This prespecified analysis of the Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO) randomized clinical trial investigates if the blood pressure–lowering effect of ultrasound renal denervation in patients with resistant hypertension is maintained after the addition of antihypertensive medication through 6 months.
Key Points
Question
Is the blood pressure (BP)–lowering effect of ultrasound renal denervation (uRDN) in patients with resistant hypertension (RHTN) maintained following blinded addition of antihypertensive medications to control BP through 6 months?
Findings
In this randomized, sham-controlled, clinical trial including 136 patients with RHTN, the BP-lowering effect of uRDN was sustained at 6 months with similar daytime systolic ambulatory BP compared with sham despite fewer medications, especially aldosterone antagonists.
Meaning
uRDN may represent an adjunctive BP-lowering therapy to medications among patients with RHTN.
Abstract
Importance
Although early trials of endovascular renal denervation (RDN) for patients with resistant hypertension (RHTN) reported inconsistent results, ultrasound RDN (uRDN) was found to decrease blood pressure (BP) vs sham at 2 months in patients with RHTN taking stable background medications in the Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO) trial.
Objectives
To report the prespecified analysis of the persistence of the BP effects and safety of uRDN vs sham at 6 months in conjunction with escalating antihypertensive medications.
Design, Setting, and Participants
This randomized, sham-controlled, clinical trial with outcome assessors and patients blinded to treatment assignment, enrolled patients from March 11, 2016, to March 13, 2020. This was an international, multicenter study conducted in the US and Europe. Participants with daytime ambulatory BP of 135/85 mm Hg or higher after 4 weeks of single-pill triple-combination treatment (angiotensin-receptor blocker, calcium channel blocker, and thiazide diuretic) with estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73 m2 or greater were randomly assigned to uRDN or sham with medications unchanged through 2 months. From 2 to 5 months, if monthly home BP was 135/85 mm Hg or higher, standardized stepped-care antihypertensive treatment starting with aldosterone antagonists was initiated under blinding to treatment assignment.
Interventions
uRDN vs sham procedure in conjunction with added medications to target BP control.
Main Outcomes and Measures
Six-month change in medications, change in daytime ambulatory systolic BP, change in home systolic BP adjusted for baseline BP and medications, and safety.
Results
A total of 65 of 69 participants in the uRDN group and 64 of 67 participants in the sham group (mean [SD] age, 52.4 [8.3] years; 104 male [80.6%]) with a mean (SD) eGFR of 81.5 (22.8) mL/min/1.73 m2 had 6-month daytime ambulatory BP measurements. Fewer medications were added in the uRDN group (mean [SD], 0.7 [1.0] medications) vs sham (mean [SD], 1.1 [1.1] medications; P = .045) and fewer patients in the uRDN group received aldosterone antagonists at 6 months (26 of 65 [40.0%] vs 39 of 64 [60.9%]; P = .02). Despite less intensive standardized stepped-care antihypertensive treatment, mean (SD) daytime ambulatory BP at 6 months was 138.3 (15.1) mm Hg with uRDN vs 139.0 (14.3) mm Hg with sham (additional decreases of −2.4 [16.6] vs −7.0 [16.7] mm Hg from month 2, respectively), whereas home SBP was lowered to a greater extent with uRDN by 4.3 mm Hg (95% CI, 0.5-8.1 mm Hg; P = .03) in a mixed model adjusting for baseline and number of medications. Adverse events were infrequent and similar between groups.
Conclusions and Relevance
In this study, in patients with RHTN initially randomly assigned to uRDN or a sham procedure and who had persistent elevation of BP at 2 months after the procedure, standardized stepped-care antihypertensive treatment escalation resulted in similar BP reduction in both groups at 6 months, with fewer additional medications required in the uRDN group.
Trial Registration
ClinicalTrials.gov Identifier: NCT02649426
Introduction
In addition to lifestyle changes and pharmacotherapy, endovascular catheter-based renal denervation (RDN) has emerged as a blood pressure (BP)–lowering treatment, but early trials reported inconsistent results.1,2 Subsequently, 3 sham-controlled trials with improved catheter designs and revised procedural technique confirmed the short-term BP-lowering efficacy of both radiofrequency and ultrasound RDN (uRDN) in the absence3,4 and the presence5 of antihypertensive medications in patients with mild-to-moderate uncontrolled hypertension.
In the sham-controlled Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE-HTN TRIO) trial, a greater reduction in 2-month daytime ambulatory systolic BP (SBP) was observed with uRDN relative to a sham procedure in patients with resistant hypertension (RHTN), despite a stable dose of a standardized, single-pill, triple combination of antihypertensive medications.6 However, the persistence of the effects of uRDN beyond 2 months in conjunction with added antihypertensive medications in patients with RHTN remains unknown. Beyond the single-pill combination therapy, a guideline-recommended, standardized, stepped-care antihypertensive treatment (SSAHT) was started in the RADIANCE-HTN TRIO trial if home BP remained uncontrolled after ascertainment of the primary end point at 2 months.6 The objectives of this prespecified analysis were to report the persistence of the BP-lowering efficacy and safety of uRDN at 6 months, as well as antihypertensive medication burden while both patients and clinical staff remained blinded to the initial study randomization.
