Abstract
Background
Newborn infants affected by hypoxic‐ischemic encephalopathy (HIE) undergo therapeutic hypothermia. As this treatment seems to be associated with pain, and intensive and invasive care is needed, pharmacological interventions are often used. Moreover, painful procedures in the newborn period can affect pain responses later in life, impair brain development, and possibly have a long‐term negative impact on neurodevelopment and quality of life.
Objectives
To determine the effects of pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia. Primary outcomes were analgesia and sedation, and all‐cause mortality to discharge.
Search methods
We searched CENTRAL, PubMed, CINAHL (Cumulative Index to Nursing and Allied Health Literature), and the trial register ISRCTN in August 2021. We also checked the reference lists of relevant articles to identify additional studies.
Selection criteria
We included randomized controlled trials (RCT), quasi‐RCTs and cluster‐randomized trials comparing drugs used for the management of pain or sedation, or both, during therapeutic hypothermia: any opioids (e.g. morphine, fentanyl), alpha‐2 agonists (e.g. clonidine, dexmedetomidine), N‐Methyl‐D‐aspartate (NMDA) receptor antagonist (e.g. ketamine), other analgesics (e.g. paracetamol), and sedatives (e.g. benzodiazepines such as midazolam) versus another drug, placebo, no intervention, or non‐pharmacological interventions.
Primary outcomes were analgesia and sedation, and all‐cause mortality to discharge.
Data collection and analysis
Two review authors independently assessed studies identified by the search strategy for inclusion. We planned to use the GRADE approach to assess the certainty of evidence. We planned to assess the methodological quality of included trials using Cochrane Effective Practice and Organisation of Care Group (EPOC) criteria (assessing randomization, blinding, loss to follow‐up, and handling of outcome data). We planned to evaluate treatment effects using a fixed‐effect model with risk ratio (RR) for categorical data and mean, standard deviation (SD), and mean difference (MD) for continuous data.
Main results
We did not find any completed studies for inclusion. Amongst the four excluded studies, topiramate and atropine were used in two and one trial, respectively; one study used dexmedetomidine and was initially reported in 2019 to be a randomized trial. However, it was an observational study (correction in 2021). We identified one ongoing study comparing dexmedetomidine to morphine.
Authors' conclusions
We found no studies that met our inclusion criteria and hence there is no evidence to recommend or refute the use of pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia.
Plain language summary
Drugs to manage pain and sedation during cooling in newborns following poor brain oxygenation at birth (hypoxic‐ischaemic encephalopathy)
Review question
Do drugs save lives, or improve pain and sedation, in newborns who have poor brain oxygenation at birth ('hypoxic‐ischaemic encephalopathy') and who are undergoing cooling?
Background
Lack of oxygen at birth may damage the brain of the newborn. Babies with less severe brain damage may make a full recovery or only have mild problems. For other babies with more serious damage, this may lead to death or to problems later in life. For instance, some of these babies develop cerebral palsy, intellectual disabilities, or other problems. We currently only have cooling as an approach to treat this condition. Cooling is achieved by the use of special helmets or, more frequently, of thermal mattresses. Cooling may cause pain, which can also have a long‐term negative impact on development and quality of life. The aim of this review was to assess if drugs can reduce pain, discomfort and mortality.
Key results
We have not identified any studies that addressed the review question. We identified four potential studies, but we excluded them due to the type of drug or study design. One study is ongoing.
How up to date is this review?
We searched for studies that were available up to August 2021.
Background
Description of the condition
Hypoxic‐ischemic encephalopathy is a leading cause of mortality and long‐term neurological sequelae, affecting more than one million newborn infants every year (Lee 2013). Standard management in neonatal intensive care units (NICUs) consists of providing supportive care to maintain cerebral perfusion and metabolic balance. Therapeutic hypothermia can improve survival without disability in infants with moderate to severe hypoxic‐ischemic encephalopathy following peripartum asphyxia (Gunn 2017; Jacobs 2013). Additional therapies are under investigation and include erythropoietin, allopurinol, xenon, topiramate, and magnesium sulfate; findings from trials on stem cell‐based interventions for hypoxic‐ischemic encephalopathy are not yet available (Bruschettini 2020). Therapeutic hypothermia should be initiated within six hours after birth and is maintained for 72 hours (Wassink 2019). So far, only late‐preterm and full‐term asphyxiated infants are treated with therapeutic hypothermia; limited results are available on therapeutic hypothermia in preterm infants (Herrera 2018; Laptook 2016). The main modalities are selective head cooling, which is performed with a helmet, and total‐body cooling, where the infant lies on a thermal mattress. The latter is more widely used because it is associated with servo‐controlled temperature regulation (Wassink 2019). Infants undergoing therapeutic hypothermia are in need of adequate management for pain and sedation due to their clinical condition and the need for intensive care, such as cooling and respiratory support. The therapeutic hypothermia by itself seems to be associated with stress and pain (Axelin 2013; Hoffman 2013; Üner 2019). Painful procedures in the newborn period can affect pain responses later in life (Walker 2019), impair brain development (Williams 2020), and possibly have a long‐term negative impact on neurodevelopment and quality of life (Chau 2019; Walker 2017).
Description of the intervention
The newborn infant undergoing therapeutic hypothermia is exposed to a higher number of painful procedures than other infants admitted to the NICU (Axelin 2013). The discomfort and pain, which the newborn infant may experience as stress, is caused by procedures (such as intubation, mechanical ventilation, or blood sampling) and might be augmented by the therapeutic hypothermia itself. Induced hypothermia produces significant stress, together with the painful procedures and interventions the asphyxiated infant often undergoes such as blood tests and ventilator treatment (Wassink 2015). It is recommended that infants treated with therapeutic hypothermia be provided with optimal analgesia and sedation (de Haan 2012; McPherson 2020; Üner 2019). However, there is no consensus regarding the use of analgesia or sedation during neonatal therapeutic hypothermia (Üner 2019). Protocols for sedation and analgesia management are rarely reported in studies on therapeutic hypothermia. To further complicate the treatment of pain and stress in this already vulnerable patient group, the encephalopathic newborn often has subtle signs of pain and neurological symptoms that can resemble symptoms of pain (Üner 2019), making the use of pain scales otherwise used in a neonatal context more difficult. Since hypothermia causes a reduction in heart rate by 10 beats per minute (BPM) with each degree Celsius, a well‐sedated newborn is expected to have a heart rate of around 100 BPM. A heart rate of greater than 110 to 120 BPM has been suggested to be a sign of inadequate sedation and analgesia (Ergenekon 2016).
Opioids have varying pharmacokinetic and pharmacodynamic profiles, and should optimally be administered in an individualized way according to the need, clinical state, and expected course of the hospitalization. Morphine can be administered by both intravenous and oral routes, whereas fentanyl and remifentanil are only used intravenously. Morphine is an agonist of the µ and k receptors (Pacifici 2016), and acts by binding to opiate receptors in the central and peripheral nervous system, exerting analgesic effect by stimulation of descending inhibitory pathways. Morphine is primarily metabolized by the liver, and pharmacokinetics differ considerably between neonates (Donato 2020). Adverse effects include miosis, pruritus, respiratory depression, constipation, urinary retention, and hypotension. Fentanyl is frequently used due to its effectiveness and high lipid solubility. It is a synthetic opioid and, compared to morphine, crosses the blood‐barrier more rapidly, and its half‐life time is shorter. Fentanyl has an onset of action of three minutes and a duration of effect of 30 minutes (Pacifici 2014; Schiller 2018). If opioids are administered to the infant for less than three days and in the absence of severe pain, a complete and abrupt cessation is recommended (Balda 2019).
Alpha‐2 agonists such as clonidine and dexmedetomidine activate the alpha‐2 receptors in the central nervous system, decreasing sympathetic activity (Donato 2020). Clonidine has analgesic properties and can reduce the need for opiates and benzodiazepines. Clearance in infants is reduced because of pathway immaturity or renal disease. Adverse effects include hypotension, rebound hypertension, atrioventricular block, and bradycardia. Contrary to opioids, alpha‐2 agonists such as clonidine do not cause respiratory depression. Though primarily used as adjunctive therapy to opioids, alpha‐2 agonists might be administered in monotherapy (O'Mara 2018).
