Abstract
Background and Aims
Decompensated cirrhotic patients with hepatitis C (HCV) are often under-represented in clinical trials. We aimed to evaluate pooled data on the efficacy and safety of sofosbuvir (SOF)-based regimens in these patients.
Methods
We conducted a systemic review and meta-analysis by searching multiple databases for studies published from October 2010 to October 2020. Outcomes of interest were sustained virologic response (SVR) and safety of SOF-based regimens in decompensated HCV patients. Two reviewers independently performed the study selection and data extraction.
Results
We included 33 studies that enrolled 5,302 HCV patients. The pooled SVR rate in decompensated patients with SOF-based regimens was 85.1% (95% CI: 82.8–87.3). Patients on SOF/velpatasvir±ribavirin achieved a significantly higher SVR (91.0%, 95% CI: 87.7–93.9) than that of SOF/ledipasvir±ribavirin [(86.3%, 95% CI: 84.6–87.8); p=0.004)], or on SOF/daclatasvir±ribavirin (82.4%, 95% CI: 78.2–86.2%; p<0.001). Adding ribavirin to SOF-based regimens (pooled SVR 84.9%, 95% CI: 81.7–87.9) did not significantly increase the SVR [(83.8% (95% CI: 76.8–89.8%; p=0.76)] in decompensated patients, which was also true in subgroup analyses for each regimen within the same treatment duration. However, adding ribavirin significantly increased the frequency of adverse events from 52.9% (95% CI: 28.0–77.1) to 89.2% (95% CI: 68.1–99.9) and frequency of severe events. The pooled incidence of hepatocellular carcinoma and case-fatality of decompensated patients were 3.1% (95% CI: 1.5–5.0) and 4.6% (95% CI: 3.1–6.3), respectively. The overall heterogeneity was high. There was no publication bias.
Conclusions
The analysis found that 12 weeks of SOF/velpatasvir without ribavirin is the preferred therapy, with a significantly higher SVR compared with other SOF-based regimens in decompensated HCV patients.
Keywords: Direct-acting antiviral, HCV liver failure, Sustained virologic response, Ribavirin
Graphical abstract
Introduction
The main cause of chronic liver disease is hepatitis C virus infection, with significant morbidity and mortality.1 Messina et al.2 observed that the disease burden based on seroprevalence had increased globally in the last 15 years. About 700,000 people die each year from complications associated with chronic hepatitis C infection that can lead to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).3,4 As interferon-based regimens are not suitable for patients with decompensated liver cirrhosis because safety concerns, the new generation of direct-acting antiviral (DAA) agents including sofosbuvir (SOF), ledipasvir (LDV), and daclatasvir (DCV) offer new hope for this special population.5 Achieving a sustained virologic response (SVR) is the current goal of the treatment of patients with HCV, defined as the inability to detect HCV RNA in plasma or serum by sensitive molecular testing after 12 (SVR12) or 24 (SVR24) weeks of treatment.6 High SVR rates and the favorable safety profile of DAA therapy in HCV patients with compensated cirrhosis have led to widespread recommendation of DAA treatment in HCV patients with decompensated disease, particularly those on transplant waiting lists and with relatively low Model For End-Stage Liver Disease scores.7–10
Guidelines of professional groups including the American Association for the Study of Liver Diseases (AASLD), Infectious Diseases Society of America (IDSA), and the European Association for the Study of the Liver (EASL) recommend the use of sofosbuvir (SOF)-based DAA regimens for HCV patients with liver decompensation. These therapies include the use of SOF and velpatasvir (VEL), ledipasvir (LDV), or daclatasvir (DCV) plus ribavirin (RBV). Health care providers should take the HCV genotype in the individual patient into consideration when selecting the regimen.6,11 Because of serious concerns associated with drug concentrations and the related risk of toxicity in patients with decompensation, DAA regimens containing protease inhibitors (e.g. grazoprevir, voxilaprevir, or glecaprevir) should be avoided in Child-Pugh B or C patients with decompensated cirrhosis.12 However, data on the comparison of the three aforementioned SOF-based regimens that would assist in selecting individualized treatment for such patients are limited. As SOF/VEL, is a pan-genotypic regimen that can be used to treat patients who have a genotype indication for SOF/LDV or SOF/DCV, comparison of the efficacy and safety of those regimens are needed to guide treatment decisions. Several recent cohort studies and randomized controlled trials (RCTs) of SOF-based DAA therapy without ribavirin have included HCV patients with decompensated liver disease. There is growing interest in investigating whether ribavirin can be removed from the regimens in this special population because of the high frequency of adverse events (AEs).13 However, data from individual studies is limited. With that in mind, we designed a meta-analysis to analyze pooled outcomes on the efficacy and safety of SOF-based regimens for HCV patients with decompensated cirrhosis. We compared the SVR and AEs of the regimens and assessed the pooled SVR benefits and AEs when adding ribavirin to the DAA treatment in the patients.
Methods
Our systematic review and meta-analysis followed a protocol developed by authors CP, HBY, and JZ and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 The search strategy, eligibility criteria, and outcomes were registered on the PROSPERO website (CRD42020149072).
Eligibility criteria
The current review included controlled or comparative studies that enrolled HCV patients with decompensated cirrhosis, which was defined with Child-Pugh scores ≥7 points, or ascites, hepatic encephalopathy, upper gastrointestinal hemorrhage in the combination of fibrosis stage 4 within 6 months before the start of DAAs treatment. Additional eligibility criteria included: (1) study patients who had received SOF-based DAA regimens and reported outcomes that included SVR, treatment-associated AEs, and the incidence of HCC, death, and liver transplantation; (2) journal articles and meeting abstracts published in English and other languages; (3) inclusion of at least 20 adult patients ≥18 years of age.
We excluded studies that met one of the following criteria: (1) enrollment of patients who received protease inhibitors; (2) treatment with only SOF or SOF combined with ribavirin; (3) inclusion of >5% of patients with coinfection of another hepatitis virus such as hepatitis B, delta or HCC, 4) lack of measurement of SVR; (5) in vitro or animal studies; and 6) lack of reported safety data. If two or more studies included the same cohort of patients, the most recent one was selected for review to avoid the analyses of the duplicated data.
Search strategy
We comprehensively and systematically searched PubMed, the Cochrane Central Register of Controlled Trials, Embase, MEDLINE, and Web of Science without language restrictions from October 2010, which is the time of the first publications on SOF DAA treatment to October 2020. The search terms were “liver cirrhosis” and “Child’s C, or Child C, or decompensated, or Child-Pugh C” and “Hepatic cirrhosis, or cirrhosis, or liver,” and “sofosbuvir.” Supplementary Table 1 summarizes the search strategy for PubMed and the other databases. We also looked at the reference lists for relevant abstracts and original research articles.
Study selection and data extraction
Two reviewers independently reviewed the titles and abstracts of the retrieved articles. Articles were selected for data extraction following review of the full text publications. Disagreements were reconciled by the consensus of the corresponding authors. For each article, two reviewers independently extracted data in duplicate using a pretested and standardized form. A third reviewer compared the content and discrepancies of the extracted data. The corresponding authors resolved inconsistencies by reviewing the full text of the articles. The extracted data were the name of first author, study type, year of publication, study country, study design, patient clinical characteristics: age, sex, body mass index, care setting, HCV RNA level, and renal function status), the severity of liver disease (Child-Pugh A/B/C), history of previous treatment for hepatitis C (treatment-naïve vs. treatment-experienced), reasons for liver transplantation before antiviral therapy (HCC non-LT vs. HCC/LT), HCV genotype, DAA regimen and treatment duration, efficacy, and safety outcomes. If the missing data in the article was not housekeeper data, we ignored it.
Assessment of outcomes
Our interests in outcomes included the treatment efficacy of SOF-based regimens assessed by SVR at 12 or 24 weeks after completion of treatment; the safety outcomes such as the frequency and percentage of AEs determined by the percentage of patients who had AEs that occurred after receiving treatment, particularly severe adverse events (SAEs) such as death, life-threatening conditions, permanent or severe disability that resulted in the patient being hospitalized, requiring extended hospital stay, or developing HCC.
