Abstract
Abstract
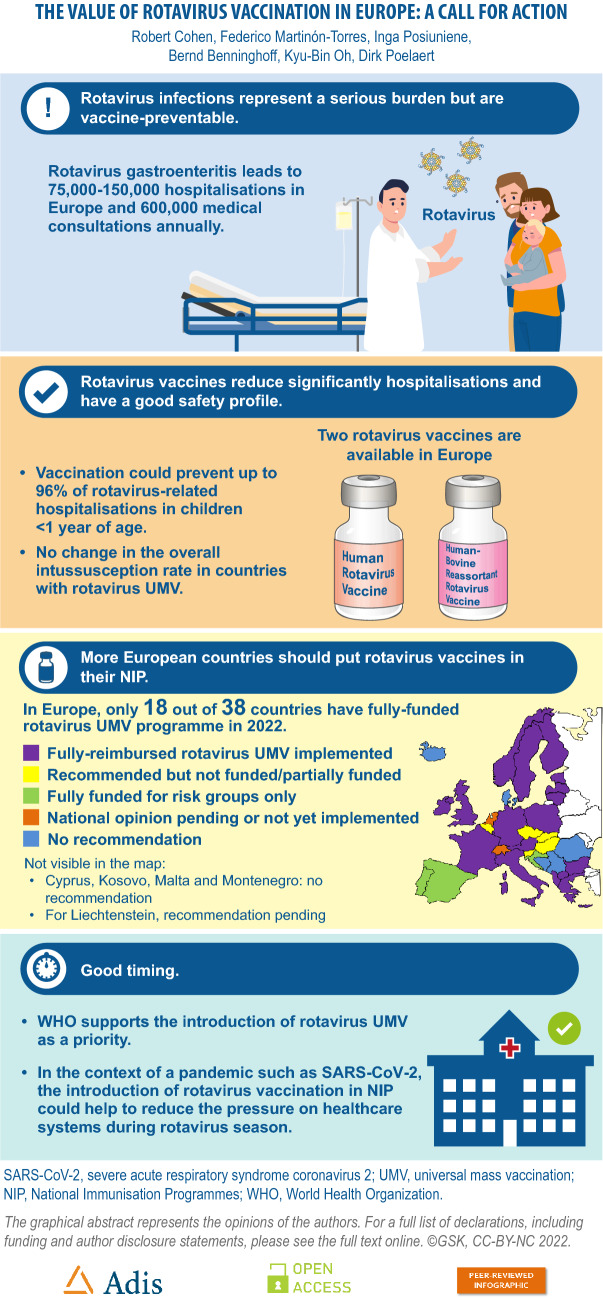
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has pushed many healthcare systems into crisis. High vaccine coverage amongst children reduces emergency room presentations, hospital admissions and deaths due to vaccine-preventable diseases, freeing up healthcare resources including polymerase chain reaction testing for patients with SARS-CoV-2. In Europe, rotavirus gastroenteritis leads to 75,000–150,000 hospitalisations and up to 600,000 medical encounters annually. Nevertheless, in 2022, only 18 countries in Europe (out of 38) have a publicly funded routine universal mass immunisation programme against rotavirus gastroenteritis. Evidence available in the last few years re-emphasises that rotavirus vaccines currently available in Europe are highly effective, preventing up to 96% of rotavirus-related hospitalisations in children less than 1 year of age (potentially 72,000–144,000 hospitalisations Europe-wide). Long-term surveillance indicates that rotavirus vaccination does not result in an overall increase in intussusception. On the contrary, increasing evidence suggests an overall reduction in intussusception in the first 12 months of life when early, high rotavirus vaccine coverage is achieved. Prevention of rotavirus gastroenteritis has marked positive impacts on parental wages and government tax revenue, with benefits extending across the whole economy. In the SARS-CoV-2 pandemic setting there is a new imperative to achieve high levels of paediatric vaccination against vaccine-preventable diseases, including rotavirus gastroenteritis. The introduction of rotavirus universal mass vaccination can be expected to reduce the number of preventable illnesses, hospitalisations and deaths caused by rotavirus gastroenteritis. Reducing vaccine-preventable diseases is particularly urgent at this time when healthcare systems are preoccupied and overwhelmed with SARS-CoV-2. Graphical abstract available for this article.
Graphical Abstract
Keywords: Rotavirus, Vaccine, Europe, Policy, Immunisation, Implementation
Key Summary Points
| Only 18 of 38 countries in Europe provide free rotavirus vaccination for all children |
| Rotavirus vaccines are highly effective and do not increase the risk of intussusception over time |
| Rotavirus universal mass vaccination (UMV) can help to reduce healthcare utilisation during and after the SARS-CoV-2 pandemic |
| Social distancing has reduced infectious disease rates but a rebound is a recognised risk |
| The WHO supports the introduction of rotavirus UMV as a priority |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21033322.
Introduction
Despite unambiguous recommendations by the World Health Organization (WHO), substantial information about the burden of rotavirus gastroenteritis in children, and unequivocal evidence of efficacy and safety, introduction of rotavirus vaccines into routine infant immunisation schedules has been slow and remains suboptimal in Europe. A review of national recommendations for rotavirus vaccination in 2018 explored possible factors contributing to low uptake of these vaccines by national health authorities [1]. Potential barriers we identified were skewed perceptions about the value versus the cost of vaccine implementation, and incomplete awareness of the burden of disease and the benefits of universal mass vaccination (UMV). In some countries, specific safety concerns contributed in those days to the reluctance of health authorities to introduce rotavirus UMV. In France, for example, the national recommendation for rotavirus vaccination was suspended in 2015 because of concerns about the occurrence of intussusception (Box 1). In July 2022, the main French health authority (Haute Autorité de Santé) issued a recommendation for rotavirus vaccination in infants aged from 6 to 24 weeks with Rotarix (two doses 1 month apart) or 6–32 weeks with RotaTeq (three doses 1 month apart). Similarly, the Spanish authorities suspended the marketing of rotavirus vaccines in 2010 because porcine circovirus (PCV) fragments were detected in the vaccines [2]. The suspension remained in place for 5 months for one rotavirus vaccine and 6 years until 2016 for the other. Since then, PCV-free rotavirus vaccines have been developed and introduced onto the market.
In this narrative review, we examine how the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic has changed the imperative for the prevention of infectious diseases in Europe. We summarise newly available evidence that describes the effectiveness of rotavirus UMV in different countries and settings; the impact of rotavirus UMV on rates of intussusception, which is the most severe potential vaccine-related adverse event; and the growing interest in the broader effects of vaccination including rotavirus vaccines on immune health. This article is a call for action to improve vaccine-induced protection of vulnerable infants and young children against rotavirus gastroenteritis, thereby freeing up healthcare resources needed to treat patients with SARS-CoV-2 infection. This literature review does not contain any new data with human participants or animals performed by any of the authors.
Box 1. Rotavirus Vaccination in France: A Lost Opportunity.
