Abstract
The Bordetella pertussis BrkA protein protects against the bactericidal activity of complement and antibody; however, some individuals mount an immune response that overcomes this bacterial defense. To further characterize this process, the bactericidal activities of sera from 13 adults with different modes of exposure to B. pertussis (infected as adults, occupational exposure, immunized with an acellular vaccine, or no identified exposure) against a wild-type strain and a BrkA complement-sensitive mutant were evaluated. All of the sera killed the BrkA mutant, suggesting past exposure to B. pertussis or cross-reactive organisms. Several samples had no or minimal activity against the wild type. All of the sera collected from the infected and occupationally exposed individuals but not all of the sera from vaccinated individuals had bactericidal activity against the wild-type strain, suggesting that some types of exposure can induce an immune response that can overcome the BrkA resistance mechanism. Adsorbing serum with the wild-type strain removed the bactericidal antibodies; however, adsorbing the serum with a lipopolysaccharide (LPS) mutant or an avirulent (bvg mutant) strain did not always result in loss of bactericidal activity, suggesting that antibodies to either LPS or bvg-regulated proteins could be bactericidal. All the samples, including those that lacked bactericidal activity, contained antibodies that recognized the LPS of B. pertussis. Bactericidal activity correlated best with the presence of the immunoglobulin G3 (IgG3) antibodies to LPS, the IgG subtype that is most effective at fixing complement.
Complement has important activities that can enhance immune clearance of bacteria. C3b deposited on the bacterial surface is a powerful opsonin, the complement cleavage products (C4a, C3a, and C5a) up-regulate the immune response, and the terminal complement components (C5 to C9) form the membrane attack complex. Insertion of the membrane attack complex into the cytoplasmic membrane can kill gram-negative bacteria (25, 36).
Complement is present in serum, but it is also exuded from the blood to mucosal surfaces (28). Mucosal pathogens have mechanisms to resist complement (6, 11, 19, 25, 36). Vibrio cholerae and Bordetella pertussis are both mucosal pathogens that produce potent toxins that contribute to the disease. Immunity to cholera does not correlate with immune responses to cholera toxin. However, the vibriocidal assay, which measures the ability of serum to kill V. cholerae by antibody-mediated complement fixation, has been correlated with immunity to cholera as well as immunity from asymptomatic colonization (11). No serologic correlate of immunity to whooping cough has been established (13, 20), and we have begun to investigate if complement might play a role in immunity to B. pertussis.
In general, the complement cascade can be activated by either of two pathways: carbohydrates on bacterial surfaces (such as lipopolysaccharide [LPS]) can activate the alternate pathway (6, 25, 36), while deposition of antibody on a bacterial surface can activate the classical or primarily antibody-dependent pathway of complement. The alternate pathway does not appear to mediate bactericidal activity against B. pertussis (7). Antibodies to B. pertussis can activate the classical pathway, but the BrkA protein confers resistance to killing by complement (7). BrkA is a 73-kDa protein with extensive homology to pertactin (7). Both promote attachment to human cells, but only BrkA mediates resistance to killing by complement (7). The resistance to complement afforded by the BrkA protein is not absolute, however. We have found that some individuals produce bactericidal antibodies that can overcome these bacterial defenses and kill the resistant strains.
LPS can mediate either protection or susceptibility to complement killing. For example, in enteric bacteria the long, highly polymerized polysaccharide (O-chain) of the LPS on smooth strains protects the bacteria from complement, while rough mutants lacking the sugar repeats are killed (10, 25, 36). The LPS of B. pertussis has a simpler structure, consisting of lipid A, core polysaccharide, and a single O-chain trisaccharide (2, 18). B. pertussis can express two forms of LPS: band A, consisting of lipid A, core, and the O-chain; or band B, a partial structure consisting of lipid A and core lacking the O-chain (4, 20, 31). The designation LOS (lipooligosaccharide) is sometimes used to indicate this distinction. Other important mucosal pathogens lacking polymerized LPS include Haemophilus influenzae, Neisseria meningitidis, Neisseria gonorrhoeae, Moraxella (formerly Branhamella) catarrhalis, Campylobacter coli, and Campylobacter jejuni (5, 31). Unlike the highly polymerized LPS of the enteric bacteria, the LPS of B. pertussis does not appear to protect from complement killing, since monoclonal antibodies to band A LPS have been shown to be bactericidal (35). Monoclonal antibodies to the outer membrane protein, pertactin, have also been shown to be bactericidal (12).
In this study we characterized human serum to identify the antibodies capable of mediating a bactericidal response to B. pertussis. Some, but not all, antibodies to LPS are bactericidal, and killing seems to correlate with the efficiency with which the Fc region of the antibody fixes complement. Antibodies to some bvg-regulated virulence factors are bactericidal.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are described in Table 1. All are derivatives of BP338. Bacteria were grown at 37°C on Bordet-Gengou agar (BGA) or in Stainer Scholte (SS) agarose supplemented with 0.15% bovine serum albumin as previously described (7, 8). The antibiotics nalidixic acid and kanamycin were used at 30 and 50 μg/ml, respectively.
TABLE 1.
Antigenic structures of the bacterial strains
Antisera.
Individual human serum samples were obtained from adult volunteers. Serum with intact complement was stored at −70°C. The 13 subjects had had different modes of exposure to B. pertussis. Subjects convalescing from clinical pertussis were participants in a “Pertussis in Families” study conducted in St. Louis, Missouri. All four (three female and one male) of these subjects were over 30 years of age and had had clinical whooping cough of over 30 days’ duration at the time of sample collection. All subjects had received antibiotic therapy during their illness. All subjects had serologic evidence of recent infection based upon their antibody response to pertussis toxin, fimbrial hemagglutinin (FHA), pertactin, and fimbriae as previously described (23). Vaccine recipients were adults between the ages of 18 and 50 years who had received one intramuscular dose of a two-component acellular vaccine containing pertussis toxoid and FHA 1 month prior to serum sampling. A single booster dose of acellular vaccine is the anticipated regimen of adolescents and adults. Four subjects had no known exposure. Two subjects (including one vaccine recipient) were laboratory workers with occupational exposure.