Assessment of the study quality and risk of bias
Two reviewers independently evaluated the quality of each study. The risk of publication bias in randomized studies was evaluated using tools from the Cochrane Collaboration.15 In each domain, studies was classified as having “low risk,” “unclear risk,” or “high risk” of bias. The risk of bias for observational studies was assessed using the modified Newcastle-Ottawa scale (NOS),16 which includes three dimensions: participant selection (maximum 4 points), comparability (maximum 2 points), and exposure or outcomes of study participants (maximum 3 points); Based on overall scores, studies were classified as high (≥7), fair (4–6), or low quality (≤4).
Statistical analysis
We performed a meta-analysis of the data using the meta and forest plot packages in R Statistics (3.6.1). The pooled SVR12 data were analyzed for efficacy outcomes. Subgroup meta-analyses of SVR12 were performed with stratification by treatment regimen, HCV genotype, treatment duration, treatment location, and decompensated liver cirrhosis. As the SVR rates in the majority of studies approached 100%, we performed a Freeman-Tukey double arcsine transformation of the combined values to stabilize the variance.17 Meta-regression was used to find difference in SVR rates between the two subgroups, and 95% confidence intervals (CIs) were calculated. The output the of meta-regression was back-transformed, and the difference between the intercept and estimate of the relevant variable was calculated. Safety data were pooled, and the analysis included the AEs, SAEs, HCC, and case-fatality rates. We then calculated the weighted difference and the pooled effect size using random-effect or fixed-effect models. To measure the overall heterogeneity across the included studies, we used the Cochrane Q test and I2 statistic, where an I2 value >50% or a Cochrane Q test p-value of <0.1 indicated significant heterogeneity. If heterogeneity was high, the random-effect model was used, otherwise a fixed-effect model was used. We used sensitivity analysis to explore the impact of individual studies on the overall results, deleting each study in turn to observe and evaluate whether the results of the remaining studies differed significantly. Publication bias was assessed by Egger’s Regression asymmetry test and funnel plots, with p<0.05 considered statistically significant.
Results
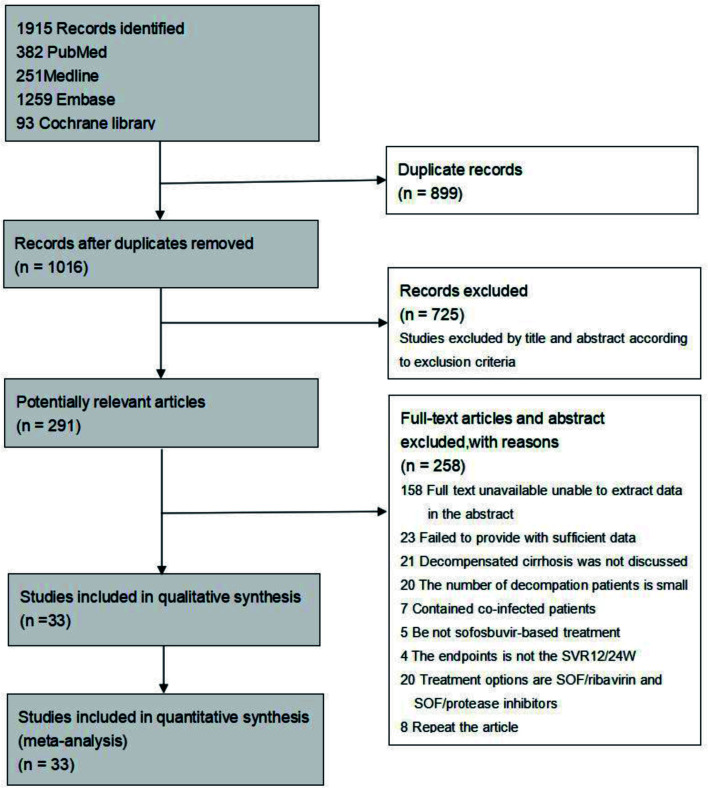
A total of 1,915 studies were identified in the initial search of the electronic databases. Of those, 33 articles met the inclusion criteria; the others were excluded. Sixteen were prospective cohort studies, nine were RCTs, and eight were retrospective analyses. Figure 1 shows the selection process and reasons for exclusion.
Fig. 1. Flow diagram of study selection.
Study characteristics
A total of 6,976 adult patients with HCV-related cirrhosis were enrolled in our meta-analysis. The characteristics of the included studies are summarized in Table 1.18–50 Studies published from 2015 to 2020 included 5,302 HCV patients with decompensated and 1,674 with compensated cirrhosis. However, the enrollment of study patients in those studies could have been before 2015. The majority of patients were Caucasian. The treatment regimens were SOF/VEL, SOF/LDV, and SOF/DCL for t 12 to 24 weeks. Ribavirin was added to the regimens in 28 studies. All RCTs were considered to be high quality studies based on the methods of randomization and allocation concealment, and were found to have low risk of bias in terms of attrition, outcome reporting, and detection. Of 24 nonrandomized cohort studies, 22 had NOS scores ≥7 and were considered to be of high quality. The remaining two studies were of medium quality with scores of 4–6 points. Comprehensive evaluation of the risk of bias is shown in Supplementary Table 2.
Table 1. Characteristics of the included studies.
| Author | Year | Study | Country | design | age | Sex Male (%) | Regimen | Duration | Genotype | SVR12/24 (DC) n/N | SVR12/24 (CC) n/N | DC/ALL (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takehara18 | 2019 | RCT | Japan | prospective | 66 (42, 83) | 39 (38) | SOF+VEL±RBV | 12 weeks | 1, 2, 3 | 94/102 | – | 100 |
| Ridruejo19 | 2019 | NO-RCT | Argentina , Brazil | prospective | 60±12 | – | SOF+DCV±RBV | 12/24 weeks | 1, 2, 3, 4 | 82/91 | 466/486 | 10 |
| Pellicelli20 | 2019 | NO-RCT | Rome, Italy | retrospective | 52.6±7.9 | – | SOF/DCV±RBV | 24 weeks | 3 | 23/28 | 199/205 | 12 |
| Sanai21 | 2018 | NO-RCT | Saudi Arabia | prospective | 61.1±10.9 | 30 (63) | SOF/LDV; SOF/LDV±RBV | 12/24 weeks | 4 | 43/48 | – | 22.5 |
| Garg22 | 2018 | NO-RCT | India | prospective | 47.2±11.51 | – | SOF+LDV±RBV; SOF+DCV±RBV | 12/24 weeks | 1, 3 | 30/41 | 33/36 | 38 |
| Young23 | 2017 | NO-RCT | European | retrospective | 56 | – | SOF+DCV±RBV | 24 weeks | 1, 3, other | 126/148 | 93/94 | 59.4 |
| Hezode24 | 2017 | NO-RCT | French | prospective | 54.3 (34–76) | – | SOF+DCV±RBV | 24 weeks | 3 | 23/43 | – | 9.3 |
| Goel25 | 2017 | NO-RCT | Indian | prospective | 45 (18–75) | – | SOF+DCV±RBV | 24 weeks | 3 | 18/22 | 28/30 | 18.8 |
