Abstract
The molecular mechanisms of resistance to genital infection with the mouse pneumonitis (MoPn) strain of Chlamydia trachomatis are unknown. A role for major histocompatibility complex class II-restricted, interleukin-12-dependent CD4+ T cells has been established, but the functional activity of these cells does not depend on secretion of gamma interferon. Here we examined the potential contribution of T-cell-mediated cytotoxicity and apoptosis to mucosal clearance of MoPn by using mice deficient in the molecular mediators of target cell lysis. Animals lacking perforin, Fas, Fas ligand, or both perforin and Fas ligand were infected genitally with C. trachomatis MoPn and monitored for expression of immunity to chlamydial antigens and clearance of MoPn from the genital mucosa. In each case, the profile of spleen cytokine production, the magnitude of the host antibody response, and the kinetics of chlamydial clearance were similar to those of genetically intact controls. Compensatory overproduction of tumor necrosis factor alpha, an alternate mediator of apoptosis in certain cell types, did not appear to account for the ability of mutant mice to resolve Chlamydia infections. These results fail to support CD4+ T-cell-mediated apoptosis or CD8+ T-cell-mediated cytotoxicity as being critical to the clearance of C. trachomatis MoPn urogenital infections.
Cellular pathways of resistance to the obligate intracellular pathogen Chlamydia trachomatis have been studied most extensively in murine models of infection and immunity. Infection of epithelial cells lining the genital mucosa with a murine strain of C. trachomatis designated mouse pneumonitis (MoPn) stimulates a vigorous host inflammatory response, immune-mediated clearance of the infection, and the induction of host resistance to reinfection. Resistance has been mapped to a type 1 subset of CD4+ T cells by monoclonal antibody-mediated subset depletion in vivo (25), by adoptive transfer of immune T-cell subsets (9, 19, 34, 40), and, more recently, by infection of mice bearing targeted mutations in immunologically relevant genes. Thus, mice deficient in T-cell receptor beta chains, major histocompatibility complex (MHC) class II proteins, or interleukin-12p40 (IL-12p40) displayed a profound delay in clearance of genital MoPn infections (29, 31). The molecular mechanism by which αβ receptor-bearing, class II-restricted CD4+ T cells function in chlamydial resistance has not been determined.
Support for a type 1 pathway of immunity to genital C. trachomatis infections spurred investigations into the in vivo relevance of the prototypic type 1 cytokine, gamma interferon (IFN-γ). Analysis of clearance kinetics in IFN-γ- or IFN-γ receptor-deficient mice revealed a requirement for IFN-γ in murine resistance to infection with human C. trachomatis serovars (21) but not with the murine MoPn strain (12, 31). This difference was recently attributed to species-specific adaptation of these chlamydial strains to host IFN-γ activity, since MoPn is resistant to murine IFN-γ but retains in vitro sensitivity to human IFN-γ (33a). The IFN-γ-driven mechanism ultimately responsible for the irreversible inhibition of human C. trachomatis growth has not been identified, but the capacity of this cytokine to up-regulate transcription of a broad array of immunologically relevant genes opens several possibilities to be explored (7). Fewer options are available for consideration with regard to the IFN-γ-independent mechanism by which CD4+ T cells mediate resistance to C. trachomatis MoPn. A partial contribution of tumor necrosis factor alpha (TNF-α) has been documented, but the relevant mechanism of action is unknown (33a). No other cytokines have been implicated in the killing of intracellular MoPn.
A potential contribution of CD8+ T cells to the elimination of MoPn from mucosal epithelial cells has also been explored. CD8+ T cells comprise roughly 35% of the lymphocytes recovered from MoPn-infected genital tissue (33) and function as professional killer cells as well as a potential source of type 1 cytokines. Although early efforts to detect cell-mediated cytotoxicity against C. trachomatis-infected target cells were unsuccessful (30), cytotoxic T lymphocytes (CTL) were implicated in the detachment of Chlamydia psittaci-infected targets from culture flasks (24) and, later, in the lysis of C. psittaci (8)- or C. trachomatis (5, 38, 39)-infected cells. However, when the in vivo activity of CTL generated against human C. trachomatis serovars was analyzed, their capacity to protect recipients was attributed to the elaboration of IFN-γ rather than to direct cell-mediated cytotoxicity (39). Similarly, CD8+-T-cell clones generated against C. trachomatis MoPn provided protection in accordance with their ability to secrete cytokines such as TNF-α (17).