Methods
Study Design
The international, multicenter, randomized, sham-controlled RADIANCE-HTN TRIO trial was conducted in 28 centers in the US and 25 centers in Europe. The full protocol, statistical analysis plan, and revision history are provided in Supplement 1, Supplement 2, and Supplement 3. Study background is available in the eAppendix in Supplement 4. The study was approved by local ethics committees or institutional review boards. All participants provided written informed consent to complete a minimum of 3-year follow-up. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Briefly, eligible participants were men or women aged 18 to 75 years with (1) RHTN defined as seated office SBP and diastolic BP (DBP) of 140/90 mm Hg or higher despite 3 or more antihypertensive medications including a diuretic and (2) an estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73 m2 or greater. Race and ethnicity were identified by direct questioning of the participant, and this information was included in the study per the US Food and Drug Administration guidance document titled “Collection of Race and Ethnicity Data in Clinical Trials.”7 The following specific categories for race and ethnicity were included: (1) American Indian or Alaska Native; (2) Asian; (3) Black, African heritage; (4) Black, Caribbean heritage; (5) Black, other heritage, specify; (6) Caucasian; (7) Hispanic or Latino; (8) multiple ethnicities, specify; (9) Native Hawaiian or other Pacific; or (10) other ethnicity not listed, specify.
At enrollment, participants were switched to a single-pill, fixed-dose, daily combination of valsartan, 160 mg (or olmesartan, 40 mg), amlodipine, 10 mg (or 5 mg in the event of severe leg edema), and hydrochlorothiazide, 25 mg. No other antihypertensive medications were allowed except β-blockers for chronic coronary syndrome or heart failure. After 4 weeks of standardized therapy, 136 participants with daytime ambulatory SBP of 135 mm Hg or higher and DBP of 85 mm Hg or higher and suitable kidney artery anatomy were randomized by computer (1:1, stratified by center using blocks of 4 or 6 and permutations of treatment within each block) to receive uRDN (n = 69) with the Paradise Renal Denervation System (ReCor Medical) or a sham procedure (n = 67).6
The procedure assignment was masked for 6 months after randomization for both patients and the clinical staff responsible for follow-up. Patients were to remain undergoing the initial antihypertensive treatment until 2 months, unless specified BP criteria were exceeded.6 From the second to the fifth month after randomization, an SSAHT was recommended in both groups if the mean BP at home was 135 mm Hg or higher (systolic) or 85 mm Hg or higher (diastolic). The SSAHT included guideline-recommended sequential addition of (1) an aldosterone antagonist (preferentially spironolactone, 25 mg/day), (2) a β1-blocker (preferentially bisoprolol, 10 mg/day), (3) a central α2-receptor agonist (clonidine, 0.1-0.2 mg/day; rilmenidine, 1-2 mg/day; or moxonidine, 0.2-0.4 mg/day), and (4) an α1-receptor blocker (prazosin, 5-10 mg/day or doxazosin, 4-8 mg/day).
From randomization to 6 months, patients had monthly visits before ingestion of their morning antihypertensive treatment to (1) undergo seated office BP, heart rate, and laboratory assessments as well as urine chemical adherence testing, (2) assess their 7-day home BP measured before each on-site visit, and (3) record adverse events and medications. Seated office BP and home BP were measured with the same validated electronic device (M10-IT [Omron Healthcare]) as previously described.3,6 Ambulatory BP measurements were performed following witnessed pill intake at baseline, 2, and 6 months as previously described (WatchBP [Microlife]),3,6 and all recordings were sent to a core laboratory (dabl Health), with treatment assignment masked.
Adherence to antihypertensive medications was assessed blinded to the treatment assignment using ultrahigh-performance liquid chromatography tandem mass spectrometry to detect drugs or their metabolites in spot urine samples collected before witnessed pill intake, at baseline, 2, and 6 months, as previously described.6 Full adherence to medications was defined as the presence of all prescribed drugs in the sample. Renal duplex ultrasound was performed at 2 and 6 months in all patients, followed by a computed tomography or magnetic resonance imaging angiogram of the kidney, if needed, to detect for renal artery stenosis.
Outcomes
Prespecified end points at 6 months included the following: (1) the number of antihypertensive medications prescribed; (2) the proportion of patients taking different classes of added antihypertensive medications, especially aldosterone antagonists; and (3) the baseline and covariate-adjusted changes in ambulatory, home, and office SBP. Other efficacy end points included change in DBP parameters and heart rate and the proportion of the patients with controlled out-of-office BP (defined as <135/85 mm Hg for both home and daytime ambulatory SBP/DBP). Safety assessments were performed and adjudicated as previously reported.6
Statistical Analysis
This was a superiority trial for which the sample size calculation was based on the change in daytime ambulatory SBP at 2 months—the primary outcome measure—as previously reported.6 The study was stopped on May 8, 2020, because the COVID-19 pandemic caused a pause in enrollment, and the prespecified evaluable sample size was met. For the present analysis, we included patients with complete baseline and 6-month ambulatory BP measurements. Continuous variables are expressed as mean (SD), unless otherwise specified, and between-group differences are expressed as mean and corresponding 2-sided 95% CIs. Comparisons between groups at baseline and 6 months were made using unpaired t tests for continuous variables and Fisher exact test for categorical variables.
Analyses of the continuous BP outcomes were done using a linear mixed model using all available data. This model included a random intercept, visit, treatment allocation, visit-by-treatment interaction, baseline measure of the outcome, and the number of antihypertensive medications as fixed effects. P values were adjusted for multiple comparisons using the Tukey-Kramer test. A similar approach was applied to binary end points with generalized linear mixed models, using log-binomial regression in place of linear regression.