In 2013, the French High Council of Public Health recommended rotavirus universal mass vaccination on the basis of evidence suggesting cost-effectiveness, with a view to future public reimbursement. Pharmacovigilance data between 2006 and 2014 identified 47 cases of acute intussusception occurring within 7 days after vaccination, and an incidence rate that was comparable to rates published in the literature. There were two deaths reported after intussusception: one death in a very premature 2-month-old infant with neurological sequelae who died at home without having received medical care, and one death in a 4-month-old who died after the third vaccine dose [79, 80]. The World Health Organization noted that no association between intussusception and the third vaccine dose has been demonstrated, and that since the third dose is usually administered at an age when the incidence of intussusception is highest, it is likely this case could be a coincidental event [79]. Although acknowledging that the absence of treatment may have contributed to one of the deaths, the High Council remained concerned that such situations could arise again, and therefore suspended the recommendation to immunise all infants against rotavirus. Each year in France there are approximately 430,000 episodes of acute gastroenteritis, 181,000 consultations, 31,000 emergency room visits, 14,000 hospitalisations and around 10 deaths due to rotavirus. A statement released by the French National Academy of Medicine in 2020 called for the re-institution of a funded national rotavirus prevention infant vaccination strategy to prevent the deleterious effects of the annual winter rotavirus epidemic that will occur at the same time as SARS-CoV-2 outbreaks during the winter season [77]. The main French health authority (Haute Autorité de Santé) in charge of establishing the vaccination policy, on July 12, 2022, recommended the vaccination against rotavirus of all infants aged 6 weeks to 6 months. The process leading to a publicly funded routine universal mass immunisation programme is underway.
Rotavirus
Without vaccination, almost all children will experience at least one episode of rotavirus gastroenteritis before the age of 5 years [3]. Globally, rotavirus caused 258 million episodes of diarrhoea and approximately 1.5 million hospitalisations in children less than 5 years of age in 2016 [4]. In this age group, rotavirus is the leading cause of diarrhoea-associated mortality worldwide and causes approximately 130,000 deaths annually [4]. Whilst the majority of rotavirus deaths occur in developing countries, the burden of rotavirus gastroenteritis in Europe is substantial. Amongst children less than 5 years of age, rotavirus gastroenteritis results in 75,000 to 150,000 hospitalisations and as many as 600,000 medical encounters in emergency departments or outpatient clinics each year [5]. The incidence of rotavirus-related hospitalisation is between 300 and 600 per 100,000 children under 5 years of age [5], a rate that is substantially higher than the rate of invasive pneumococcal disease in children of the same age in Europe prior to the introduction of pneumococcal conjugate vaccines (< 100 per 100,000) [6, 7].
Most severe rotavirus gastroenteritis and associated deaths occur in healthy children [8]. Despite attempts to identify potential risk factors for the development of severe rotavirus gastroenteritis, the European Academy of Paediatrics and the European Society for Paediatric Infectious Diseases recently concluded that the available data have found no predictors of risk of severe disease, suggesting that no specific risk groups for severe rotavirus gastroenteritis can be defined [8].
Rotavirus vaccines have been available since 2004 and are highly effective in preventing rotavirus diarrhoea [9, 10]. A Cochrane review of data from randomised controlled trials concluded that rotavirus vaccination prevents between 90% and 96% of severe rotavirus diarrhoea in children followed up for 2 years in low-mortality countries, and between 35% and 54% in high-mortality countries [10]. The WHO has recommended that rotavirus vaccination should be included in all national infant vaccination programmes since 2006 [11], and indicated in 2013 that this should be considered a priority [3]. Vaccination should commence and the series be completed as soon as possible after 6 weeks of age to induce protection before natural rotavirus infection occurs [3].
Rotavirus Vaccines
There are two rotavirus vaccines available in Europe: human rotavirus vaccine (HRV, Rotarix; GSK); and human-bovine reassortant rotavirus vaccine (HBRV, RotaTeq; Merck Sharpe & Dohme). Both were licensed in 2006 and have amassed a large body of data supporting their effectiveness and safety.
HRV is given in a two-dose series administered at least 4 weeks apart. Vaccination may be given at 6 weeks of age and should be completed preferably by 16 weeks of age and no later than age 24 weeks. HRV may be given in the same schedule to preterm infants born after at least 27 weeks gestation [12].
HBRV is given as a three-dose series with the first dose administered from 6 weeks of age until no later than age 12 weeks. There should be at least 4 weeks between doses. Vaccination should be completed preferably by 20–22 weeks of age and no later than age 32 weeks. HBRV can be given in the same schedule to preterm infants born after at least 25 weeks gestation [13].
Recommendations for Rotavirus Vaccines in Europe: What’s New in 2022?
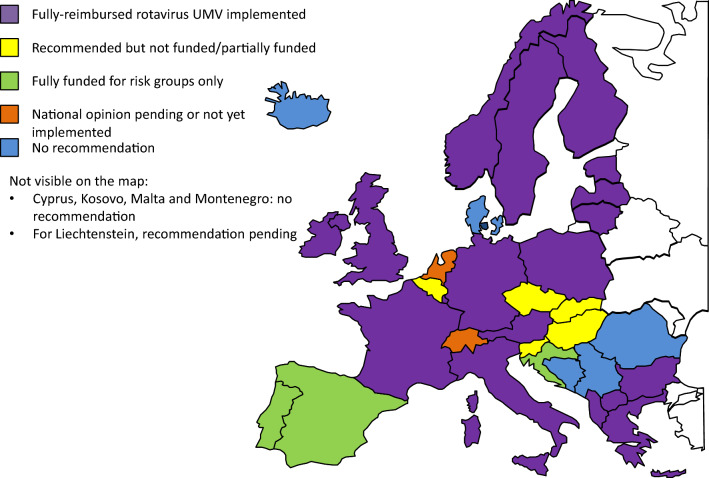
Since 2018, fully funded rotavirus UMV programmes have been instigated in Lithuania, Macedonia, Poland and Sweden. More recently, since March 2022 Greece has moved from partial to full reimbursement of rotavirus vaccination in infants (Table 1). This brings the total number of countries with a fully funded rotavirus UMV programme to 18 out of 38 countries (47%) compared to less than 40% in 2018 (Fig. 1). In Poland, where rotavirus vaccination was previously recommended but not funded [1], the health authority moved rapidly in 2020 to make rotavirus vaccination free and mandatory for children born after 31 December 2020 [14]. With 20,000–30,000 rotavirus-related hospitalisations each year in Poland, and considering that the seasonality of rotavirus coincides with peaks in infections due to the pandemic virus SARS-CoV-2, free, mandatory rotavirus UMV has been implemented to avert pressure on hospitals and prevent paralysis of the healthcare system [15].
Table 1.
