03 drugs | 4771 (28.5) | 356 (14.5) |
| ≥6 dispensations of medium- or high-dose ICS with LABA plus ≥2 short courses of OCS | 9014 (53.8) | 844 (34.4) |
| Uncontrolled asthma¶ | 13 752 (82.1) | 2059 (83.9) |
| ≥2 short courses of OCS | 12 287 (73.4) | 1746 (71.2) |
| ≥1 hospital admission for asthma | 3873 (23.1) | 1019 (41.5) |
| ≥6 dispensations of SABA | 8308 (49.6) | 1277 (52.1) |
| Asthma treatments, alone or in association, ≥3 dispensations | ||
| ICS | 14 216 (84.9) | 2114 (86.2) |
| LABA | 13 817 (82.5) | 1928 (78.6) |
| LAMA | 4173 (24.9) | 31 (1.3) |
| SABA | 11 771 (70.3) | 1968 (80.2) |
| SAMA | 3258 (19.5) | 174 (7.1) |
| LTRA | 9198 (54.9) | 1557 (63.5) |
| Theophylline | 727 (4.3) | 40 (1.6) |
| Asthma treatment combination, ≥3 dispensations | ||
| ICS–LABA | 9616 (57.4) | 1891 (77.1) |
| ICS–LABA–LAMA | 4073 (24.3) | 28 (1.1) |
| ICS or ICS–LAMA or ICS–other ATC R03+ drug | 527 (3.1) | 195 (7.9) |
| Other treatments of interest, ≥1 dispensations | ||
| OCS | 14 667 (87.6) | 2141 (87.3) |
| Azithromycin | 2879 (17.2) | 334 (13.6) |
| Antibiotics, including azithromycin (ATC J01) | 14 223 (84.9) | 1739 (70.9) |
| Topical corticosteroids | 4176 (24.9) | 630 (25.7) |
| Nasal corticosteroids | 9841 (58.8) | 1645 (67.1) |
| Nasal antihistamine drugs | 444 (2.7) | 81 (3.3) |
| Systemic antihistamine drugs | 13 336 (79.6) | 2244 (91.5) |
| Epinephrine auto-injectors | 300 (1.8) | 252 (10.3) |
Data are presented as n, mean±sd or n (%). CMUc: Couverture Maladie Universelle Complémentaire (universal medical coverage; allocation that depends on annual income); GINA: Global Initiative for Asthma; ICS: inhaled corticosteroids; LABA: long-acting β2-adrenergic agonists; LAMA: long-acting muscarinic antagonists; LTRA: leukotriene receptor antagonists; OCS: oral corticosteroids; ATC: Anatomical Therapeutic Chemical; SABA: short-acting β2-adrenergic agonists; SAMA: short-acting muscarinic antagonists. #: the Charlson Comorbidity Index was first designed to predict mortality based on a weighted score for ranges of comorbid conditions; in medico-administrative databases, it is a method used to categorise comorbidities of patients based on healthcare resource use. ¶: supplementary appendix S2. +: drugs for obstructive airway diseases.
Omalizumab exposure patterns and HCRU during exposure
The mean±sd overall follow-up period was 59.4±37.0 months in adults and 57.9±36.6 months in children. In total, 960 adults (5.7%) and eight children (0.3%) died during follow-up (242 adults and three children died during omalizumab exposure). Reasons for death are not available in the database, but 574 adults and five children died during a hospital stay, among whom 27 adults and two children had been hospitalised for asthma.
In the study population, 48.3% (n=9282; 48.9% of adults and 44.2% of children) had at least one long-term discontinuation. From the Kaplan–Meier analysis, the median treatment persistence was 51.2 (95% CI 49.3–53.4) months in adults and 53.7 (95% CI 50.6–56.4) months in children (supplementary appendix S4). The probability to pursue omalizumab in adults was 0.78 (95% CI 0.77–0.79), 0.66 (95% CI 0.65–0.67), 0.40 (95% CI 0.39–0.41) and 0.29 (95% CI 0.28–0.30) at 1, 2, 6 and 10 years from T0, respectively. In children, the probabilities were 0.89 (95% CI 0.87–0.90), 0.78 (95% CI 0.76–0.79), 0.31 (95% CI 0.28–0.34) and 0.17 (95% CI 0.12–0.21), respectively. The median (interquartile range (IQR)) MPR over this initial sequence was 99.7% (91.2–100.0%) in adults and 100.0% (92.3–100.0%) in children; 88.3% of adults (n=14 794) and 89.5% of children (n=2196) were adherent (MPR >80%).
Regarding omalizumab sequence (i.e. exposure period uninterrupted by a long-term discontinuation), 94.8% of the patients (n=18 200) had only one exposure sequence (94.7% in adults, n=15 866; 95.1% in children, n=2334), 4.9% had two (5.0% in adults, n=830; 4.5% in children, n=111) and 0.3% had more than two (0.3% in adults, n=54; 0.3% in children, n=8).
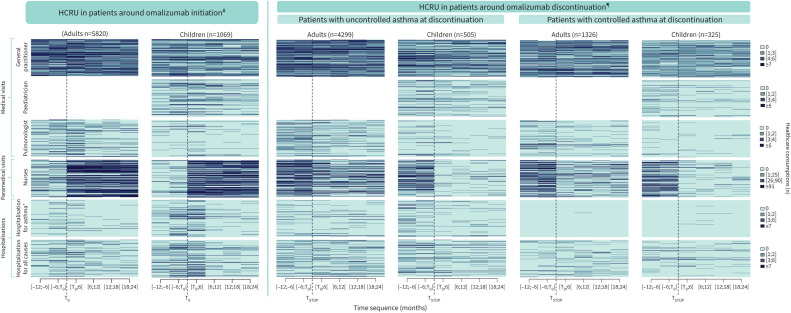
Assessment of HCRU around omalizumab initiation showed a decrease over the 2-year follow-up both in adults and children (table 2 and figure 2). Compared with before initiation ([−6;T0[ months), the number of patients with HCRU had decreased at the end of the follow-up ([18;24[ months) by 73.8% and 76.6%, respectively, in adults and in children, for hospitalisations for asthma, 27.9% and 32.5% for OCS consumption, and 1.0% and 11.6% for inhaled corticosteroids (ICS). A reduction was also observed for mean daily doses of ICS (3.7% and 21.2%, respectively, in adults and children) and of OCS in adults (22.2%) (table 3).
TABLE 2.
