Abstract
Ethical frameworks are the foundation for any research with humans or nonhuman animals. Human research is guided by overarching international ethical principles, such as those defined in the Helsinki Declaration by the World Medical Association. However, for nonhuman animal research, because there are several sets of ethical principles and national frameworks, it is commonly thought that there is substantial variability in animal research approaches internationally and a lack of an animal research ‘Helsinki Declaration’, or the basis for one. We first overview several prominent sets of ethical principles, including the 3Rs, 3Ss, 3Vs, 4Fs and 6Ps. Then using the 3Rs principles, originally proposed by Russell & Burch, we critically assess them, asking if they can be Replaced, Reduced or Refined. We find that the 3Rs principles have survived several replacement challenges, and the different sets of principles (3Ss, 3Vs, 4Fs and 6Ps) are complementary, a natural refinement of the 3Rs and are ripe for integration into a unified set of principles, as proposed here. We also overview international frameworks and documents, many of which incorporate the 3Rs, including the Basel Declaration on animal research. Finally, we propose that the available animal research guidance documents across countries can be consolidated, to provide a similar structure as seen in the Helsinki Declaration, potentially as part of an amended Basel Declaration on animal research. In summary, we observe substantially greater agreement on and the possibility for unification of the sets of ethical principles and documents that can guide animal research internationally.
Keywords: 3Rs, 3Ss, 3Vs, 4Fs, 6Ps, Ethics, Bioethics, Regulation, Morality, AAALAC, Harm-benefit-analysis
Graphical abstract
Highlights
-
•
Animal research is guided and regulated by ethical principles and moral frameworks.
-
•
Several sets of ethical principles are reviewed and consolidated into a unified set.
-
•
The Basel Declaration and several other documents contain the basis for an international Animal Research Declaration.
-
•
The Basel Declaration could, if there is interest, be amended to consolidate the sets of documents.
1. Introduction
“…what is good and right in animal research presumably does not change as one crosses national borders …” – J. Tannenbaum (2013)
Science often requires human and nonhuman animal research. Nonhuman animal research is crucial not only for understanding the brain and body in health and disease but also for developing and testing vaccines and treatments for diseases using novel approaches, such as, but not limited to, Covid-19 (Muñoz-Fontela et al., 2020). Both human and nonhuman animal research must be conducted within a robust ethical and moral framework. Such frameworks provide the necessary foundation for conducting the research in the most humane way possible, and they are commonly documented in the national legislation regulating research activity. Although all research groups work within their national guidelines, there is the perception that these guidelines vary, sometimes significantly, between countries. This can lead to difficulties when recipients of data operate under different ethical and legislative frameworks from those generating the data.
Certain facets of modern science have pivoted towards team science to achieve larger scope scientific projects that cannot be conducted by single institutions or countries. Scientific discoveries have, and can still be, achieved by individual scientists, their laboratories and institutions or focal research networks (e.g., Marie Curie, Charles Darwin, Albert Einstein, Jennifer Doudna and Emmanuelle Charpentier). However, many scientific advances now require the contributions of many scientists across the world sharing approaches, data and resources.
As some examples, the Human Genome Project (HGP) is the pre-eminent, large-scale international scientific effort that achieved the sequencing of most of the three billion base pairs in the human genome, the genetic instructions for human beings. Since its inception 30 years ago, the HGP has inspired other large-scale international genotyping efforts to map the genomes of as many animals and plants as possible (Green et al., 2015). The HGP also established working models for international collaboration and underscored the need to address the ethical and societal implications of scientific work and the advances arising from it (Collins et al., 2003). Another example is the international Human Connectome Project (HCP), which seeks to advance our understanding of the ‘wiring’ diagram of the human brain, its connectome (Van Essen et al., 2013). This required extensive international collaboration by those working on brain imaging to collect and share vast amounts of high-quality brain imaging data obtained from humans. The collaboration included sharing the analytical tools to process the data and the development of new infrastructure for data sharing platforms. The success of the HCP inspired other large-scale projects, including the PRIMatE Data Exchange (PRIME-DE) (Milham et al., 2018), which is a global consortium seeking to transform primate neuroimaging into an open and scalable science (Milham et al., 2020, 2022). Other examples of scientists working internationally with different local laws for animal research and ethical regulation abound, including successful collaborations between international groups working with nonhuman primates to advance scientific knowledge (Buckley et al., 2009; Zhou et al., 2019). Other examples include scientists working together involving rodent behavior and neurobiology aiming to ensure replicability and supporting joint work across multiple laboratories around the world (e.g., the International Brain Lab (Abbott et al., 2017)).
As part of this special issue on A Global Outlook on Nonhuman Primates in Neuroscience Research, a key objective is to develop resources and solutions to facilitate global collaboration. An international agreement to establish ethical principles to guide human medical research, the Helsinki Declaration, was established in 1964 (PP, 1964). In this article, we consider whether there is the basis for similar harmonization of ethical and regulatory principles into a global framework for research involving nonhuman animals. We first overview the Helsinki Declaration, and then summarize and critically assess several sets of ethical principles for animal research, including the 3Rs, 3Ss, 3Vs, 4Fs and 6Ps. We also provide a brief overview of current national and international ethical frameworks, many of which incorporate the 3Rs principles. We conclude by discussing whether there is a basis for an animal research equivalent to the Helsinki Declaration and the benefits of working towards such a declaration to facilitate, guide and support international collaboration that involves animal research. Although the ethical issues are relevant for all animal research, this paper and the special issue topic that it is a part of focus on nonhuman primates. Use of nonhuman primates in research is generally considered to require greater justification than use of other species. In addition, the greater resources needed for nonhuman primate research often involve global collaboration and these may require incorporating multiple national standards into study designs. All animal research needs to utilise optimal study designs and combine scientific rigor with appropriate ethical standards. However, these may be considered particularly important to improve reliability and reduce animal numbers when using nonhuman primates as model systems.
2. Overview of the Helsinki Declaration for human research
Human and nonhuman animal research raises legitimate ethical questions that need to be considered and addressed before the research can be conducted. This process is guided by ethical and moral frameworks. Such frameworks also guide the handling of regulatory issues that may arise during the research, including dealing with unexpected adverse effects and how to avoid and minimize the impact on the welfare of the animal by the research.
In 1964, the 18th General Assembly of the World Medical Association meeting, held in Helsinki, Finland, gave rise to the Helsinki Declaration on human medical research (PP, 1964). This declaration is a remarkable document that, in less than a few thousand words, establishes several succinct points that guide human medical research including, but not limited to, obtaining informed consent. Recognizing the need for a dynamic ethical framework that can remain relevant, as medical science advances and as new bioethical issues arise (Goodyear et al., 2007; Carlson et al., 2004; Riis, 2003), the document is not static. The Declaration is periodically amended to align with emerging scientific advances and bioethical issues (Levine, 1999).