Recommendations for rotavirus vaccination across Europe as of February 2021
| Country | Recommendation by national authority | Reimbursement status | Recommending government body | Medical/scientific societies that took the position to recommend rotavirus vaccination |
|---|---|---|---|---|
| Albania | All infants (UMV) | 100% | Ministry of Health, Public Health Institute | University Hospital Center ‘Mother Teresa’ Pediatrics Department |
| Austria | All infants (UMV) | 100% | Bundesministerium für Gesundheit | Austrian Society of Paediatrics & Adolescent Medicine |
| Belgium | All infants (UMV) | Small co-payment | Conseil supérieur de la Santé/Hoge Gezondheidsraad | Belgian Society of Pediatrics |
| Bosnia and Herzegovina | None | None |
Agency for Medicines and Medical Materials Institute of Public Health of the Federation of BiH |
Pediatrics Association of Republic of Srpska (Bosnia and Herzegovina entity) |
| Bulgaria | All infants (UMV) | 100%a | Expert Committee, Ministry of Health | Advisory Committee for Surveillance of Communicable Diseases |
| Croatia | Risk groups | 100% for risk groups | Croatian Institute for Public Health, Department of Epidemiology | National Committee for the Promotion and Prevention of Rotavirus Infection in Children |
| Cyprus | Included in the private sector schedule, which differs from the public sector schedule | None | Ministry of Health | Scientific Committee of Cyprus Paediatric Society for the private sector’s vaccination calendar |
| Czech Republic | All infants | None | Národní imunizační komise (National Immunization Committee, NIKO) | Česká vakcinologická společnost (Czech Vaccination Society) |
| Denmark | None | None | Sundhedsstyrelsen (National Board Health Technology Assessment) | Dansk Pædiatrisk Selskab (Danish Paediatric Society) |
| Estonia | All infants (UMV) | 100% |
Ministry of Social Affairs, Health Department State Agency of Medicines |
University of Tartu, Department of Public Health |
| Finland | All infants (UMV) | 100% |
THL, National Institute for Health and Welfare KRAR, National Advisory Committee for Vaccination |
Finnish Medical Society Duodecimi Rokotetutkimuskeskus. Tampere University Vaccine Research Centre Finnish Pediatric Association |
| France | All infants (UMV) from 2014, suspended since 2015, currently under evaluation |
100% Under evaluation |
Haute Autorité de Santé |
Association Française de Pédiatrie Ambulatoire & Groupe de Pathologie Infectieuse Pédiatrique (paediatric societies) (2012) Groupe Francophone d’Hépatologie-Gastroentéroloiey et Nutrition Pédiatriques (2007) |
| Germany | All infants (UMV) | 100% | Ständige Impfkommission (STIKO) |
Deutsche Akademie für Kinder- und Jugendmedizin e. V Gesellschaft für Neonatologie und Pädiatrische Intensivmedizin e. V |
| Greece | All infants < 6 months | 100% | Ministry of Health |
National Immunisation Committee Hellenic Paediatric Association |
| Hungary | All infants | None | Országos Epidemiológiai Központ (National Centre of Epidemiology) |
Hungarian Pediatric Association Association of Hungarian Primary Care Paediatricians |
| Iceland | None | None | Embætti landlæknis | Icelandic Pediatric Association |
| Ireland | All infants (UMV) | 100% |
Department of Health National Immunisation Advisory Committee |
Royal College of Physicians of Ireland |
| Italy | All infants from 2018 (UMV) | 100% | Ministero della Salute |
Pediatrician and Preventive Medicine Scientific societies Centro Nazionale di Epidemiologia, Sorveglianza e Promozione della Salute Società Italiana di Pediatria Federazione Italiana Medici Pediatri |
| Kosovo | None | None | Ministry of Health Kosovo | National Institute of Public Health |
| Latvia | All infants (UMV) | 100% | Centre for Disease Prevention and Control |
Slimību Profilakses un Kontroles Centrs (Center of Immunology and Prophylactics) Latvia Paediatric Association |
| Liechtenstein | Pending | – | Fürstentum Liechtenstein Amt für Gesundheit | Eidgenössische Kommission für Impffragen |
| Lithuania | All infants from 2018 (UMV) | 100% | Ministry of Health | Centre for Communicable Diseases Prevention and Control |
| Luxembourg | All infants (UMV) | 100% |
Ministère de la Santé Conseil Supérieur des Maladies Infectieuses |
Société Luxembourgeoise de Pédiatrie |
| Macedonia | All infants from 2019 (UMV) | 100% | Ministry of Health and Committee for Immunisation | Pediatric Society of Macedonia |
| Malta | None | None | Ministry of Health | Advisory committee on immunization |
| Montenegro | None | None | Institute of Public Health of Montenegro | Institute of Public Health of Montenegro |
| Netherlands | All infants (UMV) | None | Ministry of Health |
Gezondheidsraad (Health Council) Zorginstituut Nederland (national healthcare institute) |
| Norway | All infants (UMV) | 100% | Norwegian Institute of Public Health / Norsk Folkehelseinstitutt | Norwegian Child Medical Association |
| Poland | All infants from 2021 (UMV) | 100% | Ministry of Health | The Pediatric Experts Group on National Immunization; Polish Society of Vaccinology Program |
| Portugal | Risk groups from 2020 | 100% for risk groupsb | Comissão Técnica de Vacinação | Sociedade Portuguesa de Pediatria – Sociedade Portuguesa de Infecciologia pediátrica e Secção de Gastroenterologia e Nutrição Pediátrica |
| Romania | None | None | The Romanian Ministry of Health | The Romanian Pediatric Society |
| Serbia | None | None | Ministry of Health | Batut Institute |
| Slovakia | All infants | 20% | Regional Public Health Agency | Neonatal Section of Pediatric Society |
| Slovenia | All infants | None | Nacionalni inštitut za zdravje (National Institute for Public Health) | Slovenia Paediatric Society |
| Spain | Risk groups from 2019 |
100% for risk groups |
Ministry of Health, Social Services and Equality | Spanish Association of Pediatrics |
| Sweden | All infants from 2019 (UMV) | 100% | Ministry of Health | Public Health Agency Sweden |
| Switzerland | Pending | None | The Federal Office of Public Health | Swiss Society of Paediatrics Eidgenössische Kommission für Impffragen |
| United Kingdom | All infants (UMV) | 100% | Public Health England | Joint Committee on Vaccination and Immunisation |
Changes in national recommendations (since 2018) occurred in Lithuania, Macedonia, Poland, Portugal, Spain and Sweden
UMV universal mass immunisation
aLimited annual budget, re-assessed yearly based on vaccination coverage (coverage was 45% in 2020)
bRisk groups specified
Fig. 1.
Overview of recommendations for rotavirus vaccination in European countries. UMV, universal mass vaccination
Spain recently introduced a partially funded programme limited to premature infants born between weeks 25–27 and week 32 of gestation [16], which is unlikely to have any detectable impact on the overall rotavirus disease burden. There is no strong evidence that the severity of rotavirus infection is increased in specific risk groups, including preterm and immunocompromised children, strategies to vaccinate risk groups are generally troubled by issues of access and low uptake [17], and restricting rotavirus vaccination to assumed risk groups is not recommended [8]. The Health Council of the Netherlands welcomes offering universal vaccination against rotavirus through the National Immunisation Programme. In 2022, it is expected that the government will take a final decision on the implementation [18].
A decision to introduce rotavirus vaccination for risk groups in Portugal was implemented in October 2021. The risk groups are severe cardiovascular disease, hereditary metabolism disease, liver disease, kidney disease, neurological disease (strong suspicion of metabolic encephalopathy, suspicion or diagnosis of neuromuscular disease, chromosomal disorders or epileptic syndromes in the first 3 months of life and severe neonatal hypoxic-ischemic encephalopathies), very preterm (less than 32 weeks), low birth weight (less than 2500 g), congenital adrenal hyperplasia [19].
The main French health authority (Haute Autorité de Santé) in charge of establishing the vaccination policy recommended, on July 12, 2022, the vaccination against rotavirus of all infants aged 6 weeks to 6 months. The process leading to a publicly funded routine universal mass immunisation programme is underway.
Belgium and Slovakia continue to require a parental co-payment that varies from 80% of the vaccine cost in Slovakia to at most 25% in Belgium (Table 1). In Belgium, a nominal charge to parents of approximately 12 euros per dose (a standard charge for so-called category B medicinal products in Belgium [20]) has not deterred high vaccine uptake. In 2019, rotavirus vaccine coverage was 82.5% for dose 1 and 77.6% for dose 2 in Flanders, and 71.3% and 64.6%, respectively, in Brussels [21]. The latest coverage estimate available in Wallonia was 87.2% (2015) [22].
Overall, a substantial proportion of Europe’s children continue to be unprotected against rotavirus. Although there have been positive steps forward since 2018, the use of rotavirus vaccines in Europe remains suboptimal. In the following sections we present new evidence available since the 2018 review [1] that together makes a compelling argument for wider implementation of these vaccines.
