Abstract
Acquired angioedema due to C1-inhibitor deficiency (C1–INH-AAE) is a rare disease that can be diagnosed via complement testing. It often accompanies lymphoproliferative underlying diseases. Our study aimed to examine if there is a connection between complement parameters and the clinical symptoms of C1–INH-AAE, and, in case of a known underlying disease, its activity. The other question is how a connection, if proven, could help in the development of the therapeutic strategy of C1–INH-AAE patients.
In the past 30 years, out of the 3938 patients sent to the Angioedema Center with angioedema symptoms, we have diagnosed C1–INH-AAE in 19 cases. An underlying disease was diagnosed in 15 patients. Most often lymphoma (6/19 patients) and monoclonal gammopathy of undetermined significance (6/19 patients) were found.
Angioedema specific long-term prophylaxis did not result in an improvement in neither the frequency of the attacks nor in the complement parameters.
A connection has been found between the presence and activity of any underlying disease, the frequency of the angioedema attacks and the decreased level of proteins of the complement system. Decreasing complement parameters warn about the appearance or the worsening of the underlying disease. The treatment of the underlying disease brings improvement in the complement parameters.
Rituximab treatment reduced the number of attacks or completely made them disappear, and we experienced positive changes in complement parameters. Complement parameters supported the long-term efficacy of rituximab treatment for C1–INH-AAE.
The change in complement parameters predict the relapse of the underlying disease, and it is a good indicator for the prediction of angioedematous attacks. In C1–INH-AAE, it is essential to examine the patients for underlying diseases, and to regularly follow up the patient's complement parameters.
Keywords: Acquired angioedema due to C1-inhibitor deficiency, C1-inhibitor, Classical complement pathway, Lymphoproliferative diseases, Rituximab
Highlights
-
•
Angioedema attacks predict the onset of the underlying disease.
-
•
After the treatment of the underlying disease, angioedema attacks improve or disappear.
-
•
After the treatment of the underlying disease, complement parameters are normalized.
-
•
Decreasing complement parameters predict the recurrence of the angioedema.
-
•
Decreasing complement parameters predict the recurrence of the underlying disease.
Acquired angioedema due to C1-inhibitor deficiency; C1-inhibitor; Classical complement pathway; Lymphoproliferative diseases; Rituximab.
1. Introduction
Two types of bradykinin-mediated angioedemas can be distinguished: hereditary angioedema (HAE) and acquired angioedema (AAE). Both are characterized by recurrent subcutaneous and/or submucosal angioedema attacks. Both the hereditary and the acquired form can occur due to C1-inhibitor (C1–INH) deficiency (C1–INH-HAE, C1–INH-AAE) [1, 2, 3, 4, 5]. C1–INH-AAE is a rare disorder, with an approximate incidence rate of 1:500,000, the family history is negative, and the angioedema symptoms occur over the age of 40 years [2, 6, 7, 8, 9, 10, 11].
Angioedema can occur in all body regions and can lead to a life-threatening state; if the edema occurs in the larynx, it can lead to life-threatening laryngeal edema with suffocation [1, 4, 6, 9, 12, 13, 14, 15].
Several factors can have a role in the development of the symptoms; widely used angiotensin converting enzyme inhibitor (ACEIs) antihypertensives must be highlighted [9, 12, 16], but estrogen containing oral contraceptives [4, 9, 17], mechanical trauma, psychological stress, and bacterial or viral infections may also contribute to the edematous attacks [1, 4, 8].
The laboratory parameters of C1–INH-AAE are characterized by reduced total hemolytic complement (CH50), C1–INH concentration and activity and C4 level, and in 75% of the cases by decreased C1q level as well. Antibodies against C1–INH (C1–INH-Ab) can be detected in a number of patients [4, 6, 7, 8, 9, 10, 13, 16, 19].
C1–INH-AAE occurs with co-morbid lymphoma, monoclonal gammopathy of unknown significance, and other lymphoproliferative disorders or autoimmune diseases. These diseases associated with C1–INH-AAE are characterized by an increased consumption and a relative deficiency of the C1–INH protein. Depending on the disease, the consumption is due to the activation of the classic complement, coagulation, or kallikrein-kininogen pathway. All of these pathways share regulation by C1–INH. Ultimately the unregulated production of bradykinin triggers endothelial cell activation which is responsible for the increased capillary permeability and the development of angioedema. Another potential mechanism to deplete C1–INH is autoantibodies specific for C1–INH produced by malignant or dysregulated B lymphocytes [2, 6, 8, 9, 13, 16, 18, 19, 20].