Patients who had the main healthcare resource use of interest (at least one consumption) reimbursed by the health insurance, among patients exposed to omalizumab, per time sequence from the year preceding omalizumab initiation (T0) to 2 years after T0
| Time sequence (months ) | ||||||
| [−12;−6[ | [−6;T0[ | [T0;6[ | [6;12[ | [12;18[ | [18;24[ | |
| Patients within the time sequence # | ||||||
| Adults | 12 838 | 12 838 | 11 054 | 8510 | 7004 | 5820 |
| Children | 1964 | 1964 | 1782 | 1489 | 1262 | 1069 |
| Patients with ≥1 reimbursed expenditures | ||||||
| Drugs: omalizumab¶ | ||||||
| Adults | 11 054 (100.0) | 8426 (99.0) | 6956 (99.3) | 5789 (99.5) | ||
| Children | 1782 (100.0) | 1478 (99.3) | 1253 (99.3) | 1064 (99.5) | ||
| Drugs: ATC R03, including ICS | ||||||
| Adults | 11 663 (90.8) | 12 539 (97.7) | 10 189 (92.2) | 7618 (89.5) | 6295 (89.9) | 5274 (90.6) |
| Children | 1835 (93.4) | 1909 (97.2) | 1685 (94.6) | 1358 (91.2) | 1142 (90.5) | 946 (88.5) |
| Drugs: ICS | ||||||
| Adults | 10 630 (82.8) | 11 062 (86.2) | 9366 (84.7) | 7113 (83.6) | 5875 (83.9) | 4965 (85.3) |
| Children | 1675 (85.3) | 1754 (89.3) | 1556 (87.3) | 1252 (84.1) | 1037 (82.2) | 844 (79.0) |
| Drugs: OCS | ||||||
| Adults | 8604 (67.0) | 9673 (75.3) | 6554 (59.3) | 4750 (55.8) | 3778 (53.9) | 3161 (54.3) |
| Children | 1323 (67.4) | 1437 (73.2) | 1026 (57.6) | 775 (52.0) | 637 (50.5) | 528 (49.4) |
| Drugs: azithromycin | ||||||
| Adults | 1355 (10.6) | 1658 (12.9) | 1208 (10.9) | 877 (10.3) | 710 (10.1) | 592 (10.2) |
| Children | 173 (8.8) | 196 (10.0) | 151 (8.5) | 108 (7.3) | 77 (6.1) | 70 (6.5) |
| Drugs: antibiotics (ATC J01), excluding azithromycin | ||||||
| Adults | 8206 (63.9) | 8520 (66.4) | 6140 (55.5) | 4887 (57.4) | 3911 (55.8) | 3258 (56.0) |
| Children | 933 (47.5) | 987 (50.3) | 720 (40.4) | 599 (40.2) | 472 (37.4) | 415 (38.8) |
| Drugs: antihistamine drugs | ||||||
| Adults | 8560 (66.7) | 9343 (72.8) | 7408 (67.0) | 5398 (63.4) | 4369 (62.4) | 3521 (60.5) |
| Children | 1579 (80.4) | 1659 (84.5) | 1401 (78.6) | 1084 (72.8) | 889 (70.4) | 721 (67.4) |
| Medical visits: general practitioner | ||||||
| Adults | 11 763 (91.6) | 11 939 (93.0) | 10 020 (90.6) | 7673 (90.2) | 6275 (89.6) | 5229 (89.8) |
| Children | 1604 (81.7) | 1640 (83.5) | 1396 (78.3) | 1167 (78.4) | 973 (77.1) | 846 (79.1) |
| Medical visits: paediatrician | ||||||
| Children | 689 (35.1) | 809 (41.2) | 704 (39.5) | 492 (33.0) | 384 (30.4) | 296 (27.7) |
| Medical visits: pulmonologist | ||||||
| Adults | 3229 (25.2) | 5401 (42.1) | 5259 (47.6) | 2748 (32.3) | 2180 (31.1) | 1692 (29.1) |
| Children | 164 (8.4) | 270 (13.7) | 305 (17.1) | 132 (8.9) | 108 (8.6) | 94 (8.8) |
| Paramedical visits: nurses | ||||||
| Adults | 5343 (41.6) | 6753 (52.6) | 8600 (77.8) | 6564 (77.1) | 5350 (76.4) | 4441 (76.3) |
| Children | 312 (15.9) | 546 (27.8) | 1369 (76.8) | 1131 (76.0) | 919 (72.8) | 785 (73.4) |
| Hospitalisations: for all causes | ||||||
| Adults | 3747 (29.2) | 5128 (39.9) | 3923 (35.5) | 2034 (23.9) | 1641 (23.4) | 1360 (23.4) |
| Children | 614 (31.3) | 929 (47.3) | 883 (49.6) | 342 (23.0) | 280 (22.2) | 233 (21.8) |
| Hospitalisations: for asthma+ | ||||||
| Adults | 956 (7.4) | 2214 (17.2) | 1764 (16.0) | 403 (4.7) | 311 (4.4) | 262 (4.5) |
| Children | 344 (17.5) | 662 (33.7) | 662 (37.1) | 163 (10.9) | 120 (9.5) | 84 (7.9) |
| Sick leave | ||||||
| Adults | 1871 (14.6) | 2083 (16.2) | 1561 (14.1) | 1085 (12.7) | 804 (11.5) | 617 (10.6) |
Data are presented as n or n (%). ATC: Anatomical Therapeutic Chemical; ICS: inhaled corticosteroids; OCS: oral corticosteroids. #: subjects had to have a follow-up covering the entire time sequence to be accounted in a time sequence; ¶: subjects without any dispensation of omalizumab within a time sequence were in temporary discontinuation; +: as primary or related diagnosis.
FIGURE 2.
Index plot of healthcare resource use (HCRU) per semester around omalizumab initiation (T0) (from the year preceding T0 to 2 years after T0) and around omalizumab discontinuation (TSTOP) (from the year preceding TSTOP to 2 years after TSTOP). Index plots are presented per population, per nature of healthcare and per time sequence (months) at the individual level, for each subject of the population. Only patients with a complete follow-up up to 2 years after TSTOP are presented. HCRU for a given patient is represented on the same line on the overall follow-up period. The ordination of patients from an upper line to a lower line is the same for all healthcare within the same population. #: subset of patients with a 2-year follow-up available after T0 and exposed to omalizumab at 2 years; ¶: subset of patients with a 2-year follow-up available after TSTOP; +: as primary or related diagnosis.
TABLE 3.
Main daily dose of oral corticosteroids (OCS) and inhaled corticosteroids (ICS) from dispensations reimbursed by the health insurance, among patients exposed to omalizumab, per time sequence from the year preceding omalizumab initiation (T0) to 2 years after T0
| Time sequence (months) | ||||||
| [−12;−6[ | [−6;T0[ | [T0;6[ | [6;12[ | [12;18[ | [18;24[ | |
| Patients within the time sequence # | ||||||
| Adults | 12 838 | 12 838 | 11 054 | 8510 | 7004 | 5820 |
| Children | 1964 | 1964 | 1782 | 1489 | 1262 | 1069 |
| Daily dose of ICS | ||||||
| Adults | ||||||
| Patients with ≥1 dispensations# | 10 630 (82.8) | 11 062 (86.2) | 9366 (84.7) | 7113 (83.6) | 5875 (83.9) | 4965 (85.3) |
| Daily dose (µg·day−1)¶ | 1391.0±1011.2 | 1507.7±1050.5 | 1575.1±1117.1 | 1489.6±1075.1 | 1467.9±1040.9 | 1451.4±1045.8 |
| Children | ||||||
| Patients with ≥1 dispensations# | 1675 (85.3) | 1754 (89.3) | 1556 (87.3) | 1252 (84.1) | 1037 (82.2) | 844 (79.0) |
| Daily dose (µg·day−1)¶ | 608.5±511.6 | 718.9±575.1 | 680.1±559.2 | 582.0±502.0 | 544.6±491.5 | 528.9±491.3 |
| Daily dose of OCS + | ||||||
| Adults | ||||||
| Patients with ≥1 dispensations# | 8604 (67.0) | 9673 (75.3) | 6554 (59.3) | 4750 (55.8) | 3778 (53.9) | 3161 (54.3) |
| Daily dose (mg·day−1)¶ | 7.6±7.4 | 9.0±8.1 | 8.6±8.8 | 7.3±7.4 | 7.3±7.4 | 7.0±7.1 |
Data are presented as n, n (%) or mean±sd. Daily dose corresponds to the sum of ICS or OCS doses dispensed over a time sequence, divided by the number of days in the time sequence. ICS dose has been converted to beclomethasone (chlorofluorocarbon)-equivalent, based on Global Initiative for Asthma 2019 [24], and OCS to prednisone-equivalent [25, 26]. #: subjects had to have a follow-up covering the entire time sequence to be accounted in a time sequence; ¶: among subjects with at least one dispensation; +: OCS mean daily dose is not given for children considering that OCS is not a continuous treatment for this population.