Ketamine, an N‐Methyl‐d‐aspartate (NMDA) receptor antagonist, has been suggested to decrease pain and opioid consumption in infants, due to its anxiolytic and analgesic effects, with few cardiovascular and respiratory effects (Carter 2017; Saarenmaa 2001).
Paracetamol (acetaminophen) is a non‐opioid, central‐acting analgesic used to treat mild and moderate pain (Donato 2020). It is generally used to treat fever or pain, or both, and can be used for the management of patent ductus arteriosus (Ohlsson 2020). The analgesic effect of paracetamol is mediated by activation of descending serotonergic pathways, inhibition of prostaglandin synthesis, and the formation of an active metabolite influencing cannabinoid receptors (Allegaert 2017). In postoperative pain management, paracetamol might reduce pain when used in combination with morphine or fentanyl, and impact positively on decreasing opioid‐related side effects, such as abstinence syndrome (Hong 2010). The main adverse effect of paracetamol is hepatotoxicity generated by the metabolite N‐acetyl‐p‐benzoquinone imine (Donato 2020).
Midazolam is a short‐acting benzodiazepine exercising the sedative effect through binding to gamma‐aminobutyric acid (GABA) receptors (Donato 2020). It induces sedation, amnesia, and muscle relaxation, but no analgesia. Elimination of midazolam is reduced in newborn infants; when used in adults undergoing therapeutic hypothermia, there is a fivefold increase in serum levels (Ainsworth 2015). Adverse effects include hypotension and neurological irregularities (Donato 2020).
In addition to possible multi‐organ failure impacting drug pharmacokinetics due to the hypoxic insult at birth, the therapeutic hypothermia itself may impact responses to drugs in the asphyxiated infant. Therapeutic hypothermia causes redistribution of blood flow, which can significantly influence both drug distribution and clearance and could potentially impair renal excretion of drugs in humans (Ainsworth 2015). Phenobarbital administered under therapeutic hypothermia results in higher plasma concentrations and longer half‐lives than expected in normothermic newborns (Filippi 2011). Therapeutic hypothermia reduces morphine's affinity for the µ opioid receptor, making it less effective, though at the same time the clearance of morphine is lower in infants, and accumulation could occur if higher doses are used (Pacifici 2016). The use of more than one drug for pain and sedation management during therapeutic hypothermia requires considering potential pharmacological interactions. As phenobarbital is an inducer of cytochrome P450 (CYP) 3A, while midazolam is a CYP3A substrate, phenobarbital increases midazolam clearance by a factor 2.3 (Favié 2019). The co‐administration of these two drugs therefore requires dosing adjustment.
How the intervention might work
Pharmacological interventions are commonly used during neonatal therapeutic hypothermia. A large, multicenter observational study conducted in 2621 infants reported that the use of opioids was more common in the case of more severe hypoxic‐ischemic encephalopathy, although it varied widely between centers (Berube 2020). The use of morphine and benzodiazepine increased from 38% to 68% and from 40% to 53% between 2008 and 2015, respectively (Berube 2020).
The use of drugs during neonatal therapeutic hypothermia should be based on reliable pain and sedation evaluation. A Cochrane Review on scales for evaluating pain in newborn infants is under preparation (Bruschettini 2022). However, assessing the pain and effect of pain medication in the asphyxiated and hypothermic infant is associated with several difficulties. Neurological symptoms stemming from encephalopathy can mask or mimic pain symptoms, thus the assessment of pain is complex (Üner 2019). Moreover, the infant is often sedated during therapeutic hypothermia, which further complicates the pain assessment. A case report has observed that pain and agitation correlate with physiological variables such as skin conductance algesimeter during therapeutic hypothermia (Hoffman 2013). Sedation of the infant receiving active therapeutic hypothermia during transport has been found to protect against incident hypocapnia (Szakmar 2018), which could be an issue in non‐ventilated infants. A systematic review reported that the expected reduction in neonatal mortality did not appear among hypothermia‐treated infants in low‐ and middle‐income countries (Pauliah 2013). Sedation was not routinely given to hypothermia‐treated infants, possibly due to fears of respiratory compromise and a lack of facilities for providing optimal ventilatory support. This lack of satisfactory sedation might have negated the neuroprotective effects of the hypothermia treatment. Of note, the Cochrane Review on opioids in ventilated infants, which included 23 trials, concluded that it is uncertain whether morphine or fentanyl has an effect on pain, and probably have little or no effect in reducing the duration of mechanical ventilation and neonatal mortality (Bellù 2021).
The use of sedative and anticonvulsant drugs during neonatal therapeutic hypothermia should be based on the available evidence, which is scarce (Young 2016), making it difficult to recommend routine use of these agents and to identify the optimal drug (Wassink 2015). Furthermore, the risk of long‐lasting neurological sequelae of anesthetic, sedative, and analgesic drugs on the newborn infant's developing brain further complicates treatment choice (McCann 2012; Wassink 2015).
Non‐pharmacological interventions for pain management such as swaddling, sweet solutions, facilitated tucking, and non‐nutritive sucking are considered to have a low risk for adverse effects and to be effective on mild pain (Mangat 2018; Pillai Riddell 2015). These interventions engage environmental and behavioral approaches through activation of a 'gate control mechanism' preventing the pain sensation from being carried to the central nervous system (Mangat 2018). Non‐pharmacological interventions can include the infant's parents as part of the pain treatment, hence facilitating parental bonding. Of note, signs induced by therapeutic hypothermia such as shivering may be perceived by parents as infant discomfort (Craig 2020).
Why it is important to do this review
Pain and sedation need to be adequately managed in all patients. The therapeutic effect of cooling might be suboptimal if the newborn infant experiences stress and discomfort during treatment. Painful procedures and inadequate pain management in early life may lead to long‐term negative effects (Walker 2019). Drugs such as sedatives and analgesics might be used to optimize the outcome of the therapeutic hypothermia. It is important to assess which drugs should be used, as they might have different benefits, harms, and different levels of neuroprotection. Cochrane Reviews on postoperative pain (Kinoshita 2021a; Kinoshita 2021b), procedural pain (Kinoshita 2021c), sedation during mechanical ventilation (Bellù 2021; Ibrahim 2016; Romantsik 2017), prophylactic barbiturate use following perinatal asphyxia (Young 2016), and other indications in the newborn (Pirlotte 2019; Romantsik 2020), are available or are currently under preparation. However, no systematic reviews have been conducted on pain or sedation management during therapeutic hypothermia.
Objectives
To determine the effects of pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia. Primary outcomes were analgesia and sedation and all‐cause mortality to discharge.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomized controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs, and cross‐over RCTs.
Types of participants
We included studies on pharmacological interventions for pain and sedation management in late preterm (i.e. 34 to 36 weeks’ gestational age) and full‐term (i.e. more than 36 weeks’ gestational age) newborn infants undergoing therapeutic hypothermia for hypoxic‐ischemic encephalopathy (HIE) (criteria as defined in Jacobs 2013).
Types of interventions
We included studies of drugs used for the management of pain or sedation, or both, during therapeutic hypothermia. This included any opioids (e.g. morphine, fentanyl), alpha‐2 agonists (e.g. clonidine, dexmedetomidine), NMDA receptor antagonist (e.g. ketamine), other analgesics (e.g. paracetamol), and sedatives (e.g. benzodiazepines such as midazolam).
We included the following comparisons.