A major contribution of CD8+-T cell-mediated cytotoxicity to host resistance is also inconsistent with the ability of β2-microglobulin-deficient mice (23) lacking conventional αβ T-cell receptor-positive CD8+ CTL (45) to clear genital MoPn infections at a normal rate (29). Remaining γδ T-cell receptor CD8+ CTL (11) are an unlikely source of significant effector activity, since less than 5% of infiltrating T cells express this receptor genotype (33) and δ-chain-deficient mice clear genital MoPn infections at a normal rate (31). Nevertheless, a role for CD8+ T cells that recognize glycolipid antigens presented by the nonclassical CD1 molecules (4, 37) could not be ruled out, particularly given the lipid nature of dominant chlamydial antigens (22).
In an effort to provide definitive evidence for or against cell-mediated cytotoxicity as a mechanism of host resistance to the IFN-γ-insensitive MoPn strain, mice deficient in the molecular machinery of T-cell-mediated cytolysis were analyzed. At least two molecular pathways for the killing of MHC class I-compatible target cells by CD8+ T cells are relevant in this regard. The first involves release of the pore-forming protein, perforin, which perforates the target cell membrane to allow secondary penetration of the granzyme proteases that initiate DNA fragmentation and apoptosis (3). Deletion of the gene encoding perforin is sufficient to inactivate this pathway (35). The second pathway involves triggering of the target cell’s apoptotic pathway through membrane interactions between T-cell CD95L/Fas ligand and target cell CD95/Fas, a member of the TNF receptor superfamily that is up-regulated by proinflammatory cytokines such as IFN-γ (36). Activated CD4+ T cells can also trigger apoptosis through Fas-Fas ligand interactions, providing a possible mechanistic role for CD4+ T cells in MoPn resistance. Finally, CD4+- or CD8+-T cell-derived TNF-α can induce apoptosis in cells expressing the p55 TNF receptor, but death occurs only if cellular protein synthesis has been blocked (2).
The potential contribution of perforin-mediated cytotoxicity and/or Fas-mediated apoptosis to the clearance of C. trachomatis MoPn from genital epithelial cells was examined in mice carrying spontaneous or induced mutations in the genes encoding perforin, Fas, or Fas ligand. Reports from studies in other systems revealed that immunity to three other intracellular pathogens, Listeria monocytogenes (20), Leishmania major (16), and Toxoplasma gondii (13), was impacted significantly by abrogation of either pathway alone. However, the recent realization that influenza virus-specific CTL (42) and lymphokine-activated killer cells (27) may utilize either the perforin or Fas ligand pathway during target cell lysis indicated that analysis of mice with mutations in single genes may not always be sufficient to document the role of cell-mediated cytotoxicity in vivo. Therefore, we also analyzed the response of double-mutant mice lacking both the perforin and the Fas ligand pathways for lysis. Our results revealed that both single- and double-mutant mice cleared genital MoPn infections at a rate comparable to that of genetically intact controls, arguing against perforin-mediated cytolysis or Fas-mediated apoptosis as a primary mechanism for elimination of genital MoPn infections. The detection of similar histological changes in the uterine tissues of normal and mutant mice suggests that cell-mediated cytotoxicity also fails to account for the pathological consequences of chlamydial infection.
MATERIALS AND METHODS
Mice.
C57BL/6, B6.MRL-Faslpr, B6Smn.C3H-Faslgld, and C57BL/6-PfptmlSdz female mice were obtained from The Jackson Laboratory, Bar Harbor, Maine. Mice lacking both perforin and Fas ligand on a C57BL/6 by C3H F2 genetic background were the generous gift of Eckhard Podack, University of Miami. All animals were housed in an American Association for Accreditation of Laboratory Animal Care-accredited facility in filter-top cages under standard conditions for immunodeficient mouse strains and were provided food and water ad libitum.
C. trachomatis.
The MoPn strain of C. trachomatis was grown in HeLa 229 cells, and elementary bodies were purified by discontinuous density gradient centrifugation as previously described (10).
Infection of mice.