The goals of C1–INH-AAE treatment strategy are targets therapies of both angioedema attacks and the underlying disease. Angioedema attacks can be prevented by the avoidance of trigger factors [1, 4] and can be treated with the following: plasma-derived C1–INH (pdC1-INH), recombinant human C1–INH (rhC1-INH), bradykinin B2 antagonist (icatibant) and kallikrein inhibitor (ecallantide). For the prophylaxis of angioedema attacks, attenuated androgens and tranexamic acid can be used [4, 6, 8, 13, 16, 19, 21, 22]. Recent studies have proven that rituximab (anti-CD20 antibodies) can also be effective in case of serious C1–INH-AAE.
If the underlying disease is known, its treatment can reduce the number of angioedema attacks or even completely stop them [21].
While in C1–INH-HAE, there is no connection found between the quantity of specific complement components (CH50, C4, C1–INH concentration and activity) and the severity of the disease, there is no data regarding this in C1–INH-AAE in the literature.
The purpose of our prospective study was to examine if there is a connection between complement parameters and the clinical symptoms of C1–INH-AAE, and in case of a known underlying disease, its activity. The other question was that if a connection can be proven, how can it help in the development of the therapeutic strategy of C1–INH-AAE patients.
2. Materials and methods
We analyzed the clinical and laboratory data of 19 C1–INH-AAE patients recorded during follow-up visits at the Hungarian Angioedema Center of Reference and Excellence.
Our patient group consisted of patients sent to our Center between 1999–2020 with angioedema symptoms of unknown origin. The basis of the diagnosis of C1–INH-AAE is the negative family history, the time of the first appearance of the symptoms, and the results from the laboratory examination of the classical complement pathway (CH50, C1q, C4, C1–INH concentration, C1–INH functional activity, and C1–INH-Ab).
At our Center 19 patients have been diagnosed with C1–INH-AAE since 1999, and all of them were included in this study.
At the time of the diagnosis, all patients received a patient diary for keeping a record of the information about their disease and the necessary medication. If the medical history of the patient mentioned no underlying disease, further examinations (laboratory, imaging) were completed. Patients madea follow-up visit at least once a year, during which the following were recorded in the National Angioedema Registry: the trigger factors, the frequency and localization of the angioedema attacks, information about further diseases and medical interventions, plus other diseases and acute and prophylactic treatments used for angioedema attacks. During these visits, phlobotomy was performed (into serum tubes, without additives) for laboratory examinations and complement assays at every follow-up visit. After sampling, the tubes were centrifuged and blood serum was used for the laboratory examinations which were performed in maximum 2 weeks. In the meantime, the samples were stored on -20 °C.
2.1. Complement testing
To quantify antigenic C1-inhibitor levels, in-house radial immunodiffusion was performed.
The concentration of the functional C1-inhibitor was measured with a C1-inhibitor enzyme immunoassay kit (Quidel, San Diego, CA). The concentration of C1q and C1–INH-Ab were determined by in-house sandwich ELISA methods [23, 24]. CH50 (total hemolytic activity) of the classical pathway was determined with a hemolytic assay. C3 and C4 concentrations were measured by turbidimetry (Cobas Integra 400 analyzer; Roche, Switzerland).
2.2. Statistical methods
The differences between the complement parameters of the two sexes were compared with Mann-Whitney tests. The change in complement parameters in time was assessed with Pearson-correlation.
Statistical calculation was done with GraphPad Prism 7.00 software.
2.3. Ethics approval and consent to participate
The study protocol was approved by the institutional review board of Semmelweis University of Budapest, Informed consent was obtained from the participants in accordance with the Declaration of Helsinki.
3. Results
3.1. Demographics
During the examined time period, 3938 patients were sent to our Center with angioedema symptoms of undetermined origin; out of these patients, 214 were diagnosed with C1–INH deficiency. Ninety-two percent (197) were diagnosed with C1–INH-HAE, and 8% (19) with C1–INH-AAE. The ratio of hereditary and acquired C1–INH deficiency in our patient group is 11:1.
The sex ratio of the patients was even, 9 male and 10 female. The average age at the first angioedema symptom was 58 years (min: 40, max.: 83); at the time of diagnosis the average age was 60 years (min.: 41, max.: 83) and the average delay in diagnosing was 4 years (min.: 0, max.: 31). The average follow-up time was 6 years (min.: 1, max.: 16) and the patients had a follow-up visit at least once a year. The clinical manifestation of C1–INH-AAE was presented in a previous article [25].