Comparison 1. Opioids (e.g. morphine, fentanyl) versus placebo, no intervention, or non‐pharmacological interventions
Opioids versus placebo or no intervention (e.g. morphine versus placebo)
Opioids versus non‐pharmacological interventions (e.g. non‐nutritive sucking, sweet solutions (oral glucose or sucrose), swaddling, music therapy, therapeutic touch/massage, sensorial saturation, or acupuncture)
Comparison 2. Alpha‐2 agonists (e.g. clonidine, dexmedetomidine) versus placebo, no intervention, or non‐pharmacological interventions
Alpha‐2 agonists (e.g. clonidine, dexmedetomidine) versus placebo or no intervention (e.g. clonidine versus placebo)
Alpha‐2 agonists (e.g. clonidine, dexmedetomidine) versus non‐pharmacological interventions (e.g. non‐nutritive sucking, sweet solutions (oral glucose or sucrose), swaddling, music therapy, therapeutic touch/massage, sensorial saturation, or acupuncture)
Comparison 3. N‐Methyl‐D‐aspartate (NMDA) receptor antagonist (e.g. ketamine) versus placebo, no intervention, or non‐pharmacological interventions
NMDA receptor antagonist (e.g. ketamine) versus placebo or no intervention (e.g. ketamine versus placebo)
NMDA receptor antagonist (e.g. ketamine) versus non‐pharmacological interventions (e.g. non‐nutritive sucking, sweet solutions (oral glucose or sucrose), swaddling, music therapy, therapeutic touch/massage, sensorial saturation, or acupuncture)
Comparison 4. Other analgesics (e.g. paracetamol) versus placebo, no intervention, or non‐pharmacological interventions
Other analgesics (e.g. paracetamol) versus placebo or no intervention (e.g. paracetamol versus placebo)
Other analgesics (e.g. paracetamol) versus non‐pharmacological interventions (e.g. non‐nutritive sucking, sweet solutions (oral glucose or sucrose), swaddling, music therapy, therapeutic touch/massage, sensorial saturation, or acupuncture)
Comparison 5. Sedatives (e.g. benzodiazepines such as midazolam, barbiturates such as phenobarbital) versus placebo, no intervention, or non‐pharmacological interventions
Sedatives (e.g. midazolam, phenobarbital) versus placebo or no intervention (e.g. midazolam versus placebo)
Sedatives (e.g. midazolam, phenobarbital) versus non‐pharmacological interventions (e.g. non‐nutritive sucking, sweet solutions (oral glucose or sucrose), swaddling, music therapy, therapeutic touch/massage, sensorial saturation, or acupuncture)
Comparison 6. Drug type A versus drug type B (e.g. morphine versus fentanyl or morphine versus midazolam)
This could include comparisons within or between classes of interventions (opioids, alpha‐2 agonists, NMDA receptor antagonists, other analgesics, and sedatives).
We included any dose, duration, and route of administration.
We included studies where the interventions were initiated to prevent or treat pain or discomfort associated with therapeutic hypothermia, including the use of sedatives and antiseizure medications to prevent seizures.
Types of outcome measures
Outcome measures were not part of the eligibility criteria.
Primary outcomes
Analgesia and sedation assessed with validated scales in the neonatal population: the Echelle Douleur Inconfort Nouveau‐ne (EDIN) scale (Debillon 2001), COMFORTneo (COMFORTNeo 2016; van Dijk 2009), Neonatal Pain, Agitation and Sedation Scale (N‐PASS) (Hummel 2008), pain assessment tool, the Astrid Lindgren and Lund Children's Hospital's Pain and Stress Assessment Scale for Preterm and Sick Newborn Infants (ALPS‐neo) (Lundqvist 2014), Neonatal Facial Coding System (NFCS) (Grunau 1986; Peters 2003), and CRIES (Crying, Requires oxygen, Increased vital signs, Expression, Sleepless) scale (Krechel 1995). However, none of these scales are validated for assessing pain during therapeutic hypothermia. We planned to report the mean values of each scale assessed at 30 minutes, three hours, and 12 hours after the administration of the intervention. We planned to report analgesia and sedation scores separately.
All‐cause mortality to discharge.
Secondary outcomes
All‐cause neonatal mortality (death until postnatal day 28).
Sinus bradycardia (heart rate < 80 BPM).
Hypotension requiring medical therapy (inotropes, vasopressors, or fluid boluses).
Constipation, defined as difficulty in defecation causing significant distress to the newborn.
Focal gastrointestinal perforation.
Duration of mechanical ventilation (days).
In studies where infants might not be ventilated: apneic spells occurring after commencement of therapy.
In studies where infants might not be ventilated: need for mechanical ventilation occurring after commencement of therapy.
Hospital stay (days).
Time to full enteral feeding (days).
Need for gavage feeds at time of discharge.
Abnormal neurological examination at time of discharge.
Seizure, defined as a transient occurrence of signs or symptoms, or both, due to abnormal excessive or synchronous neuronal activity in the brain (Fisher 2005). The American Clinical Neurophysiology Society has defined a seizure as “a sudden, abnormal EEG (electroencephalography) event, defined by a repetitive and evolving pattern with a minimum 2 μV peak‐to‐peak voltage and duration of at least 10 seconds” (Pressler 2021; Tsuchida 2013).
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Scales of Infant Development ‐ Mental Development Index Edition II (BSID‐MDI‐II) (Bayley 1993), Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale (BSITD‐III) (Bayley 2005), Griffiths Mental Development Scale ‐ General Cognitive Index (GCI) (Griffiths 1954; Griffiths 1970) assessment greater than two standard deviations (SDs) below the mean), intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We planned to assess data separately for children aged 18 to 24 months and those aged three to five years.
Each component of major neurodevelopmental disability. We planned to assess data separately for children aged 18 to 24 months and aged three to five years.
Cognitive and educational outcomes in children aged more than five years old. We planned to assess data separately for children aged six to 12 years and those aged more than 12 years.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search for studies, including: the Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 8) in the Cochrane Library; MEDLINE via PubMed (1966 to 24 August 2021), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 24 August 2021). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for RCTs and quasi‐RCTs. We used Cochrane Neonatal's search strategy for neonates and RCTs (see Appendix 1 for the full search strategies for each database). We did not apply any language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/search/en/) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), via CENTRAL. We also searched the ISRCTN registry (www.isrctn.com/) for any unique trials not found through the CENTRAL search.
Searching other resources
We searched for errata or retractions for included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed). We planned to review the reference lists of all included articles for any relevant articles not identified by the primary search.
Data collection and analysis
We planned to collect information regarding the method of randomization, blinding, intervention, stratification, and whether the trial was single or multicenter, for each included study. We would have noted information regarding trial participants, including birth weight, gestational age, number of participants, modality of administration, and dose of drugs. We planned to analyze the clinical outcomes noted above in Types of outcome measures.
Selection of studies
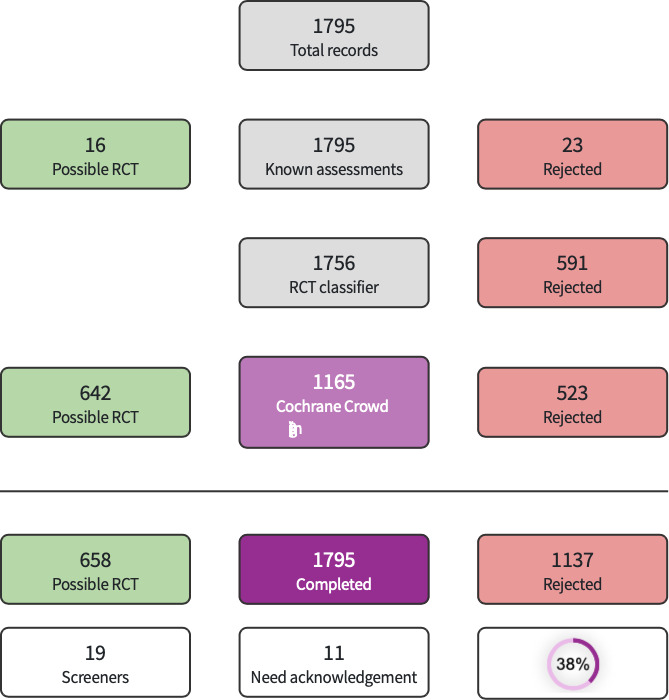
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labeled as 'an RCT' or as 'Not an RCT'; the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs, and if appropriate, Cochrane Crowd – Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, please go to the Screen4Me webpage on the Cochrane Information Specialist’s portal (community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal/searching-conducting). In addition, more detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2021.