The current version of the Helsinki Declaration has 37 points (World Medical Association, 2014). After a brief Preamble and General Principles, the document provides guidance on: 1) Risks, Burdens and Benefits; 2) Vulnerable Groups and Individuals; 3) Scientific Requirements and Research Protocols; 4) Research Ethics Committees; 5) Privacy and Confidentiality; 6) Informed Consent; 7) Use of Placebo; 8) Post-Trial Provisions; 9) Research Registration and Publication and Dissemination of Results; and, 10) Unproven Interventions in Clinical Practice. For instance, the guidance on Risks, Burdens and Benefits underscores the need for research to be assessed by an approved committee, guidance for which is provided under the section on Research Ethics Committees. The committee will conduct an analysis of the risks and burdens imposed on the subject by the research (e.g., the potential Harms) alongside the possible Benefits that the research may provide, typically in the form of the possible societal or scientific benefits, rather than an immediate benefit for the research subject which is typically not likely. This approach, often referred to as a Harm-Benefit Analysis (HBA), is also broadly supported for research with nonhuman animals (see Hartig et al., this issue).
While initially established for human medical research, and stated as such in the introductory sections, many scientists and institutions around the globe conducting human research now seek to ensure that their human research practices and regulations abide by the Helsinki Declaration. This is the case even for non-medical human research. For instance, principles that specifically apply to medical research, such as clinical trials, may not apply if the research is not a clinical trial. If the researchers working with humans abide by the Helsinki Declaration, they may report this in their publications. If so, they can include a statement about whether the research abides by the Helsinki Declaration and other local or national regulatory bodies. Research studies will also report which research committee provided approval, typically also along with the approval number.
Many of the points in the Helsinki Declaration are specific to research with human subjects, and, as such, cannot be directly applied to nonhuman animal research. However, some points, such as the need for conducting the HBA, are also explicitly required by the regulations in many countries in order for assessment committees to evaluate the harms and benefits associated with any given research project seeking approval. Hartig et al. (this issue) provide data on these analyses and approaches carried out internationally for nonhuman primate research. They note that all reporting countries (13 countries in total that conduct nonhuman primate neuroscience research and participated in the survey) conduct some form of HBA. This indicates that there is a common moral commitment that research and regulatory committees in different countries consider, regarding both the benefits of the scientific research and the impact on and the welfare of the animals.
3. What is the Basel Declaration on animal research?
The Basel Declaration on animal research was established in 2010 by over 60 scientists, initially by the Basel Declaration Society that is now Animal Research Tomorrow, the new home to the declaration (https://animalresearchtomorrow.org/en). The Declaration was established to show that science and animal welfare are mutually intertwined common principles, rather than being diametrically opposed. The Basel Declaration on animal research contains an Introduction, followed by, as shown in Table 1, Fundamental principles and a Declaration that signatories can subscribe to.
Table 1.
In what follows, we propose that the Basel Declaration on animal research can serve as a basis and a preamble to a more complete Animal Research Declaration that incorporates a unifying set of principles (Table 2) and the guidance documents already available (Table 3)..
Table 2.
Overview of Sets of Ethical Principles. The sets of principles are ordered historically and alpha numerically. See manuscript text and citations for further details.
| Sets of Ethical Principles | ||
|---|---|---|
| Set | Principles | Citation |
| 3Rs | Replacement - with other species or non-animal methods | Russell and Burch, 1959 |
| Reduction - fewest animals necessary | ||
| Refinement - use the most refined approach | ||
| 3Ss | Good Science - reduce variation using effective techniques and fewest subjects | Carol Newton (Smith and Hawkins, 2016) |
| Good Sense - the correct animal used for the correct reason (valid and translatable model) | ||
| Good Sensibilities - reduce likelihood of the animal experiencing “contingent suffering" | ||
| 3Vs (Validities) | Construct Validity - is the animal valid for the scientific objective? | Eggel and Würbel, 2021; Würbel, 2017 |
| Internal Validity - is the approach and protocol appropriately designed? | ||
| External Validity - will the work generalize to other animals or humans based on its purpose? | ||
| 4Fs (Fundamental principles) | 1. Biomedical research principle - statement on the need for biomedical research | Tannenbaum, 2017 |
| 2. Animal research principle - statement on the requirement for the animal research | ||
| 3. Medical research with human subjects - statement on the limitations of human research | ||
| 4. Animal research aims - minimize pain or distress to the animals | ||
| 6Ps (Principles) | Principle 1 - no alternative method | Beauchamp and DeGrazia, 2019 |
| Principle 2 - expected net benefit | ||
| Principle 3 - sufficient value to justify harm | ||
| Principle 4 - no unnecessary harm | ||
| Principle 5 - animal basic needs | ||
| Principle 6 - upper limits to harm | ||
Table 3.
Animal research declarations, statements and guiding documents. See links and manuscript text for further details.
| International Declarations on Human and Nonhuman Animal Research | |||
|---|---|---|---|
| Declaration | Information about | Proposing Organization | Link |
| Helsinki Declaration (human research) | Prominent international declaration on human medial research and ethics; 10 sections, 37 points | World Medical Association (WMA) | https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ |
| Basel Declaration (animal research) | Statement on animal research following Basel conference 2010, can be signed online; contains Introduction, Fundamental Principles and Declaration; 16 points together | Basel Declaration Society, now Animal Research Tomorrow | https://animalresearchtomorrow.org/en/basel-declaration |
| International Standards and Guidelines Relating to Research Animals | International standards and guidelines on animal research and ethics - online document links to two other documents (1–2 next) | International Council for Laboratory Animal Science (ICLAS) |
https://iclas.org/harmonization-committee-international-standards/ |
| 1. Use of Animals in Research and Education | Detailed statement on animal research and education; Chapter 7.8 of Terrestrial Animal Health Code; 10 articles; incorporates 3Rs | World Organization for Animal Health (OiE) | https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=0&htmfile=chapitre_aw_research_education.htm |
| 2. International Guiding Principles for Biomedical Research Involving Animals | International Guiding Principles for Animal Biomedical Research; Preamble and 10 statements | Council of International Organizations of Medical Sciences (CIOMS) & International Council for Laboratory Animal Science (ICLAS) | https://iclas.org/cioms-iclas-international-guiding-principles-for-biomedical-research-involving-animals/ |
| Additional Guidance Documents | |||
| Accreditation for Laboratory Animal Care | International accreditation sensitive to national guidelines; relies on 1. and 2. documents above | American Association for Accreditation of Laboratory Animal Care (AALAC) | https://www.aaalac.org/ |
| European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes | European animal research harmonization document, 38 articles | Council of Europe (ETS 123) | https://www.aaalac.org/pub/?id=E900CF34-9112-946E-C8A5-331F9E2897D9 |
| Guide for the Care and Use of Laboratory Animals (ILAR) | US guide for the care and use of research animals | National Research Council, U.S.A. | https://www.nap.edu/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth |
| U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training | National Institutes of Health (US) vertebrate animal research guidelines | National Institutes of Health, U.S.A. | https://olaw.nih.gov/policies-laws/gov-principles.htm |
| Non-human primate accommodation, care and use guidelines | Guidelines for nonhuman primates, integrates 3Rs and exceeds other nonhuman primate guidelines | National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) | https://nc3rs.org.uk/3rs-resources/non-human-primate-accommodation-care-and-use-guidelines |
| ARRIVE Guidelines 2.0 | Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines, including essential and recommended items | du Sert et al., PLoS Biology 2020 | https://arriveguidelines.org/ |
| PREPARE Guidelines | Planning Research and Experimental Procedures on Animals: Recommendations for Excellence | Smith et al., Lab. Anim. 2018 | https://pubmed.ncbi.nlm.nih.gov/28771074/ |
4. Overview of sets of ethical principles for animal research
Nonhuman animal research, like human research, is ethically sensitive, and most countries have legislation that regulate it (Mitchell et al., 2021) (Hartig et al., this issue). It is commonly felt that there is substantially greater variability in perspectives and approaches, and national or cultural sensitivities about animal research. This may or may not be the case (Tannenbaum, 2017). Is it possible that researchers and other stakeholders are or could be empowered by a common set of principles?