Commercially prepared guinea pig serum was used as a source of complement. It was difficult to obtain blood products that lack antibodies to B. pertussis. B. pertussis is a strict human pathogen, but it shares cross-reactive antigens with a closely related species, Bordetella bronchiseptica (a common pathogen of domestic animals), and other bacterial species. Serum from several rats and mice and ten different guinea pig samples were screened. Many of the samples reacted with B. pertussis antigens on a Western blot and had very good bactericidal activity. Sigma guinea pig serum, lot 116H9412, had only faint reactivity to a single 45-kDa protein expressed by both Bvg+ and Bvg− strains when examined by Western blotting and was used in these studies.
Radial diffusion assay to measure complement-mediated killing.
The radial diffusion assay described previously was used to measure complement-mediated killing (8, 9). Larger zones correspond to greater killing and higher titers as determined by serial dilution experiments (data not shown). In this assay, overnight cultures on BGA were harvested in SS broth to an optical density at 600 nm (OD600) of approximately 0.2, and 0.2 ml of this suspension was added to 10 ml of molten (52°C) 1% agarose in SS broth and 0.15% bovine serum albumin. The agarose was dispensed into an Integrid square petri dish and allowed to harden. Holes (3 mm in diameter) were made with an aspirator punch, and 5 μl of sample was added to each hole. The plates were incubated at room temperature until the serum diffused into the agar. The plates were overlaid with 10 ml of SS agarose without bacteria and incubated at 37°C. The resultant zones of inhibition were read 24 to 48 h later with a Bausch and Lomb metric scale and a stereomicroscope at ×7 magnification. As a point of reference, in other studies sample 13 was used in a liquid serum killing assay (9). In this assay, 107 bacteria were incubated in 20% serum for 1 h at 37°C. Complement activity was halted by diluting the organisms 10-fold in phosphate-buffered saline (PBS) containing 10 mM EDTA, and further serial dilutions were performed. About 1% of the wild-type cells survived, but only 0.01% of mutant BPM2041 cells survived. Killing has never been observed when only heat-inactivated samples were present, suggesting an obligatory role for intact complement in these assays.
Adsorption studies to selectively remove antibodies to surface antigens.
Intrinsic complement activity, which might lyse the bacteria, was removed by heating the serum at 56°C for 30 min. An approximately equal volume of serum was added to a packed pellet of live bacteria, and the antibodies were allowed to bind to the bacteria during incubation for several hours on ice. The bacteria and any antibodies bound to them were removed by centrifugation. After three incubations with fresh bacteria for a total of 24 h, the serum was filter-sterilized and stored frozen.
SDS-PAGE and immunoblotting.
Samples (5 to 10 μl) of bacterial cells suspended in PBS at an OD600 of 8 were added to equal volumes of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. The samples were boiled for 5 min prior to being subjected to discontinuous SDS-PAGE on a Mini Protean II gel system by using 1-mm spacers (Bio-Rad, Richmond, Calif.) with 10 to 15% acrylamide in the running gel and a 5% stacking gel under conditions recommended by the manufacturer. Ten percent acrylamide was used to detect proteins, and 15% gels were used for detection of LPS to get good separation in the 20-kDa range, where B. pertussis LPS migrates. Gels were blotted onto Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, Mass.) by using a Fisher (Pittsburgh, Pa.) model FB-SDB-2020 semidry blotting unit according to the manufacturer’s recommendations. Proteins were detected by chemiluminescence by using the Dupont Western blot Renaissance kit (NEN Research Products, Boston, Mass.). When antibodies to LPS were characterized, bacterial cells were solubilized by boiling for 10 min in SDS-PAGE loading buffer and incubated with proteinase K at 5 μg per ml overnight at 37°C (as recommended by the manufacturer, Sigma, St. Louis, Mo.) to remove the protein antigens.
To subtype the human antibodies by Western blotting, the blots were incubated with the human serum used at a 1:625 dilution and then with antibody specific for the Fc region of human immunoglobulin (used at a 1:150,000 dilution). All commercial antibodies were used at the dilutions recommended by the manufacturer. Human isotype- or subtype-specific mouse monoclonal antibodies were purchased from ICN, Aurora, Ohio, or Harlan Scientific, Indianapolis, Ind. Specifically, immunoglobulin A (IgA) (69-086; ICN) was diluted to 1:2,000, IgM (63-439; ICN) was diluted to 1:2,000, IgG1 (59398; ICN) was diluted to 1:500, IgG2 (070397; Harlan) was diluted to 1:500, IgG3 (59400; ICN) was diluted to 1:50, and IgG4 (91034; ICN) was diluted to 1:2,000. Peroxidase-conjugated goat anti-mouse IgG (55550; ICN) diluted to 1:69,000 was used to detect mouse monoclonal antibodies.
ELISA technique.
Enzyme-linked immunosorbent assay (ELISA) to quantitate isotype-specific antibodies to pertussis toxin, FHA, pertactin and fimbriae (types 2 and 3) was performed as previously described (21, 23, 24).
RESULTS
Characterization of bactericidal activity of human serum samples.