We included all RCTs, quasi‐RCTs, cluster‐RCTs, and cross‐over RCTs fulfilling our inclusion criteria. Two review authors (PB, YTB) reviewed the results of the search and separately selected studies for inclusion. They resolved any disagreements by discussion or by consulting a third review author when necessary.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We planned that two review authors (PB, YTB) would independently extract data from the included studies using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (EPOC 2017). We planned to pilot the form within the review team using a sample of included studies.
Data extraction would have recorded the following characteristics for each included study:
administrative details: study author(s); published or unpublished; year of publication; year in which the study was conducted; presence of vested interest; details of other relevant papers cited;
study: study design; type, duration, and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval;
participants: sex, birth weight, gestational age, number of participants;
interventions: initiation, dose, and duration of administration;
outcomes as described in Types of outcome measures.
We resolved any disagreements by discussion. For any ongoing studies identified by our search, we detailed the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date, and reported the study in the 'Characteristics of ongoing studies' table.
We contacted study investigators/authors in cases where clarification or additional data were required. Two review authors (PB, YTB) entered data into Review Manager 5 software (Review Manager 2020). We planned to replace any standard error of the mean (SEM) values by the corresponding SD.
Assessment of risk of bias in included studies
We planned for two review authors (PB, YTB) to independently assess risk of bias (low, high, or unclear) of the included trials using the Cochrane risk of bias tool RoB 1, based on the following domains (Higgins 2011).
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
We would have resolved any disagreements by discussion or by consulting a third review author. A more detailed description of risk of bias for each domain is provided in Appendix 2.
Measures of treatment effect
We would have performed statistical analyses using Review Manager 5 (Review Manager 2020), and planned to summarize data in a meta‐analysis if they were sufficiently homogeneous, both clinically and statistically.
Dichotomous data
For dichotomous data, we planned to present results using risk ratios (RR) and risk differences (RD) with 95% confidence intervals (CIs). We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) with 95% CIs if there was a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data, we planned to use the mean difference (MD) when outcomes were measured in the same way between trials. We planned to use the standardized mean difference (SMD) to combine trials that measured the same outcome but employed different methods of measurement. Where trials reported continuous data as median and interquartile range (IQR), and data passed the test of skewness, we planned to convert mean to median and estimate the SD as IQR/1.35.
Unit of analysis issues
We planned that the unit of analysis would be the participating infant in individually randomized trials, and would only have considered an infant once in the analysis. For cluster‐randomized trials, we planned that the participating neonatal unit or section of a neonatal unit or hospital would have been the unit of analysis; we would have analyzed these using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or study with a similar population, as described in Section 16.3.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021a). If we were to have used ICCs from a similar trial or from a study with a similar population, we would have reported this and conducted a sensitivity analysis to investigate the effect of variation in the ICC.
If we had identified both cluster‐randomized trials and individually randomized trials, we would only have combined the results from the two types of trials if there was little heterogeneity between study designs, and if we had considered interaction between the effect of the intervention and the choice of randomization unit to have been unlikely.
We planned to report outcome data from parallel and cross‐over trials separately. In the event that we identified cross‐over trials, in which the reporting of continuous outcome data precluded paired analysis, we would not have included these data in a meta‐analysis to avoid a unit of analysis error. Where we thought carry‐over effects existed, and where there were sufficient data, we would only have included data from the first period in the analysis (Higgins 2021b).
We planned to acknowledge any possible heterogeneity in the randomization unit, and perform a sensitivity analysis to investigate possible effects of the randomization unit on the results.
Dealing with missing data
Where feasible, we panned to carry out analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we planned to analyze all participants in the treatment group to which they had been randomized, regardless of the treatment received. If we had identified important missing data (in the outcomes) or data were unclear, we would have contacted the original investigators to request the missing data. We planned to make explicit the assumptions of any methods we used to deal with missing data. We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in our assumptions. We panned to address the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to estimate the treatment effects of individual trials and examine heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic. We would have graded the degree of heterogeneity as:
less than 25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 75%: moderate heterogeneity;
more than 75%: substantial heterogeneity.
If we were to note moderate or substantial statistical heterogeneity (I² > 50%), we planned to explore the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We intended to conduct a comprehensive search for eligible studies, and were alert for duplication of data. If we had identified 10 or more trials for meta‐analysis, we would have assessed possible publication bias by inspection of a funnel plot. If we had uncovered reporting bias that, in the opinion of the review authors, could have introduced serious bias, we would have conducted a sensitivity analysis to determine the effect of including and excluding these studies in the analysis.
Data synthesis
If we had identified multiple studies considered to be sufficiently similar, we would have performed meta‐analysis using Review Manager 5 (Review Manager 2020). For categorical outcomes, we would have calculated the typical estimates of RR and RD, each with its 95% CI; for continuous outcomes, we would have calculated the MD or the SMD, each with its 95% CI. If we had considered different pain or sedation scales to be inappropriate to be combined by means of SMD, we would have analyzed and interpreted them separately. We planned to use a fixed‐effect model to combine data where it was reasonable to assume that studies had estimated the same underlying treatment effect. If we had judged meta‐analysis to be inappropriate, we would have analyzed and interpreted individual trials separately. If there had been evidence of clinical heterogeneity, we would have attempted to provide an explanation based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We planned to explore high statistical heterogeneity in the outcomes by visually inspecting the forest plots and by removing the outlying studies in a sensitivity analysis (Higgins 2021a). If statistical heterogeneity had been significant, we would have interpreted the results of the meta‐analyses accordingly and downgraded the certainty of evidence in the summary of findings tables according to the GRADE recommendations.
We planned to consider the following groups for subgroup analysis where data were available.
Severity of hypoxic‐ischemic encephalopathy, e.g. mild, moderate, or severe encephalopathy according to Sarnat criteria (Sarnat 1976).
Indication, e.g. procedural pain, premedication, analgesia‐sedation for ventilation and for cooling.
High versus low dose.
By route of administration.
Low‐, middle‐, and high‐income settings.
We planned to restrict these analyses to the primary outcomes.
Sensitivity analysis
If we had identified substantial heterogeneity, we would have conducted sensitivity analysis to determine if the findings were affected by inclusion of only those trials considered to have used adequate methodology with a low risk of bias (selection and performance bias). We planned to report results of sensitivity analyses for primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We planned to use the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes.
Analgesia and sedation assessed at 30 minutes after the administration of the intervention with validated scales in the neonatal population: If we had judged different scales to be inappropriate to be combined by means of SMD, we would have reported the scale with the best validity and reliability for the type of population investigated (Bruschettini 2022).
Analgesia and sedation assessed at three hours after the administration of the intervention.
All‐cause mortality to discharge.
Major neurodevelopmental disability in children aged 18 to 24 months: (Jacobs 2013).
Major neurodevelopmental disability in children aged three to five years.
Two review authors (PB, YTB) planned to independently assess the certainty of the evidence for each of the outcomes listed above. We planned to consider evidence from RCTs as high certainty, downgrading the evidence by one level for serious (or two levels for very serious) limitations based on the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We planned to use GRADEpro GDT software to create a summary of findings table to report the certainty of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
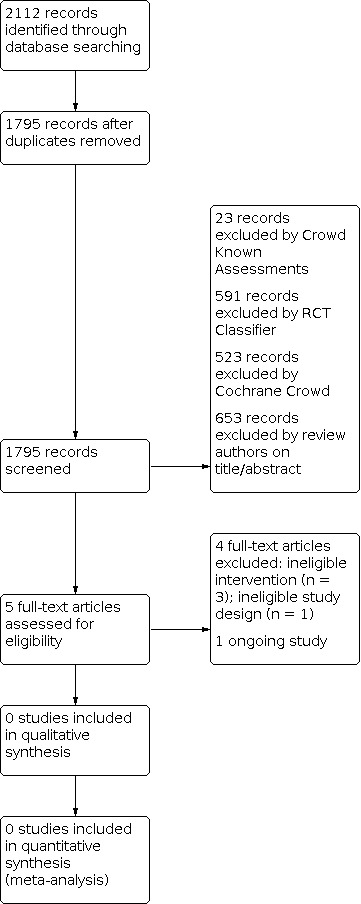
We provided results of the search in the study flow diagram (Figure 1).