New Evidence on Rotavirus Vaccine Effectiveness
Ireland
Rotavirus vaccination was introduced into the Irish national infant immunisation schedule in December 2016. The impact of vaccination was evaluated using national hospitalisation data comparing the pre-vaccination era (2010–2016) to the post-vaccination period (2017–2018). Rotavirus-attributable hospitalisations decreased by 85.5% nationally in children less than 1 year of age (p < 0.001) and by 86.5% for children less than 2 years of age (p < 0.001) [23] after the introduction of UMV.
Estonia
Rotavirus vaccination was introduced to the Estonian national infant immunisation schedule in July 2014 and the impact of vaccination was assessed using national claims data from the Estonia Health Insurance Fund. Compared to the pre-vaccination era (2007–2013), hospitalisation due to rotavirus gastroenteritis decreased by 81% in children less than 1 year of age, and by 55% in children 1–4 years of age in the post-vaccination era (2015–2018). In the post-vaccination era, children admitted to hospital with rotavirus gastroenteritis were older (median age increased from 2 to 3 years), underwent a shorter hospitalisation (median duration decreased from 3 to 2 days), and were less likely to have severe disease (70.5% versus 82.54%) compared to the pre-vaccination era [23].
United Kingdom (UK)
The UK introduced HRV into its infant immunisation schedule in 2013. Vaccine effectiveness was determined in a case–control study of laboratory-confirmed rotavirus infection. The effectiveness of two doses of HRV in preventing laboratory-confirmed rotavirus gastroenteritis was 85% (95% confidence interval [CI] 74, 91) in children less than 1 year of age, and 54% (95% CI 15, 75) in children older than 12 months [24].
Belgium
Rotavirus vaccination was introduced in Belgium in 2006. The Rotavirus Belgium Impact Study (RotaBIS) is an ongoing evaluation of the impact of vaccination that is conducted annually in 10 hospitals. Rotavirus positive cases are collected retrospectively each year allowing annual comparison with the pre-vaccination era disease burden (2005–2006). The results show a continuing impact of vaccination over the 13 years since its introduction, with a reduction in rotavirus-related hospital infections of 70% (95% CI 66, 74) after 5 years and 84% (95% CI 79, 89) after 10 years. Using a simulation model, the authors concluded that the highest vaccine impact at the commencement of a routine immunisation programme is achieved if it starts well before the upcoming rotavirus season, and achieves maximum coverage of the group in whom rotavirus transmission is highest (3- to 12-month-olds) [25].
Germany
The region of Saxony in Germany introduced rotavirus vaccination in January 2008 with the average vaccine coverage after vaccination introduction estimated to be 47.6% in children less than 5 years of age. The incidence of rotavirus-associated hospitalisations decreased by 84% in the 2012/2013 to 2016/2017 seasons compared to the pre-vaccination 2002/2003 to 2006/2007 seasons. Vaccine effectiveness over the full post-vaccination period (2007/2008 to 2016/2017 seasons) was 69% in children aged less than 5 years and 96% in children aged less than 1 year, the age group in whom protection is most needed. Twenty-six per cent of the vaccine effect resulted from herd protection [26].
No Change in the Overall Intussusception Rate in Countries with Rotavirus UMV
In 2013, the WHO Global Advisory Committee on Vaccine Safety concluded that the risk of intussusception following vaccination with HRV and HBRV was small compared to the benefits associated with prevention of the most common causes of severe acute gastroenteritis globally [27, 28]. The first rotavirus vaccine, tetravalent rhesus-human reassortant vaccine (RRV-TV RotaShield; Wyeth Lederle Vaccines), was licensed in the United States (USA) in 1998 but withdrawn in 1999 because of an observed 59-fold increase in intussusception after the first dose [29, 30]. The new HRV and HBRV vaccines are both associated with a small increased risk of intussusception in the first 7 days after vaccination that is substantially lower than that observed for RRV-TV (approximately fivefold) [31], suggestive of a class effect of rotavirus vaccines.
Recently published, long-term post-marketing surveillance data are strongly suggestive that although the risk of intussusception is slightly increased shortly after rotavirus vaccination, there is no increased long-term risk of intussusception after vaccination with HRV or HBRV. An observational study conducted in the USA using a healthcare claims database found that the risk of intussusception in fully vaccinated children less than 2 years of age was 21% lower than unvaccinated children [32]. Nationwide surveillance of hospital admissions for intussusception in the UK comparing the pre-vaccination era (2008–2013) with the post-vaccination era (2014–2018) found that there was no increase in the overall hospital admission rate for intussusception in 0- to 36-month-old children in the post-vaccination era. A significant increase in the intussusception admission rate in 8- to 16-week-old infants was offset by a significant decrease in children up until 12 months of age, such that the hospital admission rate for intussusception was significantly lower in children 0–12 months of age after introduction of the rotavirus vaccine. There was no change in disease severity and the need for surgical intervention in the post- versus the pre-vaccination era [33]. Surveillance in Ireland following the introduction of rotavirus UMV also showed no increase in the incidence of intussusception after vaccine introduction compared to the incidence prior to introduction [34].
Efforts to explain why intussusception decreases during later childhood in fully vaccinated children remain hypothetical. One hypothesis is that rotavirus vaccination could trigger intussusception in predisposed children, which means that these children develop intussusception at a younger age than they would have otherwise [35]. On the other hand, if rotavirus vaccination prevents intussusception triggered by wild-type rotavirus infection, this would reduce the overall number of intussusception cases in older children. However, it is not firmly established that rotavirus induces intussusception and the mechanism of vaccine-induced intussusception remains unexplained [33, 36].
Fears that implementation of rotavirus UMV would result in an overall increase in the incidence of intussusception in young children have not been realised. The evidence that currently available rotavirus vaccines do not increase the overall risk of intussusception is highly reassuring, and necessitates a shift in thinking about the risk–benefit of rotavirus vaccination [37]. If early, high rotavirus vaccine coverage can be achieved, it might be expected that more cases of ‘naturally occurring’ intussusception caused by rotavirus gastroenteritis would be prevented, and cases potentially caused by vaccination would be minimised, leading to an overall reduction in intussusception cases, and making the benefit–risk profile even more favourable.
For countries such as France, where concerns about intussusception motivated a withdrawal of the existing recommendation for UMV in 2015 (Box 1), long-term post-marketing surveillance suggests that it is likely that there will be no change in the total number of intussusception cases that occur in France, should rotavirus UMV be re-instituted. Since the 2015 recommendation withdrawal, additional studies conducted in France found no significant association between rotavirus vaccination and intussusception within the first 7 days of dose 1, although the vaccine coverage rate was very low. One study identified formula feeding and a history of gastroenteritis in the previous 2 weeks as risk factors for intussusception [38]. A benefit–risk evaluation of rotavirus vaccination in France found that for every intussusception death potentially triggered by vaccination, 743 deaths caused by rotavirus gastroenteritis would be prevented [38]. However, the impact of rotavirus vaccination in preventing intussusception following wild-type infection has not been quantified, potentially adding further to the benefit of vaccination over no vaccination. In 2022, the rotavirus vaccination recommendation in France has been issues by the French NITAG.
Impact of Vaccine Schedule on Effectiveness and Safety
The two available rotavirus vaccines in Europe show similar efficacy and safety profiles but differ in their posology (HRV requires two doses and HBRV requires three doses), as well as in the time frame for their administration. The scheduling of HRV and HBRV as stated in the product information is a reflection of the schedules that were evaluated during clinical development in pre-licensure trials. Some countries have harmonised both schedules into a single recommendation. For example, the US Advisory Committee on Immunization Practices (ACIP) recommends dosing at 2 and 4 months of age (+ 6 months of age for the third dose of HBRV) to align with the timing of other routinely administered vaccines [39].