Estrus synchronization in experimental mice was accomplished by subcutaneous injection of 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, Mich.) 7 days prior to infection. Animals were infected by depositing 1,500 inclusion-forming units (IFU) of C. trachomatis MoPn (equivalent to 100 50% infective doses) in 5 μl of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (pH 7.2) into the vaginal vault. The course of infection was monitored by swabbing the vaginal vault with Calgiswabs (Spectrum Medical Industries, Los Angeles, Calif.) at selected intervals followed by enumeration of recovered IFU on HeLa cell monolayers by indirect immunofluorescence as described previously (29).
Spleen cytokine assays.
Spleen cell cytokine production was measured at 18 days postinfection as described previously (31). Briefly, erythrocyte-depleted splenocytes from infected donor mice were cocultured with heat-killed (80°C, 30 min) MoPn and supernatant fluids were recovered for cytokine analyses 72 h later. Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed with purified capture and biotinylated detection monoclonal antibodies recognizing murine IL-4, IL-6, IL-10, and IFN-γ (PharMingen, San Diego, Calif.), and cytokine concentrations were calculated from standard curves generated with the corresponding recombinant cytokines. Results for IL-4 were consistently negative when an ELISA with a lower sensitivity limit of 30 pg/ml was used.
Evaluation of serum and secretory antibody responses.
Serum and secretory (vaginal wash) antibodies reactive with C. trachomatis MoPn were isotyped by ELISA with an alkaline phosphatase-conjugated anti-mouse immunoglobulin (Ig) serum (class and subclass specific; Southern Biotechnology Associates, Birmingham, Ala.) as previously described (29). ELISA titers were defined as the highest serum dilutions giving an absorbance (A405) that was at least threefold higher than that observed with preimmune serum.
RT-PCR analysis of genital tract RNA.
RNA was extracted from infected genital tracts by using Trizol (GIBCO-BRL) and subjected to reverse transcription (RT)-PCR (31) with previously described hypoxanthine phosphoribosyltransferase (HPRT) primers (15) and Clontech (Palo Alto, Calif.) TNF-α primers. Products generated after 35 cycles of amplification were electrophoresed on a 1.4% agarose gel and scanned with an AlphaImager (Alpha Innotech Corporation). The intensities of TNF-α bands were normalized to the amount of HPRT generated from each sample.
Histological evaluation of Chlamydia-infected tissues.
Mice were sacrificed during the course of immune-mediated clearance (18 days after primary genital infection) or 8 to 12 weeks later, and genital tracts were removed for fixation in 10% buffered formalin. Coded samples were submitted to Histopath of America (Millersville, Md.) for embedding, sectioning, and evaluation of hematoxylin- and eosin-stained slides by a veterinary pathologist. The intensity of the host inflammatory response was graded on a scale of 1+ to 4+ according to the following criteria: 1+, minimal response (small numbers of inflammatory cells, limited to areas adjacent to the oviduct); 2+, mild response (increased numbers of inflammatory cells, with slight thickening of the stroma and extension into surrounding adipose tissue); 3+, moderate response (pronounced presence of inflammatory cells, with obliteration of adjacent adipose tissue); and 4+, marked response (extension of the moderate-severity reaction, with obliteration of affected tissue and focal to multifocal necrosis of adjacent adipose tissue).
Statistical analyses.
Log-transformed bacterial clearance data were analyzed by Student’s t test, splenic cytokine ELISA data were analyzed by the Mann-Whitney test, and hydrosalpinx incidence was analyzed by chi-square analysis. All data are presented as means ± standard error.
RESULTS
Clearance of C. trachomatis from the genital mucosa of normal and mutant mice.