Several sets of ethical principles exist that aim to strike a balance between the argument for the necessity and benefits of the animal research and the associated welfare costs and harms to the animal. These sets of principles provide guidance on conducting research in the best way scientifically and the most humane way possible for the welfare of the research animals. We review several sets of principles and consider whether they can be replaced, reduced, refined and incorporated into a unified set.
5. The 3Rs, 3Ss, 3Vs, 4Fs and 6Ps
There is a rich history of philosophical contributions recognizing the moral importance of considering both the societal benefit and the welfare of research animals (Smith and Hawkins, 2016; Harrell et al.GT, 1977). For example, Marshall Hall (1790–1857) in the book Principles of Investigation in Physiology, considered the importance of replacing animals with observational studies, if possible. Hall also considered it important to reduce suffering and avoid unnecessary repetition by having clear objectives and outcomes (Smith and Hawkins, 2016).
The 3Rs, proposed by Russell & Burch in 1959 (Russell and Burch, 1959), have become the prominent set of principles for animal research, having been adopted in the animal research legislation by a number of countries (for a review (Smith and Hawkins, 2016; Harrell et al.GT, 1977):). The 3Rs were originally developed to promote principles that would make animal experiments more humane. The three Rs are Replacement, Reduction and Refinement. Replacement advocates replacing animal research when it is not necessary or replacing animal research with other methods and alternatives. The application and interpretation of the 3Rs has evolved over time, particularly with regards to the Replacement factor (Tannenbaum and Bennett, 2015; Council, 2003). Where replacement of animals is not considered possible, the principle of Reduction should be applied, so that the most appropriate number of animals are used. This should be combined with efforts to Refine the research approach to increase the well-being of the research animals that is still considered necessary to use (reviewed in (Tannenbaum and Bennett, 2015; Graham and Prescott, 2015):). Since their inception, the 3Rs have been seen by many as useful principles that continue to be applied in different domains of animal research (Graham and Prescott, 2015; Sneddon et al., 2017; Flecknell, 2002). There have also been challenges and critiques to the 3Rs, which we feel are appropriate for any framework to address in order to develop. It is also within the spirit of the 3Rs to gauge any set of principles in their ability to withstand Replacement, Reduction or Refinement.
The 3Ss. In 1975, the Institute of Laboratory Animal Research (ILAR) arranged an international symposium in Washington, D.C. entitled The Future of Animals, Cells, Models and Systems in Research, Development, Education and Testing. The focus of this symposium was to cover two topics: (1) the use and limitations of cell, tissue and organ cultures; and (2) the application of statistics and computer technology (Harrell et al.GT, 1977). Carol Newton was an invited speaker and introduced the concept of the 3Ss – Good Science, Good Sense and Good Sensibilities (Smith and Hawkins, 2016) (Table 1). The first S – Good Science, encapsulates the notion that the goal of all research is to do good science, and the essence of doing good science is relying on science to contribute new knowledge and progress. Good Sense is ensuring that the science uses the appropriate model available to answer the scientific question – whether these are computer (in silico), in vitro or in vivo models. Finally, Good Sensibilities is the culture of care that needs to be nurtured to do Good Science. For instance, if the science involves animal models, then it is essential that everyone involved understands the care that must be provided to the animals. Similarly, for Good Science to progress forward there should be a culture of care, regardless of the model, to ensure that there is a conscientious approach to gathering and sharing data. Newton, as a biomathematician, also underscored the importance of understanding biological variation and strong experimental design and statistical analyses that were sufficiently powered. This issue remains highly relevant and may have inspired other frameworks, such as those focusing on the validity of the animal model (3Vs, see Table 2 and next).
The 3Vs were developed recognizing that the 3Rs do not go far enough when considering the Validity of the animal model, which requires a few additional steps (Eggel and Würbel, 2021; Würbel, 2017). The 3Vs emphasize the Validation aspect of animal models. They were developed to provide guidance on how to build a protocol where the validities of the animal model can be more clearly assessed using the HBA approach, by asking the following questions: Is the animal model valid for the scientific objective (Construct Validity)? Is the model approach and the protocol designed appropriately (Internal Validity)? How likely is it that the work will generalize more broadly to other animals or humans based on its purpose (External Validity)? Yet, rather than replacement, Eggel and Würbel (2021) emphasize the internal consistency of the 3Rs and 3Vs. This suggests that the originating authors do not view the 3Vs as a replacement of the 3Rs, but rather as an extension to emphasize and guide assessment of the importance of validity in the justification for the animal research. Eggel and Würbel recommend, wherever possible, that researchers provide evidence for the 3Vs in their research with animals. This set of principles encapsulates and extends the Good Sense principle of the 3Ss (Table 2).