The risk of intussusception in healthy children increases during the first year of life, peaking at 5–7 months of age [40]. Early vaccination could reduce the overall level of intussusception potentially caused either by natural infection or vaccination. WHO therefore recommends that rotavirus vaccination should commence as soon as possible after 6 weeks of age to induce protection before natural rotavirus infection occurs [3]. The vaccine series should also be completed as soon as practical in order to maximise protection against rotavirus infection, and to also reduce the risk of vaccine-induced intussusception.
As oral vaccines, rotavirus vaccines are generally well accepted by practitioners and parents and there is good evidence that rotavirus vaccines can be co-administered with all other routinely administered paediatric vaccines [41, 42]. Nevertheless, several countries, such as Italy and the USA, have not achieved target coverage rates, possibly contributed to by the challenges posed by fitting rotavirus vaccines into the routine immunisation schedule and the lack of catch-up opportunities [43, 44]. Countries such as Italy that use a 2 + 1 schedule with routine vaccines administered at 3 and 5 months of age may find it difficult to administer the first dose of HBRV by the latest recommended age of 12 weeks, and to complete the three-dose series since the third dose requires an additional healthcare visit outside of the routine schedule [43]. Whilst HRV (two-dose series) fits more readily into a two-dose primary vaccination schedule for other vaccines, early commencement and completion of vaccination is preferable and any delay in administering the second dose could exceed the specified 24-week upper limit.
The rotavirus vaccine schedule appears to influence both coverage and the timeliness of vaccination. A review of the literature from the USA found that rotavirus vaccine coverage was 74.1% in 2016, which is lower than for most other routinely recommended vaccines. Rates of series completion ranged from as high as 90% to as low as 50%, and compliance with the ACIP-harmonised schedule was 77% for recipients of HRV and 70% for HBRV. Series completion rates and compliance rates tended to be higher in children vaccinated with HRV requiring two doses than HBRV requiring three doses [44].
A national switch from HRV to HBRV in Mexico was followed by a statistically significant decrease in rotavirus vaccine coverage from 75.6% to 61.0% (p < 0.001), and a significant decrease in series completion rate, from 93.7% to 71.1% (p < 0.001) [45]. In Belgium, 17.3% of HBRV recipients did not complete the series versus 6.8% of HRV recipients [46]. Similarly, in 2019 in Italy, 25.0% of HBRV recipients did not complete the series compared to 16.8% of HRV recipients (p = 0.022) [47].
These data suggest that whilst HRV and HBRV are both highly effective and have similar safety profiles, there may be advantages of one over the other in terms of best fit for individual vaccination schedules.
Cost-Effectiveness
The previous review of rotavirus vaccine use in Europe argued that rotavirus vaccination is undervalued in developed countries where mortality rates are low [1]. This is because cost-effectiveness studies frequently exclude herd effects, impacts on quality of life, the effect of secondary cases in households and day care centres, and the impact of work absenteeism on wages and tax revenue in their estimates. These variables are important when evaluating the national cost of rotavirus infections because the majority of rotavirus gastroenteritis cases in Europe are not admitted to hospital but are cared for at home. A study in the Netherlands using a Social Accounting Matrix to model the impact of rotavirus vaccination on the wider national economy focused on costs in terms of work absenteeism, productivity loss, sick leave and the flow-on effects on wages and tax revenue [48]. The model showed that rotavirus vaccination was associated with more economic activity, reduced health spending and higher tax revenue than no vaccination. Overall, the modelled economy with vaccination reached a surplus within 1 year after implantation of UMV compared to the model with no vaccination [48].
More studies are needed to evaluate the impact of rotavirus vaccination beyond the provision of direct medical care, incorporating the home setting where most cases are treated.
Downstream Impacts of Rotavirus Vaccination
Vaccines are designed to meet a defined public health need and to prevent disease caused by a specific pathogen. Apparently unrelated ‘downstream’ or so-called heterologous or non-specific effects of vaccines have long been recognised [49], but only recently have the mechanisms for such effects been explored, and the potential to leverage these effects for wider disease prevention considered [50]. Whether rotavirus vaccines have other non-specific impacts has been the topic of speculation.
Seizures are among the most common extraintestinal manifestation of rotavirus infection [51]. Several studies have reported a significant association between rotavirus vaccination and a lower incidence of seizures requiring hospitalisation in the 12 months following vaccination in countries including the USA, Spain, Australia and the UK [51–53]. As yet, the mechanism by which rotavirus vaccination appears to prevent seizures is not known; it remains an open question as to whether the benefits of rotavirus vaccination in reduction of seizures are due to prevention of infection in children predisposed to the neurological effects of rotavirus infection or to a true heterologous effect of the vaccine [51]. Regardless, the reduction in the incidence of seizure hospitalisation adds to the population-level benefits of rotavirus vaccination.
Wild-type rotavirus infection is thought to trigger autoimmune diseases including type 1 diabetes mellitus, coeliac disease, autoimmune uveitis and biliary atresia in genetically susceptible individuals [54, 55]. A large observational study in the USA linked rotavirus vaccination with a reduced risk of developing type 1 diabetes mellitus. After a median follow-up period of 3 years the incidence of type 1 diabetes was 41% lower (95% CI 27, 52) in children who had completed a full series of rotavirus vaccination compared to unvaccinated children [55]. Similar observations were made in Australia where the number of incident cases of type 1 diabetes decreased by 15% (95% CI 3, 25) in children 0–4 years of age after rotavirus vaccine was introduced into the routine infant immunisation schedule [56, 57]. Early evidence is also suggestive of a protective effect of rotavirus vaccination on coeliac disease [58]. However, other studies have not found an association between rotavirus vaccination and a reduced risk of autoimmune disease [58–60]. The evidence available to date is purely ecological, with confounding factors in each study.
The expanded benefits of current rotavirus vaccines on these important clinical spectra is an active research area, but the weight of current evidence is enough to reinforce the recommendation of rotavirus vaccination to all children; therefore, it has been suggested that rotavirus vaccination should be actively recommended for children genetically predisposed to coeliac disease or type 1 diabetes mellitus [54].
Implications of SARS-CoV-2 Pandemic for Vaccination
The SARS-CoV-2 pandemic has had major negative impacts on healthcare delivery. Even 2 years into the pandemic, healthcare systems continue to be overwhelmed, not only with patients with SARS-CoV-2 infection but also with shortages of staff, hospital beds and supplies [61]. Surges in respiratory specimens to be tested for SARS-CoV-2 means that polymerase chain reaction testing capacity in medical laboratories has also come under pressure [62]. Furthermore, studies have shown that, compared with the pre-pandemic period, many patients suffering illnesses other than SARS-CoV-2 infection have experienced delays in receiving care [63–66] and healthcare resource utilisation is reduced overall [67]. Such findings indicate that the impact of the pandemic is likely to continue for many years to come [61].
Rotavirus gastroenteritis peaks in winter, along with influenza and respiratory syncytial virus [68]. Winter in temperate climates presents a challenging period for healthcare systems where capacity is stretched, particularly in paediatric units, as a result of increased admission rates due to seasonal infectious disease outbreaks. Such seasonal challenges will continue to be exacerbated by the SARS-CoV-2 pandemic in the coming years [69]. Early during the pandemic, WHO highlighted the importance of maintaining routine vaccination services to prevent excess morbidity and mortality due to vaccine-preventable diseases [70]. However, arguably, little attention has been paid to the importance of maintaining high vaccine coverage rates as a mechanism to reduce the burden on the healthcare system, particularly during winter months. Rates of vaccination coverage amongst children have declined all over the world during the SARS-CoV-2 pandemic [71, 72]. In some countries in Europe, initial decreases in vaccine coverage rates were restored after either catch-up programmes or the release of statements from health authorities highlighting the importance of continued routine vaccination, illustrating that concerted efforts may be needed to get routine vaccination back on track [73, 74]. Whilst social distancing measures have improved control of a range of infectious diseases including respiratory infections and gastroenteritis during the SARS-CoV-2 pandemic [75], the risk of a rebound of infectious diseases in children is a recognised threat to global health as these measures are relaxed [72]. This is particularly the case for pathogens that all children come into contact with during the first years of life, including rotavirus. Vaccination, when available, is probably the safest way to prevent this immune debt [76].