1.
See Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
The search identified a total of 2112 search results (1795 after deduplication, Figure 1). In assessing the studies we used Cochrane’s Screen4Me workflow to help identify potential reports of randomized trials. The results of the Screen4Me assessment process can be seen in Figure 2. We then assessed the remaining 658 records left in after Screen4Me, with full‐text screening of five studies.
2.
Screen4Me summary diagram
Included studies
We did not identify any trials that matched our inclusion criteria.
We identified one ongoing study on the use of dexmedetomidine or morphine during cooling (DICE Trial: NCT04772222). Fifty infants ≥ 36 weeks' gestational age will be randomized to either dexmedetomidine (loading dose of 1 mcg/kg followed by 0.1 to 0.5 mcg/kg/h as continuous infusion) or morphine (intermittent dosing every three to four hours of 0.02 to 0.05 mg/kg/dose or continuous infusion of 0.005 to 0.01 mg/kg/h). The primary outcome is safety measures (adverse events during the first four days of life; see Ongoing studies).
Excluded studies
Following full text screening, we excluded four studies (Filippi 2018; Gill 2014; Nuñez‐Ramiro 2019; Surkov 2019). Two studies used topiramate (Filippi 2018; Nuñez‐Ramiro 2019) and one trial used atropine (Gill 2014): these drugs were not specified in the protocol of this review as they are primarily used for indications other than pain and sedation management. In Surkov 2019 infants were treated with either dexmedetomidine (n = 46) or standard care (n = 159); however, this study, though described as a randomized trial, was actually observational; a correction clarifying the design of this trial was published in 2021.
Risk of bias in included studies
No study met the eligibility criteria of this review.
Effects of interventions
No study met the eligibility criteria of this review.
Discussion
Summary of main results
We identified no eligible randomized controlled trials for inclusion on pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia.
We excluded four studies (Filippi 2018; Gill 2014; Nuñez‐Ramiro 2019; Surkov 2019). Two trials used topiramate (Filippi 2018; Nuñez‐Ramiro 2019) and one study used atropine (Gill 2014); Surkov 2019 is an observational study (personal communication with the Editor‐in‐Chief of the journal where the study was published: the manuscript will be edited), which compared infants treated with dexmedetomidine to standard care.
We identified one ongoing trial comparing dexmedetomidine to morphine administration during cooling (DICE Trial: NCT04772222). The planned sample size is 50 infants (≥ 36 weeks' gestational age). The primary outcome is adverse events during the first four days of life.
Overall completeness and applicability of evidence
We identified no eligible studies for inclusion.
Quality of the evidence
We identified no eligible studies for inclusion.
Potential biases in the review process
We used the standard methods of Cochrane Neonatal to conduct this systematic review. Our inclusive search strategy would theoretically have included all relevant studies. We applied no language restrictions. We succeeded in obtaining additional information from study authors of an eligible study, which we then excluded because of a lack of randomization (Surkov 2019, see correction in 2021, following communication between us and the Editor‐in‐Chief of the journal where this observational study is published).
Agreements and disagreements with other studies or reviews
We are not aware of any completed systematic reviews on pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia. The protocol of a review including both randomized and non‐randomized studies has been registered; this review will include also non‐pharmacological interventions (Olsson 2021). The Cochrane Review on intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit included three trials enrolling 148 neonates: the authors found insufficient data to support the use of midazolam as a sedative for neonates undergoing intensive care, and raised concerns about safety (Ng 2017). However, these three trials included very preterm infants requiring mechanical ventilation rather than late preterm infants undergoing cooling. Similarly, the Cochrane Review on opioids for newborn infants receiving mechanical ventilation included 23 trials enrolling 2023 infants (Bellù 2021); none of studies included newborn infants undergoing therapeutic hypothermia.
Authors' conclusions
Implications for practice.
We found no studies that met our inclusion criteria and hence there is no evidence to recommend or refute the use of pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia.
Implications for research.
Future research might assess the effects of pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia by using validated and adequate pain scales; and by reporting long‐term neurodevelopmental outcomes. Trials might compare different drugs (head‐to‐head comparisons) or use non‐pharmacological interventions in the control group. Observational studies might provide useful data on safety and on blood concentrations of drugs.
History
Protocol first published: Issue 7, 2021
Acknowledgements
The Methods section of this protocol is based on a standard template used by Cochrane Neonatal.
We would like to thank Cochrane Neonatal: Michelle Fiander and Jane Cracknell, Managing Editors; Colleen Ovelman, former Managing Editor; Roger Soll, and Bill McGuire, Co‐ordinating Editors, who provided editorial and administrative support.
We thank Andrea Takeda (Cochrane Central Production Service) for copy editing.
Maria Björklund (Library and ICT services, Lund University, Sweden) designed the literature searches, and Carol Friesen, former Cochrane Neonatal Information Specialist, peer reviewed the searches.
Thank you to Sivam Thanigainathan, Assistant Professor, AIIMS Jodhpur; and Marie T Berg, MD Johns Hopkins All Children's Hospital, St. Petersburg, FL for peer review of this review; and Marie Berg and David Osborn for peer review and feedback on the protocol.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Akhilanand Chaurasia, Nikolaos Sideris, Anna Noel‐Storr, Ciara Gleeson, Mohammad Aloulou, Ana‐Marija Ljubenković, Vighnesh Devulapalli, Ashutosh Kumar Singh, Muataz Kashbour, Neetu Bhadra, Amin Sharifan.
Appendices
Appendix 1. Search strategy
MEDLINE via PubMed
(((((infant, newborn[MeSH] OR newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies*[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR "low birth weight"[TIAB] OR "low birthweight"[TIAB] OR LBW[TIAB] OR infan*[TIAB] OR neonat*[TIAB]) NOT (animals [mh] NOT humans [mh]))))
AND ((hypoxic‐ischemic[Title/Abstract]) OR (encephalopathies, hypoxic ischemic[MeSH Terms]) OR (encephalopath*[Title/Abstract]) OR (asphyxia[MeSH Terms]) OR (asphyxi*[Title/Abstract])))
AND ((hypothermia, induced[MeSH Terms]) OR (hypothermia[Title/Abstract]) OR (induced hypothermia[Title/Abstract]) OR (therapeutic hypothermia[Title/Abstract]) OR (cooling[Title/Abstract]) OR (temperature management[Title/Abstract]))
AND
((randomized controlled trial[Publication Type]) OR (controlled clinical trial[Publication Type])) OR ((((randomized[Title/Abstract] OR randomly[Title/Abstract] OR randomised[Title/Abstract]) OR (placebo[Title/Abstract] OR drug therapy[Title/Abstract])) OR (groups[Title/Abstract] OR trial[Title/Abstract])) OR (((single[Title/Abstract] OR doubl*[Title/Abstract] OR tripl*[Title/Abstract] OR treb*) AND (blind*[Title/Abstract] OR mask*))))
CINAHLComplete (Ebsco)
(infant or infants or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or LBW)
AND
MH Hypoxia‐Ischemia, Brain, Neonatal OR ( hypoxic‐ischemic OR encephalopath* OR asphyxi* )
AND
MH Hypothermia, Induced OR ( hypothermia OR induced hypothermia OR therapeutic hypothermia OR temperature management OR cooling )
AND
PT randomized controlled trial OR PT controlled clinical trial OR ( randomized OR randomly OR randomised OR placebo OR drug therapy OR groups OR trial OR ) OR ( single OR doubl* OR tripl* OR treb*) AND (blind* OR mask*) )
Cochrane CENTRAL
MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET
OR infant or infants or infant’s or “infant s” or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET
AND
(MeSH descriptor: [Hypoxia‐Ischemia, Brain] explode all trees OR MeSH descriptor: [Hypoxia‐Ischemia, Brain] explode all trees OR hypoxic‐ischemic OR encephalopath* OR asphyxi*)
AND
(MeSH descriptor: [Hypothermia, Induced] explode all trees OR hypothermia OR induced hypothermia OR therapeutic hypothermia OR temperature management OR cooling)
Scopus