The 4Fs. More recently, Tannenbaum (2017) proposed a framework and several fundamental principles that emphasize the responsibility of the investigator. The 4Fs highlight that animal research is a privilege, granted by society to the research community, with the expectation that there is significant new knowledge generated and/or improvements in humans and/or other animal well-being (Tannenbaum, 2013, 2017). Fundamental Principle 1: The biomedical research principle states that research is among the most noble and imperative of human endeavors, relying on the scientific method to prevent, alleviate, and cure the pain, suffering, distress, fear, anxiety, disability, infirmity, and death associated with disease. The ethical burden of proof is also on those who think that biomedical animal research is not justified. Fundamental Principle 2: The animal research principle states that the development of knowledge necessary for the improvement of the health and well-being of humans as well as other animals may well require in vivo experimentation with nonhuman animals (US Principles, 1984: https://olaw.nih.gov/policies-laws/gov-principles.htm). Fundamental Principle 3: The principle of medical research on human subjects, we quote, “should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problems under study that the anticipated results will justify the performance of the experiment” (Nuremberg Code, 1947, para. 3: https://media.tghn.org/medialibrary/2011/04/BMJ_No_7070_Volume_313_The_Nuremberg_Code.pdf). This principle resonates with the 3Vs above. Fundamental Principle 4: Animal research should aim to minimize pain or distress to the animals in the achievement of the scientific goals of the research.
The 6Ps. Another set of principles is the 6Ps, which as the authors, Beauchamp and DeGrazia write in their 2019 book Principles of Animal Research Ethics (Beauchamp and DeGrazia, 2019; DeGrazia and Beauchamp, 2019), can be viewed as complementary to the 3Rs. The 6Ps can serve an important extension that balances the two facets society put value on: social benefit and animal welfare, subdivided into three principles each. The 6Ps include six principles that resonate with the 3Rs as well as additional principles to explicitly guide the HBA. It is worth expanding on the 6Ps framework here because it is one of the more extensive sets of ethical principles advanced to date, and because their basis in the 3Rs is also evident. We quote Beauchamp and DeGrazia directly from their book for each of the six principles to ensure their meaning is preserved and to distinguish our comments regarding the correspondences to the 3Rs.
-
•PRINCIPLES OF SOCIAL BENEFIT
-
oPrinciple 1: No-alternative method states that the “Use of animal subjects must be the sole ethically acceptable way to address a research problem whose solution offers the prospect of social benefit.” This principle resonates with the 3Rs principle of Replacement.
-
oPrinciple 2: Expected net benefit states that “The prospect of social benefit from a research study must outweigh its expected costs and risks to human beings.” This principle introduces consideration of the Benefits of the animal research to guide the HBA. The authors note that this requires an evidence-based approach to estimate, if possible, the magnitude of the benefit that could be achieved and the likelihood of achieving the benefit in relation to comparable research that has produced (or failed to produce) the benefit.
-
oPrinciple 3: Sufficient value to justify harm states that “The prospect of a net benefit for human society from a research study must be sufficiently valuable to justify expected harms to animal subjects.” This principle extends the HBA of the benefits of the research in balance with the anticipated harms to the animal.
-
o
-
•PRINCIPLES OF ANIMAL WELFARE
-
oPrinciple 4: No unnecessary harm states that “Animal subjects must not be harmed unless a particular harm is necessary for and morally justified by scientific purposes.” This principle further incorporates, into the HBA, animal welfare aspects and resonates with the 3Rs principle of Refinement, including to some extent, albeit implicitly, Reduction (using appropriate numbers of animals).
-
oPrinciple 5: Basic needs states that an “Animal subject's basic needs must be met in the conduct of studies unless failure to meet specific basic needs is necessary for and morally justified by scientific purposes.” Beauchamp and DeGrazia propose a plausible catalogue of basic needs, including access to nutritious food and clean water, safe shelter, adequate stimulation, exercise, and opportunities for species-typical functioning, sufficient rest to maintain physical and (where applicable) mental health, access to veterinary care, access to compatible conspecifics or social group members for social animals, freedom of movement with adequate space. Beauchamp and DeGrazia note that freedom from premature death is a controversial basic need. Two other needs are proposed, which need careful consideration, because many forms of biomedical research have the risk of precluding many forms of research that may require infringing on those needs. These are: (1) freedom from significant experiential harms, such as pain, distress and suffering; and (2) freedom from disease, injury and disability. Beauchamp and DeGrazia note: “Ordinarily it is wrong to harm an individual, whether human or animal, in the absence of a sufficient reason for doing so. But nearly all laboratory animal research involves foreseeable harm to subjects, and much animal research also requires not providing for some basic need. The Principle 4 and 5 are formulated with qualifying conditions that permit some harming, including not meeting basic needs, when doing so is necessary for and morally justified by the social and scientific goals of research involving animals” (see p. 20 in (Beauchamp and DeGrazia, 2019)). Notably, the Helsinki Declaration also has statements on providing for basic needs of the human subjects, included under its Vulnerable Groups and Individuals section.
-
oPrinciple 6: Upper limits to harm states that “Animal subjects must not be caused to endure severe suffering for a lengthy period of time. In rare, extraordinary cases, exceptions may be warranted if the research is necessary for and morally justified by critically important social and scientific purposes.” This principle would help to define humane endpoints for the animals in the research studies, either anticipated endpoints or those that would be implemented if unexpected adverse effects were to occur. Note that even ‘unexpected’ adverse effects can be pre-defined as those with a reasonable likelihood of occurring from the research, but which are not expected to occur in most cases.
-
o
PREPARE and ARRIVE guidelines: One area that may be lacking with the current sets of principles is that they do not have explicit principles or guidance on how animal research should be prepared and reported. Such tenets that directly address the likelihood of reproducibility of scientific research are necessary. The issue of how animal research can be planned, which includes online data analysis tools, is addressed in the PREPARE guidelines (Smith et al., 2018) and guidance is provided in the NC3Rs Experimental Design Assistant (https://www.nc3rs.org.uk/our-portfolio/experimental-design-assistant-eda#publications) (Laber et al., 2016). Guidance on how to consistently report research with nonhuman animals in publications can be found in the revised ARRIVE 2.0 principles (Percie du Sert et al., 2020). Also see Table 3 and the AAALAC reproducibility guidance (https://www.aaalac.org/accreditation-program/faqs/#Reproducibility).
Further revising of the sets of ethical principles: Another major issue is that in human and nonhuman animal research it is often difficult to replicate published results. There are several reasons why replication of a study might not be possible (Poldrack et al., 2017), some of which are that the sample sizes or the measured effects of an experiment are too small or variable. Namely, when a small sample size is combined with the inherent variability in the sensitivity of the study measures and a specific significance threshold, this can lead to a lack of replication of published results and require many more animals (including humans) to be tested to adjudicate whether the original reported results are replicable (Poldrack et al., 2017; Macleod and Mohan, 2019). Newton highlighted this issue in the 3Ss Good Science principle (Smith and Hawkins, 2016). Thus, the Reduction principle in the 3Rs may be questioned or replaced with an O for Optimal, or another R for the Right-size. This would mean that the 3Rs could actually be 2Rs and an O, or stay as 3Rs, while addressing issues that have emerged since their inception. Beauchamp and DeGrazia also indicate that the optimal number likely to lead to replicability in larger sample sizes should be used.