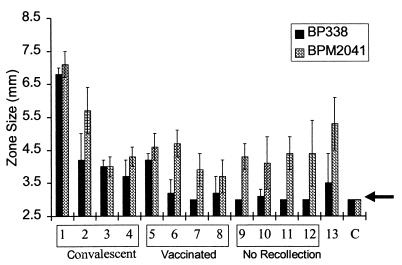
The bactericidal activity of serum from 13 adults with different modes of exposure to B. pertussis was examined by using a radial diffusion assay (Fig. 1). Samples 1 to 4 were convalescent-phase sera collected from patients diagnosed with pertussis. Samples 5 to 8 were obtained from vaccinated adults 30 days after they had received a two-component pertussis toxoid and FHA acellular vaccine. Samples 9 to 12 were obtained from individuals who had neither recollection of exposure nor disease and had not been vaccinated as adults. Two individuals, volunteers 13 and 6 (one of the acellular vaccine recipients), were laboratory workers who had been occupationally exposed to B. pertussis but had no history of clinical pertussis. Since these samples were not collected or stored in a manner that would preserve endogenous complement, 50% guinea pig serum was added as a source of complement. The heat-inactivated human samples were devoid of activity. The guinea pig serum did not have bactericidal activity (Fig. 1, sample C) when added alone but did promote killing when mixed with the human serum samples.
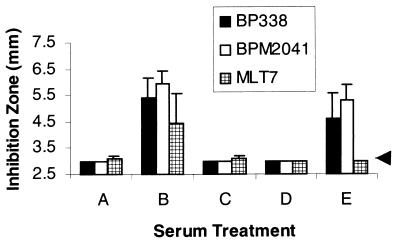
FIG. 1.
Radial diffusion assay to assess complement killing of BP338 (complement-resistant strain) and BPM2041 (BrkA−, complement-sensitive strain). Samples 1 to 4, sera from adults infected with B. pertussis; samples 5 to 8, sera from individuals vaccinated with an acellular vaccine containing pertussis toxoid and FHA; samples 9 to 12, sera from individuals with no recollection of recent disease; sample 13, serum from a laboratory worker; and C, control or guinea pig complement lacking antibodies. The arrow denotes the radius of the well, or values that correspond to no bactericidal activity.
All of the sera (Fig. 1, samples 1 to 13) killed the BrkA mutant. Previously we have shown that killing of B. pertussis by complement is antibody dependent (7), and this observation suggests that all of these adults had past exposure to B. pertussis or to cross-reactive microorganisms. Seven samples (Fig. 1, samples 6 to 12), including those collected from the four individuals with no known recent exposure to B. pertussis, lacked or had very weak bactericidal activity against the wild-type strain, suggesting that the BrkA resistance mechanism inhibited the bactericidal activity of these sera. In general, the wild-type strain (Fig. 1, BP338) was more resistant to complement than the BrkA mutant (Fig. 1; BPM2041) as demonstrated by the lack of an inhibition zone or the smaller zone diameter. This difference was not as apparent in sera collected from individuals who were convalescing from infection with B. pertussis, suggesting that infection can induce a bactericidal response strong enough to overcome the BrkA resistance mechanism.
Western blot analysis of serum samples.
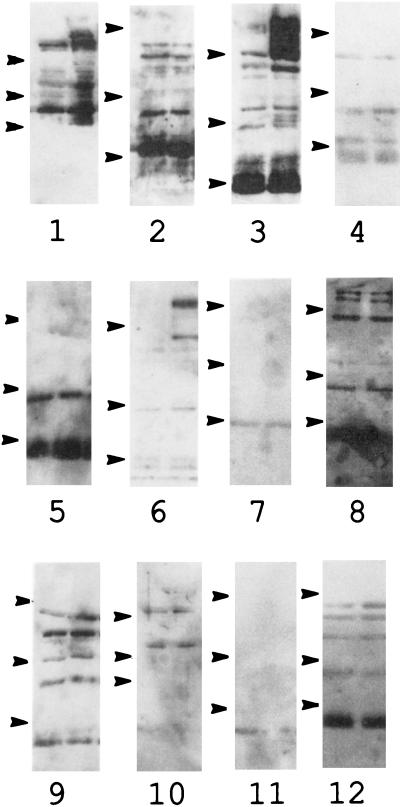
Western blot analysis was used to determine the number and identity of the antigens recognized by these sera (Fig. 2). As suggested by the presence of bactericidal activity, all of the samples recognized several B. pertussis antigens. Multiple virulence factors, including the toxins pertussis toxin, adenylate cyclase toxin, and the dermonecrotic toxin and adhesins (FHA, pertactin, tracheal colonization factor, BrkA, and fimbriae), of B. pertussis are coordinately regulated by the bvg locus. Strains with mutations in bvg are unable to produce these factors and are avirulent in animal models (38). Some of the antibodies appear to recognize proteins common to both Bvg+ and Bvg− strains (Fig. 2, samples 1 to 12, both lanes), and some are specific for proteins produced by the Bvg+ strains (Fig. 2, samples 1 to 12, right lanes).
FIG. 2.
Western blot of serum samples. Bacterial cells were separated by SDS-PAGE on a 10% acrylamide gel. Samples 1 to 12 refer to the serum samples described in the legend for Fig. 1. BP347, the Bvg− (avirulent) strain, was loaded in the left lane; BP338, the Bvg+ (virulent) strain, was loaded in the right lane. Molecular mass standards (denoted by arrows) are, from top to bottom, 114, 33.4, and 19.9 kDa.