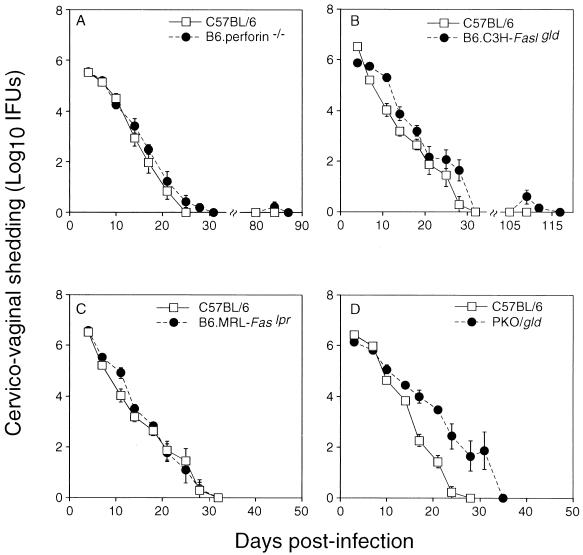
The contribution of perforin-mediated cytotoxicity and/or Fas-mediated apoptosis to immune-mediated clearance of C. trachomatis from epithelial cells of the vaginal mucosa was evaluated in a murine model of genital tract infection. Chlamydial clearance rates in normal, perforin-deficient, Faslpr mutant, and Fas ligand mutant mice as well as in double-mutant mice deficient in both perforin and Fas ligand (PKO/gld) were compared. A deficiency in perforin, Fas, or Fas ligand alone had no apparent effect on the ability of mice to clear genital MoPn infections, with clearance rates being nearly identical in control and mutant mice (Fig. 1A to C). Clearance in PKO/gld double-mutant mice was slightly delayed compared to that in the C57BL/6 control group (Fig. 1D). However, differences were marginal and probably reflected the genetic contribution of the C3H parent, since MoPn clearance in C3H mice is slightly delayed compared to that in C57BL/6 mice (with clearance usually requiring 35 days in C3H mice versus 25 to 30 days in C57BL/6 mice [unpublished data]). Failure to detect a significantly prolonged course of infection in mutant mice compared to that in normal mice does not support a major role for the perforin and/or Fas pathway in T-cell-mediated resolution of primary chlamydial infections.
FIG. 1.
Clearance of C. trachomatis MoPn from the genital mucosa of normal and mutant mice. Mice were infected with 1,500 IFU of MoPn vaginally, and chlamydial shedding was assessed twice per week. The kinetics of clearance in mice lacking perforin (A), Fas ligand (B), Fas (C), or both perforin and Fas ligand (PKO/gld) (D) are depicted versus that of normal C57BL/6 controls. Differences between groups were not statistically significant.
The potential influence of these mutations on the host’s ability to mount an acquired memory T-cell response protective against a secondary infection with C. trachomatis was assessed by rechallenging perforin- and Fas ligand-deficient mice 8 to 12 weeks after primary clearance. Comparable analyses could not be performed in PKO/gld double-mutant mice due to the development of a systemic, fatal autoimmune disease in these animals at between 12 and 16 weeks of age. Fas ligand mutant mice also developed complications consistent with their mutations in that over 50% of these animals presented with lymphadenopathy at the end of these experiments. Despite these complications, all rechallenged animals displayed evidence of an acquired immune response to C. trachomatis MoPn in that the duration and level of bacterial shedding were greatly diminished compared to those in primary infections (Fig. 1A and B).
Antibody and cytokine responses of normal and mutant mice.
Since both perforin and Fas function during the effector rather than the induction phase of T-cell cytotoxicity, mice deficient in either or both of these molecules would be expected to mount an otherwise-normal immune response. However, it is now appreciated that the developing immune system frequently compensates for the absence of a specific molecule by amplying alternate mediators with redundant or overlapping functions. The loss of the feedback signals normally provided by the missing effector pathway could also result in dysregulated immune reactivity. For these reasons, identification of compensatory mechanisms that might mask any influence of the disrupted gene becomes a relevant concern. Targeted mutation of the perforin and/or Fas effector pathway is reportedly associated with up to a threefold increase in spleen mRNA for IL-6, IL-10, IL-12p35, and/or IFN-γ (26). Compensatory enhancement of TNF-α might also be predicted, since it provides a third alternative proapoptotic pathway available to certain cytotoxic cells (1). Each of these activities was examined in single- and/or double-gene knockout mice.