6. Can these ethical sets of principles be replaced, reduced or refined?
The various sets of ethical principles could be viewed as attempts to Replace the 3Rs with alternate frameworks or any of the others available. Yet, the 3Rs appear to have regularly withstood the replacement challenge. This is because a number of the frameworks, rather than replacing the 3Rs or upending the others, note the complementary nature of the alternate principles and often incorporate or extend the 3Rs and the others. For instance, both the 6Ps and 3Vs (an extension of 3S and 4F principles) can be viewed as refinements building on the foundation of the 3Rs, including to better guide the HBA process. None of the other sets of ethical principles (3Ss, 3Vs, 4Fs, 6Ps) are necessarily replaced as such by the others, and many make common points.
In terms of Reduction, the 3Rs or 3Ss are about as compact a set of principles as possible, keeping in mind that the Helsinki Declaration currently has 37 points. The 6Ps can be viewed as either further condensed into two core values set (Social Need and Animal Welfare), or as the more extended six principles that incorporate the 3Rs and guide the HBA. The 3Vs can also extend the 3Rs in important ways with regards to explicit consideration of the validity of the animal research along three dimensions (Construct, Internal and External Validity). These facets on the validity of the animal research are not nearly as clearly articulated in any of the other sets of principles.
With regard to Refinement, each of these sets of principles we find are ultimately contemporary efforts that have led to refining and extending the original 3Rs. The 6Ps are as close to a replacement as has been made, in part, because the 6Ps explicitly incorporate the 3Rs principles. Refinement rather than Replacement is common and necessary over time, as evidenced with the historical amendments of the Helsinki Declaration for human research.
7. Unified sets of principles
We have observed that these sets of principles are complementary. They can be viewed as continuing refinement of the classical 3Rs, and are useful as a unified set of principles. The sets of principles, particularly given that they have converged in some ways around the 3Rs, are thereby a reflection of a maturing field of bioethics and the issues surrounding animal research and community values. They are arguably an improvement on extremist positions that do not support any animal research for any reason, or those that consider only the scientific progress and disregard any impact of the research on animal welfare. Surveys have shown that the public may be willing to accept certain types of animal research, provided that the research is necessary, that no alternatives exist, and that the approach taken is the most humane (Mitchell et al., 2021). Thus, these sets of ethical principles provide ethical approaches that can transcend national borders.
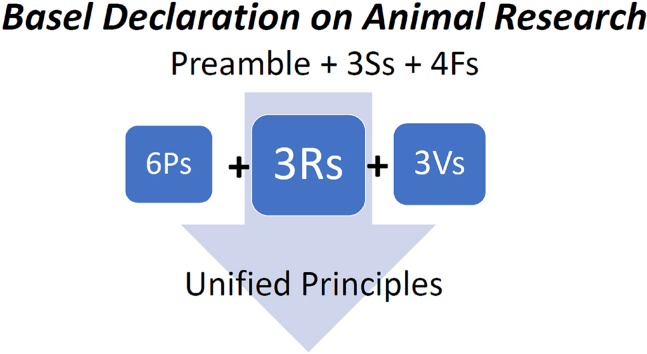
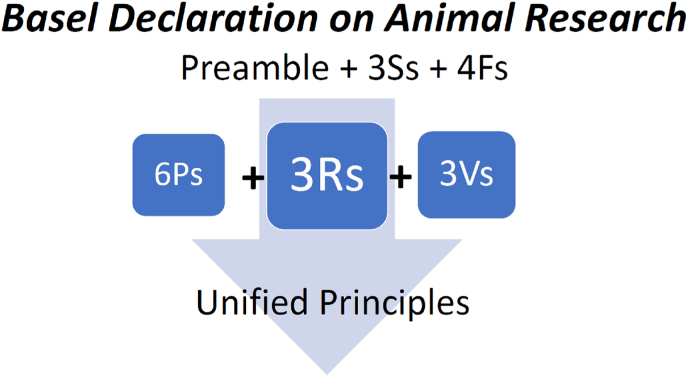
How could the principles be usefully unified? The 3Ss and 4Fs, in our view, could be easily integrated into a Preamble to an animal research declaration, such as part of the current version of the Basel Declaration. The Preamble to an Animal Research Declaration, as does the Helsinki Declaration on human research, provides the foundational basis for the research and speaks to the importance of the work and how it can be done in the most humane way possible. As such, the 3Ss can provide part of the overarching guidance on Good Science, Sense and Sensibilities, covered in greater detail and guidance by the 3Rs, 6Ps and 3Vs. Thus, we do not suggest dispensing with any of these or other sets of principles that may be available or become available. Rather, we suggest restructuring our consideration of them as a unified set. The 3Rs are prominent throughout as a core, and some might find the extension, with the HBA guidance of the 6Ps and the three validities (3Vs), very usefully integrated with the 3Ss and 4Fs as a preamble to these. The proposed unification is illustrated in Fig. 1. We avoid defining a number to the unified principles because other ethical principles may well be integrated into these. Moreover, future efforts may lead to a reduction of redundancy and a more compact unified set.
Fig. 1.
Basel Declaration and unified sets of ethical principles. The 3S and 4F principles could be an important part of the Preamble to an animal research Basel Declaration. The 3Rs are the classical core, enhanced with guidance on the HBA by the 6Ps and the three validities (3Vs). The documents listed in Table 3 and others could be consolidated into a more complete Basel Declaration, modelled after the Helsinki Declaration on human research.
8. Challenges in conducting a balanced harm-benefit-analysis (HBA)
The HBA is explicitly incorporated into the 6Ps and is implicit in many of the other sets of principles. Conducting any HBA is not, however, immune to bias even with the most objective approach. Scientists or other stakeholders may think that it can be biased towards animal harms more than scientific benefits, or vice versa. Some may be concerned that an ethics panel does not appreciate the societal benefits of the science. Others might highlight that the emphasis on animal welfare needs to be evidence-based with regards to making a positive impact on the animals’ welfare without complicating the completion of the scientific work. Also, the benefits might be difficult to appreciate by panel members if, for example, scientists struggle with or are not allowed sufficient space or time to describe their science and its potential benefits clearly enough to non-specialist panel members, or to sufficiently relay the care that they take to ensure the welfare of the animals. The value of and pitfalls in conducting the HBA are highlighted elsewhere (Hartig et al., this issue); these include a singular focus on societal benefits, which are not always possible to estimate in terms of magnitude and probability for many studies (Niemi, 2019). There are also societal harms to consider of not having the medical advances (Rollin, 2006). Another difficulty with conducting an impartial HBA is that there is inherent uncertainty in the power and sample size calculations often used to determine the optimal number of animals for a research study. Moreover, the HBA often cannot be precisely quantified, so the analysis process itself needs to build in an understanding about the level of inherent uncertainty.