Asthma control and HCRU in patients who discontinued omalizumab
Care pathways after omalizumab long-term discontinuation (TSTOP) were assessed in 7761 adults and 1082 children who had at least a 1-year follow-up period after long-term discontinuation; among them, 1829 adults and 442 children had no markers of uncontrolled asthma before discontinuation and were therefore considered to be controlled. Mean±sd age at discontinuation was similar whether asthma was controlled or not at discontinuation (respectively, 51.3±16.5 and 53.9±15.5 years for adults and 15.5±3.2 and 14.5±3.4 years for children). Median (IQR) treatment persistence in adults and children before discontinuation was, respectively, 18.2 (29.4) and 36.8 (33.0) months if asthma was controlled at discontinuation and 12.8 (23.8) and 21.4 (32.1) months if uncontrolled asthma.
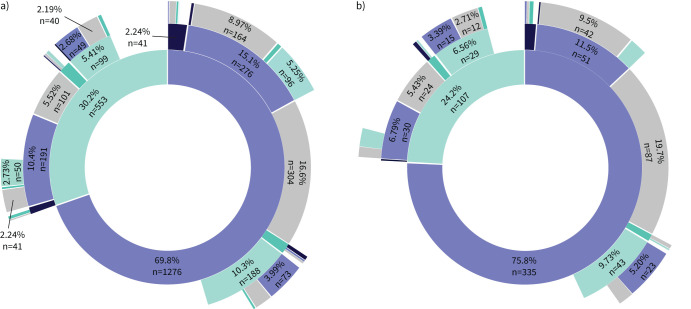
In patients with controlled asthma at discontinuation, 55.4% of adults (n=1013) and 69.9% of children (n=309) had been uncontrolled at T0 (versus 82.1% in adults and 83.9% in children at T0 for the total cohort of omalizumab treatment initiators) (table 1); 43.2% (n=791) and 36.2% (n=160), respectively, used GINA Step 4–5 treatments (versus 66.5% in adults and 46.5% in children for the overall cohort) (table 1 and supplementary appendix S5). At 1, 2 and 3 years after discontinuation, 69.8% (n=1276), 39.4% (n=720) and 24.3% (n=444) of adults, respectively, remained controlled without resuming omalizumab; follow-up ended before 3 years for 38.9% of patients (figure 3a). Among the children, that was 75.8% (n=335), 44.1% (n=195) and 32.6% (n=144), respectively; follow-up ended before 3 years for 40.7% (figure 3b). Few uncontrolled adults and children received other biotherapies after discontinuation (table 4).
FIGURE 3.
Sunburst charts of asthma control and omalizumab exposure in patients who had long-term discontinuation and had at least 1 year of follow-up after discontinuation, over a 3-year follow-up after discontinuation in a) adults (n=1829) and b) children (n=442). Patients have been categorised in four states at 1 year (first layer from the centre), 2 years (second layer) and 3 years (third layer) after omalizumab discontinuation: controlled, and unexposed to omalizumab (purple) or having resumed omalizumab (black), or uncontrolled, and unexposed to omalizumab (light green) or having resumed omalizumab (dark green). The innermost ring marks the state at 1 year after discontinuation, and the second and third concentric rings moving outward give the states from the cohort at 2 and 3 years after discontinuation. Patients with no more change of state during the continuation of the follow-up remain in the inferior ring. Patients whose follow-up has ended are represented in grey. Only sample sizes of >2% are presented.
TABLE 4.
Patients who had the main healthcare resource use of interest (at least one consumption) reimbursed by the health insurance, among patients exposed to omalizumab, per time sequence from the year preceding omalizumab discontinuation (TSTOP) to 2 years after TSTOP
| Time sequence (months ) | ||||||
| [−12;−6[ | [−6;TSTOP[ | [TSTOP;6[ | [6;12[ | [12;18[ | [18;24[ | |
| Patients within the time sequence # | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 5432 | 5432 | 5432 | 5432 | 4904 | 4299 |
| Controlled asthma at TSTOP | 1744 | 1744 | 1744 | 1744 | 1535 | 1326 |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 604 | 604 | 604 | 604 | 560 | 505 |
| Controlled asthma at TSTOP | 436 | 436 | 436 | 436 | 385 | 325 |
| Patients with ≥1 reimbursed expenditures | ||||||
| Drugs: omalizumab | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 4291 (79.0) | 5432 (100.0) | 0 (0.0) | 0 (0.0) | 197 (4.0) | 250 (5.8) |
| Controlled asthma at TSTOP | 1487 (85.3) | 1744 (100.0) | 0 (0.0) | 0 (0.0) | 75 (4.9) | 91 (6.9) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 536 (88.7) | 604 (100.0) | 0 (0.0) | 0 (0.0) | 33 (5.9) | 32 (6.3) |
| Controlled asthma at TSTOP | 417 (95.6) | 436 (100.0) | 0 (0.0) | 0 (0.0) | 12 (3.1) | 13 (4.0) |
| Drugs: ATC R03, including ICS | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 5348 (98.5) | 5342 (98.3) | 5122 (94.3) | 5112 (94.1) | 4585 (93.5) | 4023 (93.6) |
| Controlled asthma at TSTOP | 1497 (85.8) | 1435 (82.3) | 1356 (77.8) | 1326 (76.0) | 1180 (76.9) | 1062 (80.1) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 576 (95.4) | 567 (93.9) | 516 (85.4) | 510 (84.4) | 462 (82.5) | 413 (81.8) |
| Controlled asthma at TSTOP | 347 (79.6) | 318 (72.9) | 298 (68.3) | 295 (67.7) | 255 (66.2) | 218 (67.1) |
| Drugs: ICS | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 5149 (94.8) | 5146 (94.7) | 4907 (90.3) | 4863 (89.5) | 4368 (89.1) | 3835 (89.2) |
| Controlled asthma at TSTOP | 1257 (72.1) | 1243 (71.3) | 1210 (69.4) | 1180 (67.7) | 1067 (69.5) | 967 (72.9) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 532 (88.1) | 513 (84.9) | 465 (77.0) | 447 (74.0) | 410 (73.2) | 370 (73.3) |
| Controlled asthma at TSTOP | 296 (67.9) | 265 (60.8) | 240 (55.0) | 250 (57.3) | 212 (55.1) | 174 (53.5) |
| Drugs: OCS | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 4422 (81.4) | 4379 (80.6) | 3870 (71.2) | 3814 (70.2) | 3351 (68.3) | 2870 (66.8) |
| Controlled asthma at TSTOP | 341 (19.6) | 348 (20.0) | 572 (32.8) | 558 (32.0) | 472 (30.7) | 441 (33.3) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 452 (74.8) | 428 (70.9) | 352 (58.3) | 355 (58.8) | 329 (58.8) | 288 (57.0) |