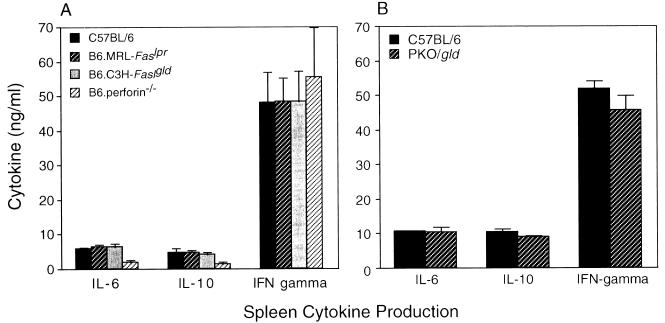
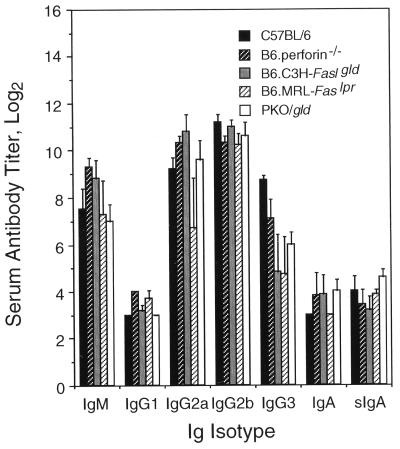
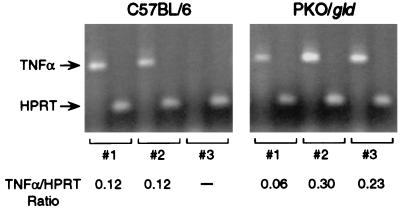
Spleen cells collected from normal mice 18 to 20 days after C. trachomatis infection and restimulated with chlamydial antigens in vitro produced substantial amounts of IL-6, IL-10, and IFN-γ but no detectable IL-4 (31) (Fig. 2). Deletion of the gene encoding perforin and/or Fas ligand did not alter this profile of cytokine secretion or the relative levels of IL-6, IL-10, and IFN-γ produced (Fig. 2). The distributions of Chlamydia-specific immunoglobulin isotypes and the titers of serum and secretory antibodies in normal and mutant mice were also similar (Fig. 3). By these criteria, amplification or dysregulation within the systemic immune response was not apparent. The potential for enhanced production of TNF-α locally was assessed in double-knockout PKO/gld mice, in which the impetus for the immune system to provide an alternate mechanism for eliminating infected cells would be greatest. TNF-α is only weakly up-regulated in the genital mucosa during MoPn infections in normal mice (31), and it was detected in only two of the three infected control mice that were examined in this experiment (Fig. 4). PKO/gld mice displayed at most a 2.5-fold increase in transcriptional activity of the TNF-α gene compared to that of the control animals (Fig. 4). Whether this increase was sufficient to compensate for the loss of both the perforin and Fas ligand apoptotic pathways is difficult to assess, but given the marginal influence of a TNF-α receptor deficiency on in vivo clearance and the apparent insensitivity of C. trachomatis to TNF-α-mediated growth inhibition in vitro (33a), a prominent role for this cytokine in host immunity to this pathogen seems unlikely. It appears instead that perforin- and/or Fas ligand-deficient mice clear genital infections at a normal rate because neither pore formation nor apoptosis is required for the intracellular killing of C. trachomatis, not because alternate pathways have successfully compensated for the missing gene(s).
FIG. 2.
Spleen cytokine responses of single-mutant (A) and double-mutant (B) mice versus those of normal controls at 18 days after MoPn infection. IL-4 was not detected in any of the culture supernatants tested. Differences between groups were not statistically significant.
FIG. 3.
Immunoglobulin isotype profiles and antibody titers of perforin-deficient, Fas mutant, Fas ligand mutant, PKO/gld, and normal mice 18 days after infection with C. trachomatis MoPn. sIgA represents the titer of secretory IgA present in the vaginal wash of each mouse. Differences between groups were not statistically significant.
FIG. 4.
RT-PCR detection of TNF-α mRNA in MoPn-infected genital tissues from normal and PKO/gld mice. Low levels of TNF-α were detected in all but one tissue sample. Between-group differences in the TNF-α/HPRT ratio were not statistically significant.
Genital tract pathology in normal and mutant mice.
The presence of substantial numbers of CD8+ T cells in the Chlamydia-infected genital mucosa (33, 43) raises the possibility that perforin or other mediators of cytolysis contribute to the development of pathology in infected tissues. In the murine genital tract, inflammation and subsequent pathological changes are most severe in the oviduct, where the development of hydrosalpinx correlates positively with infertility. To determine whether perforin- and/or Fas-mediated killing of infected epithelial cells was responsible for these tissue changes, infected genital tracts from normal and mutant mice were evaluated histologically. Inflammatory responses in normal and in perforin-, Fas-, or Fas ligand-deficient mice as well as PKO/gld mice were similar in that they consisted of a mixed neutrophil and mononuclear-cell infiltrate with an intensity ranging from 1+ to 3+ in each group of four to seven animals (data not shown). Approximately 3 months following primary clearance, genital tissues from all mice except those of the PKO/gld double-knockout strain (which died of systemic disease by 4 months of age) were submitted for analysis of residual tissue pathology. Mild to marked hydrosalpinx was detected in mice from all groups, although the proportion of affected animals ranged from 33 to 54% among mutant mice (n = 5 to 13 per group) versus 89% in control mice (n = 9). However, differences were not significant by chi-square analysis. Thus, it appears that the host inflammatory response to infection and the subsequent development of genital tract pathology cannot be attributed to the activity of T cells that function through perforin- or Fas-mediated cytolysis.