These challenges in conducting a balanced HBA do not mean that the HBA is of no value and should not be pursued. Ethical principles and guidance on the HBA are crucial for reducing bias (Brønstad et al., 2016; Laber et al., 2016). Scientists play an important role in the HBA, not just in submitting their research for evaluation and approval, but as part of the process of providing evidence for the importance of the work in ways that can be appreciated by committees including non-specialist lay members. Scientists can also look for and develop opportunities to refine their procedures alongside other developments in the scientific and animal welfare communities, some of which might help to both deliver better science without slowing down the work and have a positive benefit to the animals’ welfare. It is also important for scientists to consider improving how they describe the benefits of their science to lay individuals so that the importance of the science may be better understood (Prescott and Lidster, 2017). Some science communication and advocacy organizations, like Understanding Animal Research (https://www.understandinganimalresearch.org.uk/), conduct workshops that can help scientists to get more comfortable speaking with the public about their research. There are training and eLearning courses about animal research related to ethical principles, national legislation and conducting a balanced HBA, including training on examples taken from animal research protocols with various animal species. Not only scientists, but also animal welfare and ethics review panel members, can all benefit from guidance on contributing to and conducting a balanced HBA by using an evidence-based approach whenever possible.
If ethical evaluation is implemented with a core or possibly unified set of principles that provide clearer guidance on conducting the HBA, then the HBA assessment can be conducted within a solid scientific and animal welfare framework. The ethical principles and HBA guidance have and can continue to provide structure for discussion and debate. Society ultimately decides via its representatives and legislation what is allowed and what is not. Any discussion is more meaningful and effective if it is grounded in a guiding set of principles not tied to any specific country.
9. The role of Animal Care and Use Committees
The Helsinki Declaration for human research in the section on Research Ethics Committees recognizes the importance of regulatory committees and provides guidance on conducting a balanced review. Several of the animal research documents in Table 2, Table 3 explicitly recognize the important moral role of animal research review committees. The committees have the moral charge to review and approve animal research and to oversee the protection of animal welfare. Some countries (e.g., the United Kingdom (UK) and European Union (EU)) assign the HBA to a local and/or national ethical review body. The US and China assign the approval of animal research applications to Institutional Animal Care and Use Committees (IACUC), which are protocol review and compliance committees (Mitchell et al., 2021); also see Hartig et al., this issue. These committees are tasked with conducting impartial reviews that are free of conflicts-of-interest (COIs). Any COIs should be declared and recognized as is common in any scientific committee. By following a set of ethical principles and guidance for review of applications, including on conducting the HBA, bias in the evaluation and approval process for human or animal research can be minimized. The committees also need to abide by local and national legislation and regulation.
10. Incorporating ethical principles into the fabric of legislation
The UK was one of the first countries to establish legislation for the protection of animals used in research, in the Cruelty to Animals Act (1876). Since then, The Animal (Scientific Procedures) Act 1986 has governed the use of animals in research in the UK. This Act limits the amount of pain, suffering, distress, and lasting harm to which laboratory animals may be subjected. These laws continue to be legislatively reviewed and revised. In the United States, in 1985, the US Congress enacted the Improved Standards for Laboratory Animals Act on Animal Welfare, replacing the Animal Welfare Act of 1966. In Canada, the Council on Animal Care advanced a Guide to the Care and Use of Experimental Animals. Australia has animal protection legislation in their Code for the Care and Use of Animals for Scientific Purposes (2013). In New Zealand, The Animal Welfare Act has operated since 1999. For further information on the history of animal regulations, guidance and legal protections internationally see (Rollin, 2006).
The EU is one of the few organizations that has enacted legislation that serves to regulate the animal research conducted across its different member states (Brønstad et al., 2016). The EU Directive 2010/63/EU requires all member states to enact national legislation that meets the requirements set out in the Directive. The Directive, unlike the Helsinki Declaration, is a lengthy document. It has six Chapters and sixty-six articles and is a revision of the previous EU Directive 86/609/EEC. The EU Directive 2010/63/EU explicitly incorporates the 3Rs and requires that all research applications to regulatory committees address the three principles. The Directive also provides constraints on the re-use of animals in multiple research studies or protocols. In individual cases where re-use is permitted, this may lead to a Reduction in animal numbers. It also stipulates that animals should be socially housed whenever possible, and formalizes the minimal enclosures allowed by animal species (Annex III, section B). The EU Directive ensures that for all animal research there is a designated veterinarian and regulatory animal welfare oversight body. It guides the conduction of the HBA and provides reporting obligations. It also requires that the maximum “severity” that animals experience during the research is reported after completion of each study. This “severity” classification attempts to summarize the harms experienced by animals as (Annex VIII): ‘non-recovery’ (procedures entirely under general anesthesia without recovery); ‘mild’, ‘moderate’ or ‘severe’. The EU, UK and a number of other countries annually report their animal research numbers by species and by class of severity and are also publishing non-technical project summaries with regards to the scientific benefits, anticipated animal harms and the 3Rs considerations of the animal research.
Countries that might not have explicit animal research and protection legislation often emulate the regulatory process and HBA conducted in countries that do (see Hartig et al., this issue for international HBA approaches in nonhuman primate research). This is done, in part, because publishing scientific papers in peer-reviewed scientific journals requires that a local oversight body has evaluated the necessity for the animal research and approved the research application. While the HBA is not formalized in every country, Hartig et al. (this issue) show how countries surveyed across North America, Europe and Asia consider the HBA, in particular with regards to nonhuman primate neuroscience research. This survey stands as a resource for information relevant for understanding international animal research practices and regulation. By expanding this survey to all animal species and more of the countries in the southern hemisphere, the HBA approach could be a common international framework as an overarching moral imperative for research involving nonhuman animals. Unifying the ethical sets of principles into an international declaration could encourage a harmonised approach to the use of animals in research.