At the time of diagnosis, our patients showed a complement pattern that is a characteristic of acquired C1–INH-AAE. CH50, C4, C1–INH concentration and activity were decreased in all patients. Most patients (12/19) had a decreased C1q and 11/19 patients had C1–INH-Ab (5 patients had IgG, 6 patients had IgA, 5 patients had IgM type C1–INH-Ab).
Four patient (P1, P9, P10, P14) died during follow-up, but their death was not related to C1–INH-AAE. After two years of examinations, one patient (P15) was lost to follow-up.
3.2. Treatment of angioedema attacks
The most common cause (6 patients) of angioedema attacks was the use of ACEI medications, which were stopped and an antihypertensive with an alternative mechanism of action was selected. Mechanical trauma as a trigger factor was reported by 5 patients, one patient marked specific foods as the trigger of the angioedema attacks.
Twelve subjects used acute treatment for attacks. PdC1–INH (10/12), rhC1-INH (3/12) and icatibant (6/12) treatment were effective in the cessation of the angioedema attacks. A dosage of 55UI/kg of PDC1-INH, greater than the approved dose of 20UI/kg [8], of pdC1-INH was used for patient 10 (P10) due to lack of efficacy of lower dosage. As preprocedural prophylaxis, pdC1-INH was given to 2 patients before a dental procedure, 1 patient received icatibant before a dental procedure, 1 patient received rhC1-INH before an abdominal hernia operation. The procedures were successful, and angioedema did not occur.
As long-term prophylaxis, 1 patient (P7) received attenuated androgen (danazol), and 2 patients received tranexamic acid (P10, P12). Despite long-term prophylaxis, all three patients experienced angioedema attacks. The Mann-Whitney test was used to compare each complement parameter during and without long-term prophylaxis. P7, the patient taking danazol, showed a marginally significant increase in the level of C1q (p = 0.0212) during prophylaxis; the patients taking tranexamic acid (P10, P12) showed no significant change in any complement parameter (CH50, C1q, C4, C3, C1–INH antigenic level and functional activity, or C1–INH-Ab).
3.3. Underlying diseases and treatment
Six patients (P2, P5, P6, P13, P14, P18) were diagnosed with lymphoma; 6 patients (P3, P8, P10, P11, P16, P17) had MGUS, 3 patients (P7, P9, P12) had other malignant diseases (myeloma multiplex, chronic lymphocytic leukemia, or basal cell adenoma) as an underlying disease. In case of 8 patients (P2, P3, P7, P8, P9, P11, P16, P17) the underlying disease did not require treatment, 5 patients received rituximab treatment (P6, P10, P13, P14, P18); one patient (P5) received rituximab treatment and autologous stem cell transplantation; one patient (P12) received VAD (vincristin, doxorubicin, dexamethasone) chemotherapeutic treatment, autologous stem cell transplantation due to the underlying disease and received rituximab treatment for the recurrent angioedema attacks.
In case of 4 patients with underlying diseases (P10, P12, P13, P14) the long-term follow-up made it possible to compare the number of angioedema attacks prior to and after the treatment of the underlying disease. In addition, the number of angioedema attacks/year can be compared between patients who got treatment for their underlying disease and those who were not treated this way (Figure 1). Patients who did not have an underlying disease were excluded from this evaluation.
Figure 1.
Yearly number of angioedema attacks before and after underlying disease treatment (TR).
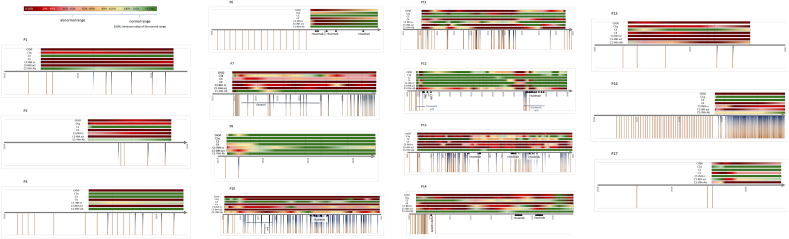
Complement parameters were investigated to see if they are reliable factors to predict the change of the severity of C1–INH-AAE and the underlying disease. Complement parameters changed over time during the multiannual follow-up which is shown on Figure 2. Out of the nineteen patients, four are not shown, since P4 and P5 did not have attacks, and P18 and P19 were evaluated in the last 12 months, therefore they were excluded from the investigation due to the small amount of data available.
Figure 2.