| Fox26 | 2017 | NO-RCT | US | retrospective | – | – | SOF+LDV±RBV | 8/12 weeks | 1 | 1,133/1,299 | – | 21.5 |
| Dalgard27 | 2017 | NO-RCT | Scandinavia | retrospective | 54 | – | SOF+LDV+RBV; SOF+DCV±RBV | 12–24 weeks | 3 | 20/24 | 103/110 | 12.7 |
| Alonso28 | 2017 | NO-RCT | Spain | retrospective | 55 (8) | – | SOF+DCV±RBV; SOF+LDV±RBV | 12/24 weeks | 3 | 38/42 | 157/166 | 20.2 |
| Poordad29 | 2016 | NO-RCT | Spain | prospective | 58 (19–75) | – | SOF+DCV+RBV | 12 weeks | 1, 2, 3, 4 | 39/48 | – | 42.5 |
| Foster30 | 2016 | NO-RCT | UK | prospective | 54 (28–79) | 297 (73) | SOF+DCV±RBV; SOF+LDV±RBV | 12 weeks | 1, 3, other | 329/409 | – | 87.6 |
| Cheung31 | 2016 | NO-RCT | England | prospective | 54 (28–79) | – | SOF+DCV±RBV; SOF+LDV±RBV | 12 weeks | 1, 3, other | 317/406 | – | 82.8 |
| Backus32 | 2016 | NO-RCT | US | prospective | 61.66±6.0 | – | SOF+LDV±RBV | 8/12 weeks | 1 | 117/133 | – | 3.0 |
| Abaalkhail33 | 2016 | NO-RCT | Middle East | prospective | 59.5 | 13 (54) | SOF+LDV±RBV | 12/24 weeks | 4 | 20/24 | 36/37 | 21.6 |
| Leroy34 | 2016 | NO-RCT | France | prospective | 55 | – | SOF+DCV±RBV | 24 weeks | – | 78/93 | – | 100 |
| Petersen35 | 2016 | NO-RCT | European | prospective | – | – | SOF+DCV±RBV | 24 weeks | 1a, 1b, 3 | 123/147 | – | 100 |
| Flamm36 | 2019 | NO-RCT | France, USA | prospective | 55 (39–77) | 26 (81) | SOF+VEL+RBV | 12 weeks | 1, 2, 3, other | 25/32 | – | 100 |
| Zhang37 | 2019 | NO-RCT | Cambodian | retrospective | 59 (55–65) | 41 (38) | SOF+DCV±RBV | 12/24 weeks | – | 89/107 | – | 100 |
| El-Sherif38 | 2018 | RCT | US | prospective | 59 (54–62) | – | SOF+LDV+RBV; SOF+VEL±RBV | 12/24 weeks | 1, 2, 3, 4 | 509/594 | – | 100 |
| Abd Alla39 | 2018 | RCT | Egypt | prospective | 19–72 | – | SOF+LDV | 24 weeks | 4 | 42/50 | 25/25 | 66.7 |
| Welzel40 | 2016 | RCT | Germany | prospective | 57.0 (27–87) | – | SOF+DCV±RBV | 24 weeks | 1, 2, 3, 4, 5 | 131/165 | 200/223 | 34 |
| Manns41 | 2016 | RCT | Germany | prospective | 58 (54–62) | 113 (68) | SOF+LDV+RBV | 12 weeks | 1, 4 | 131/160 | 65/67 | 47.9 |
| Curry42 | 2015 | RCT | USA | prospective | 58 (43–72) | 186 (70) | SOF+VEL±RBV | 12 weeks | 1, 2, 3, 4, 6 | 234/267 | – | 100 |
| Troland43 | 2017 | RCT | Greater Glasgow | prospective | 49.4 (7.1) | – | SOF+DCV+RBV | 12 weeks | 3 | 21/25 | 24/26 | 43.1 |
| Bansal44 | 2017 | RCT | India | prospective | 50 (35–70) | – | SOF+DCV+RBV | 24 weeks | – | 31/32 | 42/42 | 17.2 |
| Liu45 | 2018 | RCT | China | prospective | 60 (27–85) | – | SOF+LDV±RBV; SOF+DCV±RBV; SOF+VEL±RBV | 12/24 weeks | 1, 2, 3, 4, 6 | 39/43 | 118/127 | 8.3 |
| Atsukawa46 | 2020 | NO-RCT | Japan | prospective | 65 (43–86) | 38 (59) | SOF+VEL | 12 weeks | 1, 2 | 61/64 | – | 100 |
| Gheorghe47 | 2020 | NO-RCT | Romania | retrospective | 61 (35–83) | – | SOF+LDV±RBV | 12/24 weeks | 1b | 174/209 | 123/140 | 100 |
| Tahata48 | 2020 | NO-RCT | Japan | prospective | 68 (40–87) | 43 (52) | SOF+VEL | 12 weeks | 1, 2, 3 | 74/82 | – | 42.2 |
| Takaoka49 | 2020 | NO-RCT | Japan | retrospective | 68 (62–72) | 39 (54) | SOF+VEL | 12 weeks | 1, 2 | 69/72 | – | 100 |
| Zhang50 | 2020 | NO-RCT | Cambodia | prospective | 60.2 (55.5–65.8) | – | SOF+DCV | 12/24 weeks | 1, 2, 6 | 175/264 | 2,235/2,494 | 2.9 |
N, total number of patients included in the study; n, number of patients with sustained viral response at 12/24 weeks after the end of treatment. DC, decompensated cirrhosis; SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; DCV, daclatasvir; RBV, ribavirin; RCT, random effects model.
Overall treatment outcomes of patients with decompensated cirrhosis
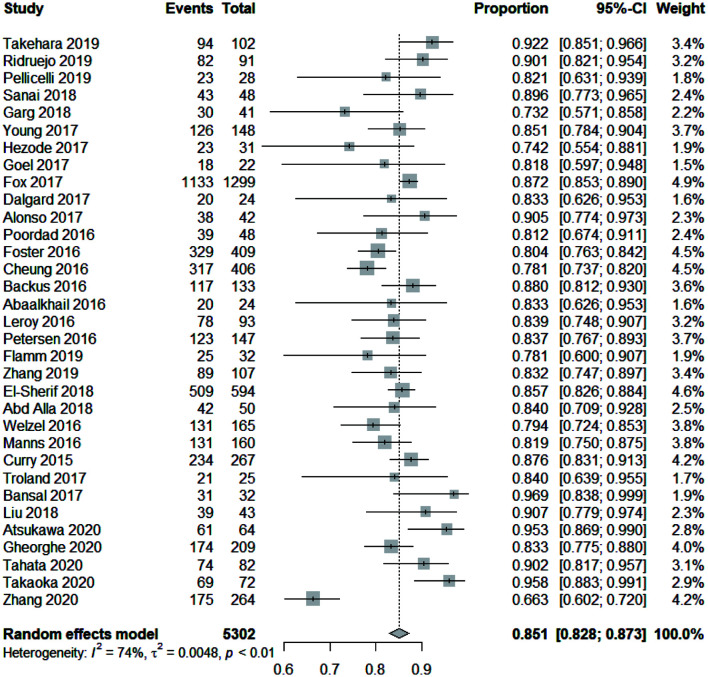
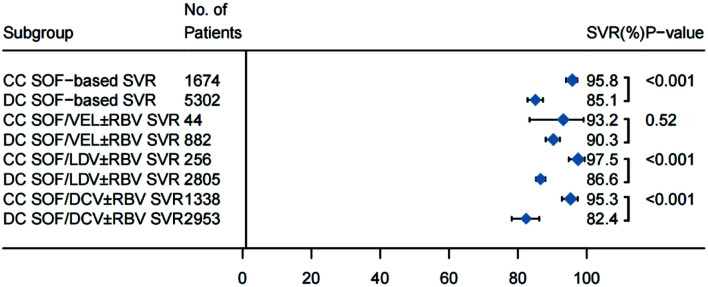
The treatment of cirrhotic patients with HCV with the aforementioned DAA regimens yielded high cure rates. The pooled SVR12 rate for all 5,302 patients with decompensated hepatitis C cirrhosis in the 33 studies was 85.1% (95% CI: 82.8–87.3). The random-effect model was used in the analysis because the I2=74% (χ2=0.0048, p<0.01). The forest plots of SVR12 rates are shown in Figure 2. For comparison of pooled SVR rates in HCV patients with cirrhosis at the compensated versus decompensated stages, we compiled the SVR data from 14 studies including 1,674 patients with compensated cirrhosis and 5,302 with decompensated cirrhosis (Fig. 3). A significantly higher pooled SVR rate was found in the compensated group [95.8% (95% CI: 94.0–97.3) vs. 85.1% (95% CI: 82.8–87.3); p<0.001)] (Supplementary Fig. 1). In a meta-regression model, the pooled SVR rate of patients with decompensated cirrhosis treated with DAA was 10.1% (95% CI: 6.6–13.6) lower than that of patients with compensated cirrhosis.
Fig. 2. Forest plots of SVR12 rates of all hepatitis C patients with cirrhosis.
The dotted vertical line and the diamond show the summary effect (random-effect model); outer edges show the 95% confidence intervals (CIs). SVR, sustained virologic response.
Fig. 3. Treatment outcomes of patients with compensated versus decompensated cirrhosis.
Horizontal bars are 95% confidence intervals (CIs) The box size indicates relative sample size. Two-tailed p-values <0.05 are significant (meta-regression).