We also assessed treatment effects (change in BP parameters, heart rate, and eGFR) using analysis of covariance (or covariance based on ranks) with uRDN vs sham as the main effect and baseline value as a covariate. Analyses for prespecified subgroups were performed using linear regression analyses with change in daytime ambulatory SBP at 6 months as the dependent variable. Baseline BP, treatment group, subgroup, and treatment group by subgroup interaction term were included as independent variables in the models. Adjusted mean daytime ambulatory SBP by subgroup and P value for the treatment by subgroup interaction term are presented. All analyses were performed using SAS software, version 9.4 (SAS Institute). A P value < .05 (2-sided) was considered significant.
Results
A total of 65 of 69 participants (94.2%) in the uRDN group and 64 of 67 participants (95.5%) in the sham group had ambulatory blood pressure measurements at 6 months and were included in the analysis (Figure 1). Baseline characteristics were similar across both study groups and did not differ from those of the intention-to-treat population (eTable 1 in Supplement 4). Participants identified with the following race and ethnicity groups: 1 American Indian or Alaska Native (0.8%), 1 Arabic (0.8%), 2 Asian (1.6%); 1 Asian/Persian (0.8%), 25 Black (19.4%); 90 Caucasian (69.8%); 4 Hispanic or Latino (3.1%); 1 Portuguese (0.8%), and 4 unknown (3.1%). Included in the study was a total of 65 of 69 participants (94.2%) in the uRDN group and 64 of 67 participants (95.5%) in the sham group (mean [SD] age, 52.4 [8.3] years; 104 male [80.6%]; 25 female [19.4%]). Participants had a mean (SD) eGFR of 81.5 (22.8) mL/min/1.73 m2. Blinding was maintained at 6 months between the 2 groups (eTable 2 in Supplement 4).
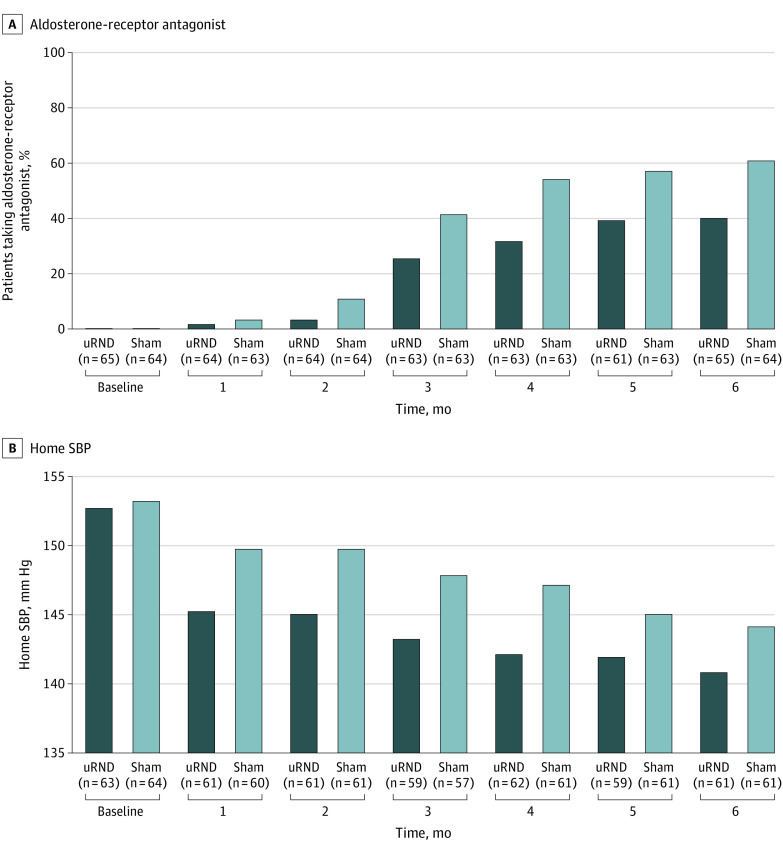
Figure 1. Participant Flow Chart.
uRDN indicates ultrasound renal denervation.
Burden of and Adherence to Medications
The single-pill combination treatment remained unchanged at the same dosage throughout the 6-month follow-up in 50 of 65 patients (76.9%) in the uRDN group and 53 of 64 patients (82.8%) in the sham group. Fewer antihypertensive medications were added from baseline to 6 months in the uRDN group (mean [SD], 0.7 [1.0] medications) vs the sham group (mean [SD], 1.1 [1.1] medications; P = .045) (Table 1 and eFigure 1 in Supplement 4). The most frequently added antihypertensive medication was an aldosterone antagonist (predominantly spironolactone) (Table 1). However, an aldosterone antagonist was less frequently added at each monthly visit in the uRDN group vs the sham group through 6 months (uRDN group, 26 of 65 patients [40.0%] vs sham group, 39 of 64 patients [60.9%]; P = .02; overall odds ratio [OR], 0.4; 95% CI, 0.2-0.7; P < .001) (Figure 2 and eTable 3 in Supplement 4). There was no difference between the 2 groups in the use of the other recommended antihypertensive medications at 6 months. Full adherence to medications was high at 6 months (uRDN group, 40 of 56 patients [71.4%] vs sham group, 43 of 55 patients [78.2%]; P = .41) (eFigure 2 in Supplement 4).