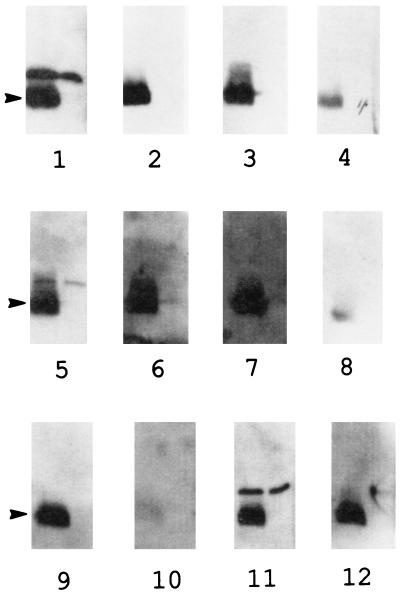
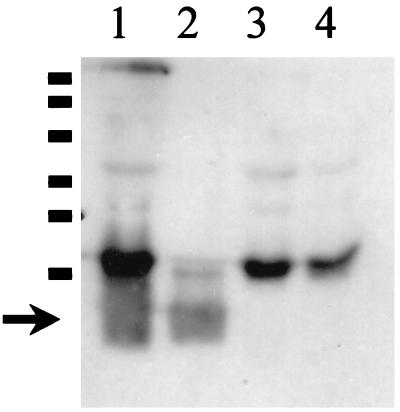
The presence of antibodies to LPS was also examined by Western blotting. Proteinase K treatment was used to eliminate comigrating protein antigens. Most of the samples reacted with an approximately 20-kDa proteinase K-resistant band (Fig. 3) that was present in the wild-type strain (BP338) but absent in mutant MLT7 (37), which fails to express band A LPS. Some sera reacted with proteinase K-resistant bands in both strains, suggesting that they were not band A (full-length) LPS.
FIG. 3.
Western blot after proteinase K treatment. BP338 (wild type), in the left lane, and MLT7 (LPS mutant), in the right lane, were treated with proteinase K and subjected to SDS-PAGE on a 15% acrylamide gel. The gel was transferred and blotted with serum from samples 1 to 12. The arrow denotes the LPS band, which migrates a little faster than the 20-kDa protein marker.
Adsorption to remove antibodies to surface determinants.
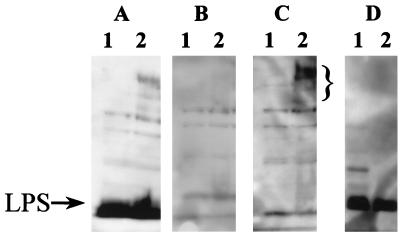
Sample 13 was chosen for further characterization because it had good bactericidal activity and was our most abundant sample. In the Western analysis, this serum gave an especially strong reaction to LPS, as demonstrated by the reactivity to a proteinase K-resistant band (Fig. 4D, lane 2) that was lacking in the LPS mutant MLT7 (data not shown). In addition, this sample contained antibodies to several high-molecular-weight Bvg+ antigens (Fig. 4A and C; compare lanes 2 and lanes 1).
FIG. 4.
Western blots of adsorbed serum sample 13. A through C, lane 1, BP347 (avirulent mutant); lane 2, BP338 (wild type) separated by SDS-PAGE on a 10% gel. A, blotted with serum sample 13; B, blotted with sample 13 absorbed with BP338, the Bvg+ (virulent) strain; C, blotted with sample 13 adsorbed with BP347, the Bvg− (avirulent) strain. D, lane 1, BP338 whole cells; lane 2, BP338 cells treated with proteinase K separated by SDS-PAGE on a 15% gel and blotted with sample 13 adsorbed with MLT7 (LPS mutant strain). The bracket denotes the high-molecular-weight Bvg-specific antigens, and the bottom of the bracket corresponds to the migration of the 84-kDa marker. The arrow indicates the 19.9-kDa marker as well as LPS.
Since this sample recognized many bacterial antigens, it is not possible to attribute bactericidal activity to any single class of antibodies without fractionating the sample. Adsorption was used to selectively remove antibodies to surface determinants. Heat-inactivated serum was added to a packed bacterial pellet, and the antibodies were allowed to bind to the bacteria. The bacteria and any antibodies bound to them were removed by centrifugation. This treatment did not result in proteolysis of the immunoglobulin molecules, since treated and untreated serum samples were identical in Western blots probed with goat anti-human IgG (data not shown). Adsorption with bacterial mutants lacking specific surface determinants should enrich for antibodies that recognize the missing bacterial components.
Adsorption with BP338, a wild-type strain, would be predicted to remove all antibodies to surface determinants present on wild-type B. pertussis. When compared to control serum (Fig. 4A), serum adsorbed with BP338 (Fig. 4B) lacked antibodies to several bands with molecular masses of greater than 80 kDa as well as some smaller bands, including the LPS band. Similarly, incubation with BP347, a Bvg− mutant, should remove antibodies to surface determinants common to virulent and avirulent strains, but not antibodies to Bvg+ antigens. After adsorption with this strain, the intensity of the LPS band was reduced; however, the Bvg+ high-molecular-weight bands were still present (Fig. 4C). Incubation with MLT7, the LPS mutant, should give a pattern very similar to that of BP338 but with retention of the LPS antibodies. The higher-molecular- weight Bvg+ bands were lost after this treatment, but the intensity of the LPS band was not changed (Fig. 4D).
Bactericidal activity of adsorbed serum.
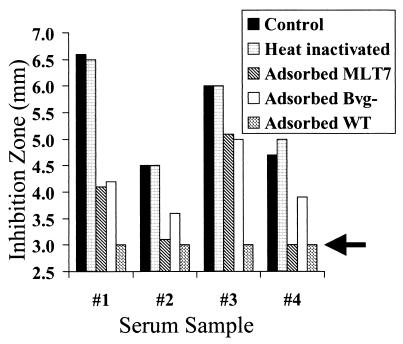
The heat inactivation step used to destroy endogenous complement had only a slight effect on the bactericidal antibody activity. In one assay with the wild-type strain, the untreated sample gave a zone size of 6.3 mm by using the endogenous human complement and a zone size of 6.2 mm after heat inactivation when guinea pig serum was added as a source of complement. The absorbed sera were characterized for bactericidal activity (Fig. 5). Serum that was adsorbed with the Bvg+ strain, BP338 (Fig. 5C), or the Bvg− strain, BP347 (Fig. 5D), lacked bactericidal activity against either the wild-type strain or the BrkA mutant serum-sensitive strain. These results suggest that a shared epitope is the target of the bactericidal antibodies. Pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, FHA, pertactin, pili, BrkA, and tracheal colonization factor are not shared epitopes and could not have been the source of bactericidal antibodies in this sample. In contrast, the sera adsorbed with MLT7, the LPS mutant (Fig. 5E), retained bactericidal activity against the wild-type and serum-sensitive strains but MLT7 was protected, suggesting that the terminal trisaccharide of band A LPS is at least one target of bactericidal antibodies.