When we analyzed the two groups following stratification by DAA regimen (Fig. 3, Supplementary Table 3), The pooled SVR rates remained significantly lower in the decompensated patients who received SOF/LDV±RBV [86.6% (95% CI: 85.3–88.0) vs. 97.5% (95% CI: 94.8–99.4); p<0.001)] (Supplementary Fig. 2), or SOF/DCL±RBV [82.4% (95% CI: 78.2–86.2) vs. 95.3% (95% CI: 92.8–97.4); p<0.001)] (Supplementary Fig. 3). When compared with patients with compensated cirrhosis, decompensated cirrhotic patients had a significantly lower pooled SVR rate [10.14% (95% CI: 5.8–16.4) with SOF/LDV±RBV and 12.0% (95% CI: 6.7–17.2)] with SOF/DCV±RBV. The combined SVR rates were similar in both groups (compensated vs. decompensated) when patients received SOF/VEL±RBV [93.2% (95% CI: 83.4–99.1) vs. 90.3% (95% CI: 88.1–92.2); p=0.52)] (Supplementary Fig. 4).
Subgroup SVR analysis by DAA regimens in decompensated patients
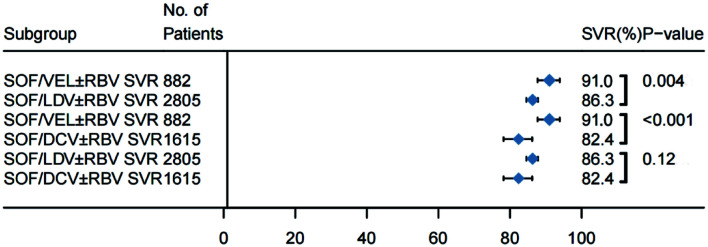
Further analysis with stratification by DAA regimen indicated that SOF/VEL±RBV had a significantly higher SVR12 rate than those of other regimens in decompensated cirrhotic patients. Nineteen of the 33 studies included a total of 2,805 patients with decompensated cirrhosis who were treated with SOF/LDV±RBV. Nineteen included 1,615 with decompensated cirrhosis and treated with SOF/DCV±RBV, and eight included 882 patients with decompensated cirrhosis treated with SOF/VEL±RBV. The pooled SVR rates (Fig. 4) of HCV patients decompensated cirrhosis treated with SOF/VEL±RBV was 91.0% (95% CI: 87.7–93.9), which was significantly higher than that of patients treated with SOF/LDV±RBV [(86.3% (95% CI: 84.6–87.8)] or SOF/DCV±RBV [82.4% (95% CI: 78.2–86.2)] (Supplementary Fig. 5). In the meta-regression model, patients treated with SOF/VEL±RBV had 8.3% (95% CI: 2.1–14.5) and 6.4% (95% CI: 3.2–9.8) higher SVR rates than patients treated with SOF/DCV±RBV and SOF/LDV±RBV, respectively.
Fig. 4. SVR12 rates of patients with decompensated cirrhosis on different regimens.
Horizontal bars are 95% confidence intervals (CIs); box size indicates relative sample size. (n=36). Two-tailed p-values <0.05 are significant (meta-regression).
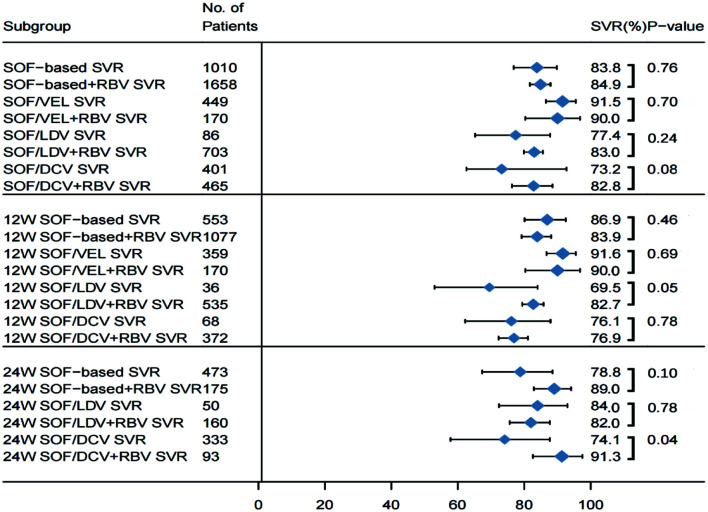
The impact of ribavirin on SVR in decompensated patients
To analyze the effect of ribavirin on treatment outcomes in HCV patients with decompensated cirrhosis, we compared the pooled SVR rates of 1,010 who received SOF-based DAA without RBV and 1,658 with RBV. In the meta-regression analysis (Fig. 5), the SVR rates in patients treated with RBV [83.8% (95% CI: 76.8–89.8)] and without RBV [84.9% (95% CI: 81.7–87.9)]; were not significantly different (p=0.76) (Supplementary Fig. 6). Subgroup analysis of different DAA regimens found that at both 12 versus 24 weeks of treatment, the effectiveness of achieving SVR was similar with or without use of RBV. The pooled data indicated that when the three regimens (SOF/LDV, SOF/DV, or SOF/VEL) were compared, differences in the enhancement of the SVR rate with the addition RBV to each therapy after 12 or 24 weeks of treatment were not significant. All regimens had similar pooled SVRs in this special population. Among those who received SOF/VEL, adding RBV (n=170) did not significantly increase the SVR rate compared with the patients (n=449) treated without RBV [90.0% (95% CI: 80.3–96.8) vs. 91.5% (95% CI: 86.5–95.5); p=0.70] (Supplementary Fig. 7).
Fig. 5. Outcomes of decompensated patients treated with and without RBV.
Horizontal bars are 95% confidence intervals (CIs); box size indicates relative sample size. (n=36). Two-tailed p-values <0.05 are significant (meta-regression).
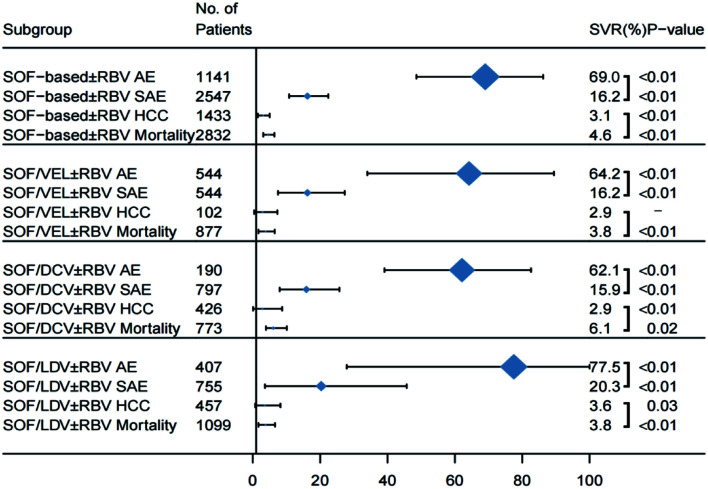
Overall safety outcomes of DAA therapy in decompensated patients
Of the 33 studies reviewed, 8/33, 15/33, 7/33, and 16/33 studies that reported safety data in terms of AEs, SAEs, HCC, and case-fatality in the total of 1,141, 2,547, 1,433, and 2,832 study patients, respectively (Fig. 6). The overall heterogeneity across the studies of the four safety outcomes was high, with an I2 of >50% for each assessment. A random-effect model was used for the analysis of AEs, SAEs, HCC, and case-fatality across the studies. The compiled percentage of the AEs (i.e. one or more AE including headache, dizziness, nausea, vomiting, diarrhea, and the others reported by the investigators) was 69.0% (95% CI: 48.6–86.2). In addition, the percentage of patients who discontinued DAA treatment because of AEs (Supplementary Table 4) was 3.2% (95% CI: 1.5–5.2). The pooled percentage of SAEs, naïve in the onset of acute myocardial infarction, chronic obstructive pulmonary disease, epilepsy, mania, and others, was 16.2% (95% CI: 10.8–22.4). The pooled percentage of HCC was 3.1% (95% CI: 1.5–5.0) and that of death was 4.6% (95% CI: 3.1–6.3) in decompensated patients who received DAA therapy, respectively (Fig. 6).