Table 1. Number and Type of Antihypertensive Medications at 6 Months in the Analysis Population.
| Characteristic | No./total No. (%) | P value | |
|---|---|---|---|
| uRDN group (n = 65) | Sham group (n = 64) | ||
| No. of medications at 6 mo, mean (SD) | 3.8 (1.0) | 4.1 (1.1) | .08a |
| Change in No. of medications: 6 mo − baseline, mean (SD) | 0.7 (1.0) | 1.1 (1.1) | <.05a |
| No. of antihypertensive medications at 6 mo | |||
| 1 | 1/65 (1.5) | 0/64 (0) | .37b |
| 2 | 2/65 (3.1) | 1/64 (1.6) | |
| 3 | 26/65 (40.0) | 22/64 (34.4) | |
| 4 | 22/65 (33.8) | 18/64 (28.1) | |
| ≥5 | 14/65 (21.5) | 23/64 (35.9) | |
| Types of medication at 6 mo within patients on medications | |||
| Components of the triple combination therapy | |||
| Calcium channel blocker | 64/65 (98.5) | 63/64 (98.4) | <.99b |
| Angiotensin II antagonist | 63/65 (96.9) | 64/64 (100) | .50b |
| Diuretic | 62/65 (95.4) | 61/64 (95.3) | <.99b |
| Standardized stepped-care antihypertensive treatment: | |||
| Spironolactonec | 26/65 (40.0) | 39/64 (60.9) | .02d |
| β-Blocker | 22/65 (33.8) | 25/64 (39.1) | .54d |
| Centrally acting α2-receptor agonists | 3/65 (4.6) | 6/64 (9.4) | .32b |
| α1-Receptor blocker | 3/65 (4.6) | 4/64 (6.3) | .72b |
| Other added medication | |||
| Vasodilator | 1/65 (1.5) | 0/64 (0) | <.99b |
Abbreviations: uRDN, ultrasound renal denervation.
P value from Wilcoxon test comparing the renal denervation group to the sham group.
Fisher exact test.
Two patients received eplerenone in the uRDN group instead of spironolactone. Higher dose of spironolactone (50 or 75 mg) used in 5 of 24 patients (19%) in the uRDN group vs 12 of 39 patients (30.8%) in the sham group.
χ2 Test.
Figure 2. Percentage of Patients Taking an Aldosterone-Receptor Antagonist and Corresponding 7-Day Home Systolic Blood Pressure (SBP) Measurements.
Data were captured monthly from randomization to 6 months in the ultrasound renal denervation (uRDN) group (n = 65) and the sham group (n = 64) in the analysis population. The mean of 7-day home SBP values is shown. The number of patients with available data in each group is shown in parentheses below the x-axis. A, An aldosterone-receptor antagonist was much less frequently added at each monthly visit in the uRDN group by physicians blinded to the randomization compared with the sham group though 6 months (overall odds ratio, 0.4; 95% CI, 0.2-0.7; absolute risk difference: −15.1%; 95% CI, −23.9% to −6.2%; P < .001). B, The overall decrease in home SBP from baseline to 1, 2, 3, 4, 5, and 6 months was greater in the uRDN group than in the sham group (estimated overall between-group difference: −4.3 mm Hg; 95% CI, −8.1 to −0.5 mm Hg; P = .03 in a mixed linear model adjusting for baseline and medications added).
Blood Pressure
The mean (SD) daytime ambulatory SBP at 6 months decreased by 11.8 (14.2) mm Hg from baseline reaching 138.3 (15.1) mm Hg in the uRDN group (−2.4 [16.6] mm Hg from month 2) and decreased by 12.3 (14.2) mm Hg from baseline in the sham group, reaching 139.0 (14.3) mm Hg (−7.0 [16.7] mm Hg from month 2) (eTable 4 and eFigure 3 in Supplement 4). The overall change in daytime ambulatory SBP from baseline to 6 months was −2.5 mm Hg lower with uRDN vs sham (95% CI, −6.7 to 1.7 mm Hg; P = .25) in a mixed linear model (Table 2). There was no heterogeneity in the between-group difference in daytime ambulatory SBP changes from baseline according to sex, race and ethnicity, age, abdominal circumference, baseline BP, and geography (eFigure 4 in Supplement 4). The changes in 24-hour and nighttime ambulatory SBP did not significantly differ between the 2 groups and were consistent with the changes in daytime SBP (eTable 4 in Supplement 4).
Table 2. Linear Mixed Models for Repeated Measures From Baseline Through 6 Months for Daytime Ambulatory, Home, and Office Systolic Blood Pressure (SBP) in the Ultrasound Renal Denervation Group and the Sham Group in the Analysis Population.
| Outcome | Mean (95% CI) | P value |
|---|---|---|
| Daytime ambulatory SBP (mm Hg)a | ||
| Treatment difference: model excluding interaction termb | −2.7 (−6.9 to 1.6) | .22 |
| Treatment difference: model including visit by group interaction termc | −2.5 (−6.7 to 1.7) | .25 |
| Home SBP (mm Hg)d | ||
| Treatment difference: model excluding interaction termb | −4.4 (−8.3 to −0.6) | .02 |
| Treatment difference: model including visit by group interaction termc | −4.3 (−8.1 to −0.5) | .03 |
| Office SBP (mm Hg)d | ||
| Treatment difference: model excluding interaction termb | −3.1 (−8.1 to 1.9) | .22 |
| Treatment difference: model including visit by group interaction termc | −2.9 (−7.9 to 2.0) | .24 |
Abbreviation: SBP, systolic blood pressure.
Daytime: months 2 and 6 included in analysis.
Estimates from a linear regression model (with compound symmetry covariance structure) including treatment group, baseline blood pressure value, and number of medications at visit as fixed effects.
Estimates from a linear regression model (with compound symmetry covariance structure) including treatment group, visit, interaction between treatment group and visit, baseline blood pressure value, and number of medications at visit as fixed effects.
Home and office: months 1, 2, 3, 4, 5, and 6 included in analyses.