( ( TITLE‐ABS‐KEY ( ( infant OR infants OR infantile OR infancy OR newborn* OR "new born" OR "new born" OR "newly born" OR neonat* OR baby* OR babies OR premature OR premature OR prematurity OR preterm OR preterm OR "pre term" OR premises ) ) OR TITLE‐ABS‐KEY ( ( "low birth weight" OR "low birthweight" OR lbw OR nicu ) ) ) ) AND ( ( TITLE‐ABS‐KEY ( ( hypoxic‐ischemi* OR encephalopath* OR asphyxi* ) ) AND TITLE‐ABS‐KEY ( ( hypothermia OR induced AND hypothermia OR therapeutic AND hypothermia OR temperature AND management OR cooling ) ) ) ) not TITLE‐ABS‐KEY ( animals AND not AND humans )
AND
TITLE‐ABS‐KEY ( randomized OR randomly OR randomised OR placebo OR drug AND therapy OR groups OR trial OR ( single OR doubl* OR tripl* OR treb* ) AND ( blind* OR mask* ) )
Web of Science
TOPIC: (( infant OR infants OR infantile OR infancy OR newborn* OR "new born" OR "new born" OR "newly born" OR neonat* OR baby* OR babies OR premature OR premature OR prematurity OR preterm OR preterm OR "pre term" OR premises OR "low birth weight" OR "low birthweight" OR lbw OR nicu )) AND TOPIC: (( hypoxic‐ischemi* OR encephalopath* OR asphyxi* )) AND TOPIC: (( hypothermia OR induced AND hypothermia OR therapeutic AND hypothermia OR temperature AND management OR cooling )) NOT TOPIC: ((animals NOT humans)) AND
TOPIC: (TITLE‐ABS‐KEY ( randomized OR randomly OR randomised OR placebo OR drug AND therapy OR groups OR trial OR ( single OR doubl* OR tripl* OR treb* ) AND ( blind* OR mask* ) ))
Clinicaltrials.gov
Other terms
(Hypoxic* OR ischemi* OR encephalopaht* OR hypothermia OR induced AND hypothermia OR therapeutic AND hypothermia OR temperature AND management OR cooling )
AND
randomized OR randomly OR randomised OR placebo OR drug therapy OR groups OR trial OR ((single OR doubl* OR tripl* OR treb*) AND (blind* OR mask*))
Filter: Child (birth‐17 years)
Appendix 2. Risk of bias tool
We will use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we will seek information regarding the method of randomization, blinding, and reporting of all outcomes of all infants enrolled in the trial. We will assess each criterion listed below as being at a low, high, or unclear risk of bias. Two review authors will separately assess each study. Any disagreements will be resolved by discussion. We will enter our findings in the risk of bias table in the 'Characteristics of included studies' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorize the method used to generate the allocation sequence as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorize the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorize the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or class of outcomes. We will categorize the methods as:
low risk, high risk, or unclear risk for participants; and
low risk, high risk, or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorize the methods used to blind outcome assessment. We will assess blinding separately for different outcomes or class of outcomes. We will categorize the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomized participants); reasons for attrition or exclusion where reported; and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorize the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we will compare the prespecified outcomes against the outcomes reported in the published results. If the study protocol was not published in advance, we will contact the study authors to gain access to the protocol. We will assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would be expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns we had about other sources of bias (e.g. whether there was a potential source of bias related to the specific study design, or whether the trial was stopped early due to some data‐dependent process). We will assess whether each study is free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we will explore the impact of the level of bias by undertaking sensitivity analyses.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Filippi 2018 | Ineligible intervention |
| Gill 2014 | Ineligible intervention |
| Nuñez‐Ramiro 2019 | Ineligible intervention |
| Surkov 2019 | Observational study, as reported in the correction in 2021 (the original publication in 2019 reported as a randomized trial) |
Characteristics of ongoing studies [ordered by study ID]
NCT04772222.
| Study name | Dexmedetomidine Use in Infants Undergoing Cooling Due to Neonatal Encephalopathy (DICE Trial) |
| Methods | Randomized controlled trial |
| Participants | 50 infants ≥ 36 weeks' gestational age treated with therapeutic hypothermia |
| Interventions | Dexmedetomidine (loading dose of 1 mcg/kg followed by 0.1 to 0.5 mcg/kg/h as continuous infusion) or morphine (intermittent dosing every 3 to 4 hours of 0.02 to 0.05 mg/kg/dose or continuous infusion of 0.005 to 0.01 mg/kg/h). |
| Outcomes | Primary outcome: safety measures (adverse events during the first 4 days of life). Secondary outcomes: plasma levels of dexmedetomidine. Other outcomes: Number of participants who experience shivering, number of participants who require respiratory support, days to full oral feedings, generalized motor assessment scores 7 days after weaned of study drug, generalized motor assessment scores at 3 to 4 months of age, Hammersmith infant neurological exam scores at 6 to 9 months of age, test of motor performance scores at 3 to 4 months of corrected age, Peabody Developmental Motor Skills at 6 to 9 months of age, ages and stages questionnaire at 6 to 9 months of age. |
| Starting date | 1 August 2021 |
| Contact information | Mariana Baserga, MD: mariana.baserga@hsc.utah.edu Carrie Rau, RN: carrie.rau@hsc.utah.edu |
| Notes |
Differences between protocol and review
None.
Contributions of authors
Conceiving the protocol: PB, MB, YTB, EO
Designing the review: PB, MB, YTB, GS, EO
Co‐ordinating the review: MB
Data collection for the review: PB, YTB
Screening search results: PB, YTB
Organizing retrieval of papers: PB, GS, YTB, EO
Screening retrieved papers against eligibility criteria: PB, YTB
Appraising quality of papers: PB, YTB
Extracting data from papers: PB, YTB
Writing to authors of papers for additional information: GS
Data management for the review: MB, GS, EO
Entering data into Review Manager 5: PB, YTB
Interpretation of data: MB, GS, EO
Providing a methodological and a clinical perspective: MB
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
MB is employed by this organization.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden, Sweden
Cochrane Sweden is supported from Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
Declarations of interest
PB has no interests to declare.
MB has no interests to declare.
GS has no interests to declare.
YTB has no interests to declare.
EO has no interests to declare.
New
References
References to studies excluded from this review
Filippi 2018 {published data only}
- Filippi L, Fiorini P, Catarzi S, Berti E, Padrini L, Landucci E, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): a feasibility study. Journal of Maternal-Fetal & Neonatal Medicine 2018;31(8):973-80. [DOI: 10.1080/14767058.2017.1304536] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gill 2014 {published data only}
- Gill H, Thoresen M, Smit E, Davis J, Liu X, Dingley J, et al. Sedation management during therapeutic hypothermia for neonatal encephalopathy: atropine premedication for endotracheal intubation causes a prolonged increase in heart rate. Resuscitation 2014;85(10):1394-8. [DOI: 10.1016/j.resuscitation.2014.07.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nuñez‐Ramiro 2019 {published data only}
- Nuñez-Ramiro A, Benavente-Fernández I, Valverde E, Cordeiro M, Blanco D, Boix H, et al, on behalf of the Hypotop Study Group. Topiramate plus cooling for hypoxic-ischemic encephalopathy: a randomized, controlled, multicenter, double-blinded trial. Neonatology 2019;116(1):76-84. [DOI: 10.1159/000499084] [PMID: ] [DOI] [PubMed] [Google Scholar]
Surkov 2019 {published data only}
- Surkov D. CORRECTION: Using of dexmedetomidine in term neonates with hypoxic-ischemic encephalopathy [Застосування дексмедетомідину в доношених новонароджених з гіпоксично-ішемічною енцефалопатією]. Medical Perspectives-Medicni Perspektivi [Internet] 2021;26(3):230. [DOI: 10.26641/2307-0404.2021.3.242347] [journals.uran.ua/index.php/2307-0404/article/view/242347] [DOI] [Google Scholar]
- Surkov D. Using of dexmedetomidine in term neonates with hypoxic-ischemic encephalopathy. Intensive care medicine experimental 2018;6(Suppl 2):0486. [Google Scholar]
- Surkov D. Using of dexmedetomidine in term neonates with hypoxic-ischemic encephalopathy [Застосування дексмедетомідину в доношених новонароджених з гіпоксично-ішемічною енцефалопатією]. Medical Perspectives-Medicni Perspektivi 2019;2(24):332019. [DOI: 10.26641/2307-0404.2019.2.170123] [DOI] [Google Scholar]
References to ongoing studies
NCT04772222 {published data only}
- Dexmedetomidine use in infants undergoing cooling due to neonatal encephalopathy (DICE Trial). clinicaltrials.gov/ct2/show/NCT04772222 (first received 26 February 2021).