Fig. 6. Pooled safety results of patients with decompensated cirrhosis.
Dotted vertical line and diamond show the summary effect (random-effect model); outer edge shows 95% confidence intervals (CIs). AEs, adverse reactions; SAEs, serious adverse reactions; HCC, hepatocellular carcinoma.
In patients who were treated with SOF/VEL±RBV, the reported percentages of AEs, SAEs, HCC, and mortality were 64.2% (95% CI: 34.0–89.4), 16.2% (95% CI: 7.5–27.3), 2.9% (95% CI: 0.4–7.3), and 3.8% (95% CI: 1.7–6.5); respectively (Supplementary Fig. 8). In patients treated with SOF/DCV±RBV, the percentages of AEs, SAEs, HCC, and mortality were 62.1% (95% CI: 39.1–82.6), 15.9% (95% CI: 8.0–25.7), 2.9% (95% CI: 0.1–8.7), and 6.7% (95% CI: 3.9–10.1); respectively. Lastly, the percentages of AEs, SAEs, HCC, and mortality in patients treated with SOF/LDV±RBV were 77.5% (95% CI: 27.9–100), 20.3% (95% CI: 3.6–45.7), 3.6% (95% CI: 0.7–8.2), and 3.8% (95% CI: 1.7–6.6); respectively.
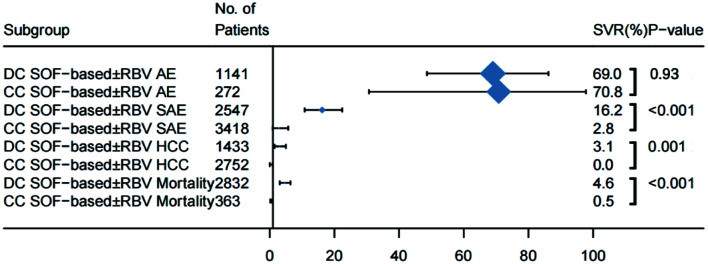
Safety profiles of DAAs in decompensated versus compensated patients
The difference in incidence of AEs in decompensated and compensated patients treated with DAAs [69.0% (95% CI: 48.6–86.2) vs. 70.8% (95% CI: (30.7–97.8); p=0.93)] was not significant. However, patients with decompensated cirrhosis had a significantly higher frequency of SAEs [2% (95% CI: 10.8–22.4) vs. 2.8% (95% CI: 0.9–5.7%); p<0.001], incidence of HCC [3.1% (95% CI: 1.5–5.0) vs. 0.0% (95% CI: 0.0–0.9); p=0.001); and case-fatality rate [4.6% (95% CI: 3.1–6.3) vs. 0.5% (95% CI: 0.2–0.); p<0.001) on DAA therapy (Supplementary Figs. 9–11, and Supplementary Table 5).
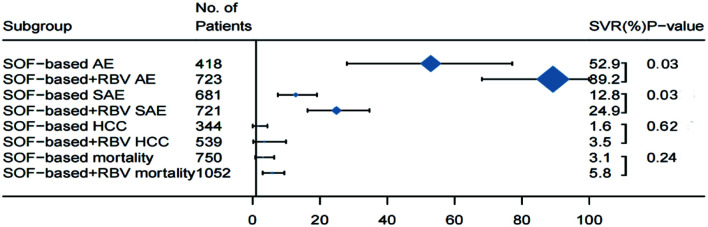
Safety analysis in decompensated patients treated with or without RBV
To gain a better understanding of the safety profile in decompensated patients treated with regimens containing RBV, we compared the pooled safety data with those of patients on DAA therapy without RBV (Fig. 7). Patients who were treated with RBV (n=723) had a significantly higher frequency of AEs [89.2% (95% CI: 68.1–99.9) vs. 52.9% (95% CI: 28.0–77.7); p=0.03] (Supplementary Fig. 12), compared with those without RBV treatment (n=418). The frequency of SAEs in patients treated with RBV (n=721) was also significantly higher than that of patients (n=681) who were given DAAs without RBV [24.9% (95% CI: 16.3–34.7) vs. 12.8% (95% CI: 7.5–19.1); p=0.03] (Supplementary Fig. 13). In the meta-regression analysis, the frequencies of AEs and SAEs in patients treated with RBV increased by 33.3% (95% CI: 8.9–57.8) and 11.1% (95% CI: 1.7–23.9), respectively. The adverse effects associated with adding RBV and extending treatment duration are shown in Supplementary Table 5. There were no significant differences (p>0.05) in the frequencies of HCC and case-fatality rates between the two treatment methods (Fig. 8). In patients treated with SOF/VEL (Supplementary Fig. 14), the frequency of AEs was significantly higher in those with RBV than in those without RBV [50.2% (95% CI: 18.9–81.4) vs. 91.1% (95% CI: 85.4–95.6); p=0.008). The results are consistent with the overall safety profile for patients with RBV treatment.
Fig. 7. Safety profiles for decompensated patients treated with and without RBV.
Horizontal bars are 95% confidence intervals (CIs); box size indicates relative sample size. (n=36). Two-tailed p-values <0.05 are significant (meta-regression).
Fig. 8. Safety profiles for decompensated and compensated patients treated with and without RBV.
Horizontal bars are 95% confidence intervals (CIs); box size indicates relative sample size. (n=36). Two-tailed p-values <0.05 are significant (meta-regression).
Publication bias
A sensitivity analysis of the effect on the overall results by excluding individual studies showed that the pooled SVR was not significantly changed by exclusion of any one of the 33 studies, indicating the robustness of the current analysis. Egger’s funnel plots (Supplementary Fig. 15) showed that the meta-analysis had no significant publication bias (p=0.83) as the plot of the included studies was symmetrical (Supplementary Fig. 16).
Discussion
SOF-based regimens are recommended by AASLD-IDSA and EASL guidelines for the treatment of decompensated cirrhotic patients with HCV. Both guidelines suggest SOF/VEL+RBV for 12 weeks (preferred) or SOF/VEL without RBV for 24 weeks (for RBV contraindications or poor tolerance) for decompensated patients. In addition, the AASLD and IDSA guidelines also recommend SOF/LDV+RBV for 12 weeks (preferred) or SOF/VEL without RBV for 24 weeks (for RBV contraindications or poor tolerance). The recommendations are based on the findings of a few pivotal or cohort studies including SOLAR-1, SOLAR-2, ASTRAL-4, and HCV-TARGET, and the US chorionic hepatitis cohort study.8,41,42,51 However, the meta-analysis data was not available when the guidelines were published.
To the best of our knowledge, this study including 33 RCTs or cohort study data is the first meta-analysis for HCV patients with decompensated cirrhosis. We found that SOF-based regimes in patients with decompensated cirrhosis had a 10.1% (95% CI: 6.6–13.6) points lower pooled SVR rate than that in patients with compensated disease. However, SVR rates in the two groups (compensated vs. decompensated) were similar when patients received SOF/VEL±RBV therapy (93.2% vs. 90.3%; p=0.52), which suggests that decompensated status did not negatively impact the SVR achieved with the SOF/VEL regimen. A subgroup analyses confirmed that SOF/VEL±RBV therapy had a significantly higher SVR rate (91.0) for decompensated cirrhotic patients compared with SOF/LDV±RBV regimens (86.3%, p=0.004), or SOF/DCV±RBV (82.4%, p<0.001). The findings further support SOF/VEL±RBV as the first-line treatment in this subpopulation. Our study provides new evidence to address the discrepancy between the EASL and AASLD-IDSA guidelines for the use of SOF/LDV±RBV for decompensated patients. Most important, the current meta-analysis demonstrated that adding RBV to SOF/LDV or SOF/VEL regimens failed to improve the pooled SVRs, but RBV increased the frequency and severity of AEs. Pooled data pointed in the direction of using 12 weeks of SOF/VEL for decompensated patients without RBV as the optimal regimen. The pooled SVR of 359 patients was 91.6. Lastly, our meta-analysis showed that decompensated patients on DAAs had a higher frequency of SAEs, incidence of HCC, and case-fatality rate compared with those of compensated patients. The findings further support the guideline approaches of close monitoring during the DAA treatment. In patients who could not tolerate VEL for HCV treatment, the alternative might be SOF/LDV without ribavirin for 12 weeks or SOF/DCV with ribavirin for 24 weeks.
