Abstract
Background:
Hematuria is one of the most common symptoms in nephrology and urology. Due to the lack of extensive meta-analysis studies on the epidemiology of hematuria in Iran, this study was conducted to determine the epidemiological status of hematuria in Iran.
Methods:
In Sep 2020, researchers studied six international databases such as PubMed, ISI/WOS, ProQuest, Embase, Scopus, and Google Scholar for English papers and Iranian databases (SID and MagIran) for Persian papers. Joanna Briggs Institute (JBI) checklist was used to review and control the quality of articles. Heterogeneity between studies was assessed by Cochran's test and its composition using I2 statistics.
Results:
After several screening phase, the number of 25 article included to the final analysis. The prevalence of hematuria in the general population and children, in Iran were estimated at 16.4% (95% CI, - 0.05–37.9) and 1.6% (95% CI, 0.9–2.3) respectively. The odds ratio (OR) of women to men in the prevalence of hematuria in the general population 1.74, 95% CI: 1.20–2.52, P=0.003, patients with beta-thalassemia major 2.02, 95% CI: 1.11–3.65, P=0.020, children 2.61, 95% CI: 1.19–5.71, P=0.016, the elderly 1.50, 95% CI: 1.15–1.94, P=0.002, and taxi drivers 3.73, 95% CI: 2.58–5.38, P<0.001 was obtained.
Conclusion:
The prevalence of hematuria in the general population is relatively high. Hematuria is a good predictor for detecting of bladder cancer and Idiopathic hypercalciuria and the physician should attention to microscopic hematuria.
Keywords: Epidemiology, Microscopic hematuria, Systematic review, Meta-analysis, Iran
Introduction
Hematuria is one of the most common symptoms in nephrology and urology (1), observed to be self-limiting or chronic (2). According to the guidelines of the American Urological Association, published in 2012 and reviewed in 2016, hematuria refers to the presence of three or more red blood cells in a collected urine sample (without benign cause of the hematuria) (3), according to the severity of the disease, it is classified into two categories: microscopic and macroscopic (4, 5). Hematuria is seen in urinary tract sediment examination for a long time (6) and can be considered as an isolated finding associated with other renal abnormalities or as part of systemic disease (7). Hematuria can also is a sign of other diseases such as malignancies (8). For this reason, for accurate assessment of the individual, cross-sectional imaging using computed tomography and cystoscopy in patients aged 35 years and older, physical examination, urinalysis, and referral to an urologist are recommended (3, 9).
Hematuria is most often self-limiting, resolving with treatment of the underlying infection. Treatment of the underlying infectious etiology is paramount to preventing or attenuating the course of hematuria (10).
Numerous factors such as inflammation, bladder or prostate infection, urological malignancies, kidney stones, past or current smoking, old age, occupational exposure, and the use of anti-inflammatory drugs can play a role in causing hematuria (2, 11, 12).
The choice of a diagnostic protocol for patients with microscopic hematuria has wide clinical and economic implications, due to the high diagnostic costs, ultrasound with cystoscopy is more cost effective for diagnosis (13).
In Iran the prevalence of hematuria in general population is varies, as Bahrami et al reported 12.6% of hematuria and Mansour-Ghanaei et al reported 34.1% (14, 15). Moreover, the American Urological Association has recently reported that the prevalence of hematuria varies from 0% to 56% and is associated with factors such as age, sex, estimated population, source of referral, and clinical status (16). Occasionally asymptomatic microscopic hematuria can be companion by macroscopic attacks, in addition can be cause of proteinuria, hypertension and chronic renal failure, and ordinarily leads to end-stage renal disease (ESRD)(1). These symptoms may occur at any time between the first and seventh decade of life (8). Approximately 3.6% of patients with hematuria will develop urinary tract malignancy in the future (16). In patients with advanced chronic renal disease who also have hematuria, the rate of renal function decline is more (6). Many studies have tested the prevalence of microscopic hematuria with varying percentages in a general screening population. In a similar study, out of 37 people with urological malignancies, 16 had to have a urine test again, of which 13 had hematuria (3). The prevalence of hematuria is significantly higher in elderly people who took regular doses of aspirin than in older people who did not take aspirin (17).
Our knowledge about global and regional burden of hematuria is very limited, but in a recent study of the Global Burden of Disease (GBD) published on chronic kidney disease (CKD), valuable information has been obtained. Globally, in 2017, 1.2 million people worldwide died of CKD. The prevalence of CKD worldwide has increased by 29.3% since 1990. In Iran, the age- standardized prevalence of CKD was 10924 per 100000 in 2017 and the age- standardized death rate was 16.6 per 100000 (18).