Changes of complement parameters, angioedema attacks and treatments during long term follow-up. Colour scale: green colors mean that the given parameter was in the normal range; from yellow to red, the values are further away from the normal range. Under the time axis, brown lines mean the attacks, and blue half-lines mean the treatments. Horizontal blue lines show a long-term prophylaxis, with the medication named under. Black star: diagnosis of the underlying disease. Black triangles show the type of the underlying disease and its time points. The deceased patient's timelines end without an arrow. CH50: total hemolytical complement; C1–INH cc: C1-inhibitor antigenic concentration; C1–INH act: C1-inhibitor activity; MZL: marginal zone lymphoma; MGUS: monoclonal gammopathy of undetermined significance; CLL: chronic lymphoid leukemia; MM: myeloma multiplex; VAD: chemotherapy of vincristine, doxorubicin, dexamethasone; ASCT: autologous stem cell therapy.
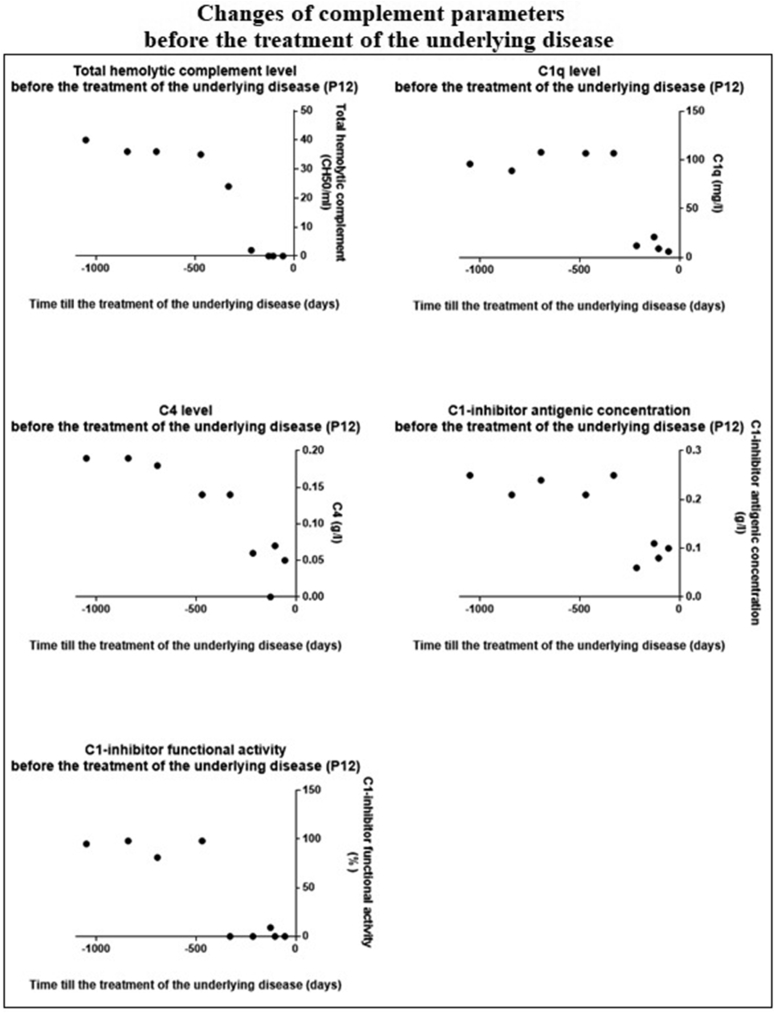
3.4. Underlying disease appearance and complement parameters
The significant decrease we found in the complement parameters predicted the development of the underlying disease. Figure 3 shows this connection in the case of P12.
Figure 3.
Changes of complement parameters before the treatment of the underlying disease. 0 of X axis: treatment of the underlying disease.