DISCUSSION
Mutation of the genes that encode the primary molecular mediators of CD4+- or CD8+-T-cell lysis had little to no effect on clearance of C. trachomatis MoPn from the genital mucosa. The kinetics of chlamydial shedding in perforin-, Fas-, and Fas ligand-deficient mice were comparable to that of controls during primary as well as secondary infections. A slight delay in clearance by PKO/gld mice, deficient in both perforin and Fas ligand, probably reflected the contribution of background genes from the C3H gld donor strain, which clears MoPn infections more slowly than the C57BL/6 mouse strain. Indeed, if the resolution of genital Chlamydia infections depended on a functional Fas and/or perforin cytolytic pathway, the course of infection in PKO/gld double-mutant mice should have been prolonged dramatically, as seen in mice lacking other molecules critical to the host immune response. For example, mice deficient in the MHC class II glycoproteins exhibited high levels of chlamydial shedding for over 90 days (29), while mice lacking the type 1 T-cell-inducing cytokine, IL-12, remained infected for nearly 50 days (31). Moreover, absence of the perforin and/or Fas ligand pathway had no effect on the intensity of inflammation in infected genital tissue or on the subsequent development of uterine pathology. Therefore, it appears that the ability to generate C. trachomatis-specific CD8+ CTL in vitro does not necessarily imply a functional role for these cells in immune-mediated elimination of infected epithelial cells in vivo. It also appears unlikely that Fas-mediated apoptosis accounts for the critical role of CD4+ T cells in the clearance of C. trachomatis MoPn.
The ability of mice deficient in perforin and/or Fas or Fas ligand to mount an efficacious immune response to genital Chlamydia infections could not be attributed to compensatory amplification of alternative effector pathways. Both normal and mutant animals generated comparable systemic cytokine and antibody responses to chlamydial antigens, and they displayed similar levels of IgA in their vaginal secretions. TNF-α, a major candidate for immunological compensation in mice lacking both perforin and Fas ligand (1), was increased no more than twofold in some, but not all, Chlamydia-infected PKO/gld mice. The failure to detect significant enhancement of pathways capable of substituting for the loss of perforin and/or Fas suggests that immune-mediated resolution of C. trachomatis MoPn infections occurs independently of T-cell-mediated cytotoxicity or apoptosis. However, we cannot rule out a potential contribution of the TNF-related apoptosis-inducing ligand (TRAIL) (41), TNF receptor-related apoptosis-mediating protein (TRAMP) (6), or other newly defined proapoptotic mediators for which similar analytical systems are not yet available.
The inability to detect a role for T-cell-mediated apoptosis in chlamydial clearance from the genital mucosa may reflect an antiapoptotic action of the invading organism. It was recently demonstrated that infection with C. trachomatis protected HeLa cells against apoptosis induced by TNF-α, perforin, Fas antibody, or exposure to one of two apoptosis-inducing chemicals, staurosporine and etoposide (14). The protection afforded by infection required chlamydial but not host protein synthesis and occurred at a step upstream of caspase-3 activation and cytochrome c release. While the precise mechanism of Chlamydia-induced interference with the natural progression of the apoptotic cascade has not been defined, the ability of chlamydiae to evade this mechanism of immunological control is consistent with the findings reported herein.