| Controlled asthma at TSTOP | 75 (17.2) | 88 (20.2) | 132 (30.3) | 135 (31.0) | 115 (29.9) | 80 (24.6) |
| Drugs: other biotherapies¶ | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 16 (0.3) | 72 (1.3) | 68 (1.4) | 39 (0.9) |
| Controlled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 3 (0.2) | 9 (0.5) | 5 (0.3) | 3 (0.2) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 5 (0.9) | 0 (0.0) |
| Controlled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Drugs: azithromycin | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 769 (14.2) | 882 (16.2) | 839 (15.4) | 835 (15.4) | 748 (15.3) | 630 (14.7) |
| Controlled asthma at TSTOP | 105 (6.0) | 96 (5.5) | 115 (6.6) | 106 (6.1) | 85 (5.5) | 93 (7.0) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 57 (9.4) | 60 (9.9) | 40 (6.6) | 48 (7.9) | 38 (6.8) | 41 (8.1) |
| Controlled asthma at TSTOP | 18 (4.1) | 19 (4.4) | 15 (3.4) | 16 (3.7) | 12 (3.1) | 8 (2.5) |
| Drugs: antibiotics (ATC J01), excluding azithromycin | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 3815 (70.2) | 3717 (68.4) | 3439 (63.3) | 3391 (62.4) | 3003 (61.2) | 2588 (60.2) |
| Controlled asthma at TSTOP | 703 (40.3) | 727 (41.7) | 830 (47.6) | 783 (44.9) | 653 (42.5) | 586 (44.2) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 317 (52.5) | 277 (45.9) | 267 (44.2) | 275 (45.5) | 240 (42.9) | 217 (43.0) |
| Controlled asthma at TSTOP | 116 (26.6) | 139 (31.9) | 125 (28.7) | 136 (31.2) | 111 (28.8) | 105 (32.3) |
| Drugs: antihistamine drugs | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 3359 (61.8) | 3329 (61.3) | 3144 (57.9) | 3154 (58.1) | 2778 (56.6) | 2437 (56.7) |
| Controlled asthma at TSTOP | 1028 (58.9) | 997 (57.2) | 993 (56.9) | 924 (53.0) | 803 (52.3) | 682 (51.4) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 453 (75.0) | 439 (72.7) | 384 (63.6) | 395 (65.4) | 349 (62.3) | 331 (65.5) |
| Controlled asthma at TSTOP | 233 (53.4) | 220 (50.5) | 206 (47.2) | 222 (50.9) | 188 (48.8) | 153 (47.1) |
| Medical visits: general practitioner | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 5025 (92.5) | 4988 (91.8) | 4932 (90.8) | 4864 (89.5) | 4388 (89.5) | 3831 (89.1) |
| Controlled asthma at TSTOP | 1513 (86.8) | 1497 (85.8) | 1499 (86.0) | 1480 (84.9) | 1310 (85.3) | 1117 (84.2) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 509 (84.3) | 497 (82.3) | 469 (77.6) | 475 (78.6) | 435 (77.7) | 371 (73.5) |
| Controlled asthma at TSTOP | 320 (73.4) | 306 (70.2) | 317 (72.7) | 316 (72.5) | 271 (70.4) | 220 (67.7) |
| Medical visits: paediatrician | ||||||
| Children | ||||||
| Uncontrolled asthma at TSTOP | 200 (33.1) | 192 (31.8) | 142 (23.5) | 139 (23.0) | 109 (19.5) | 86 (17.0) |
| Controlled asthma at TSTOP | 106 (24.3) | 82 (18.8) | 51 (11.7) | 48 (11.0) | 29 (7.5) | 21 (6.5) |
| Medical visits: pulmonologist | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 2478 (45.6) | 2587 (47.6) | 1930 (35.5) | 1604 (29.5) | 1359 (27.7) | 1089 (25.3) |
| Controlled asthma at TSTOP | 517 (29.6) | 464 (26.6) | 315 (18.1) | 255 (14.6) | 210 (13.7) | 186 (14.0) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 94 (15.6) | 104 (17.2) | 60 (9.9) | 52 (8.6) | 40 (7.1) | 38 (7.5) |
| Controlled asthma at TSTOP | 43 (9.9) | 36 (8.3) | 25 (5.7) | 16 (3.7) | 15 (3.9) | 10 (3.1) |
| Paramedical visits: nurses | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 3634 (66.9) | 4036 (74.3) | 2790 (51.4) | 2746 (50.6) | 2401 (49.0) | 2097 (48.8) |
| Controlled asthma at TSTOP | 1143 (65.5) | 1225 (70.2) | 710 (40.7) | 705 (40.4) | 582 (37.9) | 534 (40.3) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 356 (58.9) | 387 (64.1) | 134 (22.2) | 105 (17.4) | 102 (18.2) | 92 (18.2) |
| Controlled asthma at TSTOP | 265 (60.8) | 258 (59.2) | 67 (15.4) | 61 (14.0) | 56 (14.5) | 42 (12.9) |
| Hospitalisations: for all causes | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 1996 (36.7) | 2077 (38.2) | 1919 (35.3) | 1806 (33.2) | 1473 (30.0) | 1306 (30.4) |
| Controlled asthma at TSTOP | 312 (17.9) | 323 (18.5) | 331 (19.0) | 354 (20.3) | 290 (18.9) | 249 (18.8) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 217 (35.9) | 188 (31.1) | 150 (24.8) | 145 (24.0) | 117 (20.9) | 104 (20.6) |
| Controlled asthma at TSTOP | 61 (14.0) | 45 (10.3) | 50 (11.5) | 56 (12.8) | 51 (13.2) | 38 (11.7) |
| Hospitalisations: for asthma+ | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 886 (16.3) | 862 (15.9) | 649 (11.9) | 515 (9.5) | 399 (8.1) | 339 (7.9) |
| Controlled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 16 (0.9) | 30 (1.7) | 19 (1.2) | 17 (1.3) |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 139 (23.0) | 111 (18.4) | 59 (9.8) | 63 (10.4) | 46 (8.2) | 34 (6.7) |
| Controlled asthma at TSTOP | 0 (0.0) | 0 (0.0) | 5 (1.1) | 10 (2.3) | 10 (2.6) | 2 (0.6) |
| Sick leave | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 804 (14.8) | 792 (14.6) | 738 (13.6) | 686 (12.6) | 545 (11.1) | 473 (11.0) |
| Controlled asthma at TSTOP | 189 (10.8) | 198 (11.4) | 189 (10.8) | 194 (11.1) | 163 (10.6) | 114 (8.6) |
Data are presented as n or n (%). ATC: Anatomical Therapeutic Chemical; ICS: inhaled corticosteroids; OCS: oral corticosteroids. #: subjects had to have a follow-up covering the entire time sequence to be accounted in a time sequence; ¶: reslizumab, mepolizumab, benralizumab, dupilumab; +: as primary or related diagnosis.