Additional references
Ainsworth 2015
- Ainsworth SB. Effects of therapeutic hypothermia on medications. In: Neonatal formulary 7: drug use in pregnancy and the first year of life. 7th edition. West Sussex: John Wiley & Sons, 2015. [ISBN: 978-1-118-81959-3] [Google Scholar]
Allegaert 2017
- Allegaert K, den Anker J. Perinatal and neonatal use of paracetamol for pain relief. Seminars in Fetal & Neonatal Medicine 2017;22(5):308-13. [DOI: 10.1016/j.siny.2017.07.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Axelin 2013
- Axelin A, Cilio MR, Asunis M, Peloquin S, Franck LS. Sleep-wake cycling in a neonate admitted to the NICU: a video-EEG case study during hypothermia treatment. Journal of Perinatal & Neonatal Nursing 2013;27(3):263-73. [DOI: 10.1097/JPN.0b013e31829dc2d3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Balda 2019
- Balda RC, Guinsburg R. Evaluation and treatment of pain in the neonatal period [Avaliação e tratamento da dor no período neonatal]. Revista Pediátrica - Publicação Oficial da Sociedade Brasileira de Pediatria 2019;9(1):43-52. [DOI: 10.25060/residpediatr-2019.v9n1-13] [DOI] [Google Scholar]
Bayley 1993
- Bayley N. Bayley scales of infant development–II. San Antonio, TX: Psychological Corporation, 1993. [Google Scholar]
Bayley 2005
- Bayley N. Bayley scales of infant and toddler development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2005. [Google Scholar]
Bellù 2021
- Bellù R, Romantsik O, Nava C, Waal KA, Zanini R, Bruschettini M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013732. [DOI: 10.1002/14651858.CD013732] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berube 2020
- Berube MW, Lemmon ME, Pizoli CE, Bidegain M, Tolia VN, Cotten CM, et al. Opioid and benzodiazepine use during therapeutic hypothermia in encephalopathic neonates. Journal of Perinatology 2020;40(1):79–88. [DOI: 10.1038/s41372-019-0533-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bruschettini 2020
- Bruschettini M, Romantsik O, Moreira A, Ley D, Thébaud B. Stem cell-based interventions for the prevention of morbidity and mortality following hypoxic-ischaemic encephalopathy in newborn infants. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013202. [DOI: 10.1002/14651858.CD013202.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruschettini 2022
- Bruschettini M, Olsson E, Persad E, Garratt A, Roger S. Clinical rating scales for assessing pain in newborn infants. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: MR000064. [DOI: 10.1002/14651858.MR000064] [DOI] [Google Scholar]
Carter 2017
- Carter BS, Brunkhorst J. Neonatal pain management. Seminars in Perinatology 2017;41(2):111-6. [DOI: 10.1053/j.semperi.2016.11.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chau 2019
- Chau C, Ranger M, Bichin M, Park M, Amaral R, Chakravarty M, et al. Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: neonatal pain and genetic variation. Frontiers in Behavioral Neuroscience 2019;13:51. [DOI: 10.3389/fnbeh.2019.00051] [DOI] [PMC free article] [PubMed] [Google Scholar]
COMFORTNeo 2016
- Dijk M. Comfort assessment NeoScale version 5; 2016. Available at www.comfortassessment.nl/web/files/8414/7936/9137/COMFORT_Neo_scale___final_version_2016_11_17.pdf.
Craig 2020
- Craig AK, James C, Bainter J, Evans S, Gerwin R. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. Journal of Maternal-Fetal & Neonatal Medicine 2020;33(17):2889-96. [DOI: 10.1080/14767058.2018.1563592] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Debillon 2001
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36-41. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Haan 2012
- Haan T, Bijleveld Y, Lee J, Groenendaal F, den Broek M, Rademaker C, et al. Pharmacokinetics and pharmacodynamics of medication in asphyxiated newborns during controlled hypothermia. The PharmaCool multicenter study. BMC Pediatrics 2012;22(12):45. [DOI: 10.1186/1471-2431-12-45] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donato 2020
- Donato J, Rao K, Lewis T. Pharmacology of common analgesic and sedative drugs used in the neonatal intensive care unit. Clinics in Perinatology 2019;46(4):673-92. [DOI: 10.1016/j.clp.2019.08.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC resources for review authors, 2017. Available at epoc.cochrane.org/resources/epoc-resources-review-authors.
Ergenekon 2016
- Ergenekon E. Therapeutic hypothermia in neonatal intensive care unit: challenges and practical points. Journal of Clinical Neonatology 2016;5(1):8-17. [DOI: 10.4103/2249-4847.173271] [DOI] [Google Scholar]
Favié 2019
- Favié LM, Groenendaal F, den Broek MP, Rademaker CM, Haan TR, Straaten HL, et al, on behalf of the PharmaCool study group. Phenobarbital, midazolam pharmacokinetics, effectiveness, and drug-drug interaction in asphyxiated neonates undergoing therapeutic hypothermia. Neonatology 2019;116(2):154-62. [DOI: 10.1159/000499330] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippi 2011
- Filippi L, la Marca G, Cavallaro G, Fiorini P, Favelli F, Malvagia S, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia 2011;52(4):794-801. [DOI: 10.1111/j.1528-1167.2011.02978.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fisher 2005
- Fisher RS, Emde BW, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470-2. [DOI: 10.1111/j.0013-9580.2005.66104.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 17 October 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Griffiths 1954
- Griffiths R. The abilities of babies: a study of mental measurement. London, UK: University of London Press, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years. London, UK: Child Development Research Center, 1970. [Google Scholar]
Grunau 1986
- Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 1986;76(3):277-86. [DOI: 10.1016/S0304-3959(98)00046-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gunn 2017
- Gunn A, Laptook A, Robertson N, Barks J, Thoresen M, Wassink G, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatric Research 2017;81(1-2):202-9. [DOI: 10.1038/pr.2016.198] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrera 2018
- Herrera TI, Edwards L, Malcolm WF, Smith PB, Fisher KA, Pizoli C, et al. Outcomes of preterm infants treated with hypothermia for hypoxic-ischemic encephalopathy. Early Human Development 2018;125:1-7. [DOI: 10.1016/j.earlhumdev.2018.08.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Higgins 2021b
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hoffman 2013
- Hoffman K, Bromster T, Hakansson S, Van den Berg J. Monitoring of pain and stress in an infant with asphyxia during induced hypothermia: a case report. Advances in Neonatal Care 2013;13(4):252-61. [DOI: 10.1097/ANC.0b013e31829d8baf] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hong 2010
- Hong JY, Kim WO, Koo BN, Cho JS, Suk EH, Kil HK. Fentanyl-sparing effect of acetaminophen as a mixture of fentanyl in intravenous parent-/nurse-controlled analgesia after pediatric ureteroneocystostomy. Anesthesiology 2010;113(3):672-7. [DOI: 10.1097/ALN.0b013e3181e2c34b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology: Official Journal of the California Perinatal Association 2008;28(1):55-60. [DOI: 10.1038/sj.jp.7211861] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2016
- Ibrahim M, Jones LJ, Lai NM, Tan K. Dexmedetomidine for analgesia and sedation in newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD012361. [DOI: 10.1002/14651858.CD012361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2021a
- Kinoshita M, Borges do Nascimento IJ, Styrmisdóttir L, Bruschettini M. Systemic opioid regimens for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD015016. [DOI: 10.1002/14651858.CD015016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2021b
- Kinoshita M, Stempel KS, Borges do Nascimento IJ, Bruschettini M. Systemic opioids versus other analgesics and sedatives for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014876. [DOI: 10.1002/14651858.CD014876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2021c
- Kinoshita M, Olsson E, Borys F, Bruschettini M. Opioids for procedural pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD015056. [DOI: 10.1002/14651858.CD015056] [DOI] [Google Scholar]
Krechel 1995
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Pediatric Anesthesia 1995;5(1):53-61. [DOI: 10.1111/j.1460-9592.1995.tb00242.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Laptook 2016