FIG. 5.
Bactericidal antibodies in sample 13. Bactericidal activities against BP338 (wild-type strain), BPM2041 (BrkA complement-sensitive mutant strain), and MLT7 (LPS mutant strain) were tested by using the radial diffusion assay as described in the legend for Fig. 1. Each sample contained 50% guinea pig serum and 50% test serum (described in Fig. 4). A, PBS control; B, heat-inactivated serum sample 13; C, serum sample 13 adsorbed with wild-type, Bvg+ (virulent), strain; D, serum sample 13 adsorbed with the Bvg− (avirulent) strain; E, serum sample 13 adsorbed with MLT7 (LPS mutant strain). The killing by the heat-inactivated serum (panel B) was found to be significantly different from those by the samples shown in panels A, C, and D for all three strains when tested by t test. The killing by the serum adsorbed with MLT7 (panel E) was found to be significantly different from those by the samples shown in panels A, C, and D for strains BP338 and BPM2041 but not for strain MLT7 when tested by t test.
Quantitative ELISA.
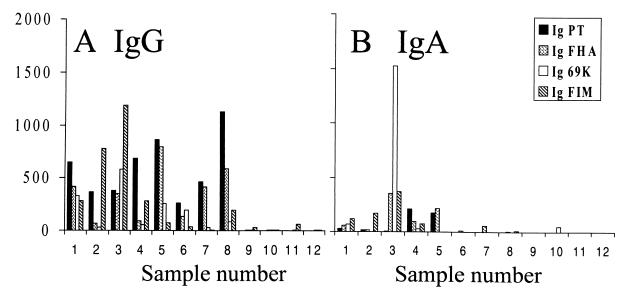
ELISA was used to quantitate the levels of isotype-specific antibodies to pertussis toxin, FHA, pertactin, and fimbriae and to assess the effect of the adsorption on serum antibody levels (Fig. 6). The individuals with no known exposure to pertussis had low levels of or no IgG or IgA antibodies to these four determinants. The infected and vaccinated individuals all had a significant IgG response to pertussis toxin. Antibodies to pertussis toxin did not appear to be bactericidal, since some samples (Fig. 6, samples 7 and 8) had high levels of antibodies to pertussis toxin but lacked bactericidal activity (Fig. 1, samples 7 and 8). Similarly, antibodies to FHA did not appear to be bactericidal since all of the vaccinated individuals (volunteers 5 to 8) had high titers to FHA yet only sample 5 possessed bactericidal activity.
FIG. 6.
Serum titers of IgG (A) and IgA (B) to pertussis toxin (PT), FHA, pertactin (69K), and fimbriae (FIM) were determined as previously described (21, 23, 24).
Bactericidal antibodies in other sera.
Preliminary adsorption studies were performed to characterize the bactericidal antibodies in the serum samples from the convalescent-phase sera, samples 1 to 4. The limited amounts of these samples and the comparatively large volumes needed for the bactericidal assays did not permit as extensive a characterization as that performed with sample 13, and the values from one experiment are reported. The heat inactivation step reduced killing for the wild-type strain for some of the samples, but activity against BPM2041, the serum-sensitive mutant, was retained. In all cases, adsorption with the wild-type strain (BP338) removed the bactericidal activity. In contrast to serum sample 13, collected from the occupationally exposed individual (Fig. 5), all of the convalescent-phase sera retained bactericidal activity after adsorption with the Bvg− mutant, BP347 (Fig. 7), suggesting that these antibodies recognized Bvg-regulated virulence factors. In addition, samples 1 and 3 and to a small extent sample 2 retained bactericidal activity after adsorption with the LPS mutant, MLT7 (Fig. 7).
FIG. 7.
Bactericidal antibodies in convalescent-phase serum. The radial diffusion assay was used to determine the bactericidal activity against BPM2041, the BrkA mutant. Untreated and adsorbed samples from infected individuals (volunteers 1 to 4) were supplemented with 50% guinea pig serum and tested for bactericidal activity. Control, untreated serum; adsorbed MLT7, adsorbed with LPS mutant; adsorbed Bvg−, adsorbed with avirulent strain, BP347; adsorbed WT, adsorbed with wild-type strain, BP338.
Antibodies to LPS appeared to mediate bacterial killing in samples 1, 2, 3, and 13; however, samples without bactericidal activity also contained antibodies to LPS (Fig. 3). We examined the class and subtypes of antibodies to LPS. Figure 8 shows a representative Western blot demonstrating a positive antibody response to LPS, as indicated by the diffuse bands present in lanes 1 and 2 (containing whole wild-type cells and proteinase K-treated wild-type cells, respectively) that are absent in lanes 3 and 4 (containing whole LPS mutant cells and proteinase K-treated LPS mutant cells, respectively). In addition, some bands were common to both strains, suggesting the existence of an IgG3 response to common antigens.
FIG. 8.