At the end of omalizumab exposure ([−6;TSTOP[), HCRU in patients with controlled asthma at TSTOP was lower than in those with uncontrolled asthma, for all studied items (tables 4 and 5, and figure 2). In addition, HCRU before TSTOP in adults and children with controlled asthma at TSTOP was lower than HCRU before T0 ([−6;T0[) observed in the overall cohort of omalizumab treatment initiators, for all items except nursing care, in line with omalizumab administration (tables 2 and 3). Rates of OCS users before T0 were 75.3% and 73.2%, respectively, for adults and children; they were 20.0% and 20.2%, respectively, before TSTOP in adults and children with controlled asthma. OCS mean daily doses in adults were 9.0±8.1 mg day−1 before T0 and 2.5±1.2 mg day−1 before TSTOP. The longitudinal analysis of HCRU after discontinuation showed a global rate maintenance, both in adults and children with controlled asthma at TSTOP (tables 4 and 5, figure 2, and supplementary appendix S6). Notably, rates of OCS users in adults before TSTOP and at 2 years after discontinuation were, respectively, 20.0% and 33.3%; rates in children were, respectively, 20.2% and 24.6%. OCS mean daily doses in adults were 2.5±1.2 mg·day−1 before TSTOP and 4.3±3.5 mg day−1 at 2 years. Lastly, rates of hospitalisation for asthma at 2 years were 1.3% in adults and 0.6% in children (versus none before TSTOP).
TABLE 5.
Main daily dose of oral corticosteroids (OCS) and inhaled corticosteroids (ICS) from dispensations reimbursed by the health insurance, among patients exposed to omalizumab, per time sequence from the year preceding omalizumab discontinuation (TSTOP) to 2 years after TSTOP
| Time sequence (months ) | ||||||
| [−12;−6[ | [−6;TSTOP[ | [TSTOP;6[ | [6;12[ | [12;18[ | [18;24[ | |
| Patients within the time sequence # | ||||||
| Adults | ||||||
| Uncontrolled asthma at TSTOP | 5432 | 5432 | 5432 | 5432 | 4904 | 4299 |
| Controlled asthma at TSTOP | 1744 | 1744 | 1744 | 1744 | 1535 | 1326 |
| Children | ||||||
| Uncontrolled asthma at TSTOP | 604 | 604 | 604 | 604 | 560 | 505 |
| Controlled asthma at TSTOP | 436 | 436 | 436 | 436 | 385 | 325 |
| Daily dose of ICS | ||||||
| Adults | ||||||
| Patients with ≥1 dispensations# | ||||||
| Uncontrolled asthma at TSTOP | 5149 (94.8) | 5146 (94.7) | 4907 (90.3) | 4863 (89.5) | 4368 (89.1) | 3835 (89.2) |
| Controlled asthma at TSTOP | 1257 (72.1) | 1243 (71.3) | 1210 (69.4) | 1180 (67.7) | 1067 (69.5) | 967 (72.9) |
| Daily dose (µg·day−1)¶ | ||||||
| Uncontrolled asthma at TSTOP | 1623.6±1133.6 | 1629.0±1119.8 | 1568.6±1118.3 | 1558.4±1096.0 | 1538.0±1070.9 | 1522.6±1082.8 |
| Controlled asthma at TSTOP | 1086.4±861.7 | 1084.5±830.4 | 1064.7±814.2 | 1072.6±817.2 | 1092.5±840.7 | 1082.9±864.3 |
| Children | ||||||
| Patients with ≥1 dispensations# | ||||||
| Uncontrolled asthma at TSTOP | 532 (88.1) | 513 (84.9) | 465 (77.0) | 447 (74.0) | 410 (73.2) | 370 (73.3) |
| Controlled asthma at TSTOP | 296 (67.9) | 265 (60.8) | 240 (55.0) | 250 (57.3) | 212 (55.1) | 174 (53.5) |
| Daily dose (µg·day−1)¶ | ||||||
| Uncontrolled asthma at TSTOP | 730.5±661.0 | 764.6±666.1 | 672.2±656.0 | 714.9±682.4 | 696.3±631.1 | 688.4±556.0 |
| Controlled asthma at TSTOP | 421.5±315.8 | 422.2±336.0 | 456.7±412.1 | 507.6±445.7 | 471.8±392.8 | 526.8±414.9 |
| Daily dose of OCS+ | ||||||
| Adults | ||||||
| Patients with ≥1 dispensations# | ||||||
| Uncontrolled asthma at TSTOP | 4422 (81.4) | 4379 (80.6) | 3870 (71.2) | 3814 (70.2) | 3351 (68.3) | 2870 (66.8) |
| Controlled asthma at TSTOP | 341 (19.6) | 348 (20.0) | 572 (32.8) | 558 (32.0) | 472 (30.7) | 441 (33.3) |
| Daily dose (µg·day−1)¶ | ||||||
| Uncontrolled asthma at TSTOP | 9.6±9.2 | 10.2±9.5 | 10.6±10.4 | 10.0±9.7 | 9.6±9.6 | 9.8±9.8 |
| Controlled asthma at TSTOP | 2.4±0.9 | 2.5±1.2 | 3.9±4.0 | 4.1±3.7 | 4.2±3.4 | 4.3±3.5 |
Data are presented as n, n (%) or mean±sd. Daily dose corresponds to the sum of ICS or OCS doses dispensed over a time sequence, divided by the number of days in the time sequence. ICS dose has been converted to beclomethasone (chlorofluorocarbon)-equivalent, based on Global Initiative for Asthma 2019 [24], and OCS to prednisone-equivalent [25, 26]. #: subjects had to have a follow-up covering the entire time sequence to be accounted in a time sequence; ¶: among subjects with at least one dispensation; +: OCS mean daily dose is not given for children considering that OCS is not a continuous treatment for this population.
HCRU before TSTOP in children with uncontrolled asthma at TSTOP was also lower than before T0 for the overall cohort of children who initiated omalizumab, substantially for hospitalisations for asthma (33.7% and 18.4%, respectively, before T0 and TSTOP) and for drug use, except for ICS daily doses (tables 2–5). In adults with uncontrolled asthma at TSTOP, although drug use was higher before TSTOP compared with before T0 in the overall cohort, rate of hospitalisations for asthma was lower (17.2% before T0, 15.9% before TSTOP). The longitudinal analysis of HCRU up to 2 years after discontinuation also showed a global rate maintenance in patients with uncontrolled asthma at TSTOP (tables 4 and 5, figure 2, and supplementary appendix S6).
In terms of mortality, 2.8% of adults with controlled asthma at TSTOP (n=51) died at follow-up versus 6.7% of adults with uncontrolled asthma at TSTOP (n=398). 35 out of 51 deaths in adults with controlled asthma at TSTOP occurred during a hospitalisation (68.6%) versus 240 out of 398 deaths in adults with uncontrolled asthma at TSTOP (60.3%). None of the 35 hospitalisations that resulted in death were due to asthma in adults with controlled asthma at TSTOP, while 10 out of the 240 hospitalisations that resulted in death in adults with uncontrolled asthma at TSTOP were asthma hospitalisations (4.2%). No death at follow-up was identified in children with controlled asthma at TSTOP, while six deaths occurred in children with uncontrolled asthma at TSTOP (0.9%), including four during hospitalisation, one of which was due to asthma.
Discussion
Our study constitutes the first description of omalizumab real-life exposure patterns in both adults (n=16 750) and children (n=2453), based on the SNDS database, with >10 years of follow-up. This cohort of asthma patients represents the largest ever described in the literature. The SNDS database covers nearly all asthma patients in France, ensuring a comprehensive unbiased assessment. This real-life study focuses on omalizumab exposure and long-term discontinuations, and provides information on asthma control persistence after discontinuation. Lastly, it describes changes in HCRU in patients treated with omalizumab and in patients who discontinued omalizumab, before and after initiation and discontinuation, focusing on healthcare resources related to asthma including medicines and hospitalisations considered as proxies of treatment impact and benefits.