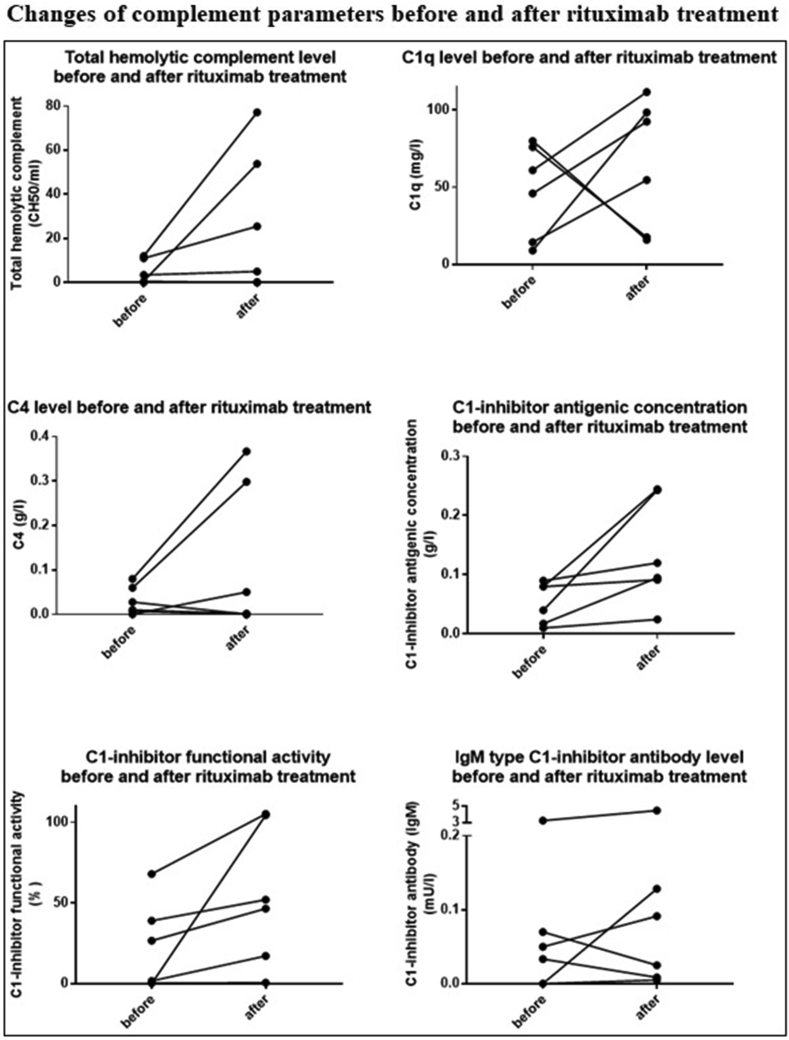
3.5. Rituximab treatment and complement parameters
Following rituximab treatment, the attacks of 3 patients (P6, P12, P14) completely ceased; for P10, the frequency of the attacks did not change; for P13, the number of attacks decreased. P5 did not have attacks even prior to the treatment; thus the change in attack frequency could not be assessed.
In case of the patients, who got rituximab treatment (P5, P6, P10, P12, P13, P14) the value of CH50, C1q, C4, C1–INH concentration, C1–INH activity significantly increased and IgM type C1–INH-Ab level significantly decreased (p < 0,05) after treatment with rituximab (Figure 4.).
Figure 4.
Changes of complement parameters before and after rituximab treatment.
4. Discussion
There are limited data in the medical literature related to the long-term follow-up of C1–INH-AAE patients; most of these data primarily focus on the clinical symptoms and the treatment. With larger patient number and longer follow-up period, this report provides evidence that the clinical symptoms of C1–INH-AAE patients, the frequency of the angioedema attacks, the appearance and activity of the underlying disease, and the treatment of these diseases show a connection with the complement parameters.
Regarding demographic data, the ratio of men and women was equal and sex did not influence the clinical picture or the complement parameters, as it was predictable from existing literature data [26].
While in C1–INH-HAE, mechanical trauma is the most common trigger factor, in C1–INH-AAE, the administration of ACEI medication is the most common provoking factor. This difference may be the result of the fact that C1–INH-AAE starts in older age, when using antihypertensive medication is more common. Following our previously published national protocol, we concomitantly stop the ACEI medication and perform complement testing in case of every angioedema patient [27]. This rationale supported by the fact that 6 of our patients had been taking ACEI medication prior to the diagnosis.
All three on demand treatments (pdC1-INH, rhC1-INH, icatibant) successfully ceased the edematous attacks, similarly to what Bork and Bouillet has experienced [26, 28].
One of our patients needed an increased dose of pdC1-INH (max 55 UI/kg) for the cessation of the attacks. This patient had C1–INH-Ab. In a previous study, similar observations were made that patients with C1–INH-Ab need a larger dose (even 9 times larger) of pdC1-INH. The reason for this may be that antibodies inactivate the administered C1–INH. Since we detected C1–INH-Ab in 11 of our patients, and only one of these patients needed more than the recommended 20 UI/kg pdC1-INH for the treatment of the angioedema attacks, it is possible that other mechanisms may also play a role in the different therapeutic responses [11, 26, 28, 29].