Western blotting performed to detect anti-IgG3 antibodies to LPS. A 15% acrylamide gel was loaded as follows: lane 1, BP338 (wild-type) cells; lane 2, proteinase K-treated BP338; lane 3, MLT7 (LPS mutant) cells; lane 4, proteinase K-treated MLT cells subjected to SDS-PAGE. The gel was transferred, blotted with serum from sample 1 and then with mouse anti-human IgG3 followed by goat anti-mouse IgG, and developed for the chemiluminescent signal. Protein markers at 206, 125, 88, 47.7, 34.9, and 29.6 kDa are indicated from top to bottom. The arrow denotes migration of the LPS band.
IgM antibodies activate complement very well, while IgA is not effective at activating complement. In humans, IgG3 activates complement better than IgG1, which in turn activates it better than IgG2. IgG4 does not activate complement. As seen in Table 2, almost all of the samples had IgG1 antibodies to LPS. Most of the samples with good bactericidal activity against the wild-type strain also had IgM or IgG3 antibodies to LPS. None of the samples had IgA antibodies to LPS.
TABLE 2.
Isotypes and subtypes of antibodies to B. pertussis whole cells and LPS
| Serum sample no.a | IgM
|
IgG3
|
IgG1
|
IgG2
|
IgG4
|
IgA
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellsb | LPSc | Cells | LPS | Cells | LPS | Cells | LPS | Cells | LPS | Cells | LPS | |
| 1 | + | − | + | + | + | + | + | − | + | − | + | − |
| 2 | + | + | + | + | + | + | − | − | + | − | + | − |
| 3 | + | + | + | + | + | + | + | +/− | + | + | + | − |
| 4 | + | − | + | + | + | − | + | − | − | − | + | − |
| 13 | + | − | + | + | + | + | + | +/− | + | + | + | − |
| 5 | +/− | − | +/− | − | + | + | − | − | + | + | + | − |
| 6 | − | − | − | − | + | + | − | − | − | − | − | − |
| 7 | + | − | − | − | + | + | + | − | + | − | + | − |
| 8 | + | − | + | − | + | − | + | − | + | − | + | − |
| 9 | + | − | + | − | + | + | + | − | − | − | + | − |
| 10 | − | − | − | − | + | − | + | − | − | − | − | − |
| 11 | − | − | + | − | + | + | + | − | + | − | + | − |
| 12 | + | − | +/− | − | + | + | + | − | − | − | + | − |
Samples 1 through 5 and sample 13 had bactericidal activity against the wild-type strain and the complement-sensitive mutant, and samples 6 through 12 had activity against only the complement-sensitive mutant.
Whole cells of wild-type BP338 and LPS mutant MLT7 were boiled in SDS loading buffer and transferred to polyvinylidene difluoride membranes for Western blotting as described in the legend for Fig. 8.
The wild-type strain BP338 and the LPS mutant strain MLT7 were treated with proteinase K to remove protein antigens as described in the legend for Fig. 8, and antibodies to the terminal trisaccharide of LPS were identified as reactivity at the appropriate molecular mass, which is present in BP338 but lacking in MLT7.
DISCUSSION
A pertussis vaccine could prevent disease either by neutralizing toxin activities of B. pertussis or by eliminating the microbe. The current whole-cell and the new acellular vaccines seem to protect from severe disease but not necessarily from infection by the bacterium (1, 13, 23, 29, 30). They probably function by reducing the bacterial load or neutralizing toxin effects. However, elimination of the bacteria, if possible, is a better vaccine strategy, since infected individuals with subclinical disease can infect susceptible individuals who could develop fulminating disease.
We have examined human serum for the presence of antibodies that promote killing by complement, an immune response that could eliminate the bacteria from infected individuals. While the BrkA protein of B. pertussis confers resistance to killing by the antibody-dependent pathway of complement (7), some individuals generate an immune response that can overcome this resistance mechanism. Our sample size was small, but this study suggests that infected individuals are more likely to generate a bactericidal response than adults vaccinated with the two-component vaccine.
B. pertussis has multiple antigenic states. The bvgAS genes positively control expression of multiple virulence factors, including pertussis toxin, adenylate cyclase toxin, dermonecrotic toxin, FHA, pertactin, tracheal colonization factor, BrkA, and fimbriae. The adsorption studies with a Bvg− mutant suggest that antibodies to Bvg-regulated proteins can be bactericidal. While we have not identified which Bvg-regulated proteins elicit bactericidal activity, bactericidal activity did not correlate with the presence of antibodies to either pertussis toxin or FHA or with immunization with a vaccine containing only these two antigens. Several explanations could account for this. These samples could contain only antibodies incapable of activating complement (IgG4 or IgA). Alternatively, pertussis toxin and FHA may not be capable of acting as bactericidal targets. We favor the latter explanation, since pertussis toxin is secreted and FHA is only loosely associated with the bacterial surface. Surface association is a property that is essential for directing the membrane attack complex to the membrane. Of the protein antigens that are being considered for the expanded-spectrum acellular vaccines, only pertactin and fimbriae are surface localized. Pertactin has been shown to elicit bactericidal antibodies (12), and acellular vaccines containing pertactin have been reported to give better protection than vaccines lacking this component (13, 30). Its role as a bactericidal target needs to be evaluated further in vaccine recipients that have received this antigen.
In addition to Bvg-regulated antigens, bactericidal antibodies to LPS accounted for some of the bactericidal activity in the sera from individuals convalescing from natural infection. LPS has been intentionally removed from the new acellular vaccines because of the potential toxicity of the lipid A component. Our studies suggest that antibodies to the terminal trisaccharide appear to be sufficient to elicit bactericidal activity. If the toxic lipid A portion of LPS could be separated from the protective portion, this antigen may be safe for vaccine use.