In Taiwan, hematuria was significantly associated with sex, age, BMI, and urbanization (19). In Chronic Renal Insufficiency Cohort (CRIC) Study, hematuria was associated with a significantly higher risk of CKD progression and death in the first 2 years of follow-up. Individuals with hematuria were more likely to be Hispanic (22% vs. 9.5%, respectively), lower mean eGFR (40.2 vs. 45.3 ml/min/1.73 m2), higher levels of urinary albumin >1.0 g/day (36% vs. 10%), and have diabetes (56% vs. 48%) (20).
Hematuria has a major effect on global health, both as a direct cause of morbidity and death and as an important risk factor for CKD. Studies on the epidemiology of hematuria in Iran are limited and there is not accurate information about the epidemiology of hematuria. Otherwise, the results of other studies have shown different estimates in the general population, children and other groups. Due to the lack of extensive meta-analysis studies on the epidemiology of hematuria in Iran, this study was conducted to determine the epidemiological status of hematuria in Iran.
Methods
Study protocol
The present study was a systematic review of the microscopic hematuria and its risk factors in Iran. The reporting method of the present study is based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) checklist (21). PRISMA is an evidence-based minimum set of items aimed at helping authors to report a wide array of systematic reviews and meta-analyses. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research.
Search strategy
In Sep 2020, researchers studied six international databases such as PubMed, Embase, ProQuest, ISI/WOS, Scopus, and Embase for English papers and Iranian databases (MagIran and SID) for Persian papers. The selected keywords for databases included “Hematuria”, “Epidemiology”, “Prevalence”, “Frequency”, “occurrence”, “Incidence”, “Risk factor” and “Iran”. Two researchers examined the articles separately. The collected articles was entered into EndNote, X7 software and duplicate articles were automatically deleted.
Inclusion and exclusion criteria
Inclusion criteria
All articles done on the prevalence and risk factors related to microscopic hematuria without time and language restrictions in Iran.
Exclusion criteria
Articles whose full text was not available, also, conferences paper, seminars, and dissertations.
Quality assessment
The Joanna Briggs Institute (JBI) checklist was used to assess the quality of the articles (22). The purpose of this appraisal was to assess the methodological quality of a study and to determine the extent to which a study has addressed the possibility of bias in its conduct, design, and analysis. The checklist has nine questions that are marked with the answer “Yes”, “No”, “Not applicable” and “Not clear”. If the score was more than 7, the article was of high quality, if it was between 4 and 6, it was of medium quality; and if it was less than 4, it was of low quality. The results of the quality assessment presented in Table 1.
Table 1:
JBI critical appraisal checklist applied for included studies
| Author Name/Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Quality points | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mansour-Ghanaei, 2019(15) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | 7/9 | High |
| Motamed, 2018(23) | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | 6/9 | Medium |
| Akhavan Sepahi, 2017(24) | Yes | Yes | Yes | Yes | Yes | Not clear | No | No | Not clear | 5/9 | Medium |
| Esteghamati, 2017(25) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Not clear | 4/9 | Medium |
| Moghtaderi, 2013(26) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | 5/9 | Medium |
| Safaei, 2013(27) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Not clear | 7/9 | Medium |
| Shafi, 2013(28) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | 8/9 | High |
| Madani, 2012(29) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9/9 | High |
| Ahmadi, 2012(9) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 8/9 | High |
| Barahimi, 2011(14) | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | No | Not clear | 6/9 | Medium |
| Salehi, 2011(30) | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | No | No | 6/9 | Medium |
| Mahdavi-Mazdeh, 2010(31) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Not clear | 8/9 | High |
| Emamghorashi, 2010(32) | Yes | Yes | Yes | Yes | Yes | No | No | No | Not clear | 5/9 | Medium |
| Akhavan Sepahi, 2010(33) | Yes | Yes | Yes | Not clear | Yes | Not clear | No | No | Not clear | 4/9 | Medium |
| Fallahzadeh, 2010(34) | Yes | Yes | Yes | Yes | Yes | Not clear | Yes | No | No | 6/9 | Medium |
| Shajari, 2009(35) | Yes | Yes | Yes | Yes | Yes | No | No | No | No | 5/9 | Medium |
| Badeli, 2009(36) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Not clear | 6/9 | Medium |
| Derakhshan, 2008(37) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 7/9 | High |
| Ahmadzadeh, 2008(38) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Not clear | 6/9 | Medium |
| Sadeghi, 2008(39) | Yes | Yes | Yes | Yes | No | No | Yes | No | Not clear | 4/9 | Medium |
| Esfahani, 2007(40) | Yes | Yes | Yes | Not clear | No | Yes | No | No | Not clear | 4/9 | Medium |
| Safary, 2005(41) | Yes | Yes | Yes | Yes | Yes | Not clear | No | No | Not clear | 5/9 | Medium |
| Ghasemi, 2004(42) | Yes | Yes | Yes | Yes | No | No | No | No | Not clear | 4/9 | Medium |
| Vazirian, 2004(43) | Yes | Yes | Yes | Yes | No | No | Yes | No | Not clear | 4/9 | Medium |
| Shahidi, 2001(44) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | 7/9 | High |
Q1. Sample was representative?