11. An animal research Basel Declaration and supporting framework are available
There is already the basis for an animal research declaration like the Helsinki Declaration on human research. It just so happens at this point in time to be distributed across a number of different sets of documents. The overview in Table 3 provides citations and links to these documents. For instance, the International Council for Laboratory Animal Science (ICLAS) has a document that links to the World Health Organization for Animal Research (OiE) highlighting their detailed statement on animal research and education. The other document linked to by ICLAS is on International Guiding Principles for Animal Biomedical Research, which includes a Preamble and 10 statements. This is as close to a Helsinki Declaration set of principles as we have seen. Both of these documents are used by AAALAC International to accredit any country seeking to establish animal research based on a common set of international principles. AAALAC International also recognizes national and cultural sensitivities in its accreditation. The Basel Declaration, in our view, contains an important Preamble to an animal research declaration, and when all three sets of documents are considered, they, in essence, become structurally very much like the Helsinki Declaration, with a preamble and key sets of principles.
It seems then, that the framework for the animal research equivalent to the Helsinki Declaration is already available. The problem is that without consolidation of these documents, there is not a single document that can be referred to but several. Thereby, as with the sets of ethical principles reviewed earlier, we also recommend consolidation of the relevant sets of documents into the next potential amendment of the Basel Declaration (Fig. 1). Parts of the EU Directive and other international documents and legislation available may also provide useful points that could be consolidated. It would not, however, be in the spirit of the Helsinki Declaration to simply collate many documents, because the declaration is succinct and provides clear guidance that crosses borders.
12. The benefits of further developing the Basel Declaration for animal research
It is not necessary to institute an animal research ‘Helsinki Declaration’ in the same way that the Helsinki Declaration itself is not a mandate for all human research. It can be voluntarily adopted by those that choose to do so. Some might also see the Basel Declaration as the equivalent to the Helsinki Declaration and appreciate its current succinctness and elegance. One might also argue that the human research Helsinki Declaration is not necessary if there are human research regulations and legal protections already in place at the local or national levels. The problem, however, is that without a global declaration for animal research there are tremendous hurdles for scientists who seek to collaborate or directly work in parallel with research groups conducting animal research in different countries. This will become an increasingly more complicated problem to navigate for global initiatives, if for example scientists from different countries working under different animal research regulations are not permitted to collect and use animal research data together. Another issue is that a mutually agreed on global declaration could help to provide common guidance to ensure better replicability of animal research findings and improve the quality of the scientific data while also reducing animal numbers when groups work in isolation or under substantially varying welfare conditions, animal oversight and regulation. One reason why the 3Rs are being widely adopted is that they not only promote good welfare, but also good science. There are valid concerns where animals in poor health, stressed unnecessarily and undergoing poorly conducted procedures generate poor data quality (Mitchell et al., 2021; Prescott and Lidster, 2017). The effects of the laboratory environment on research outcomes has been highlighted by several authors (e.g. (Mogil, 2017; Hylander et al., 2022)). Addressing these concerns brings together improved welfare, often by reducing uncontrolled stress, and optimal study design. There is an argument to be made about scientifically introduced heterogeneity in animal results (Richter, 2017), but that is different from heterogeneity that is introduced because of welfare issues leading to poor quality science. Working towards agreement on a global declaration does not need to impinge on geographical and cultural sensitivities, and this effort can establish common ground to increase rigor and reproducibility (Bliss-Moreau et al., 2021) while reducing animal numbers.
There are a few ways to overcome this barrier. The less ideal option (currently practiced by many scientists) is to have scientists only collaborate intellectually, with the animal component of their research being conducted in a specific country. However, even an intellectual collaboration involving data obtained from animal research can still be viewed as involvement in the animal research itself. Another option is that the scientists in different countries separately conduct the research under their own regulatory frameworks and funding requirements. This can work to some extent, but for large initiatives that may require international funding, it may become too complicated for any scientist or group of scientists to navigate the common ground and differences in regulations. There is also very little data to inform this process, thereby collections of articles in the scientific literature, such as this one, can help to generate information and provide sustainable resources to support international scientific collaboration involving animal research (Hartig et al., this issue). Nevertheless, one promising effort to overcome this barrier is to have an international animal research declaration. The Basel Declaration, even in its current form, aims to facilitate and provide common ground that recognizes the core values (i.e., societal benefit and animal welfare) that many countries and scientists might find useful to subscribe to and sign. If they do, then there would be at least a general ethical framework to facilitate global collaboration involving animal research. Once this is established there are practical steps and frameworks available to further guide the collaborative research internationally (Mitchell et al., 2021) (Hartig et al., this issue).
13. Ideas on global oversight
We have considered how global oversight could be facilitated by an AAALAC International accreditation (Table 3). This would provide somewhat of a common basis, but also accepts the individual oversight of the country where the research is occurring and is dependent on the laws and regulations in that nation. However, there may be concern of over-regulation imposing further barriers for researchers to overcome in order to work with animal research groups in other countries, so there are implementational issues that would need to be addressed. This requires an overarching implementational framework that would incorporate ethical principles and common ground amongst the guiding regulations of each nation, as proposed by Hartig et al. (this issue). Any of these ideas, if they prove useful for facilitating and guiding international collaboration would be helped with an animal research declaration.
14. How could the Basel Declaration on animal research be further developed?
As our analysis of ethical sets of principles and documents show, there is substantial common ground in ethical principles across the countries and organizations that generated these documents. The EU has broken ground in how an international animal research directive can be achieved, and the 3Rs have survived the test of time, being implemented in the legislative frameworks of several countries. The sets of principles have also expanded and we suggest a unifying approach (Fig. 1) as a cornerstone for any global animal research declaration. This paper serves to link the various documents and advocates for applying a conceptual unification of the current sets of ethical principles, such that any scientist, institution, country or global collaboration could use these as guidance. However, we suggest here, that there might also be interest for the society that generated the Basel Declaration to consider interacting with the organizations that generated some of the other documents into advancing an amended, more complete, Basel Declaration, further modelled after the Helsinki Declaration that inspired it. This effort could study and consider the relevant documents and consolidate them into what may prove to be a guiding animal research Declaration. As we have considered, the documents for this amendment are there and the time is ripe for the Basel Declaration, for example, to enshrine and guide, the next natural amendment of a global Animal Research Declaration inspired by the Helsinki Declaration.