LPS has been reported to induce antibodies of all IgG classes (15, 16, 17, 19, 39). In some diseases the subtype of the IgG that participates in the response to LPS has been shown to have important clinical implications. In Southeast Asia, melioidosis, caused by Burkholderia pseudomallei, is often a fatal disease. In one study, survivors of systemic melioidosis were found to have an IgG3 response to the LPS of B. pseudomallei. In contrast, individuals who succumbed, who experienced less severe local infection, or who had serologic evidence of previous asymptomatic infection failed to produce IgG3 antibodies to LPS but had detectable IgG1 and IgG2 responses to LPS (15). Serum with IgG3 antibodies to LPS was more effective at mediating opsonic killing of B. pseudomallei. In another example, IgG1 and IgG3 responses to LPS have been shown to be harmful. Guillain-Barré syndrome is a rare neurological disease thought to result when an infection triggers an autoimmune response against peripheral nerves. The LPS of C. jejuni contains a ganglioside-like epitope that appears to induce autoantibodies (17, 39). Guillain-Barré syndrome was observed in individuals who generated an IgG1 or IgG3 response to LPS following C. jejuni infection but was not observed in individuals who generated an IgG2 response (17, 39). In animal models neurologic damage has been shown to be complement-dependent, and IgG3 and IgG1 antibodies fix complement better than IgG2 antibodies, suggesting a molecular basis for the IgG subtype association.
The bactericidal activity against B. pertussis LPS was also associated with the presence of certain IgG subtypes, specifically the presence of IgG3, the subtype that is most efficient at fixing complement. The lack of an IgG2 response was expected since IgG2 antibodies are T cell independent. Cross-linking of B-cell receptors (surface IgM) is required (14), and that occurs only with polyvalent antigens. B. pertussis LPS is not polyvalent. The other IgG subtypes require T-cell help, and the choice is determined by the cytokine milieu when the response is generated. High levels of antibody production and the IgG1, IgG2, and IgG4 subclasses are associated with interleukin 4 cytokine production and the Th2 (helper) T-cell response, while gamma interferon production and the Th1 T-cell response promote IgG3 antibody production (6, 33). Several recent reports have suggested a role for Th1 help in immunity to pertussis (3, 22, 32, 34). A tendency toward a Th1 response has been shown to occur in infected individuals (34) and with some, but not all, vaccines (3). Consistent with this, in our study, most individuals had an IgG1 response to B. pertussis LPS but only infected individuals had an IgG3 response. The Th1 response has also been correlated with more effective immune clearance in a mouse model of pertussis (22, 32). Whether increased bactericidal clearance is responsible for the enhanced immune response remains to be determined.
The ideal pertussis vaccine, one that prevents carriage of the bacteria as well as disease, seems to be possible to achieve, but it may require a new approach to vaccine development. It is well recognized that an immune response can be beneficial, harmful, or even neutral. We have found that antibodies can be beneficial or neutral in terms of promoting bactericidal activity to B. pertussis. Previous attempts to identify an antibody correlate of immunity to pertussis have not met with much success (1, 13, 30). This preliminary study suggests that it may be possible to obtain this important information, but it is not enough to determine what antigens are recognized by the Fab region of the antibody. This information must be correlated with the effector functions that can be performed by the constant region. Assessing the development of functional immunity, such as neutralization of toxin activity, blocking of bacterial adherence, and opsonization, as well as activation of complement and bacterial killing, needs to be considered when designing the next generation of acellular vaccines. In addition, the ability to distinguish a protective from a nonprotective immune response has taken on a greater importance following the appearance of the recent report that suggests vaccine use may select for antigenic variants of B. pertussis (26).
We have described a simple and sensitive method by which to characterize bactericidal antibodies in human serum samples. Applying this technique to a larger human sample will generate important information on the ability of specific antigens to generate a bactericidal immune response and their possible roles in immunity to pertussis.
ACKNOWLEDGMENTS
This work was supported by grant RO1 AI38415 to A.A.W. and grants NO1-AI-05051 and NO1-AI-45250, the Saint Louis University VTEU contracts.
We thank Mark Peppler for supplying us with the mutant strain MLT7 and Nancy Sirota for technical assistance.
REFERENCES
- 1.Ad Hoc Group for the Study of Pertussis Vaccines. Placebo-controlled trial of two acellular pertussis vaccines in Sweden—protective efficacy and adverse events. Lancet. 1988;i:955–960. [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello C M, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodeur B R, Martin D, Hamel J, Shahin R D. Antigenic analysis of the saccharide moiety of the lipooligosaccharide of Bordetella pertussis. Springer Semin Immunopathol. 1993;15:205–215. doi: 10.1007/BF00201101. [DOI] [PubMed] [Google Scholar]
- 5.Campagnari A A, Spinola S M, Lesse A J, Kwaik Y A, Mandrell R E, Apicella M A. Lipooligosaccharide epitopes shared among Gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990;8:353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 6.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez R C, Weiss A A. Susceptibility of Bordetella pertussis strains to antimicrobial peptides. Antimicrob Agents Chemother. 1996;40:1041–1043. doi: 10.1128/aac.40.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez R C, Weiss A A. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol Lett. 1998;163:57–63. doi: 10.1111/j.1574-6968.1998.tb13026.x. [DOI] [PubMed] [Google Scholar]
- 10.Frank M M. The mechanism by which microorganisms avoid complement attack. Curr Opin Immunol. 1992;4:14–19. doi: 10.1016/s0952-7915(92)90117-w. [DOI] [PubMed] [Google Scholar]