Several study limitations should be discussed. The meta-analysis did not include the study by Lu et al.52 that was cited by EASL guidelines to support the use of RBV combined with the SOF/VEL regimen. In that study, the odds ratio of SVR in decompensated patients who received SOF/VEL with RBV (n=1,135) and without RBV(n=2,996) were 0.48 (95% CI: 0.27–0.86) and 0.13 (95% CI: 0.07–0.24), respectively. We did not include Lu et al.52 in this meta-analysis because it did not report the number of patients who achieved a SVR in the regimen we studied, which met the exclusion criteria of our study. In their study, patient data were compiled together including the first, second, and the third generation of DAA treatment for decompensated cirrhotic patients and the use of RBV was associated with higher SVR in a multivariate model.52 Although the current analysis did not show the enhancement of SVR when adding RBV to genotype 3 patients with liver decompensation, the number of patients in comparison was limited to less than 70 patients. Further meta-analysis is needed to confirm these findings when more genotype 3 studies are available. Another general concern for a meta-analysis is the significant heterogeneity of studies included in the meta-analysis. To minimize such impact on the analysis, we had performed comparisons of SVRs for the subgroups including 12-week therapy vs. 24-week therapy, Asian vs. non-Asian patients, and RCT vs. non-RCT studies. Based on the subgroup analyses, there were no heterogeneity sources in our study. Furthermore, the majority of published studies were non-RCTs. We did not assess pooled data for RCTs because two RCTs used the SOF/VEL±RBV regimen and had relatively small sample sizes. Other possible selection biases inherit in a meta-analysis design included data from studies in different practice settings, patient enrollment criteria, or baseline values at enrollment. Finally, most of the studies were uncontrolled single-arm studies, which limited the ability to draw firm conclusions about the safety and effectiveness of the protocol. Despite the above limitations, the strength of our meta-analysis lies in its exhaustive literature research, well-defined approach for data selection and extraction, comprehensive statistical analyses, reporting in accord with PRISMA statements, and no significant evidence of publication bias.
In conclusion, our meta-analysis showed that SOF/VEL±RBV regimens had a significantly higher pooled SVR rate (91.0%) for decompensated cirrhotic patients compared with SOF/LDV±RBV (86.3%, p=0.004) and SOF/DCV±RBV (82.4% p<0.001). In addition, patients treated with SOF/VEL without RBV for 12 weeks achieved an SVR of 91.5%, which was similar to that of SOF/VEL+RBV (90.0). There was no data on SOF /VEL±RBV for 24 weeks, and adding RBV to SOF-based regimens increased the overall frequency of AEs or SAEs, our results suggest that SOF/VEL regimen without RBV was the best option in the clinical setting for HCV patients with liver decompensation when considering the efficacy and the AEs. Our findings have very important clinical implications that may serve as the evidence base for selecting SOF/VEL as the first-line treatment without RBV and potentially change future guidelines or the standard of clinical practice. SOF/LDV should be avoided because of the inferior efficacy in decompensated patients when compared with SOF/VEL. In patients who must be treated with SOF/LDV (n=36), it was not clear whether 24 weeks of therapy had a significantly higher SVR rate than that achieved with 12 weeks of therapy (n=50) because of the relatively small sample size. However, adding RBV had no significant impact on the SVR with either 12 or 24 weeks of SOF/LDV therapy. Our analysis also highlighted the need for future studies in decompensated patients who failed SOF/VEL (about 10) as the SVR rates were significantly lower in decompensated patients than in noncirrhotic patients.
Supporting information
The combined SVR of SOF-based regimen in the treatment of patients with compensated cirrhosis was higher than that in the decompensated period: 95.8% (95% CI: 94.0–97.3) vs. 85.1% (95% CI: 82.8–87.3), p<0.0001. SOF, sofosbuvir; DC, decompensated cirrhosis; CC, Compensatory cirrhosis; SVR, sustained virologic response.
The combined SVR of SOF/LDV regimen in the treatment of patients with compensated cirrhosis was higher than that in the decompensated period: 97.5% (95% CI: 94.8–99.4) vs. 86.6% (95% CI: 85.3–88.0), p<0.0001. SOF, sofosbuvir; LDV, ledipasvir; RBV, ribavirin; SVR: sustained virologic response; DC, decompensated cirrhosis; CC, compensated cirrhosis.
The combined SVR of SOF/DCV regimen in the treatment of patients with compensated cirrhosis was significantly higher than that in the decompensated period: 95.3% (95% CI: 92.8–97.4) vs. 82.4% (95% CI: 78.2–86.2), p<0.0001. SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavirin; SVR: sustained virologic response; DC, decompensated cirrhosis; CC, compensated cirrhosis.
There was no statistical difference between the combined SVR and the decompensated phase of SOF/VEL regimen in the treatment of patients with compensated liver cirrhosis (93.2%, 95% CI: 83.4–99.1% vs. 90.3%, 95% CI: 88.1–92.2%, p=0.5238). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; DC, decompensated cirrhosis; CC, compensatory cirrhosis; SVR, sustained virologic response.
The SVR rates were 91.0% (95% CI: 87.7–93.9) with SOF/VEL, n=882; 86.3% (95% CI: 84.6–87.8), n=2,805 with SOF/LDV; and 82.4% (95% CI: 78.2–86.2) with SOF/DCV, n=1,615; p=0.0023. SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; DCV, daclatasvir; RBV, ribavirin; SVR, sustained virologic response.
There was no statistically significant difference in the SVR in groups treated with a SOF/DAA regimen with RBV and without RBV: 83.8% (95% CI: 76.8–89.8) vs. 84.9% (95% CI: 81.7–87.9), p=0.7634. SOF, sofosbuvir; RBV, ribavirin; DAA, direct-acting antiviral; SVR, sustained virologic response.
There was no statistically significant difference in in the SVR rates with and without RBV: 91.5% (95% CI: 86.5–95.5) vs. 90.0% (95% CI: 80.3–96.8), p=0.7019. SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; SVR, sustained virologic response.
AE 64.2% (95% CI: 34–89.4). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse events.
The incidence of SAEs was significantly higher in patients with decompensated hepatitis C cirrhosis than that in compensated patients: 16.2% (95% CI: 10.8–22.4) vs. 2.8% (95% CI: 0.9–5.7) p<0.0001). SOF, sofosbuvir; SAE, serious adverse events; DC, decompensated cirrhosis; CC, compensated cirrhosis.
The incidence of death was significantly higher in patients with decompensated hepatitis C cirrhosis than that in compensated patients: 3.1% (95% CI: 1.5–5.0) vs. 0.0% (95% CI: 0.0–0.9), p=0.0014. SOF, sofosbuvir; DC, decompensated cirrhosis; CC, compensatory cirrhosis; HCC, hepatocellular carcinoma.
The incidence of combined deaths was significantly higher in patients with decompensated than in compensated hepatitis C cirrhosis: 4.6% (95% CI: 3.1–6.3) vs. 0.5% (95% CI: 0.2–0.8), p<0.0001). SOF: sofosbuvir; DC: decompensated cirrhosis; CC, compensated cirrhosis; Mortality, death.
Mortality was increased if RBV was added to SOF-based DAA treatment: 89.2% (95% CI: 68.1–99.9) vs. 52.9% (95% CI: 28.0–77.1), p=0.0253. SOF, sofosbuvir; RBV, ribavirin; SVR, sustained virologic response; AE, adverse events.
The SAEs associated with SOF-based DAA treatment were increased when RBV was added: 24.9% (95% CI: 16.3–34.7) vs. 12.8 (95% CI: 7.5–19.1), p=0.0255. SOF, sofosbuvir; RBV, ribavirin; SVR, sustained virological response; SAE, serious adverse events.
The AE associated with treatment increased when RBV was added to SOF/VEL: 91.1% (95% CI: 85.4–95.6) vs. 50.2% (95% CI: 18.9–81.4), p=0.0077). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse events.
There was no publication bias in the studies included in the meta-analysis (p=0.9218).
The funnel chart is roughly symmetrical, indicating that there was no publication bias.