The molecular mechanism(s) by which CD4+ T cells mediate resistance to C. trachomatis MoPn remains elusive. While several potentially relevant pathways for elimination of pathogen-infected cells have been ruled out, few have been identified as being important. The dependence of the clearance reaction on MHC class II glycoproteins and IL-12 strongly suggests a critical role for type 1 CD4+ T cells, but their precise mechanism of action is unclear. A contribution of T-cell-derived TNF-α was suggested by the consistent but marginal delay in clearance associated with mutation of the p55 TNF-α receptor, but a role for this mediator in target cell apoptosis is not supported by the ability of MoPn-infected cells to resist TNF-α-mediated killing in vitro (14, 33a). Alternatively, TNF-α may contribute to host resistance indirectly by activating NF-κB-inducible genes required for the expression of an effective immune response, such as the vascular adhesion molecules VCAM-1 and ICAM-1 and the proinflammatory cytokines IL-8 and IL-12 (28). However, while these events may augment the recruitment of reactive cells to sites of infection, they do not account for the ultimate elimination of Chlamydia-infected epithelial cells. The prototypic type 1 cytokine, IFN-γ, and the plethora of potentially relevant IFN-γ-inducible genes are required to prevent systemic dissemination of genital MoPn infections but not to control infection in genital epithelial cells, at least in the C57BL/6 inbred host (12, 31). Requisite roles for other cytokines expressed in the genital mucosa during chlamydial infection, such as IL-6 and IL-10, are unlikely, given the normal clearance rates of IL-6- and IL-10-deficient mice (reference 32 and unpublished data). While the relative contributions of IL-13 and IL-15 have yet to be examined, available evidence does not provide strong support for a cytokine-dependent mechanism of chlamydial clearance. Coupled with the present results that indicate the lack of a requirement for direct cell-mediated cytotoxicity or apoptosis, few clues exist to explain the means by which CD4+ T cells mediate the clearance of C. trachomatis MoPn from genital epithelial cells.
Recent data from this and other bacterial systems suggest that the molecular mechanisms of pathogen resistance may actually differ from one cell type to another. For example, a role for perforin was apparent in CD8+-T cell-dependent clearance of Listeria monocytogenes from the spleen, but not in its clearance from the liver, where CD8+-T cell-mediated cytotoxicity was found to be strictly Fas dependent (20). IFN-γ was required to prevent systemic dissemination of C. trachomatis MoPn by macrophages (12, 31) but not to resolve infection in genital epithelial cells. The IFN-γ-dependent mechanism responsible for control of macrophage chlamydial infections has not been fully resolved (18), but it does not appear to rely on an apoptotic signaling pathway, since mice carrying mutations in the perforin, Fas, or Fas ligand gene never displayed clinical signs of systemic chlamydial disease and bacteria could not be recovered from the organs of clinically ill PKO/gld mice (data not shown). In addition to being able to infect genital epithelial cells and macrophages, C. trachomatis also has the capacity to infect epithelial cells of the conjunctival, respiratory, and intestinal mucosae, sites for which even less is known about the molecular mechanisms of host resistance. Recent evidence documenting a role for IL-6 in host resistance to pulmonary (44) but not genital (32) infections with C. trachomatis MoPn lends credence to the possibility that the cellular and molecular events associated with infection of different tissues and/or cell types are not identical. Coupled with the potential variety of effector-cell phenotypes and the redundancy of the host immune system, which tends to mask the influence of deleted genes, the definition of the molecular mechanisms of immune-mediated resistance to all C. trachomatis strains presents an interesting challenge.
ACKNOWLEDGMENTS
We are indebted to John Carlson and Jos Van Putten for performing densitometry of TNF-α gels and to Robert Evans for assistance with graphics.