Q2. Participants appropriately recruited?
Q3. Sample size was adequate?
Q4. Study subjects and the setting described?
Q5. Data analysis conducted?
Q6. Objective, standard criteria, reliably used?
Q7. Appropriate statistical analysis used?
Q8. Confounding factors/subgroups/differences identified and accounted?
Q9. Subpopulations identified using objective criteria?
Screening studies
The initial search for the articles was performed independently by two people (S.H and M.K). Screening of the studies, extracting results, and assessing the quality control of studies was done separately by two people (M.K and F.H). If there were no agreement between the two, the team leader (S.H) would give the final opinion on the article.
Data extraction from
All final papers submitted to the study process were prepared for data extraction by a pre-prepared checklist. This checklist included the author's first name, year of publication, study period, place of study, and point estimate.
Statistical analysis
Heterogeneity between studies was assessed by Cochran's test (with a significance level of less than 0.1) and its composition using I2 statistics. If the I2 was higher than 50%, it indicates high heterogeneity. In the case of heterogeneity, the random-effects model was used with the inverse variance method. The prevalence of hematuria was calculated based on the percentage or the amount reported in the studies. The results of prevalence were reported as the pooled estimates and the 95% confidence intervals (CIs). Meta-regression was used to examine the impact of moderator variables on prevalence of hematuria using regression-based techniques. Meta-regression was done to explore the sources of potential heterogeneity using the following variables: publication year and sample size. The odds ratio (OR) index was used to compare prevalence of hematuria in groups. Publication bias was investigated using Egger test. All analyzes were performed using CMA version 2 statistical software.
Results
Description of the study search
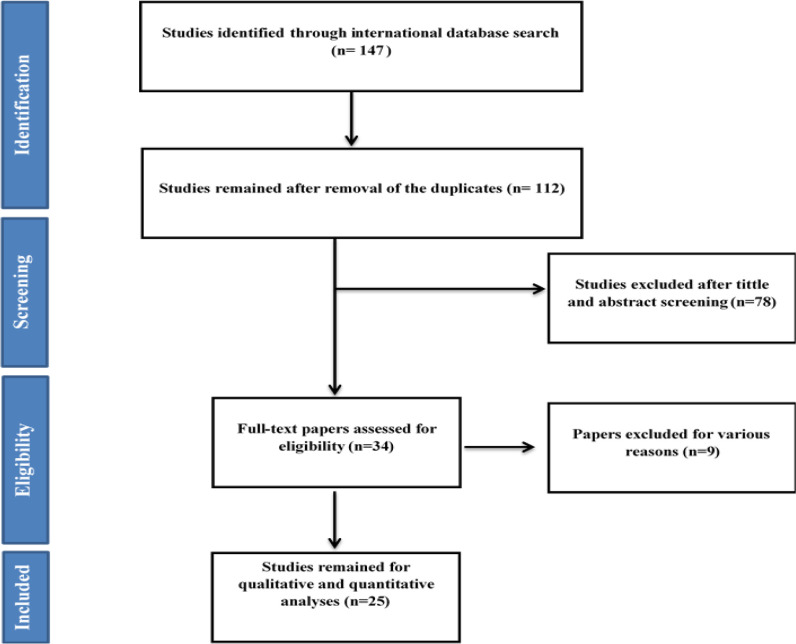
After searching all international databases, 147 articles were found, which after removing 35 duplicate articles, the 112 article entered the review stage in terms of title and abstract. After reviewing the titles and abstracts of the articles, 34 articles entered the next stage, in which the full text of the articles was reviewed and 25 articles entered the final analysis. References to imported articles were also reviewed to add relevant studies. Articles were excluded from the study for a variety of reasons, including insufficient information (15), irrelevant population (71), and duplication of results (n=1). The flowchart of the included studies is shown in Fig. 1.