Omalizumab exposure patterns and HCRU during exposure
In our population, approximately half of the adults and children (49% and 44%, respectively) had at least one omalizumab long-term discontinuation, with a median treatment persistence before discontinuation of 4.5 years (51 months in adults and 54 months in children). Most patients were adherent: 88% of adults and 89% of children had a MPR >80%. Over two-thirds of patients (66% of adults and 78% of children) continued omalizumab treatment at 2 years and more than one-third were still on omalizumab treatment at 6 years (40% in adults and 31% in children). Very few patients (<5%) had more than one exposure sequence to omalizumab.
Other real-life studies have also recently examined omalizumab patterns of exposure in current practice [3, 5, 6, 11, 12, 27–29]. Two of these were performed using reimbursed healthcare expenditure databases and defined omalizumab discontinuation as a gap in use ≥90 days [11, 12]. However, comparison with these studies is impossible since our study was the first to define long-term discontinuation as a gap in use ≥1 year rather than ≥90 days, as such long-term discontinuations are more likely to be intentional.
We captured HCRU in real-life before and after omalizumab initiation. Apart from nursing care, that probably reflects visits for omalizumab administration, we observed a decrease in the assessed HCRU following omalizumab initiation and over time, both in adults and children. This was particularly pronounced for hospitalisations for asthma (75% reduction at 2 years) and OCS use both in terms of number of use (30% reduction) as well as of daily doses in adults (20% reduction), in line with the literature [30]. This considerable decrease in HCRU may be attributed to the direct and indirect beneficial effects of omalizumab, including better asthma control in responders.
Asthma control and HCRU in patients who discontinued omalizumab
Several clinical trials [31–33] and small real-life studies [27–29] have demonstrated that omalizumab decreases the incidence of asthma exacerbations. A recent randomised, double-blind, placebo-controlled, clinical trial (XPORT) also evaluated the persistence of omalizumab benefits in adult asthma patients withdrawing from long-term omalizumab treatment compared with patients continuing omalizumab over a 1-year follow-up period [34]. These results showed that patients who pursued omalizumab had significantly better asthma control: 67% of patients who continued treatment had no exacerbation versus 48% of those who discontinued (OR 0.45, 95% CI 0.24–0.83). However, the authors highlighted the relatively large percentage of patients without any exacerbations in the discontinuation arm, suggesting that omalizumab discontinuation may be a safe option in a subset of treated asthmatic patients.
Our study is the first real-life report that assessed asthma control at discontinuation, and the persistence of control after discontinuation, in a large cohort of 7761 adults and 1082 children over a 3-year follow-up period. Among adults with controlled asthma at discontinuation, 70%, 39% and 24% were still controlled and had not resumed omalizumab at 1, 2 and 3 years after discontinuation, respectively. Although these rates of asthma control are solely based on HCRU, they confirm the large percentage of patients who remained controlled 1 year after omalizumab discontinuation, in line with the XPORT trial results, but also up to 2 and 3 years. Of note, patients who discontinued omalizumab therapy while asthma was controlled had fewer markers of uncontrolled asthma and GINA Step 4–5 treatments before omalizumab initiation, compared with the whole cohort, suggesting that those patients had less severe asthma at omalizumab initiation.
The comparison of HCRU over the 6-month period before omalizumab discontinuation with before initiation provides three major findings: 1) for adults and children with controlled asthma at discontinuation, the rate of all studied HCRU at the end of exposure was reduced; 2) a decrease of HCRU was also observed for children with uncontrolled asthma at discontinuation, with a substantial reduction in hospitalisations for asthma; and 3) adults with uncontrolled asthma at discontinuation had a slightly higher rate of HCRU at the end of omalizumab exposure for the studied drugs, except for the proportion of patients hospitalised for asthma. These results may reflect different levels of response to treatment, some patients with uncontrolled asthma at discontinuation being probably partial responders or having drug escape. Lastly, the level of HCRU after discontinuation was maintained over the 2-year follow-up, both in adults and children, irrespective of asthma control at omalizumab discontinuation. Stable HCRU after discontinuation may reflect a potential effect of omalizumab on asthma natural history but also education, changes in lifestyle and better medical follow-up associated with initiation of a biologic.
Main results in children and differences with adults
Some clinical trials have shown the benefits of omalizumab therapy in children with severe allergic asthma [30]. Our study includes the largest paediatric cohort ever studied (2453 children), with a well-balanced proportion of school-age children (6–11 years old) and adolescents. The results highlight some important features regarding the burden of early-life asthma and HCRU. Compared with adults, more children had hospitalisations related to asthma prior to omalizumab initiation (37% versus 17%). This either reflects more severe asthma or that children are more likely to be hospitalised for severe asthma management. However, severe exacerbations with hospital admission are a main feature in this population [3, 5, 6]. Finally, children also tend to have a slightly longer treatment persistence before discontinuation (median 53.7 versus 51.2 months in our study).
We estimated that 31% of children would pursue omalizumab therapy 6 years after initiation. Recently, the retrospective ANCHORS study found that 21% of 484 children initiating omalizumab were treated for up to 6 years [3]. The authors recorded a decrease of 86% in exacerbations and of 85% in OCS courses during the first year of treatment. Our large real-life study confirms and extends these data, with a 77% decrease in hospitalisations for asthma and 32% fewer patients on OCS 2 years after omalizumab initiation.
Deschildre et al. [4] published the first large real-life study evaluating treatment discontinuation in children. Treatment was discontinued by the treating physician because of stable good control in 35 out of 100 children (6–18 years old) with severe allergic asthma (treatment duration 25–72 months). Eight of them resumed omalizumab therapy for worsening asthma. A nested case–control study reported no significant difference between omalizumab discontinuation (n=30) versus maintenance (n=30) for the rate of asthma exacerbations or asthma control, contrary to the results observed in studies conducted in adults. Our present study shows that, compared with adults, more children with controlled asthma who discontinued omalizumab remained controlled and did not resume omalizumab therapy at 1, 2 and 3 years after discontinuation (respectively, 76%, 44% and 33% of children and 70%, 39% and 24% of adults).
Limitation
Study limitations mainly result from the use of reimbursed healthcare expenditure data. Asthma characteristics and comorbidities were identified through mapping algorithms which may lead to selection and measurement biases. More specifically, only 67% of adults and 46% of children were found to have used GINA Step 4–5 before T0, which leads to the assumption that this might have been underestimated. The same assumption can be made for the asthma control algorithm: using the GINA definition for uncontrolled asthma [24], frequent exacerbation criteria corresponded to OCS use and hospitalisations for asthma, and poor symptom control has been approached through the use of short-acting β2-adrenergic agonists. Of note, most algorithms have been used in prior studies; although this does not guarantee their validity, it does ensure that they were designed and optimised following discussion with clinicians and experts from the SNDS databases and reviewers. Another limitation is that measurement of omalizumab exposure was solely based on its dispensation; this is mitigated by the fact that, in most cases, omalizumab was injected by healthcare professionals. However, the assessment of the exposure to Anatomical Therapeutic Chemical class R03 drugs (drugs for obstructive airway diseases) and OCS is subject to measurement bias, given adherence issues in asthma patients [35, 36]. In addition, patterns of exposure for the most recent years would have been influenced by the availability of novel biologic agents for the treatment of severe eosinophilic asthma. Mepolizumab was the first available in France from June 2015 in a compassionate programme (“Temporary Authorisation for Use”) prior to its commercialisation in February 2018 [37]. Finally, due to the nature of the source data, the exact reasons for omalizumab discontinuation remain unknown.