- Laptook AR. Birth asphyxia and hypoxic-ischemic brain injury in the preterm infant. Clinics in Perinatology 2016;43(3):529-45. [DOI: 10.1016/j.clp.2016.04.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric Research 2013;74(Suppl 1):50-72. [DOI: 10.1038/pr.2013.206] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundqvist 2014
- Lundqvist P, Kleberg A, Edberg AK, Larsson BA, Hellström-Westas L, Norman E. Development and psychometric properties of the Swedish ALPS-Neo pain and stress assessment scale for newborn infants. Acta Paediatrica 2014;103(8):833-9. [DOI: 10.1111/apa.12672] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangat 2018
- Mangat A, Oei JL, Chen K, Quah-Smith I, Schmölzer G. A review of non-pharmacological treatments for pain management in newborn infants. Children 2018;5(10):130. [DOI: 10.3390/children5100130.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2012
- McCann ME, Soriano SG. General anesthetics in pediatric anesthesia: influences on the developing brain. Current Drug Targets 2012;13:944-51. [DOI: 10.2174/138945012800675768.] [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2020
- McPherson C, O'Mara K. Provision of sedation and treatment of seizures during neonatal therapeutic hypothermia. Neonatal Network 2020;39(4):227-35. [DOI: 10.1891/0730-0832.39.4.227] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD002052. [DOI: 10.1002/14651858.CD002052] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane centralised search service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;133:130-39. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Mara 2018
- O'Mara K, Weiss MD. Dexmedetomidine for sedation of neonates with HIE undergoing therapeutic hypothermia: a single-center experience. American Journal of Perinatology Reports 2018;8(3):e168-73. [DOI: 10.1055/s-0038-1669938] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohlsson 2020
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD010061. [DOI: 10.1002/14651858.CD010061.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 2021
- Olsson E, Bruschettini M, Thernström Blomqvist Y, Bäcke P. IPSNUT - Interventions for the management of Pain and Sedation in Newborns undergoing Therapeutic hypothermia for hypoxic-ischemic encephalopathy - A systematic review. PROSPERO 2020 CRD42020205755. Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020205755.
Pacifici 2014
- Pacifici GM. Clinical pharmacology of analgesics in infants and the pharmacologic management of pain in neonates. Medical Express 2014;1(1):105-15. [DOI: 10.5935/MedicalExpress.2014.03.03] [DOI] [Google Scholar]
Pacifici 2016
- Pacifici GM. Metabolism and pharmacokinetics of morphine in neonates: a review. Clinics 2016;71(8):474-80. [DOI: 10.6061/clinics/2016(08)11] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauliah 2013
- Pauliah SS, Shankaran S, Wade A, Cady EB, Thayyil S. Therapeutic hypothermia for neonatal encephalopathy in low- and middle-income countries: a systematic review and meta-analysis. PLOS ONE 2013;8:e58834. [DOI: 10.1371/journal.pone.0058834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2003
- Peters JW, Koot HM, Grunau RE, Boer J, Druenen MJ, Tibboel D, et al. Neonatal Facial Coding System for assessing postoperative pain in infants: item reduction is valid and feasible. Clinical Journal of Pain 2003;19(6):353-63. [DOI: 10.1097/00002508-200311000-00003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pillai Riddell 2015
- Pillai Riddell RR, Racine NM, Gennis HG, Turcotte K, Uman LS, Horton RE, et al. Non‐pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD006275. [DOI: 10.1002/14651858.CD006275.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirlotte 2019
- Pirlotte S, Beeckman K, Ooms I, Van Rompaey B, Cools F. Pharmacological interventions for the prevention of pain during endotracheal suctioning in ventilated neonates. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013355. [DOI: 10.1002/14651858.CD013355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pressler 2021
- Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia 2021;62(3):615-28. [DOI: 10.1111/epi.16815] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Romantsik 2017
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for sedation and analgesia for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD012468. [DOI: 10.1002/14651858.CD012468.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romantsik 2020
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for pain in non-ventilated infants. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013104. [DOI: 10.1002/14651858.CD013104.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saarenmaa 2001
- Saarenmaa E, Neuvonen PJ, Huttunen P, Fellman V. Ketamine for procedural pain relief in newborn infants. Archives of Disease in Childhood - Fetal and Neonatal Edition 2001;85(1):F53-6. [DOI: 10.1136/fn.85.1.f53] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarnat 1976
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology 1976;33(10):696-705. [DOI: 10.1001/archneur.1976.00500100030012] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schiller 2018
- Schiller RM, Allegaert K, Hunfeld M, den Bosch GE, den Anker J, Tibboel D. Analgesics and sedatives in critically ill newborns and infants: the impact on long-term neurodevelopment. Journal of Clinical Pharmacology 2018;58:S140-50. [DOI: 10.1002/jcph.1139] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Szakmar 2018
- Szakmar E, Kovacs K, Meder U, Bokodi G, Szell A, Somogyvari Z, et al. Asphyxiated neonates who received active therapeutic hypothermia during transport had higher rates of hypocapnia than controls. Acta Paediatrica 2018;107:1902-8. [DOI: 10.1111/apa.14159] [DOI] [PubMed] [Google Scholar]
Thomas 2021
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2021;133:140-51. [DOI: 10.1016/j.jclinepi.2020.11.003vcvc] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuchida 2013
- Tsuchida TN, Wusthoff CJ, Shellhaas RA, Hahn CD, Sullivan JE, Nguyen S, et al, American Clinical Neurophysiology Society Critical Care Monitoring Committee. American Clinical Neurophysiology Society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. Journal of Clinical Neurophysiology 2013;30(2):161-73. [DOI: 10.1097/WNP.0b013e3182872b24] [PMID: ] [DOI] [PubMed] [Google Scholar]
Üner 2019
- Üner IL, Johansen T, Dahle J, Persson M, Stiris T, Andresen JH. Therapeutic hypothermia and N-PASS; results from implementation in a level 3 NICU. Early Human Development 2019;137:104828. [DOI: 10.1016/j.earlhumdev.2019.104828] [PMID: ] [DOI] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DW, Anand KJ, Guldemond F, Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clinical Journal of Pain 2009;25(7):607-16. [DOI: 10.1097/AJP.0b013e3181a5b52a] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2017
- Walker SM. Translational studies identify long-term impact of prior neonatal pain experience. Pain 2017;158:S29-42. [DOI: 10.1097/j.pain.0000000000000784] [DOI] [PubMed] [Google Scholar]
Walker 2019
- Walker SM. Long-term effects of neonatal pain. Seminars in Fetal & Neonatal Medicine 2019;24(4):101005. [DOI: 10.1016/j.siny.2019.04.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wassink 2015
- Wassink G, Lear C, Gunn K, Dean J, Bennet L, Gunn A. Analgesics, sedatives, anticonvulsant drugs, and the cooled brain. Seminars in Fetal & Neonatal Medicine 2015;20(2):109-14. [DOI: 10.1016/j.siny.2014.10.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wassink 2019
- Wassink G, Davidson J, Dhillon S, Zhou K, Bennet L, Thoresen M, et al. Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy. Current Neurology and Neuroscience 2019;19(2):2. [DOI: 10.1007/s11910-019-0916-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2020
- Williams MD, Lascelles BD. Early neonatal pain—a review of clinical and experimental implications on painful conditions later in life. Frontiers in Pediatrics 2020;8:30. [DOI: 10.3389/fped.2020.00030] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2016
- Young L, Berg M, Soll R. Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD001240. [DOI: 10.1002/14651858.CD001240.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