Fig. 1:
Flowchart of the included studies in systematic review
Description of the entered studies
The characteristics of the included studies (9, 14, 15, 23–44) presented in Table 2. Based on the results obtained from the geographical explanation, 6 studies in Fars Province(23, 30, 32, 34, 35, 37), 3 studies in Tehran Province(29, 31, 40), 3 studies in Guilan Province(15, 27, 36), 2 studies in Isfahan Province(14, 44), 2 studies in Qom Province(24, 33), 2 studies in Mazandaran Province(9, 28), and one study in the Hormozgan(25), Bushehr(42), Gorgan (Golestan Province)(26), Khuzestan(38), Zahedan (Sistan & Baluchestan Province)(39), Hamedan(41), and Kermanshah(43).
Table 2:
Basic characteristics of the studies included in the review
| Order | Author, year | location | Sample size | Population | Age (mean or range) | Prevalence of hematuria | Age category | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Gender | Total | ||||||||
| Male | Female | ||||||||
| 1 | Shahidi 2001(44) | Isfahan | 1000 | Adult | - | - | - | 2.6 % | |
| 2 | Vazirian 2004(43) | Kermansh | 6831 | Children | Primary school | - | - | 0.25% | |
| 3 | Ghasemi 2004(42) | Boushehr | 2047 | Children | 6–13 y | (18/1035) 1.74 % | (28/1012) 2.77 % | (43/2047) 2.1% | - |
| 4 | Safary 2005(41) | Hamadan | 800 | Children | 10–11 y | - | - | 4.1% | |
| 5 | Esfahani 2007(40) | Tehran | 28 | Idiopathic Hypercalciuria | 6–11 | (2/16) 12.5% | (1/12) 8.33% | (3/28) 10.71% | |
| 6 | Sadeghi 2008(39) | Zahedan | 1169 | Children | 7–15 y | 1 | 2.1 | 7–9 = 1.59 10–12= 1.48 13–15= 1.54 |
|
| 7 | Ahmadzadeh 2008(38) | Khuzestan | 15 | Idiopathic Hypercalciuria | 6–12 y | - | - | (10/15) 66% | |
| 8 | Derakhshan 2008(37) | Fars | 108 | Beta-Thalassemia | 4–24 y for thalassemia intermediate | - | - | (17/50) 34% | In all thalassemia: (19/108) 17.6% |
| 3–24 y for thalassemia major | - | - | (2/58) 3.44 % | ||||||
| 9 | Badeli 2009(36) | Guilan | 1520 | Children | 4–6 y | - | - | 0.85% | |
| 10 | Shajari 2009(35) | Fars | 1601 | Children | 6–7 | - | - | 1% | |
| 11 | Fallahzadeh 2010(34) | Fars | 500 | Beta-Thalassemia Major | 6m – 32y | 19/256 | 34/244 | (53/500) 10.6% | < 20= 9.8 >20= 20 |
| 12 | Akhavan Sepahi 2010(33) | Qom | 100 | Urolithiasis (renal stone) | <14 y | - | - | (70/100) 70% | |
| 13 | Emamghorashi 2010(32) | Fars | 1068 | Children | 7–11 y | - | - | 3.3 % | |
| 14 | Mahdavi-Mazdeh 2010(31) | Tehran | 30747 | Taxi Drivers | 18–86 | (546/30254) 1.8% | (32/502) 6.4% | (578/30747) 1.87% | |
| 15 | Salehi 2011(30) | Fars | 216 | Bladder Cancer | - | - | - | (183/216) 84.7% | |
| 16 | Barahimi 2011(14) | Isfahan | 1400 | General population >30 | >30 | 7.2 % | 14.6 % | 12.6% | |
| 17 | Ahmadi 2012(9) | Mazandaran | 112 | Bladder Cancer | - | - | - | 49.1% | |
| 18 | Madani 2012(29) | Tehran | Hypercalciuria | 40.7% | |||||
| 19 | Shafi 2013(28) | Mazandaran | 175 | Bladder Cancer | - | - | - | (111/175) 63.4% | >40 63.4% (99/156) <40 = 63.1% (12/19) |
| 20 | Safaei 2013(27) | Guilan | 19 | Idiopathic Hypercalciuria | - | (2/11) 18.1% | (1/8) 12.5% | (3/19) 15.78% | |
| 21 | Moghtaderi 2013(26) | Gorgan | 3000 | Children | 6–14 | (8/1800) 0.44 % | (27/1200) 2.25 % | (35/3000) 1.16% | |
| 22 | Esteghamati 2017(25) | Hormozgan | 153 | Idiopathic Hypercalciuria | 2m–14y | - | - | (28/153) 18.3% | |
| 23 | Akhavan Sepahi 2017(24) | Qom | 199 | Children with Nephrolithiasis | < 14 y | - | - | 38.7% | |
| 24 | Motamed 2018(23) | Fars | 756 | Bladder Cancer | - | 79.3 % | 78.9 % | (598/756) 79.1% | |
| 25 | Mansour-Ghanaei 2019(15) | Guilan | 10520 | General population >35 | 35–70 | (1431/4887) 29.3% | (2151/5633) 38.1 | (3585/10520) 34.1% | |
Prevalence of hematuria in Iran in different groups
General population
Based on the results of 3 studies (14, 15, 44), the overall prevalence of hematuria in the general population was estimated at 16.4% (95 % CI, −0.05–37.9, I2=99.8%, P<0.001) (Fig. 2).