The introduction of rotavirus UMV provides countries with a mechanism to reduce the winter burden of infectious disease. Poland has taken steps to this end, making rotavirus mandatory and free in response to the SARS-CoV-2 pandemic. The French National Academy of Medicine called for the re-institution of a funded national rotavirus prevention strategy to curb the annual winter rotavirus epidemic that will occur at the same time as SARS-CoV-2 outbreaks [77]. The Dutch Paediatric Association has also called for the rapid introduction of universal rotavirus vaccination in order to free up hospital resources for patients with SARS-CoV-2, and to smooth the expected winter peak in disease [78]. Eradication of SARS-CoV-2 is not feasible in the short term given current vaccine options and outbreaks are likely to continue to occur. It is imperative that vaccination rates against all vaccine-preventable diseases are maximised before physical social distancing measures are fully relaxed.
Conclusion
In 2022, there is compelling evidence that rotavirus vaccines used in UMV in Europe are highly effective in preventing rotavirus gastroenteritis, do not increase the overall incidence of intussusception in early childhood, and are cost-effective, contributing positively to economic productivity, wages and tax revenue. Considering a maximum vaccine effectiveness of 96% in children aged less than 1 year [26], Europe-wide rotavirus vaccination could potentially prevent between 72,000 and 144,000 hospitalisations [5].
This article is a call for action: immunisation has decisively benefitted society in general. Yet preventing stagnation in efforts to reach all people in need, especially in countries with the lowest coverage and the greatest number of unvaccinated children, is an essential future perspective. The world cannot afford to turn the clock back on immunisation. We can expect ever more innovative vaccines that will offer additional opportunities to reduce mortality and improve the quality of life for every person, but specifically for those infants and young children who do not have a voice. We need to be their strong advocates.
In the current pandemic setting, there is a new urgency to achieve high levels of paediatric vaccination against vaccine-preventable diseases, including rotavirus gastroenteritis. The introduction of rotavirus UMV can be expected to reduce the number of preventable illnesses, hospitalisations and deaths caused by rotavirus gastroenteritis at this time when healthcare systems are preoccupied and overwhelmed with SARS-CoV-2.
Acknowledgements
Trademark Statement
Rotarix is a trademark owned by or licensed to GSK. RotaTeq is a trademark of Merck Sharpe & Dohme.
Funding
This work was supported by GlaxoSmithKline Biologicals SA who covered all costs associated with the development and publication of this manuscript.
Medical Writing, Editorial, and Other Assistance
The authors thank the Business & Decision Life Sciences platform for editorial assistance, design support and manuscript coordination, on behalf of GSK. Joanne Wolter (independent on behalf of GSK) provided medical writing support.
Author Contributions
All authors participated in the conception of the review or the identification of the eligible studies. All authors critically reviewed the manuscript and provided final approval on the submitted version.
Disclosures
Robert Cohen reports grant for clinical study conduct from GSK, MSD, Pfizer and Sanofi outside of the present work. Robert Cohen also reports fees for participation in congresses/conferences from GSK, MSD and Pfizer outside of the present work. Robert Cohen further reports he has been involved in advisory boards organised by GSK, MSD or Pfizer. Federico Martinón-Torres reports grants from Jansen, MSD and AstraZeneca to conduct research/clinical trials outside of the present work. Federico Martinón-Torres also reports personal fees from Novavax, Seqirus, Sanofi/Pasteur, MSD, Pfizer and GSK outside of the present work. Federico Martinón-Torres further reports other non-financial relation or fees paid to his institution from Ablynx, Biofabri, Jansen, GSK, Regeneron, Medimmune, Pfizer, MSD, Sanofi/Pasteur, Novavax, Novartis, Seqirus, Roche and Abbott and Instituto de Salud Carlos III. Kyu-Bin Oh, Inga Posiuniene, Bernd Benninghoff and Dirk Poelaert are employees of GSK and hold shares of GSK. All authors declare no other financial or non-financial relationships or activities.
Compliance with Ethics Guidelines
This literature review does not contain any new data with human participants or animals performed by any of the authors.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Robert Cohen and Federico Martinón-Torres equally contributed.
Change history
12/10/2022
A Correction to this paper has been published: 10.1007/s40121-022-00740-7
References
- 1.Poelaert D, Pereira P, Gardner R, Standaert B, Benninghoff B. A review of recommendations for rotavirus vaccination in Europe: arguments for change. Vaccine. 2018;36(17):2243–2253. doi: 10.1016/j.vaccine.2018.02.080. [DOI] [PubMed] [Google Scholar]
- 2.Díez-Domingo J, Garcés-Sánchez M, Giménez-Sánchez F, Colomina-Rodríguez J, Martinón-Torres F. What have we learnt about rotavirus in Spain in the last 10 years? An Pediatr (Engl Ed) 2019;91(3):166–179. doi: 10.1016/j.anpedi.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64. [PubMed]
- 4.Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. ECDC Expert opinion on rotavirus vaccination in infancy. Stockholm: ECDC; 2017.
- 6.Hausdorff WP, Siber G, Paradiso PR. Geographical differences in invasive pneumococcal disease rates and serotype frequency in young children. Lancet. 2001;357(9260):950–952. doi: 10.1016/S0140-6736(00)04222-7. [DOI] [PubMed] [Google Scholar]
- 7.Calbo E, Díaz A, Cañadell E, et al. Invasive pneumococcal disease among children in a health district of Barcelona: early impact of pneumococcal conjugate vaccine. Clin Microbiol Infect. 2006;12(9):867–872. doi: 10.1111/j.1469-0691.2006.1502_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Dornbusch HJ, Vesikari T, Guarino A, LoVecchio A, Hadjipanayis A, Koletzko B. Rotavirus vaccination for all children or subgroups only? Comment of the European Academy of Paediatrics (EAP) and the European Society for Paediatric Infectious Diseases (ESPID) recommendation group for rotavirus vaccination. Eur J Pediatr. 2020;179(9):1489–1493. doi: 10.1007/s00431-020-03608-5. [DOI] [PubMed] [Google Scholar]
- 9.Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32(5):435–444. doi: 10.1097/QCO.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman H, Henschke N, Hungerford D, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2021;11(11):Cd08521. doi: 10.1002/14651858.CD008521.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotavirus vaccines: an update. Relevé Épidémiologique Hebdomadaire / Section D'hygiène Du Secrétariat De La Société Des Nations = Weekly Epidemiological Record/Health Section of the Secretariat of the League of Nations. 2009;84(50):533–40. [PubMed]
- 12.Rotarix. Summary of Product Characteristics (24/03/2020). https://www.ema.europa.eu/en/medicines/human/EPAR/rotarix; 2020. Accessed 04 Mar 2021.
- 13.RotaTeq. Summary of Product Characteristics (14/09/2020). https://www.ema.europa.eu/en/medicines/human/EPAR/rotateq; 2020. Accessed 04 Mar 2021.
- 14.Dziennik Urzędowy Ministra Zdrowia [Official Journal of the Ministry of Health]. Komunikat Głównego Inspektora Sanitarnego w sprawie Programu Szczepień Ochronnych na rok 2021. Warsaw, December 22, 2020 Item 117.