- 11.Glass R I, Svennerholm A-M, Khan M R, Huda S, Huq M I, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–241. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 12.Gotto J W, Eckhardt T, Reilly P A, Scott J V, Cowell J L, Metcalf III T N, Mountzouros K, Gibbons J J, Jr, Siegel M. Biochemical and immunological properties of two forms of pertactin, the 69,000-molecular-weight outer membrane protein of Bordetella pertussis. Infect Immun. 1993;61:2211–2215. doi: 10.1128/iai.61.5.2211-2215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16(4th Suppl.):S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hienzel F P. Antibodies. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practices of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 36–57. [Google Scholar]
- 15.Ho M, Schollaardt T, Smith M D, Perry M B, Brett P J, Chaowagul W, Bryan L E. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect Immun. 1997;65:3648–3653. doi: 10.1128/iai.65.9.3648-3653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam D, Wretlind B, Ryd M, Lindberg A A, Christensson B. Immunoglobulin subclass distribution and dynamics of Shigella-specific antibody responses in serum and stool samples in shigellosis. Infect Immun. 1995;63:2054–2061. doi: 10.1128/iai.63.5.2054-2061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs B C, Endtz H P, van der Meché F G A, Hazenberg M P, de Klerk M A, van Doorn P A. Humoral immune responses against Campylobacter jejuni lipopolysaccharides in Guillain-Barré syndrome and Miller Fisher syndrome. J Neuroimmunol. 1997;79:62–68. doi: 10.1016/s0165-5728(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 18.Lebbar S, Charoff M, Szabó L, Mérienne C, Szilógyi L. Structure of the hexasaccharide proximal to the hydrophobic region of lipopolysaccharides present in Bordetella pertussis endotoxin preparations. Carbohydr Res. 1994;259:257–275. doi: 10.1016/0008-6215(94)84061-x. [DOI] [PubMed] [Google Scholar]
- 19.Losonsky G A, Yunyongyin J, Lim V, Reymann M, Lim Y L, Wasserman S S, Levine M M. Factors influencing the secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun. 1996;64:10–15. doi: 10.1128/iai.64.1.10-15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin D, Peppler M S, Brodeur B R. Immunological characterization of the lipooligosaccharide B band of Bordetella pertussis. Infect Immun. 1992;60:2718–2725. doi: 10.1128/iai.60.7.2718-2725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meade B D, Deforest A, Edwards K M, Romani T A, Lynn R, O’Brien C H, Swartz C B, Reed G F, Deloria M A. Description and evaluation of serologic assays used in a multicenter trial of acellular pertussis vaccines. Pediatrics. 1995;96:570–575. [PubMed] [Google Scholar]
- 22.Mills K H G, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mink C M, Sirota N M, Nugent S. Outbreak of pertussis in a fully immunized adolescent and adult population. Arch Pediatr Adolesc Med. 1994;148:153–157. doi: 10.1001/archpedi.1994.02170020039006. [DOI] [PubMed] [Google Scholar]
- 24.Mink C M, O’Brien C H, Wassilak S, Deforest A, Meade B D. Isotype and antigen specificity of pertussis agglutinins following whole-cell pertussis vaccination and infection with Bordetella pertussis. Infect Immun. 1994;62:1118–1120. doi: 10.1128/iai.62.3.1118-1120.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffitt M C, Frank M M. Complement resistance in microbes. Springer Semin Immunopathol. 1994;15:327–344. doi: 10.1007/BF01837364. [DOI] [PubMed] [Google Scholar]
- 26.Mooi F R, van Oirschot H, Heuvelman K, van der Heide H G J, Gaastra W, Willems R J L. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun. 1998;66:670–675. doi: 10.1128/iai.66.2.670-675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mountzouras K T, Kimura A, Cowell J L. A bactericidal monoclonal antibody specific for the lipooligosaccharide of Bordetella pertussis reduces colonization of the respiratory tract of mice after aerosol infection with B. pertussis. Infect Immun. 1992;60:5316–5318. doi: 10.1128/iai.60.12.5316-5318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson C G A, Erjefalt I, Alkner U, Baumgarten C, Grief L, Gustafsson B, Luts A, Pipkorn U, Sundler F, Svensson C, Wollmer P. Plasma exudation as a first line respiratory mucosal defence. Clin Exp Allergy. 1991;21:17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 29.Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979;1:401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- 30.Plotkin S A, Cadoz M. The acellular pertussis vaccine trials: an interpretation. Pediatr Infect Dis J. 1997;16:508–517. doi: 10.1097/00006454-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Preston A, Mandrell R E, Gibson B W, Apicella M A. The lipooligosaccharide of Gram-negative bacteria. Crit Rev Microbiol. 1996;22:139–180. doi: 10.3109/10408419609106458. [DOI] [PubMed] [Google Scholar]
- 32.Redhead K, Watkins J, Barnard A, Mills K H G. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romagnani S. Th1 and Th2 in human disease. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 34.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H G. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 35.Shahin R D, Hamel J, Leef M L, Brodeur B R. Analysis of protective and nonprotective monoclonal antibodies specific for Bordetella pertussis lipooligosaccharide. Infect Immun. 1994;62:722–725. doi: 10.1128/iai.62.2.722-725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor P W. Complement-mediated killing of susceptible Gram-negative bacteria: an elusive mechanism. Exp Clin Immunogenet. 1992;9:48–56. [PubMed] [Google Scholar]
- 37.Turcotte M L, Martin D, Brodeur B R, Peppler M S. Tn5-induced lipopolysaccharide mutations in Bordetella pertussis that affect outer membrane function. Microbiology. 1997;143:2381–2394. doi: 10.1099/00221287-143-7-2381. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss A A, Melton A R, Walker K E, Andraos-Selim C, Meidl J J. Use of the promoter fusion transposon Tn5 lac to identify mutations in the Bordetella pertussis vir-regulated genes. Infect Immun. 1989;57:2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuki N, Ichihashi Y, Taki T. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J Neuroimmunol. 1995;60:161–164. doi: 10.1016/0165-5728(95)00052-4. [DOI] [PubMed] [Google Scholar]