In contrast, the overall decrease in home SBP from baseline to 1, 2, 3, 4, 5, and 6 months was greater with uRDN compared with sham (estimated overall between-group difference: −4.3 mm Hg; 95% CI, −8.1 to −0.5 mm Hg; P = .03) in a mixed linear model adjusting for baseline and number of medications (Table 2 and Figure 2). A similar trend was observed for office SBP changes (Table 2). All other BP parameters are reported in eTable 5 in Supplement 4.
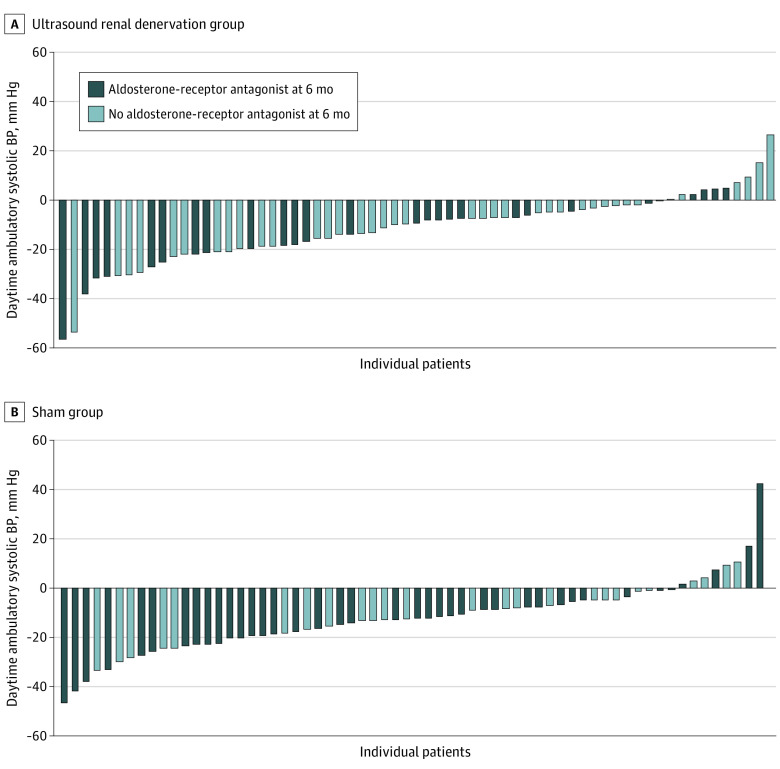
Individual patient changes in daytime ambulatory SBP at 6 months displayed large between-participant variability in both groups (Figure 3). In the mixed linear model, out-of-office BP control (home and daytime ambulatory) was achieved more frequently with uRDN compared with sham (OR, 10.0; 95% CI, 2.7-37.2; P = .03 and OR, 1.8; 95% CI, 0.9-3.6; P = .07, respectively) (eTable 6 in Supplement 4). There was a larger mean (SD) decrease in daytime ambulatory heart rate in the sham group (−7.3 [9.6] beats/minute) than in the uRDN group (−1.9 [8.8] beats/minute; P = .04) perhaps reflecting the more frequent use of β-blockers (eTable 7 in Supplement 4).
Figure 3. Individual Changes in Daytime Ambulatory Systolic Blood Pressure (BP) From Baseline to 6 Months in the Analysis Population.
A, Ultrasound renal denervation group (n = 65). B, Sham group (n = 64).
Safety Outcomes
As previously reported, 3 adverse events occurred after uRDN up to 30 days after the procedure (eTable 8 in Supplement 4).6 Other cardiovascular or kidney events through 6 months were reported in 4 patients in each group (eTable 8 in Supplement 4). eGFR decreased slightly and similarly from baseline to 6 months in the 2 groups (eTable 9 in Supplement 4). No new kidney artery stenosis of 50% or greater was detected on noninvasive imaging in either group at 6 months.
Discussion
The prespecified analysis of the RADIANCE-HTN TRIO randomized clinical trial demonstrated that the BP-lowering effect of uRDN was maintained through 6 months after initiation of the recommended SSAHT in patients with RHTN who had persistent elevation of BP at 2 months after the procedure. As suggested by the SSAHT escalation protocol, higher home BP values caused physicians, who were kept blinded to randomization assignment up to 6 months, to add antihypertensive medications, especially aldosterone antagonists, to background initial triple antihypertensive therapy in order to lower BP. This occurred to a greater extent in the sham group. However, the protocolized escalation of antihypertensive medications resulted in a similar ambulatory SBP reduction at 6 months in both the uRDN and the sham groups, with fewer additional medications required in the uRDN group.
Demonstration of the durability of the BP-lowering effect of uRDN in a randomized trial remains a methodological challenge, given the clinical need to add pharmacological treatment(s) in the case of persistent, or recurrent, uncontrolled hypertension after more than 2 months of follow-up.8,9 Prior trials of RDN that enrolled patients with mild hypertension at low risk of hypertension-related complications allowed investigators to maintain stable medication regimens until assessment of the primary BP efficacy end point between 2 and 6 months.3,4,5 The risk of a hypertension-related complication within 6 months is much higher for patients with RHTN,10 as reported at 6 months in the Renal Denervation in Patients with Uncontrolled Hypertension (SYMPLICITY HTN-3) trial.1 Therefore, in the RADIANCE-HTN TRIO trial, we chose to initiate a guideline-recommended SSAHT protocol early after ascertainment of the primary efficacy end point at 2 months, for safety, ethical, and regulatory reasons. The low proportion of hypertension-related complications at 6 months in the TRIO cohort (6.2%) supports our decision to initiate the SSAHT protocol early compared with the SYMPLICITY HTN-3 trial (15.7%) and has been recommended by the Hypertension Academic Research Consortium.9 However, this study design complicates the ascertainment of the RDN-related BP lowering effects over time, especially if there is an imbalance in antihypertensive medications sequentially added by physicians and/or taken by patients through the institution of SSAHT.