- 15.Polish News. The Ministry of Health wants mandatory vaccination of children against rotavirus. Sept 04 2020. https://www.polishnews.co.uk/the-ministry-of-health-wants-mandatory-vaccination-of-children-against-rotavirus/; 2020. Accessed 04 Mar 2021.
- 16.Consejo Interterritorial Del Sistema Nacional De Salud Vacunación Específica En Menores Y Adolescentes (<18 Años) Con Condiciones De Riesgo. Calendario recomendado año 2021. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/docs/CalendarioVacunacion_GRinfantil.pdf; 2021. Accessed 04 Mar 2021.
- 17.Doherty M, Schmidt-Ott R, Santos JI, et al. Vaccination of special populations: protecting the vulnerable. Vaccine. 2016;34(52):6681–6690. doi: 10.1016/j.vaccine.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 18.The Hague Health Council of the Netherlands. Rotavirus vaccination 2021 - Publication no 2021/31 2021. https://www.healthcouncil.nl/documents/advisory-reports/2021/06/30/vaccination-against-rotavirus; 2021. Accessed 12 May 2022.
- 19.Serviço Nacional de Saúde [National Health Service]. Programa Nacional de Vacinação 2020: Vacinação contra gastroenterite por rotavírus de crianças pertencentes a grupos de risco [National Vaccination Program 2020: Vaccination against rotavirus gastroenteritis of children belonging to risk groups]. https://www.sip-spp.pt/media/2cgdhusi/rotavi-rus_grupos_risco.pdf; 2021. Accessed 12 May 2022.
- 20.CBiP. Commented Directory of Medicines. https://www.cbip.be/fr/chapters/13?frag=11445&trade_family=23762; 2021. Accessed 20 May 2021.
- 21.Kind en Gezin. Region and Local Health Consultation (LOGO). Figures and reports. https://www.kindengezin.be/gezondheid-en-vaccineren/vaccinaties/; 2021. Accessed 07 May 2021.
- 22.Lajot A, Wyndham-Thomas C, Matthijnssens J, Van Ranst M. Rotavirus. Rotavirus epidemiological surveillance 2017–2018 and 2018–2019 seasons. Sciensano, VPD Annual Report. https://www.sciensano.be/en/biblio/epidemiologische-surveillance-van-het-rotavirus-seizoenen-2017-2018-en-2018-2019; 2018. Accessed 12 May 2022.
- 23.Kõivumägi K, Toompere K, Soeorg H, et al. Acute gastroenteritis hospitalizations after implementation of universal mass vaccination against rotavirus. Vaccine. 2020;38(13):2879–2886. doi: 10.1016/j.vaccine.2020.01.098. [DOI] [PubMed] [Google Scholar]
- 24.Walker JL, Andrews NJ, Atchison CJ, et al. Effectiveness of oral rotavirus vaccination in England against rotavirus-confirmed and all-cause acute gastroenteritis. Vaccine X. 2019;1:100005. [DOI] [PMC free article] [PubMed]
- 25.Standaert B, Strens D, Pereira P, Benninghoff B, Raes M. Lessons learned from long-term assessment of rotavirus vaccination in a high-income country: the case of the rotavirus vaccine Belgium Impact Study (RotaBIS) Infect Dis Ther. 2020;9(4):967–980. doi: 10.1007/s40121-020-00345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietsch C, Liebert UG. Rotavirus vaccine effectiveness in preventing hospitalizations due to gastroenteritis: a descriptive epidemiological study from Germany. Clin Microbiol Infect. 2019;25(1):102–106. doi: 10.1016/j.cmi.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Rotavirus surveillance worldwide – 2009. Wkly Epidemiol Rec. 2011;86(18):174–6. [PubMed]
- 28.Global Advisory Committee on Vaccine Safety, 12–13 June 2013. Relevé épidémiologique hebdomadaire / Section d'hygiène du Secrétariat de la Société des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2013;88(29):301–12.
- 29.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. New Engl J Med. 2001;344(8):564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007. [PubMed] [Google Scholar]
- 31.Rosillon D, Buyse H, Friedland LR, Ng S-P, Velázquez FR, Breuer T. Risk of intussusception after rotavirus vaccination: meta-analysis of postlicensure studies. Pediatr Infect Dis J. 2015;34(7):763–768. doi: 10.1097/INF.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 32.Burke RM, Tate JE, Dahl RM, Aliabadi N, Parashar UD. Does rotavirus vaccination affect longer-term intussusception risk in US infants? J Pediatric Infect Dis Soc. 2020;9(2):257–260. doi: 10.1093/jpids/piz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGeoch LJ, Finn A, Marlow RD. Impact of rotavirus vaccination on intussusception hospital admissions in England. Vaccine. 2020;38(35):5618–5626. doi: 10.1016/j.vaccine.2020.06.078. [DOI] [PubMed] [Google Scholar]
- 34.Burns HE, Collins AM, Fallon UB, Marsden PV, Ni Shuilleabhain CM. Rotavirus vaccination impact, Ireland, implications for vaccine confidence and screening. Eur J Public Health. 2020;30(2):281–285. doi: 10.1093/eurpub/ckz238. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen L, Morens D, Elixhauser A, Gerber M, Van Raden M, Blackwelder W. Effect of rotavirus vaccination programme on trends in admission of infants to hospital for intussusception. Lancet. 2001;358(9289):1224–1229. doi: 10.1016/S0140-6736(01)06346-2. [DOI] [PubMed] [Google Scholar]
- 36.Willame C, Cheuvart B, Aris E, Vetter V, Cohet C. Association between rotavirus gastroenteritis and intussusception: suggested evidence from a retrospective study in claims databases in the United States. Hum Vaccin Immunother. 2021;17(1):269–277. doi: 10.1080/21645515.2020.1770514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetter V, Pereira P, Benninghoff B. Rotavirus vaccination and intussusception: a paradigm shift? Hum Vaccin Immunother. 2021;17(1):278–282. doi: 10.1080/21645515.2020.1770035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledent E, Arlegui H, Buyse H, et al. Benefit versus risk assessment of rotavirus vaccination in France: a simulation and modeling analysis. BioDrugs. 2018;32(2):139–152. doi: 10.1007/s40259-018-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Table 1 Recommended child and adolescent immunization schedule for ages 18 years or younger, United States, 2021. https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html; 2021. Accessed 05 Mar 2021.