REFERENCES
- 1.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson E A, Bleackley R C. Mechanisms of lysis by cytotoxic T cells. Crit Rev Immunol. 1995;15:359–384. doi: 10.1615/critrevimmunol.v15.i3-4.90. [DOI] [PubMed] [Google Scholar]
- 4.Balk S P, Burke S, Polischuk J E, Frantz M E, Yang L, Porcelli S, Colgan S P, Blumberg R S. β2-Microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 5.Beatty P R, Stephens R S. CD8+ T lymphocyte-mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–4595. [PubMed] [Google Scholar]
- 6.Bodmer J L, Burns K, Schneider P, Hofmann K, Steiner V, Thome M, Bornand T, Hahne M, Schroter M, Becker K, Wilson A, French L E, Browning J L, MacDonald H R, Tschopp J. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas (Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 7.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 8.Buzoni-Gatel D, Guilloteau L, Bernard F, Bernard S, Chardes T, Rocca A. Protection against Chlamydia psittaci in mice conferred by Lyt-2+ T cells. Immunology. 1992;77:284–288. [PMC free article] [PubMed] [Google Scholar]
- 9.Cain T K, Rank R G. Local Th1-like responses are induced by intravaginal infection of mice with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1995;63:1784–1789. doi: 10.1128/iai.63.5.1784-1789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa I, Bix M, Liao N-S, Zijlstra M, Jaenisch R, Raulet D. Most gamma delta T cells develop normally in β2-microglobulin-deficient mice. Proc Natl Acad Sci USA. 1992;89:653–657. doi: 10.1073/pnas.89.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkers E Y, Yap G, Scharton-Kersten T, Charest H, Butcher B A, Caspar P, Heiny S, Sher A. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol. 1997;159:1903–1908. [PubMed] [Google Scholar]
- 14.Fan T, Lu H, Hu H, Shi L, McClarty G, Nance D, Greenberg A, Zhong G. Inhibition of apoptosis in Chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzinelli R R, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 16.Huang F P, Xu D, Esfandiari E O, Sands W, Wei X Q, Liew F Y. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J Immunol. 1998;160:4143–4147. [PubMed] [Google Scholar]
- 17.Igietseme J U, Magee D M, Williams D M, Rank R G. Role for CD8+ T cells in antichlamydial immunity defined by chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igietseme J U, Perry L L, Ananaba G A, Uriri I M, Ojior O O, Kumar S N, Caldwell H D. Chlamydial infection in inducible nitric oxide synthase knockout mice. Infect Immun. 1998;66:1282–1286. doi: 10.1128/iai.66.4.1282-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, TH1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 20.Jensen E R, Glass A A, Clark W R, Wing E J, Miller J F, Gregory S H. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect Immun. 1998;66:4143–4150. doi: 10.1128/iai.66.9.4143-4150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson M, Schön K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny G E. Antigens of the mycoplasmatales and chlamydiae. In: Sela M, editor. The antigens. III. New York, N.Y: Academic Press, Inc.; 1975. pp. 449–478. [Google Scholar]
- 23.Koller B H, Smithies O. Inactivating the β2-microglobulin locus in mouse embryonic stem cells by homologous recombination. Proc Natl Acad Sci USA. 1989;86:8932–8935. doi: 10.1073/pnas.86.22.8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammert J K. Cytotoxic cells induced after Chlamydia psittaci infection in mice. Infect Immun. 1982;35:1011–1017. doi: 10.1128/iai.35.3.1011-1017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landers D V, Erlich K, Sung M, Schachter J. Role of L3T4-bearing T-cell populations in experimental murine chlamydial salpingitis. Infect Immun. 1991;59:3774–3777. doi: 10.1128/iai.59.10.3774-3777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laochumroonvorapong P, Wang J, Liu C-C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee R K, Spielman J, Zhao D Y, Olsen K J, Podack E R. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- 28.May M J, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 29.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavia C S, Schachter J. Failure to detect cell-mediated cytotoxicity against Chlamydia trachomatis-infected cells. Infect Immun. 1983;39:1271–1274. doi: 10.1128/iai.39.3.1271-1274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 32.Perry L L, Feilzer K, Caldwell H D. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infec Immun. 1998;66:1265–1269. doi: 10.1128/iai.66.3.1265-1269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 33a.Perry, L. L., et al. J. Immunol., in press.
- 34.Ramsey K H, Rank R G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi L, Mai S, Israels S, Browne K, Trapani J A, Greenberg A H. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shustov A, Nguyen P, Finkelman F, Elkon K B, Via C S. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-γ production. J Immunol. 1998;161:2848–2855. [PubMed] [Google Scholar]
- 37.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 38.Starnbach M N, Bevan M J, Lampe M F. Murine cytotoxic T lymphocytes induced following Chlamydia trachomatis intraperitoneal or genital tract infection respond to cells infected with multiple serovars. Infect Immun. 1995;63:3527–3530. doi: 10.1128/iai.63.9.3527-3530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 40.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas W D, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195–2200. [PubMed] [Google Scholar]
- 42.Topham D, Tripp R, Doherty P. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 43.Van Voorhis W C, Barrett L K, Sweeney Y T, Kuo C C, Patton D L. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:647–650. doi: 10.1093/infdis/174.3.647. [DOI] [PubMed] [Google Scholar]
- 44.Williams D M, Grubbs B G, Darville T, Kelly K, Rank R G. A role for interleukin-6 in host defense against murine Chlamydia trachomatis infection. Infect Immun. 1998;66:4564–4567. doi: 10.1128/iai.66.9.4564-4567.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. β2-Microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]