Between the uRDN and sham groups, we observed an imbalance in the monthly prescription of antihypertensive medications by physicians kept blinded to randomization assignment, most notably, the addition of an aldosterone antagonist to those receiving background initial triple antihypertensive therapy. The large imbalance observed in this trial was somewhat unexpected in comparison with the open-label Renal Denervation in Hypertension (DENER-HTN) trial, which included patients with similar clinical characteristics and applied a similar SSAHT protocol. In the DENERHTN trial, there was no imbalance in the number of medications (5.3 medications) and proportion of patients receiving spironolactone (79%) in between RDN and control groups at 6 months,11 and thus, a 5.9-mm Hg greater decrease in daytime ambulatory SBP in favor of RDN was reported.11 In contrast, in the RADIANCE-HTN TRIO trial, the more intensified SSAHT regimen in the sham vs the uRDN group prescribed by physicians blinded to uRDN or sham assignment likely contributed to the progressive narrowing of the between-group difference in daytime ambulatory SBP changes from baseline, although both treatment groups had greater than 10-mm Hg decreases from baseline.
In contrast to the ambulatory BP monitoring, in-home BP recordings obtained in the week before monthly study visits, the uRDN group achieved lower home SBP by approximately 4 mm Hg over the 6-month follow-up when compared with the sham group, and a greater odds of out-of-office BP control was achieved with uRDN as assessed by home vs ambulatory BP measurements. This moderate disagreement may be due to the more reproducible BP measurements achieved from one visit to another with home BP monitoring done monthly by the patient in very standardized conditions and in the same environment in contrast with ambulatory BP monitoring, which may differ between recordings due different environmental conditions several months apart.12 Witnessed medication intake immediately after the start of ambulatory BP measurements may have also reduced the between-group difference in daytime ambulatory SBP by standardizing exposure to antihypertensive medications.
Altogether, our results demonstrate the maintenance of a BP-lowering effect of uRDN at 6 months in the RADIANCE-HTN TRIO trial, mirroring and reinforcing the results of the RADIANCE-HTN SOLO trial at 6 months.13 They are, however, at variance with those of the Renal Denervation on Quality of 24-hour BP Control by Ultrasound in Resistant Hypertension (REQUIRE) trial conducted in Japan and South Korea,14 which did not have the same rigorous design as the RADIANCE-HTN trials. The REQUIRE trial showed BP reduction at 3 months with uRDN similar to other sham-controlled studies but unexpectedly greater BP reductions in the sham group due to various study design issues, similar to those reported in the SYMPLICITY HTN-3 trial.8,15
If maintained in the long term, as highlighted by the 3-year report of the Global SYMPLICITY Registry16 as well as the 36-month results of the RADIANCE-HTN SOLO17 and Global Clinical Study of Renal Denervation with the Symplicity Spyral Multielectrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy (SPYRAL ON-MED) study,18 the BP reduction achieved after uRDN, especially in patients with RHTN who are at high risk of a cardiovascular event,10 is of a magnitude previously associated with a reduction in stroke, coronary heart disease, heart failure, and all-cause mortality for antihypertensive drug therapy.19 In patients with RHTN, the magnitude of the SBP response to RDN especially in responders has been associated with improved long-term clinical outcome after a median follow-up of 48 months.20
In patients with RHTN, antihypertensive medication therapy with a triple antihypertensive therapy, including a renin-angiotensin system blocker, a calcium channel blocker, and a diuretic (optimally given in a single pill) and followed by protocolized stepped-care escalation, remains the mainstay of therapy recommended by guidelines to control BP.21,22 In the RADIANCE-HTN TRIO trial, when this medical regimen was more intensified in the sham group, a similar BP reduction could be still achieved at 6 months than with the alternative strategy including prior uRDN. Whether a similar BP control with more intensified medical therapy would be maintained in the long term, especially in real-life conditions, remains an open question. Indeed, relying exclusively on the intensification of antihypertensive treatment regimen may be limited by both medication-related adverse effects (especially with spironolactone,23 which is largely underused by physicians24) and of nonadherence especially in real-life conditions.25 Indeed, full adherence to medications was high in our trial (>70%) favored by the use of the single-pill triple combination;26 it was much higher than in the DENERHTN trial (approximately 50%),11 in which patients were treated with free combinations of medications. Even though a single-pill triple-combination treatment may not be available in all clinical settings, one of the critical themes of the RADIANCE-HTN TRIO trial is that simplification of the clinical regimen through combination therapy is a critical first step in treating patients with RHTN (upstream of consideration of RDN).
Limitations
The limitations of the RADIANCE-HTN TRIO study have been previously discussed.6 Briefly, one limitation is the short duration of follow-up to assess longer-term durability of the BP lowering effect of uRDN and its safety in patients with RHTN, although extended follow-up in unblinded conditions after 6 months is planned. Because we favored the internal validity of this explanatory study to demonstrate in optimal conditions the BP-lowering efficacy of uRDN in patients with RHTN, it limits27 the external applicability of the study results to everyday practice. However, because BP is a continuous variable and the BP-lowering effect of both uRDN and radiofrequency-based RDN is similar in trials across the whole spectrum of hypertension severity, our findings could apply to a large fraction of patients with RHTN, but the BP-lowering effect may be variable for a single individual patient. Further, as in all other trials, there was large between-patient variation in the BP response to uRDN plus SSAHT as well as to the SSAHT alone, some of which may be attributed to variable renal nerve ablation, medication adherence, prevailing state of sympathetic hyperactivity or other factors. At present, there is still no reliable periprocedural marker of successful uRDN.28 We included patients with RHTN and eGFR greater than 40 mL per minute, of whom only 11% had eGFR rates less than 60 mL per minute. Finally, patients with hypertension and comorbidities (including heart failure with reduced ejection fraction, postmyocardial infarction, left ventricular dysfunction, type 2 diabetes, and chronic kidney disease) who were excluded from our trial would still need aldosterone antagonists29,30,31 (and β-blockers for heart failure and left ventricular dysfunction)32 to improve their cardiovascular and kidney prognosis.