- 40.Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS ONE. 2013;8(7):e68482. doi: 10.1371/journal.pone.0068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics. 2008;122(5):e1062–e1066. doi: 10.1542/peds.2008-1059. [DOI] [PubMed] [Google Scholar]
- 42.Vesikari T, Karvonen A, Borrow R, et al. Results from a randomized clinical trial of coadministration of RotaTeq, a pentavalent rotavirus vaccine, and NeisVac-C, a meningococcal serogroup C conjugate vaccine. Clin Vaccin Immunol. 2011;18(5):878–884. doi: 10.1128/CVI.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinelli D, Fortunato F, Marchetti F, Prato R. Rotavirus vaccine administration patterns in Italy: potential impact on vaccine coverage, compliance and adherence. Hum Vaccin Immunother. 2021;17(5):1546–1551. doi: 10.1080/21645515.2020.1816109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghaswalla PK, D'Angelo J, Abu-Elyazeed R. Rotavirus vaccination in the US: a systematic review of vaccination coverage and completion. Hum Vaccin Immunother. 2021;17(3):872–879. doi: 10.1080/21645515.2020.1794440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luna-Casas G, Juliao P, Carreño-Manjarrez R, et al. Vaccine coverage and compliance in Mexico with the two-dose and three-dose rotavirus vaccines. Hum Vaccin Immunother. 2019;15(6):1251–1259. doi: 10.1080/21645515.2018.1540827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbe M, Berger N, Blommaert A, et al. Sustained low rotavirus activity and hospitalisation rates in the post-vaccination era in Belgium, 2007 to 2014. Euro Surveill. 2016;21(27):30273. doi: 10.2807/1560-7917.ES.2016.21.27.30273. [DOI] [PubMed] [Google Scholar]
- 47.Aquilani S, Dari S, Fiasca F. Assessing rotavirus vaccination coverage and compliance after two years of local experience in Italy. Ann Ig. 2020;32(4):433–435. doi: 10.7416/ai.2020.2367. [DOI] [PubMed] [Google Scholar]
- 48.Kotsopoulos N, Haitsma G, Connolly MP, Standaert B. Estimating the money flow in the economy attributed to rotavirus disease and vaccination in the Netherlands using a Social Accounting Matrix (SAM) framework. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):603–612. doi: 10.1080/14737167.2020.1693269. [DOI] [PubMed] [Google Scholar]
- 49.Mayr A. Taking advantage of the positive side-effects of smallpox vaccination. J Vet Med B Infect Dis Vet Public Health. 2004;51(5):199–201. doi: 10.1111/j.1439-0450.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 50.Laupèze B, Del Giudice G, Doherty M, Van der Most R. Vaccination as a preventative measure contributing to immune fitness. NPJ Vaccines. 2021;6(1):93. doi: 10.1038/s41541-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gómez-Rial J, Sánchez-Bátan S, Rivero-Calle I, et al. Rotavirus infection beyond the gut. Infect Drug Resist. 2019;12:55–64. doi: 10.2147/IDR.S186404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salas A, Pardo-Seco J, Cebey-López M, et al. Impact of rotavirus vaccination on childhood hospitalizations for seizures: heterologous or unforeseen direct vaccine effects? Vaccine. 2019;37(25):3362–3368. doi: 10.1016/j.vaccine.2019.04.086. [DOI] [PubMed] [Google Scholar]
- 53.Hungerford DJ, French N, Iturriza-Gómara M, Read JM, Cunliffe NA, Vivancos R. Reduction in hospitalisations for acute gastroenteritis-associated childhood seizures since introduction of rotavirus vaccination: a time-series and change-point analysis of hospital admissions in England. J Epidemiol Community Health. 2019;73(11):1020–1025. doi: 10.1136/jech-2019-213055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gómez-Rial J, Rivero-Calle I, Salas A, Martinón-Torres F. Rotavirus and autoimmunity. J Infect. 2020;81(2):183–189. doi: 10.1016/j.jinf.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 55.Rogers MAM, Basu T, Kim C. Lower incidence rate of type 1 diabetes after receipt of the rotavirus vaccine in the United States, 2001–2017. Sci Rep. 2019;9(1):7727. doi: 10.1038/s41598-019-44193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perrett KP, Jachno K, Nolan TM, Harrison LC. Association of rotavirus vaccination with the incidence of type 1 diabetes in children. JAMA Pediatr. 2019;173(3):280–282. doi: 10.1001/jamapediatrics.2018.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrett K, Jachno K, Nolan T. Coding error in analysis of rotavirus vaccination and type 1 diabetes. JAMA Pediatr. 2019;173(9):894–895. doi: 10.1001/jamapediatrics.2019.2463. [DOI] [PubMed] [Google Scholar]
- 58.Hemming-Harlo M, Lähdeaho ML, Mäki M, Vesikari T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J. 2019;38(5):539–541. doi: 10.1097/INF.0000000000002281. [DOI] [PubMed] [Google Scholar]
- 59.Vaarala O, Jokinen J, Lahdenkari M, Leino T. Rotavirus vaccination and the risk of celiac disease or type 1 diabetes in Finnish children at early life. Pediatr Infect Dis J. 2017;36(7):674–675. doi: 10.1097/INF.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 60.Inns T, Fleming KM, Iturriza-Gomara M, Hungerford D. Paediatric rotavirus vaccination, coeliac disease and type 1 diabetes in children: a population-based cohort study. BMC Med. 2021;19(1):147. doi: 10.1186/s12916-021-02017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers LC, Liu VX. The COVID-19 pandemic strikes again and again and again. JAMA Netw Open. 2022;5(3):e221760. doi: 10.1001/jamanetworkopen.2022.1760. [DOI] [PubMed] [Google Scholar]
- 62.Durant TJS, Peaper DR, Ferguson D, Schulz WL. Impact of COVID-19 pandemic on laboratory utilization. J Appl Lab Med. 2020;5(6):1194–1205. doi: 10.1093/jalm/jfaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czeisler M, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440–9. [DOI] [PMC free article] [PubMed]
- 66.Mogharab V, Ostovar M, Ruszkowski J, et al. Global burden of the COVID-19 associated patient-related delay in emergency healthcare: a panel of systematic review and meta-analyses. Global Health. 2022;18(1):58. doi: 10.1186/s12992-022-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3):e045343. doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7(8):e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 69.Editorial. Too long to wait: the impact of COVID-19 on elective surgery. Lancet Rheumatol. 2021;3(2):e83. [DOI] [PMC free article] [PubMed]
- 70.World Health Organization. Guidance on routine immunization services during COVID-19 pandemic in the WHO European Region. 20 March 2020. Geneva: World Health Organization https://apps.who.int/iris/bitstream/handle/10665/334123/WHO-EURO-2020-1059-40805-55114-eng.pdf; 2020. Accessed 8 Mar 2021.
- 71.Bramer CA, Kimmins LM, Swanson R, et al. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care Improvement Registry, May 2016-May 2020. Am J Transpl. 2020;20(7):1930–1931. doi: 10.1111/ajt.16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldman AG, O'Leary ST, Isakov LD. The risk of resurgence in vaccine preventable infections due to COVID-related gaps in immunization. Clin Infect Dis. 2021;73(10):1920–1923. doi: 10.1093/cid/ciab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Middeldorp M, van Lier A, van der Maas N, et al. Short term impact of the COVID-19 pandemic on incidence of vaccine preventable diseases and participation in routine infant vaccinations in the Netherlands in the period March-September 2020. Vaccine. 2021;39(7):1039–1043. doi: 10.1016/j.vaccine.2020.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonald HI, Tessier E, White JM, et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveill. 2020;25(19):2000848. doi: 10.2807/1560-7917.ES.2020.25.19.2000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. [DOI] [PubMed]
- 76.Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Académie nationale de médecine. Covid-19: an opportunity to vaccinate infants against rotavirus infections. Press release from the French National Academy of Medicine. July 22, 2020. https://www.academie-medecine.fr/covid-19-an-opportunity-to-vaccinate-infants-against-rotavirus-infections/?lang=en; 2020. Accessed 05 Mar 2021. [DOI] [PMC free article] [PubMed]
- 78.Wilschut J, Bruijning-Verhagen P, Postma M. Rotavirusvaccinatie voor alle jonge kinderen. Ned Tijdschr Geneeskd. 2022;166:D6337. [PubMed] [Google Scholar]
- 79.World Health Organization. Statement on risks and benefits of rotavirus vaccines Rotarix and RotaTeq. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/rotavirus-vaccines/related-links; 2022. Accessed 23 May 2022.
- 80.Haut Conseil de la Santé Publique: Avis relatif a la vaccination des nourissons vis-à-vis des gestroentérites à rotavirus. 21 avril 2015. https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspa20150421_rotavirussusprecovaccnourrisson.pdf; 2015. Accessed 05 Mar 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.