Acknowledgments
We thank Dr Lu Yin (Medical Research and Biometrics Center, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Fuwai Hospital) for her assistance with data analyses. The study was selected by AASLD as a poster presentation, the abstract was available to the public electronically on the AASLD website in early October 2021 and published in the October 2021 supplement of Hepatology.
Abbreviations
- AE
adverse event
- CC
compensated liver cirrhosis
- DAA
direct-acting antiviral
- DC
decompensated liver cirrhosis
- DCV
daclatasvir
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LDV
ledipasvir
- RBV
ribavirin
- SAE
serious adverse event
- SOF
sofosbuvir
- SVR
sustained virological response
- VEL
velpatasvir
Data sharing statement
All data are available upon request
References
- 1.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveria Andrade LJ, D’Oliveira A, Melo RC, De Souza EC, Costa Silva CA, Paraná R. Association between hepatitis C and hepatocellular carcinoma. J Glob Infect Dis. 2009;1(1):33–37. doi: 10.4103/0974-777X.52979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Fan X, Deng H, Zhang X, Zhang K, Li N, et al. Efficacy and safety of glecaprevir/pibrentasvir for chronic hepatitis C virus genotypes 1-6 infection: A systematic review and meta-analysis. Int J Antimicrob Agents. 2019;54(6):780–789. doi: 10.1016/j.ijantimicag.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Kamal-Yanni M. Hepatitis C drug affordability. Lancet Glob Health. 2015;3(2):e73–e74. doi: 10.1016/S2214-109X(14)70365-1. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver; Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73(5):1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Hesamizadeh K, Sharafi H, Rezaee-Zavareh MS, Behnava B, Alavian SM. Next Steps Toward Eradication of Hepatitis C in the Era of Direct Acting Antivirals. Hepat Mon. 2016;16(4):e37089. doi: 10.5812/hepatmon.37089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Poordad F, Shiffman ML, Ghesquiere W, Wong A, Huhn GD, Wong F, et al. Daclatasvir and sofosbuvir with ribavirin for 24 weeks in chronic hepatitis C genotype-3-infected patients with cirrhosis: a Phase III study (ALLY-3C) Antivir Ther. 2019;24(1):35–44. doi: 10.3851/IMP3278. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152(5):1090–1099.e1. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 12.Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 13.Hézode C, Bronowicki JP. Ideal oral combinations to eradicate HCV: The role of ribavirin. J Hepatol. 2016;64(1):215–225. doi: 10.1016/j.jhep.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2016;70:68–89. doi: 10.1016/j.jclinepi.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7(5):e2195. doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takehara T, Sakamoto N, Nishiguchi S, Ikeda F, Tatsumi T, Ueno Y, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54(1):87–95. doi: 10.1007/s00535-018-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridruejo E, Cheinquer H, Marciano S, Mendizabal M, Piñero F, Wolff FH, et al. B.A.R.C.O.S. (Brazilian Argentine Hepatitis C Collaborative Observational Study): Effectiveness and clinical outcomes of HCV treatment with daclatasvir and sofosbuvir with or without ribavirin. J Viral Hepat. 2019;26(10):1200–1209. doi: 10.1111/jvh.13148. [DOI] [PubMed] [Google Scholar]
- 20.Pellicelli A, Messina V, Giannelli V, Distefano M, Palitti VP, Vignally P, et al. High Efficacy and Safety of Flat-Dose Ribavirin Plus Sofosbuvir/Daclatasvir in Genotype 3 Cirrhotic Patients. Gut Liver. 2020;14(3):357–367. doi: 10.5009/gnl18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanai FM, Altraif IH, Alswat K, AlZanbagi A, Babatin MA, AlMousa A, et al. Real life efficacy of ledipasvir/sofosbuvir in hepatitis C genotype 4-infected patients with advanced liver fibrosis and decompensated cirrhosis. J Infect. 2018;76(6):536–542. doi: 10.1016/j.jinf.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Garg G, Dixit VK, Shukla SK, Singh SK, Sachan S, Tiwari A, et al. Impact of Direct Acting Antiviral Drugs in Treatment Naïve HCV Cirrhosis on Fibrosis and Severity of Liver Disease: A Real Life Experience from a Tertiary Care Center of North India. J Clin Exp Hepatol. 2018;8(3):241–249. doi: 10.1016/j.jceh.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young J, Weis N, Hofer H, Irving W, Weiland O, Giostra E, et al. The effectiveness of daclatasvir based therapy in European patients with chronic hepatitis C and advanced liver disease. BMC Infect Dis. 2017;17(1):45. doi: 10.1186/s12879-016-2106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hézode C, Lebray P, De Ledinghen V, Zoulim F, Di Martino V, Boyer N, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, for hepatitis C virus genotype 3 in a French early access programme. Liver Int. 2017;37(9):1314–1324. doi: 10.1111/liv.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel A, Bhargava R, Rai P, Aggarwal R. Treatment of chronic genotype-3 hepatitis C virus infection using direct-acting antiviral agents: An Indian experience. Indian J Gastroenterol. 2017;36(3):227–234. doi: 10.1007/s12664-017-0763-3. [DOI] [PubMed] [Google Scholar]
- 26.Fox DS, McGinnis JJ, Tonnu-Mihara IQ, McCombs JS. Comparative treatment effectiveness of direct acting antiviral regimens for hepatitis C: Data from the Veterans administration. J Gastroenterol Hepatol. 2017;32(6):1136–1142. doi: 10.1111/jgh.13652. [DOI] [PubMed] [Google Scholar]
- 27.Dalgard O, Weiland O, Noraberg G, Karlsen L, Heggelund L, Färkkilâ M, et al. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study. PLoS One. 2017;12(7):e0179764. doi: 10.1371/journal.pone.0179764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso S, Riveiro-Barciela M, Fernandez I, Rincón D, Real Y, Llerena S, et al. Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort. J Viral Hepat. 2017;24(4):304–311. doi: 10.1111/jvh.12648. [DOI] [PubMed] [Google Scholar]
- 29.Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, et al. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63(5):1493–1505. doi: 10.1002/hep.28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64(6):1224–1231. doi: 10.1016/j.jhep.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65(4):741–747. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64(2):405–414. doi: 10.1002/hep.28625. [DOI] [PubMed] [Google Scholar]
- 33.Abaalkhail F, Elsiesy H, Elbeshbeshy H, Shawkat M, Yousif S, Ullah W, et al. Treatment of Patients With Hepatitis C Virus Infection With Ledipasvir-Sofosbuvir in the Liver Transplant Setting. Transplantation. 2017;101(11):2739–2745. doi: 10.1097/TP.0000000000001907. [DOI] [PubMed] [Google Scholar]
- 34.Leroy V, Hezode C, Metivier S, Tateo M, Conti F, Nguyen-Khac E, et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV infection and decompensated cirrhosis: Interi renchysis of a french multicentre compassionate use program. The International Liver Congress™, EASL - European Association for the Study of the Liver, 2016 April 13-17, Barcelona, Spain.
- 35.Petersen J, Welzel TM, Herzer K, Ferenci P, Gschwantler M, Cornberg M, et al. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of chronic HCV infection in patients with decompensated cirrhosis: Results of a European multicenter compassionate use program. The International Liver Congress™, EASL - European Association for the Study of the Liver, 2016 April 13-17, Barcelona, Spain.
- 36.Flamm S, Lawitz E, Borg B, Charlton M, Landis C, Reddy R. High efficacy and improvement in CPT class with sofosbuvir/velpatasvir plus ribavirin for 12 weeks in patients with CPT C decompensated cirrhosis. The International Liver Congress™, EASL – European Association for the Study of the Liver, 2019 April 10-14, Vienna, Austria.