Conclusions
This study, based on the French healthcare database system (SNDS), represents the largest report of omalizumab real-life use in the almost exhaustive treated population of asthma patients in France, including data from 19 203 patients and covering >10 years of follow-up. We found a similar median omalizumab treatment persistence of 4.5 years before long-term discontinuation in both adults and children. HCRU related to asthma, especially hospitalisations and OCS use, decreased during omalizumab exposure, compared with HCRU measured before treatment initiation, in both adults and children. In addition, in patients who discontinued omalizumab while asthma was controlled, we confirm the large percentage of patients who had persistent asthma control 1 year after discontinuation in real-life, in line with previously published results, but also up to 2 and 3 years. Lastly, we showed the long-term maintenance of low HCRU in adults and children who discontinued omalizumab while asthma was controlled, notably for OCS use and hospitalisations for asthma.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix 1: Study design ERJ-03130-2021.Appendix_1 (591.1KB, jpg)
Appendix 2: Main mapping algorithms used to identify comorbid conditions and to assess asthma characteristics ERJ-03130-2021.Appendix_2 (252.6KB, pdf)
Appendix 3: Reconstitution of omalizumab exposure ERJ-03130-2021.Appendix_3 (459.2KB, jpg)
Appendix 4: Time to omalizumab first long-term discontinuation ERJ-03130-2021.Appendix_4 (568.2KB, jpg)
Appendix 5: Asthma characteristics at omalizumab initiation (T0) and discontinuation (TSTOP) in patients who discontinued omalizumab ERJ-03130-2021.Appendix_5 (202.7KB, pdf)
Appendix 6: Longitudinal evolution of HCRU over the 2-year follow-up after omalizumab discontinuation ERJ-03130-2021.Appendix_6 (1.8MB, jpg)
Shareable PDF
Acknowledgements
The authors would like to thank Sara Guillemin from RCTs (Lyon, France) for her valuable comments and suggestions to improve the quality of the paper.
Footnotes
Author contributions: All authors conceived and designed the study. A. Rigault and A. Lajoinie performed the statistical analyses. A. Lajoinie wrote the first version of the manuscript. All authors commented on the results and contributed to the manuscript.
Conflict of interest: The study was supported by Novartis, which was the data controller of the study. D. Kamar and C. Thonnelier are employees of Novartis. Novartis was involved in the study design, data analysis, decision to publish and preparation of the manuscript. D. Kamar additionally reports consulting fees and support for attending meetings from Ividata Life Sciences providing services for Novartis. A. Lajoinie and A. Rigault are employees of RCTs. RCTs did not fund the study but was in charge of designing and performing the study as data processor. A. Rigault was an employee of the French health insurance until 1 September 2020. M. Humbert, A. Bourdin, C. Taillé, A. Deschildre and M. Molimard received honoraria paid by Novartis as members of the study Independent Scientific Committee. M. Humbert also reports consulting fees from AstraZeneca, Chiesi, GSK, Novartis and Sanofi; lecture honoraria from AstraZeneca, GSK, Novartis and Sanofi; outside the submitted work. A. Bourdin and C. Taillé also report lecture honoraria from AstraZeneca, Chiesi, GSK, Novartis and Sanofi; outside the submitted work. A. Deschildre also reports grants from Fondation du Souffle; Conseil Régional Hauts-de-France program 2014–2018 (grant ARCiR 2015-284); consulting fees from Novartis, ALK, GSK, Sanofi, Regeneron, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science and Stallergenes Greer; lecture honoraria from Novartis, ALK, GSK, Sanofi, Aimmune Therapeutics, DBV Technologies, Nestlé Health Science and Boehringer Ingelheim; support for attending congresses from ALK, Sanofi, Boehringer Ingelheim, Stallergenes Greer, Novartis, AstraZeneca, Meda, DBV Technologies, Aimmune and Nutricia; acted on the Data Safety Monitoring Board (DSMB) for the BOOM study (ClinicalTrials.gov identifier: NCT04045301; Lead Investigator: Philippe Bégin, Montreal, QC, Canada); outside the submitted work. M. Molimard also reports consulting fees from Novartis and Stallergenes; participation on advisory board for Banook; outside the submitted work.
Support statement: This work was supported by Novartis Pharma. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Pradère P, Garcia G, Humbert M, et al. . Omalizumab: what have we learned after ten years of prescription? Rev Mal Respir 2016; 33: 117–127. doi: 10.1016/j.rmr.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 2.Incorvaia C, Mauro M, Makri E, et al. . Two decades with omalizumab: what we still have to learn. Biologics 2018; 12: 135–142. doi: 10.2147/BTT.S180846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieto García A, Garriga-Baraut T, Plaza Martín AM, et al. . Omalizumab outcomes for up to 6 years in pediatric patients with severe persistent allergic asthma. Pediatr Allergy Immunol 2021; 32: 980–991. doi: 10.1111/pai.13484 [DOI] [PubMed] [Google Scholar]
- 4.Deschildre A, Roussel J, Drumez E, et al. . Omalizumab discontinuation in children with severe allergic asthma: an observational real-life study. Allergy 2019; 74: 999–1003. doi: 10.1111/all.13678 [DOI] [PubMed] [Google Scholar]
- 5.Deschildre A, Marguet C, Salleron J, et al. . Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J 2013; 42: 1224. doi: 10.1183/09031936.00149812 [DOI] [PubMed] [Google Scholar]
- 6.Deschildre A, Marguet C, Langlois C, et al. . Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J 2015; 46: 856. doi: 10.1183/09031936.00008115 [DOI] [PubMed] [Google Scholar]
- 7.Nopp A, Johansson SGO, Adédoyin J, et al. . After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy 2010; 65: 56–60. doi: 10.1111/j.1398-9995.2009.02144.x [DOI] [PubMed] [Google Scholar]
- 8.Molimard M, Mala L, Bourdeix I, et al. . Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med 2014; 108: 571–576. doi: 10.1016/j.rmed.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 9.European Network of Centres for Pharmacoepidemiology and Pharmacovigilance . ENCePP Guide on Methodological Standards in Pharmacoepidemiology: 5.1 Overview. 2021. www.encepp.eu/standards_and_guidances/methodologicalGuide5_1.shtml Date last accessed: 3 May 2022.