Three of our patients received long-term angioedema prophylaxis; for the indication, concomitant diseases, underlying diseases, the side effects of medications (dislipidemia, hematological and liver malignancies, hypertension, and thrombosis) [6, 19] and the preferences of the patient were taken into account. We could not prove objectively (regarding the number of attacks or the complement parameters) the positive effects of long-term prophylaxis (danazol, tranexamic acid) in case of our patients. According to the literature, 94.1% of patients taking danazol experience a medical status improvement [6, 25].
Regarding complement parameters, we could assess a slightly significant difference in the level of 1-1 complement parameter in 2 patients, so the advantageous effect of these cannot be unequivocally stated.
58% of our patients were diagnosed with an underlying disease, this data in the literature varies between 70-93% [4, 7, 9, 26]. Regarding the type of the underlying disease, we assessed similar incidence rates to the data found in the literature. 32% of our patients were diagnosed with a lymphoproliferative disorder, while the literature mentions 27–64% [1, 8, 9, 15, 26]. MGUS was found in 53% of our patients, while other studies reported 32–48% [4, 10, 15, 26]. Besides following-up the C1–INH-AAE of our patients, they are regularly monitored for the underlying disease as well. 4 of our MGUS patients developed lymphoma during the follow-up years.
The angioedema attacks did not become less frequent is patients, who did not get treatment for their underlying disease. Apart from one patient (P10), the number of attacks decreased after the treatment of the underlying disease; in 3 out of 5 cases the subjects were symptom-free. In one patient (P12), angioedema symptoms did not appear until 9 years after the treatment of the underlying disease (autologous stem cell transplantation and VAD chemotherapy) (P12/1 on Figure 1); the symptoms reoccurred while no examination confirmed the return of the underlying disease. After rituximab treatment (P12/2 on Figure 1), the angioedema episodes ceased.
To sum up, following rituximab therapy the underlying disease went into remission, and the frequency of the angioedema attacks decreased as well. It also reduced the number of angioedema attacks, or even ceased them, if the rituximab therapy was only introduced because of the angioedema attacks. In cases, where we could not diagnose any lymphoproliferative underlying disease, it is possible that a lymphoproliferative process was already present in a very early stage and that the rituximab treatment solved the source of the problem.
One of our patients (P12) received rituximab treatment only because of C1–INH-AAE (frequent angioedema attacks). During the 4-year-long follow-up period, he did not experience an angioedema attack. This is in line with the observation that rituximab treatment may be effective in cases where no underlying disease can be diagnosed (just yet) as a root cause of C1–INH-AAE [1, 4].
4.1. Changes of complement parameters
The timelines of the individual patients show that the complement parameters do not change or get worse without the treatment of the underlying disease (P1, P2, P3, P7, P11, P15, P16, P17).
We found only one exception: in case of P8, after one dosage of pdC1-INH the angioedema attacks disappeared and the complement parameters went back to normal.
Patients who got treatment for the angioedema attacks (P6, P10, P12, P13, P14) show improving complement results.
Statistical calculation confirms the significant decrease of complement parameters as the patients get closer to the time of the treatment of the underlying disease (we assume that if the underlying disease needs treatment then it is getting more and more severe with time and it is the most severe just before the treatment). This verifies that the complement parameters, and thus the classical complement pathway have close connection with the underlying disease.
The effect of rituximab on the underlying disease and the angioedema attacks was supported by the results of complement testing. All the analyzed complement parameters changed positively, i.e. CH50, C4, C1q, C1–INH antigenic concentration and activity increased, and we found a negative correlation between the frequency of the attacks and the complement values. Levi have published a similar observation for 10 patients following rituximab treatment [18].
Our new observation was that complement parameters predicted both the appearance of the angioedema episodes the increase in their frequency and the recurrence of the underlying disease.
5. Conclusions
Based on our examination, we can prove the statement that the thorough exploration of the underlying disease is the key in C1–INH-AAE, since its treatment decreases or ceases the development of angioedema attacks.
The follow-up of the complement parameters is essential in the prediction of the underlying disease. Decreasing complement parameters is a warning sign of the appearance or the worsening of the underlying disease. The treatment of the underlying disease brings improvement in the complement parameters as well. Rituximab can be effective even in cases where the underlying disease was not explored or is in remission, but the angioedema episodes occur frequently.
Previously there was no study about C1–INH-AAE that statistically evaluated the connection between the typical complement profile and the treatment of the related underlying disease in a bigger data set; therefore, our work contains several new observations from many aspects.
The monitoring of complement parameters can help in developing a long-term treatment strategy for physicians treating C1–INH-AAE and the underlying disease. This monitoring could provide further objective data for proving the effectiveness of the therapy and its monitoring, and in case of C1–INH-AAE, complement parameters could be used as a predicting biomarker, in contrast to C1–INH-HAE, where no connection can be made between the severity of the disease and the complement parameters.