CRediT authorship contribution statement
Christopher I. Petkov: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. Paul Flecknell: Conceptualization, Writing – review & editing. Kathy Murphy: Conceptualization, Resources. Michele A. Basso: Conceptualization, Resources, Writing – review & editing. Anna S. Mitchell: Writing – original draft, Writing – review & editing. Renee Hartig: Conceptualization, Writing – review & editing. Sally Thompson-Iritani: Conceptualization, Resources, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Supported by Wellcome Trust (WT092606AIA), National Institutes of Health USA (R01–DC04290) and European Research Council (ERC Consolidator Grant, MECHIDENT).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100060.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Abbott L.F., et al. An international laboratory for systems and computational neuroscience. Neuron. 2017;96(6):1213–1218. doi: 10.1016/j.neuron.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp T.L., DeGrazia D. Oxford University Press; 2019. Principles of Animal Research Ethics. [Google Scholar]
- Bliss-Moreau E., et al. Improving rigor and reproducibility in nonhuman primate research. Am. J. Primatol. 2021;83(12) doi: 10.1002/ajp.23331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brønstad A., et al. Current concepts of harm–benefit analysis of animal experiments–report from the AALAS–FELASA working group on harm–benefit analysis–part 1. Lab. Anim. 2016;50(1):1–20. doi: 10.1177/0023677216642398. _suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M.J., et al. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325(5936):52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Carlson R.V., et al. The revision of the Declaration of Helsinki: past, present and future. Br. J. Clin. Pharmacol. 2004;57(6):695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S., et al. The Human Genome Project: lessons from large-scale biology. Science. 2003;300(5617):286–290. doi: 10.1126/science.1084564. [DOI] [PubMed] [Google Scholar]
- Council N.R. 2003. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. [PubMed] [Google Scholar]
- DeGrazia D., Beauchamp T.L. Beyond the 3 Rs to a more comprehensive framework of principles for animal research ethics. ILAR J. 2019;1:1–164. doi: 10.1093/ilar/ilz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggel M., Würbel H. Internal consistency and compatibility of the 3Rs and 3Vs principles for project evaluation of animal research. Lab. Anim. 2021;55(3):233–243. doi: 10.1177/0023677220968583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknell P. Replacement, reduction, refinement. ALTEX-alternatives to Animal Experimentation. 2002;19(2):73–78. [PubMed] [Google Scholar]
- Goodyear M.D., et al. British Medical Journal Publishing Group; 2007. The Declaration of Helsinki. [Google Scholar]
- Graham M.L., Prescott M.J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 2015;759:19–29. doi: 10.1016/j.ejphar.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.D., et al. Human genome project: twenty-five years of big biology. Nature. 2015;526(7571):29–31. doi: 10.1038/526029a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell G., et al. In: Proceedings of Symposium on Human Perspectives. GT H., editor. National Academy of Sciences; Washington, DC: 1977. Future of animals, cells, models and systems in research, development, education and testing; pp. 16–24. [Google Scholar]
- Hylander B.L., et al. Using mice to model human disease: understanding the roles of baseline housing-induced and experimentally imposed stresses in animal welfare and experimental reproducibility. Animals. 2022;12(3):371. doi: 10.3390/ani12030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber K., et al. Recommendations for addressing harm–benefit analysis and implementation in ethical evaluation–report from the AALAS–FELASA working group on harm–benefit analysis–part 2. Lab. Anim. 2016;50(1_Suppl. l):21–42. doi: 10.1177/0023677216642397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.J. Mass Medical Soc; 1999. The Need to Revise the Declaration of Helsinki. [DOI] [PubMed] [Google Scholar]
- Macleod M., Mohan S. Reproducibility and rigor in animal-based research. ILAR J. 2019;60(1):17–23. doi: 10.1093/ilar/ilz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., et al. An open resource for non-human primate imaging. Neuron. 2018;100(1):61–74. doi: 10.1016/j.neuron.2018.08.039. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M., et al. Accelerating the evolution of nonhuman primate neuroimaging. Neuron. 2020;105(4):600–603. doi: 10.1016/j.neuron.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M., et al. Toward next-generation primate neuroscience: a collaboration-based strategic plan for integrative neuroimaging. Neuron. 2022;110(1):16–20. doi: 10.1016/j.neuron.2021.10.015. [DOI] [PubMed] [Google Scholar]
- Mitchell A.S., et al. International primate neuroscience research regulation, public engagement and transparency opportunities. Neuroimage. 2021;229 doi: 10.1016/j.neuroimage.2020.117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S. Laboratory environmental factors and pain behavior: the relevance of unknown unknowns to reproducibility and translation. Lab. Anim. 2017;46(4):136–141. doi: 10.1038/laban.1223. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fontela C., et al. Animal models for COVID-19. Nature. 2020;586(7830):509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi S.M. Harm-benefit analyses can be harmful. ILAR J. 2019;60(3):341–346. doi: 10.1093/ilar/ilaa016. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N., et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J. Cerebr. Blood Flow Metabol. 2020;40(9):1769–1777. doi: 10.1177/0271678X20943823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 2017;18(2):115. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pp R. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br. Med. J. 1964;2(5402) doi: 10.1136/bmj.2.5402.177. 177-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott M.J., Lidster K. Improving quality of science through better animal welfare: the NC3Rs strategy. Lab. Anim. 2017;46(4):152–156. doi: 10.1038/laban.1217. [DOI] [PubMed] [Google Scholar]
- Richter S.H. Systematic heterogenization for better reproducibility in animal experimentation. Lab. Anim. 2017;46(9):343–349. doi: 10.1038/laban.1330. [DOI] [PubMed] [Google Scholar]
- Riis P. Thirty years of bioethics: the Helsinki declaration 1964-2003. New Rev. Bioeth. 2003;1(1):15–25. doi: 10.1080/1740028032000131396. [DOI] [PubMed] [Google Scholar]
- Rollin B.E. The regulation of animal research and the emergence of animal ethics: a conceptual history. Theor. Med. Bioeth. 2006;27(4):285–304. doi: 10.1007/s11017-006-9007-8. [DOI] [PubMed] [Google Scholar]
- Russell W.M.S., Burch R.L. Methuen; 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Smith A.J., Hawkins P. Good science, good sense and good sensibilities: the three Ss of Carol Newton. Animals. 2016;6(11):70. doi: 10.3390/ani6110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.J., et al. PREPARE: guidelines for planning animal research and testing. Lab. Anim. 2018;52(2):135–141. doi: 10.1177/0023677217724823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon L.U., et al. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017;220(17):3007–3016. doi: 10.1242/jeb.147058. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J. Animal Models for the Study of Human Disease. Elsevier; 2013. Ethics in biomedical animal research: the key role of the investigator; pp. 1–34. [Google Scholar]
- Tannenbaum J. Animal Models for the Study of Human Disease. Elsevier; 2017. Ethics in biomedical animal research: the key role of the investigator; pp. 1–44. [Google Scholar]
- Tannenbaum J., Bennett B.T. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. JAALAS. 2015;54(2):120–132. [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., et al. The Wu-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014;81(3):14–18. [PubMed] [Google Scholar]
- Würbel H. More than 3Rs: the importance of scientific validity for harm-benefit analysis of animal research. Lab. Anim. 2017;46(4):164–166. doi: 10.1038/laban.1220. [DOI] [PubMed] [Google Scholar]
- Zhou Y., et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature. 2019;570(7761):326–331. doi: 10.1038/s41586-019-1278-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.