- 10.Palmaro A, Moulis G, Despas F, et al. . Overview of drug data within French health insurance databases and implications for pharmacoepidemiological studies. Fundam Clin Pharmacol 2016; 30: 616–624. doi: 10.1111/fcp.12214 [DOI] [PubMed] [Google Scholar]
- 11.Li P, Kavati A, Puckett JT, et al. . Omalizumab treatment patterns among patients with asthma in the US Medicare population. J Allergy Clin Immunol Pract 2020; 8: 507–515. doi: 10.1016/j.jaip.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Ke X, Kavati A, Wertz D, et al. . Real-world clinical characteristics, treatment patterns, and exacerbations in US patients with asthma newly treated with omalizumab. Clin Ther 2018; 40: 1140–1158. doi: 10.1016/j.clinthera.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 13.Tuppin P, Rudant J, Constantinou P, et al. . Value of a national administrative database to guide public decisions: from the Système National d'Information Interrégimes de l'Assurance Maladie (SNIIRAM) to the Système National des Données de Santé (SNDS) in France. Rev Epidemiol Sante Publique 2017; 65: Suppl. 4, S149–S167. doi: 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Bannay A, Chaignot C, Blotiere P-O, et al. . The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care 2016; 54: 188–194. doi: 10.1097/MLR.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 15.Bannay A, Chaignot C, Blotière P-O, et al. . Système national d'information inter-régimes de l'assurance maladie (Sniiram) chaîné au Programme de médicalisation des systèmes d'information (PMSI) et score de Charlson. [National health insurance inter-scheme information system (Sniiram) linked to the information systems medicalization program (PMSI) and Charlson score.] Rev Epidemiol Sante Publique 2014; 62: Suppl. 4, S124. doi: 10.1016/j.respe.2014.05.027 [DOI] [Google Scholar]
- 16.Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. . The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract 2019; 7: 1477–1487. doi: 10.1016/j.jaip.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 17.Molimard M, Thonnelier C, Le Gros V, et al. . Impact de Xolair sur la consommation de soins en France: une analyse des données de l'EGB. [Impact of Xolair on healthcare consumption in France: an analysis of EGB data.] Rev Malad Respir 2016; 33: A9–A10. doi: 10.1016/j.rmr.2015.10.023 [DOI] [Google Scholar]
- 18.Caisse Nationale de l'Assurance Maladie des Travailleurs Salariés (CNAMTS) . Méthode de repérage des pathologies et d'affectation des dépenses aux pathologies (CNAMTS). [Method for identifying pathologies and allocating expenses to pathologies (CNAMTS).] 2018. www.ameli.fr/l-assurance-maladie/statistiques-et-publications/etudes-en-sante-publique/cartographie-des-pathologies-et-des-depenses/methode.php Date last accessed: 3 October 2019. [PubMed]
- 19.Dib F, de Rycke Y, Guillo S, et al. . Impact of a population-based asthma management program in France (Sophia Asthme): a matched controlled before-and-after quasi-experimental study using the French health insurance database (SNDS). Pharmacoepidemiol Drug Saf 2019; 28: 1097–1108. doi: 10.1002/pds.4842 [DOI] [PubMed] [Google Scholar]
- 20.de Rycke Y, Dib F, Guillo S, et al. . Evaluation médico-économique du programme d'accompagnement des patients asthmatiques Sophia Asthme. Deuxième année de déploiement. [Medico-economic evaluation of the support program for asthmatic patients Sophia Asthme. Second year of deployment.] 2019. www.ameli.fr/sites/default/files/2019-01_rapport-asthme-a-2-ans-vaguea_evaluations-du-service-sophia_assurance-maladie.pdf Date last accessed: 13 July 2022.
- 21.Novartis . Xolair (omalizumab). Summary of product characteristics. 2015. www.ema.europa.eu/en/documents/product-information/xolair-epar-product-information_en.pdf Date last accessed: 3 October 2019.
- 22.Galozy A, Nowaczyk S, Sant'Anna A, et al. . Pitfalls of medication adherence approximation through EHR and pharmacy records: definitions, data and computation. Int J Med Inform 2020; 136: 104092. doi: 10.1016/j.ijmedinf.2020.104092 [DOI] [PubMed] [Google Scholar]
- 23.The Professional Society for Health Economics and Outcomes Research (ISPOR) . Medication adherence and persistence. 2015. www.ispor.org/member-groups/special-interest-groups/medication-adherence-and-persistence Date last accessed: 25 October 2019.
- 24.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2019. Available from: http://ginasthma.org/
- 25.Cutrera R, Baraldi E, Indinnimeo L, et al. . Management of acute respiratory diseases in the pediatric population: the role of oral corticosteroids. Ital J Pediatr 2017; 43: 31. doi: 10.1186/s13052-017-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asare K. Diagnosis and treatment of adrenal insufficiency in the critically ill patient. Pharmacotherapy 2007; 27: 1512–1528. doi: 10.1592/phco.27.11.1512 [DOI] [PubMed] [Google Scholar]
- 27.Molimard M, de Blay F, Didier A, et al. . Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med 2008; 102: 71–76. doi: 10.1016/j.rmed.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 28.Grimaldi-Bensouda L, Zureik M, Aubier M, et al. . Does omalizumab make a difference to the real-life treatment of asthma exacerbations? Results from a large cohort of patients with severe uncontrolled asthma. Chest 2013; 143: 398–405. doi: 10.1378/chest.12-1372 [DOI] [PubMed] [Google Scholar]
- 29.Humbert M, Taillé C, Mala L, et al. . Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J 2018; 51: 1702523. doi: 10.1183/13993003.02523-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agache I, Beltran J, Akdis C, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 31.Walker S, Monteil M, Phelan K, et al. . Anti-IgE for chronic asthma in adults and children. Cochrane Database Syst Rev 2006; 2: CD003559. doi: 10.1002/14651858.CD003559.pub3 [DOI] [PubMed] [Google Scholar]
- 32.Normansell R, Walker S, Milan SJ, et al. . Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014; 1: CD003559. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humbert M, Beasley R, Ayres J, et al. . Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60: 309–316. doi: 10.1111/j.1398-9995.2004.00772.x [DOI] [PubMed] [Google Scholar]
- 34.Ledford D, Busse W, Trzaskoma B, et al. . A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol 2017; 140: 162–169. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 35.Lindmark SW, Sandström L, Selberg J, et al. . Treatment adherence among adults with asthma – a report from the OLIN asthma cohort. Eur Respir J 2020; 56: Suppl. 64, 5169. doi: 10.1183/13993003.congress-2020.5169 [DOI] [Google Scholar]
- 36.Demoly P, Paggiaro P, Plaza V, et al. . Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev 2009; 18: 105–112. doi: 10.1183/09059180.00001209 [DOI] [PubMed] [Google Scholar]
- 37.Taillé C, Chanez P, Devouassoux G, et al. . Mepolizumab in a population with severe eosinophilic asthma and corticosteroid dependence: results from a French early access programme. Eur Respir J 2020; 55: 1902345. doi: 10.1183/13993003.02345-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Appendix 1: Study design ERJ-03130-2021.Appendix_1 (591.1KB, jpg)
Appendix 2: Main mapping algorithms used to identify comorbid conditions and to assess asthma characteristics ERJ-03130-2021.Appendix_2 (252.6KB, pdf)
Appendix 3: Reconstitution of omalizumab exposure ERJ-03130-2021.Appendix_3 (459.2KB, jpg)
Appendix 4: Time to omalizumab first long-term discontinuation ERJ-03130-2021.Appendix_4 (568.2KB, jpg)
Appendix 5: Asthma characteristics at omalizumab initiation (T0) and discontinuation (TSTOP) in patients who discontinued omalizumab ERJ-03130-2021.Appendix_5 (202.7KB, pdf)
Appendix 6: Longitudinal evolution of HCRU over the 2-year follow-up after omalizumab discontinuation ERJ-03130-2021.Appendix_6 (1.8MB, jpg)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03130-2021.Shareable (435.2KB, pdf)