Conclusions
In this prespecified analysis of the RADIANCE-HTN TRIO randomized clinical trial, in patients with RHTN initially randomly assigned to uRDN or a sham procedure and who had persistent elevation of BP at 2 months after the procedure, protocolized escalation of antihypertensive medications resulted in similar BP reduction at 6 months in both groups, with fewer additional medications, especially aldosterone antagonists, required in the uRDN group. The maintenance of a BP-lowering effect of uRDN at 6 months mirror the results of the RADIANCE-HTN SOLO trial at 6 months13 and therefore, demonstrated persistent BP-lowering efficacy of uRDN across a broad spectrum of patients with mild hypertension to severe and RHTN.
Trial Protocol
Statistical Analysis Plan
Amendments to Trial Protocol
eAppendix. Abbreviations, Organization, and Study Background
eFigure 1. Percentage of Patients on Fewer, the Same, or More (+1 to + ≥3) Antihypertensive Medications Added to the Baseline Standardized Triple Medication Therapy at Each Monthly Visit From Randomization to 6 Months in the Ultrasound Renal Denervation Group (uRDN; n = 65) and the Sham Group (n=64) in the Analysis Population
eFigure 2. Adherence to Antihypertensive Medications at Baseline, 2, and 6 Months the Ultrasound Renal Denervation (uRDN) Group and the Sham Group in the Analysis Population
eFigure 3. Twenty-Four Hour Ambulatory Profiles of Systolic Blood Pressure (SBP) at Baseline, 2, and 6 Months in the Ultrasound Renal Denervation Group (left) and the Sham Group (right) in the Analysis Population
eFigure 4. Forest Plot of Differences Between the Ultrasound Renal Denervation Group and the Sham Group in Daytime Ambulatory Systolic Blood Pressure Changes Across Prespecified Subgroups
eTable 1. Baseline Demographics and Clinical Characteristics of the Analysis Population
eTable 2. Bang Blinding Index at Hospital Discharge and 6 Months (Analysis Population)
eTable 3. Percentage of Patients Who Received an Aldosterone Receptor Antagonist Through 6 Months in the Analysis Population
eTable 4. Change in Ambulatory, Home, and Office Systolic Blood Pressure at 6 Months in Patients With Ultrasound Renal Denervation (n=65) and Sham (n=64) in the Analysis Population
eTable 5. Change in Ambulatory, Home, and Office Diastolic Blood Pressure (DBP) at 6 Months Following Ultrasound Renal Denervation (uRDN) or Sham Procedure (Analysis Population)
eTable 6. Generalized Linear Mixed Models for Repeated Measures for Controlled Blood Pressure (BP) Rates for Daytime Ambulatory, Home, and Office (Analysis Population)
eTable 7. Change in Ambulatory, Home, and Office Heart Rate at 6 Months Following Ultrasound Renal Denervation (uRDN) or Sham Procedure (Analysis Population)
eTable 8. Incidence of Major Adverse Events and Other Prespecified Safety Events in All Randomized Subjects Through 6 Months follow-up
eTable 9. Estimated Glomerular Filtration Rate (eGFR) and Serum Creatinine at Baseline and 6 Months (for Subjects With Data at Both Time Points)
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Bhatt DL, Kandzari DE, O’Neill WW, et al. ; SYMPLICITY HTN-3 Investigators . A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 2.Azizi M, Sapoval M, Gosse P, et al. ; Renal Denervation for Hypertension (DENERHTN) investigators . Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957-1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 3.Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335-2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 4.Böhm M, Kario K, Kandzari DE, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators . Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444-1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 5.Kandzari DE, Böhm M, Mahfoud F, et al. ; SPYRAL HTN-ON MED Trial Investigators . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346-2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 6.Azizi M, Sanghvi K, Saxena M, et al. ; RADIANCE-HTN investigators . Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476-2486. doi: 10.1016/S0140-6736(21)00788-1 [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration . Collection of race and ethnicity data in clinical trials. Accessed October 15, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials
- 8.Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76(5):1410-1417. doi: 10.1161/HYPERTENSIONAHA.120.15745 [DOI] [PubMed] [Google Scholar]
- 9.Kandzari DE, Mahfoud F, Weber MA, et al. Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the hypertension academic research consortium. Circulation. 2022;145(11):847-863. doi: 10.1161/CIRCULATIONAHA.121.057687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642. doi: 10.1161/CIRCULATIONAHA.111.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azizi M, Pereira H, Hamdidouche I, et al. ; DENERHTN Investigators . Adherence to antihypertensive treatment and the blood pressure–lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) Trial. Circulation. 2016;134(12):847-857. doi: 10.1161/CIRCULATIONAHA.116.022922 [DOI] [PubMed] [Google Scholar]
- 12.Stergiou GS, Palatini P, Parati G, et al. ; European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability . 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens. 2021;39(7):1293-1302. doi: 10.1097/HJH.0000000000002843 [DOI] [PubMed] [Google Scholar]