Declarations
Author contribution statement
Zsofia Polai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zsuzsanna Balla; Lilian Varga; Szabolcs Benedek: Contributed reagents, materials, analysis tools or data.
Henriette Farkas: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Henriette Farkas was supported by Nemzeti Kutatási, Fejlesztési és Innovaciós Alap [124557].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare the following conflict of interests:
H. Farkas has received honoraria and travel grants from CSL Behring, Shire/Takeda, Swedish Orphan Biovitrum, Octapharma, Kalvista and Pharming; and/or served as a consultant for these companies and has participated in clinical trials/registries for BioCryst, CSL Behring, Pharming, Pharvaris and Shire.
Zs. Balla has participated in clinical trials of CSL Behring, Pharvaris and Takeda.
The other authors have declared that no conflict of interest exists.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the medical professionals working in the day-to-day care of these patients, without whose help this study could not have been possible. Namely Judit Bali, Lászlóné Kertész, Andrásné Dóczi and Éva Zsuzsanna Szendrei.
References
- 1.Kazandjieva J., Christoff G. Angioedema as a systemic disease. Clin. Dermatol. 2019;37:636–643. doi: 10.1016/j.clindermatol.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Balla Z., Ignácz B., Varga L., et al. How angioedema quality of life questionnaire can help physicians in treating C1-inhibitor deficiency patients? Clin. Rev. Allergy Immunol. 2021;61:50–59. doi: 10.1007/s12016-021-08850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu M.A., Perego F., Zanichelli A., et al. Angioedema phenotypes: disease expression and classification. Clin. Rev. Allergy Immunol. 2016;51:162–169. doi: 10.1007/s12016-016-8541-z. [DOI] [PubMed] [Google Scholar]
- 4.Otani I.M., Banerji A. Acquired C1 inhibitor deficiency. Immunol. Allergy Clin. 2017;37:497–511. doi: 10.1016/j.iac.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Busse P.J., Christiansen S.C. Hereditary angioedema. N. Engl. J. Med. 2020;382:1136–1148. doi: 10.1056/NEJMra1808012. [DOI] [PubMed] [Google Scholar]
- 6.Leru P.M., Anton V.F., Bumbea H. Nine year follow-up of a rare case of angioedema due to acquired C1-inhibitor deficiency with late onset and good response to attenuated androgen. Allergy Asthma Clin. Immunol. 2018;14:69. doi: 10.1186/s13223-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y., Wang C. Where we are with acquired angioedema due to C1 inhibitor deficiency: a systematic literature review. Clin. Immunol. 2021;230 doi: 10.1016/j.clim.2021.108819. [DOI] [PubMed] [Google Scholar]
- 8.Gobert D., Bouillet L., Armengol G., et al. Acquired angioedema due to C1-inhibitor deficiency: CREAK recommendations for diagnosis and treatment. Rev. Med. Interne. 2020;41:838–842. doi: 10.1016/j.revmed.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Sobotkova M., Zachova R., Hakl R., et al. Acquired angioedema with C1 inhibitor deficiency: occurrence, clinical features, and management: a nationwide retrospective study in the Czech republic patients. Int. Arch. Allergy Immunol. 2021;182:642–649. doi: 10.1159/000512933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S., Konala V.M., Kyaw T., et al. An unusual case of acquired angioedema and monoclonal gammopathy of renal significance in a middle-aged caucasian female. J. Investig. Med. High Impact Case Rep. 2020;8 doi: 10.1177/2324709620912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanichelli A., Azin G.M., Wu M.A., et al. Diagnosis, course, and management of angioedema in patients with acquired C1-inhibitor deficiency. J. Allergy Clin. Immunol. Pract. 2017;5:1307–1313. doi: 10.1016/j.jaip.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Farkas H., Veszeli N., Kajdácsi E., et al. “Nuts and bolts” of laboratory evaluation of angioedema. Clin. Rev. Allergy Immunol. 2016;51:140–151. doi: 10.1007/s12016-016-8539-6. [DOI] [PubMed] [Google Scholar]