- 37.Zhang M. Safety and efficacy of Daclatasvir/sofosbuvir in HCV-infected decompensated cirrhosis patients: A Cambodian retrospective cohort study. Hepatol Int. 2019 [Google Scholar]
- 38.El-Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, et al. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology. 2018;154(8):2111–2121.e8. doi: 10.1053/j.gastro.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Abd Alla MDA, El Awady MK, Dawood RM, Elhawary MA, Al-Azhari SS, Galal AGM. Hepatitis C virus serologic relapse after treatment with direct-acting antivirals is dependent on viral RNA levels in peripheral blood mononuclear cells and the grade of liver cirrhosis. Arch Virol. 2018;163(10):2765–2774. doi: 10.1007/s00705-018-3922-7. [DOI] [PubMed] [Google Scholar]
- 40.Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65(11):1861–1870. doi: 10.1136/gutjnl-2016-312444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16(6):685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 42.Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 43.Troland D, Fox R, Morris AJ, Priest M. 12 weeks of sofosbuvir, daclatasvir and ribavirin for GT3 patients with cirrhosis. J Hepatol. 2017;66(1):S304. [Google Scholar]
- 44.Ricardo AC, Chen J, Toth-Manikowski SM, Meza N, Joo M, Gupta S, et al. Hispanic ethnicity and mortality among critically ill patients with COVID-19. PLoS One. 2022;17(5):e0268022. doi: 10.1371/journal.pone.0268022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu CH, Huang YJ, Yang SS, Chang CH, Yang SS, Sun HY, et al. Generic sofosbuvir-based interferon-free direct acting antiviral agents for patients with chronic hepatitis C virus infection: a real-world multicenter observational study. Sci Rep. 2018;8(1):13699. doi: 10.1038/s41598-018-32060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atsukawa M, Tsubota A, Kondo C, Toyoda H, Nakamuta M, Takaguchi K, et al. Real-World Clinical Application of 12-Week Sofosbuvir/Velpatasvir Treatment for Decompensated Cirrhotic Patients with Genotype 1 and 2: A Prospective, Multicenter Study. Infect Dis Ther. 2020;9(4):851–866. doi: 10.1007/s40121-020-00329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gheorghe LS, Preda C, Iliescu L, Istratescu D, Chifulescu AE, Pop CS, et al. Efficacy and Safety of Ledispavir/Sofosbuvir with or without Ribavirin in patients with Decompensated Liver Cirrhosis and Hepatitis C Infection: a Cohort Study. J Gastrointestin Liver Dis. 2020;29(3):385–390. doi: 10.15403/jgld-2448. [DOI] [PubMed] [Google Scholar]
- 48.Tahata Y, Hikita H, Mochida S, Kawada N, Enomoto N, Ido A, et al. Sofosbuvir plus velpatasvir treatment for hepatitis C virus in patients with decompensated cirrhosis: a Japanese real-world multicenter study. J Gastroenterol. 2021;56(1):67–77. doi: 10.1007/s00535-020-01733-4. [DOI] [PubMed] [Google Scholar]
- 49.Takaoka Y, Miura K, Morimoto N, Ikegami T, Kakizaki S, Sato K, et al. Real-world efficacy and safety of 12-week sofosbuvir/velpatasvir treatment for patients with decompensated liver cirrhosis caused by hepatitis C virus infection. Hepatol Res. 2021;51(1):51–61. doi: 10.1111/hepr.13576. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, O’Keefe D, Iwamoto M, Sann K, Kien A, Hang V, et al. High sustained viral response rate in patients with hepatitis C using generic sofosbuvir and daclatasvir in Phnom Penh, Cambodia. J Viral Hepat. 2020;27(9):886–895. doi: 10.1111/jvh.13311. [DOI] [PubMed] [Google Scholar]
- 51.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66(4):1090–1101. doi: 10.1002/hep.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu M, Wu KH, Li J, Moorman AC, Spradling PR, Teshale EH, et al. Adjuvant ribavirin and longer direct-acting antiviral treatment duration improve sustained virological response among hepatitis C patients at risk of treatment failure. J Viral Hepat. 2019;26(10):1210–1217. doi: 10.1111/jvh.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The combined SVR of SOF-based regimen in the treatment of patients with compensated cirrhosis was higher than that in the decompensated period: 95.8% (95% CI: 94.0–97.3) vs. 85.1% (95% CI: 82.8–87.3), p<0.0001. SOF, sofosbuvir; DC, decompensated cirrhosis; CC, Compensatory cirrhosis; SVR, sustained virologic response.
The combined SVR of SOF/LDV regimen in the treatment of patients with compensated cirrhosis was higher than that in the decompensated period: 97.5% (95% CI: 94.8–99.4) vs. 86.6% (95% CI: 85.3–88.0), p<0.0001. SOF, sofosbuvir; LDV, ledipasvir; RBV, ribavirin; SVR: sustained virologic response; DC, decompensated cirrhosis; CC, compensated cirrhosis.
The combined SVR of SOF/DCV regimen in the treatment of patients with compensated cirrhosis was significantly higher than that in the decompensated period: 95.3% (95% CI: 92.8–97.4) vs. 82.4% (95% CI: 78.2–86.2), p<0.0001. SOF, sofosbuvir; DCV, daclatasvir; RBV, ribavirin; SVR: sustained virologic response; DC, decompensated cirrhosis; CC, compensated cirrhosis.
There was no statistical difference between the combined SVR and the decompensated phase of SOF/VEL regimen in the treatment of patients with compensated liver cirrhosis (93.2%, 95% CI: 83.4–99.1% vs. 90.3%, 95% CI: 88.1–92.2%, p=0.5238). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; DC, decompensated cirrhosis; CC, compensatory cirrhosis; SVR, sustained virologic response.
The SVR rates were 91.0% (95% CI: 87.7–93.9) with SOF/VEL, n=882; 86.3% (95% CI: 84.6–87.8), n=2,805 with SOF/LDV; and 82.4% (95% CI: 78.2–86.2) with SOF/DCV, n=1,615; p=0.0023. SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir; DCV, daclatasvir; RBV, ribavirin; SVR, sustained virologic response.
There was no statistically significant difference in the SVR in groups treated with a SOF/DAA regimen with RBV and without RBV: 83.8% (95% CI: 76.8–89.8) vs. 84.9% (95% CI: 81.7–87.9), p=0.7634. SOF, sofosbuvir; RBV, ribavirin; DAA, direct-acting antiviral; SVR, sustained virologic response.
There was no statistically significant difference in in the SVR rates with and without RBV: 91.5% (95% CI: 86.5–95.5) vs. 90.0% (95% CI: 80.3–96.8), p=0.7019. SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; SVR, sustained virologic response.
AE 64.2% (95% CI: 34–89.4). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse events.
The incidence of SAEs was significantly higher in patients with decompensated hepatitis C cirrhosis than that in compensated patients: 16.2% (95% CI: 10.8–22.4) vs. 2.8% (95% CI: 0.9–5.7) p<0.0001). SOF, sofosbuvir; SAE, serious adverse events; DC, decompensated cirrhosis; CC, compensated cirrhosis.
The incidence of death was significantly higher in patients with decompensated hepatitis C cirrhosis than that in compensated patients: 3.1% (95% CI: 1.5–5.0) vs. 0.0% (95% CI: 0.0–0.9), p=0.0014. SOF, sofosbuvir; DC, decompensated cirrhosis; CC, compensatory cirrhosis; HCC, hepatocellular carcinoma.
The incidence of combined deaths was significantly higher in patients with decompensated than in compensated hepatitis C cirrhosis: 4.6% (95% CI: 3.1–6.3) vs. 0.5% (95% CI: 0.2–0.8), p<0.0001). SOF: sofosbuvir; DC: decompensated cirrhosis; CC, compensated cirrhosis; Mortality, death.
Mortality was increased if RBV was added to SOF-based DAA treatment: 89.2% (95% CI: 68.1–99.9) vs. 52.9% (95% CI: 28.0–77.1), p=0.0253. SOF, sofosbuvir; RBV, ribavirin; SVR, sustained virologic response; AE, adverse events.
The SAEs associated with SOF-based DAA treatment were increased when RBV was added: 24.9% (95% CI: 16.3–34.7) vs. 12.8 (95% CI: 7.5–19.1), p=0.0255. SOF, sofosbuvir; RBV, ribavirin; SVR, sustained virological response; SAE, serious adverse events.
The AE associated with treatment increased when RBV was added to SOF/VEL: 91.1% (95% CI: 85.4–95.6) vs. 50.2% (95% CI: 18.9–81.4), p=0.0077). SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse events.
There was no publication bias in the studies included in the meta-analysis (p=0.9218).
The funnel chart is roughly symmetrical, indicating that there was no publication bias.