- 13.Azizi M, Schmieder RE, Mahfoud F, et al. ; RADIANCE-HTN Investigators . Six-month results of treatment-blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation. 2019;139(22):2542-2553. doi: 10.1161/CIRCULATIONAHA.119.040451 [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Yokoi Y, Okamura K, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45(2):221-231. doi: 10.1038/s41440-021-00754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persu A, Kjeldsen S, Staessen JA, Azizi M. Renal denervation for treatment of hypertension: a second start and new challenges. Curr Hypertens Rep. 2016;18(1):6. doi: 10.1007/s11906-015-0610-9 [DOI] [PubMed] [Google Scholar]
- 16.Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40(42):3474-3482. doi: 10.1093/eurheartj/ehz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rader F, Kirtane AJ, Wang Y, et al. ; Collaborators . Durability of blood pressure reduction after ultrasound renal denervation: 3-year follow-up of the treatment arm of the randomised RADIANCE-HTN SOLO trial. EuroIntervention. 2022;EIJ-D-22-00305. Published online 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399(10333):1401-1410. doi: 10.1016/S0140-6736(22)00455-X [DOI] [PubMed] [Google Scholar]
- 19.Rahimi K, Bidel Z, Nazarzadeh M, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration . Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397(10285):1625-1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fengler K, Reimann P, Rommel KP, et al. Comparison of long-term outcomes for responders vs nonresponders following renal denervation in resistant hypertension. J Am Heart Assoc. 2021;10(21):e022429. doi: 10.1161/JAHA.121.022429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams B, Mancia G, Spiering W, et al. ; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 22.Carey RM, Calhoun DA, Bakris GL, et al. ; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council . Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90. doi: 10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31(1):3-15. doi: 10.1097/HJH.0b013e3283599b6a [DOI] [PubMed] [Google Scholar]
- 24.Egan BM, Zhao Y, Li J, et al. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62(4):691-697. doi: 10.1161/HYPERTENSIONAHA.113.01448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhry NK, Kronish IM, Vongpatanasin W, et al. ; American Heart Association Council on Hypertension; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Medication adherence and blood pressure control: a scientific statement from the American Heart Association. Hypertension. 2022;79(1):e1-e14. doi: 10.1161/HYP.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to single-pill vs free-equivalent combination therapy in hypertension: a systematic review and meta-analysis. Hypertension. 2021;77(2):692-705. doi: 10.1161/HYPERTENSIONAHA.120.15781 [DOI] [PubMed] [Google Scholar]
- 27.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62(5):464-475. doi: 10.1016/j.jclinepi.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud F, Azizi M, Ewen S, et al. Proceedings from the 3rd European Clinical Consensus Conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41(16):1588-1599. doi: 10.1093/eurheartj/ehaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152-161. doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakris GL, Agarwal R, Anker SD, et al. ; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 31.Pitt B, Filippatos G, Agarwal R, et al. ; FIGARO-DKD Investigators . Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252-2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 32.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
Amendments to Trial Protocol
eAppendix. Abbreviations, Organization, and Study Background
eFigure 1. Percentage of Patients on Fewer, the Same, or More (+1 to + ≥3) Antihypertensive Medications Added to the Baseline Standardized Triple Medication Therapy at Each Monthly Visit From Randomization to 6 Months in the Ultrasound Renal Denervation Group (uRDN; n = 65) and the Sham Group (n=64) in the Analysis Population
eFigure 2. Adherence to Antihypertensive Medications at Baseline, 2, and 6 Months the Ultrasound Renal Denervation (uRDN) Group and the Sham Group in the Analysis Population
eFigure 3. Twenty-Four Hour Ambulatory Profiles of Systolic Blood Pressure (SBP) at Baseline, 2, and 6 Months in the Ultrasound Renal Denervation Group (left) and the Sham Group (right) in the Analysis Population
eFigure 4. Forest Plot of Differences Between the Ultrasound Renal Denervation Group and the Sham Group in Daytime Ambulatory Systolic Blood Pressure Changes Across Prespecified Subgroups
eTable 1. Baseline Demographics and Clinical Characteristics of the Analysis Population
eTable 2. Bang Blinding Index at Hospital Discharge and 6 Months (Analysis Population)
eTable 3. Percentage of Patients Who Received an Aldosterone Receptor Antagonist Through 6 Months in the Analysis Population
eTable 4. Change in Ambulatory, Home, and Office Systolic Blood Pressure at 6 Months in Patients With Ultrasound Renal Denervation (n=65) and Sham (n=64) in the Analysis Population
eTable 5. Change in Ambulatory, Home, and Office Diastolic Blood Pressure (DBP) at 6 Months Following Ultrasound Renal Denervation (uRDN) or Sham Procedure (Analysis Population)
eTable 6. Generalized Linear Mixed Models for Repeated Measures for Controlled Blood Pressure (BP) Rates for Daytime Ambulatory, Home, and Office (Analysis Population)
eTable 7. Change in Ambulatory, Home, and Office Heart Rate at 6 Months Following Ultrasound Renal Denervation (uRDN) or Sham Procedure (Analysis Population)
eTable 8. Incidence of Major Adverse Events and Other Prespecified Safety Events in All Randomized Subjects Through 6 Months follow-up
eTable 9. Estimated Glomerular Filtration Rate (eGFR) and Serum Creatinine at Baseline and 6 Months (for Subjects With Data at Both Time Points)
Nonauthor Collaborators
Data Sharing Statement