- 13.Belbézier A., Boccon-Gibod I., Bouillet L. Efficacy of lanadelumab in acquired angioedema with C1-inhibitor deficiency. J. Allergy Clin. Immunol. Pract. 2021;9:2490–2491. doi: 10.1016/j.jaip.2021.01.040. [DOI] [PubMed] [Google Scholar]
- 14.MacBeth L.S., Volcheck G.W., Sprung J., et al. Perioperative course in patients with hereditary or acquired angioedema. J. Clin. Anesth. 2016;34:385–391. doi: 10.1016/j.jclinane.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Abdulkareem A., D'Souza R.S., Mundorff J., et al. Refractory abdominal pain in a patient with chronic lymphocytic leukemia: Be wary of acquired angioedema due to C1 esterase inhibitor deficiency. Case Rep Hematol. 2018 doi: 10.1155/2018/7809535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesenak M., Brndiarova M., Banovcin P., et al. Successful use of recombinant human C1-INH in a patient with acquired angioedema due to C1 inhibitor deficiency and an unusually high titer of anti-C1-inhibitor autoantibodies. J. Investig. Allergol. Clin. Immunol. 2021;31:255–256. doi: 10.18176/jiaci.0635. [DOI] [PubMed] [Google Scholar]
- 17.Marbán Bermejo E., Caballero T., López-Trascasa M., et al. Acquired angioedema with anti-C1-inhibitor autoantibodies during assisted reproduction techniques. J. Investig. Allergol. Clin. Immunol. 2018;28:62–64. doi: 10.18176/jiaci.0213. [DOI] [PubMed] [Google Scholar]
- 18.Levi M., Cohn D., Zeerleder S., et al. Long-term effects upon rituximab treatment of acquired angioedema due to C1-inhibitor deficiency. Allergy. 2019;74:834–840. doi: 10.1111/all.13686. [DOI] [PubMed] [Google Scholar]
- 19.Bonnin A.J., DeBrosse C., Moncrief T., et al. Case report presenting the diagnostic challenges in a patient with recurrent acquired angioedema, antiphospholipid antibodies and undetectable C2 levels. Allergy Asthma Clin. Immunol. 2018;14:24. doi: 10.1186/s13223-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sbattella M., Zanichelli A., Ghia P., et al. Splenic marginal zone lymphomas in acquired C1-inhibitor deficiency: clinical and molecular characterization. Med. Oncol. 2018;35:118. doi: 10.1007/s12032-018-1183-7. [DOI] [PubMed] [Google Scholar]
- 21.Baird D., Craig T.J., Miller J.J. Atypical presentation of acquired angioedema. Cutis. 2018;101:E14–E16. [PubMed] [Google Scholar]
- 22.Longhurst H.J., Zanichelli A., Caballero T., et al. Comparing acquired angioedema with hereditary angioedema (types I/II): findings from the Icatibant Outcome Survey. Clin. Exp. Immunol. 2017;188:148–153. doi: 10.1111/cei.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delamarche C., Berger F., Pouplard A., et al. An ELISA technique for the measurement of C1q in cerebrospinal fluid. J. Immunol. Methods. 1988;114:101–106. doi: 10.1016/0022-1759(88)90160-3. [DOI] [PubMed] [Google Scholar]
- 24.Mészáros T., Füst G., Farkas H., et al. C1-inhibitor autoantibodies in SLE. Lupus. 2010;19:634–638. doi: 10.1177/0961203309357059. [DOI] [PubMed] [Google Scholar]
- 25.Pólai Z., Balla Z., Andrási N., et al. A follow-up survey of patients with acquired angioedema due to C1-inhibitor deficiency. J. Intern. Med. 2021;289:547–558. doi: 10.1111/joim.13182. [DOI] [PubMed] [Google Scholar]
- 26.Bork K., Staubach-Renz P., Hardt J. Angioedema due to acquired C1-inhibitor deficiency: spectrum and treatment with C1-inhibitor concentrate. Orphanet J. Rare Dis. 2019;14:65. doi: 10.1186/s13023-019-1043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balla Z., Zsilinszky Z., Pólai Z., et al. The importance of complement testing in acquired angioedema related to angiotensin-converting enzyme inhibitors. J. Allergy Clin. Immunol. Pract. 2021;9:947–955. doi: 10.1016/j.jaip.2020.08.052. [DOI] [PubMed] [Google Scholar]
- 28.Bouillet-Claveyrolas L., Ponard D., Drouet C., et al. Clinical and biological distinctions between type I and type II acquired angioedema. Am. J. Med. United States. 2003:420–421. doi: 10.1016/s0002-9343(03)00396-6. [DOI] [PubMed] [Google Scholar]
- 29.Farkas H. Current pharmacotherapy of bradykinin-mediated angioedema. Expet Opin. Pharmacother. 2013;14:571–586. doi: 10.1517/14656566.2013.778826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.