Fig. 2:
The prevalence of hematuria in Iranian general population
Children
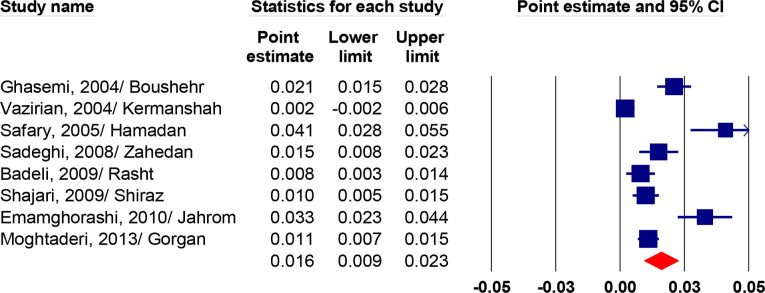
According to the results of eight studies(26, 32, 35, 36, 39, 41–43) that reported the prevalence of hematuria in children, the prevalence in Iran was 1.6% (95 % CI, 0.9–2.3, I2=90.2 %, P<0.001) (Fig. 3).
Fig. 3:
The prevalence of hematuria in Iranian children
Idiopathic Hypercalciuria
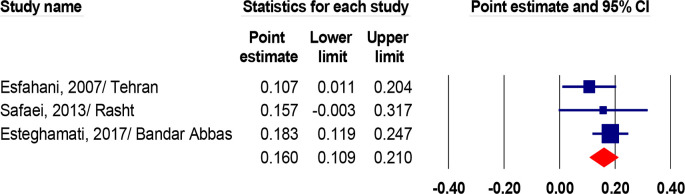
Based on three studies in the population (25, 27, 29), the prevalence of hematuria was estimated at 16% (95% CI, 10.9–21.0, I2=82.1 %, P<0.001). (Fig. 4).
Fig. 4:
The prevalence of hematuria in Idiopathic hypercalciuria
Meta- regression
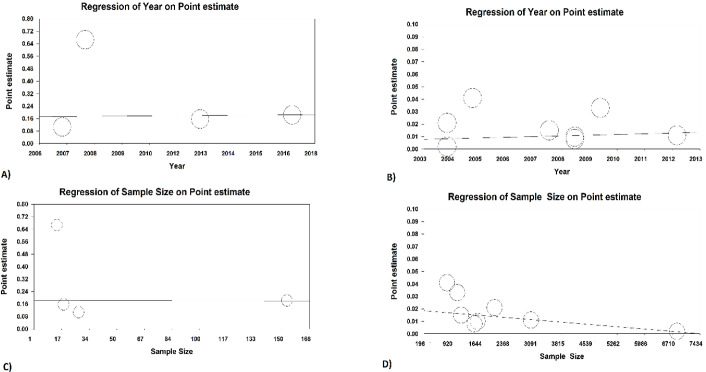
The results of meta-regression for sample size showed that there was a negative significant association between sample size and prevalence of hematuria in children (Reg coef= −0.0001, P<0.001). There was no significant association between year of publication and prevalence of hematuria in children (Reg coef= 0.0001, P=0.061). Moreover, no significant association between sample size (Reg coef= 0.0001, P=0.856).and year of publication (Reg coef= −0.0002, P=0.964) was observed in prevalence of hematuria in Idiopathic Hypercalciuria group (Fig. 5).
Fig. 5:
Result of meta-regression for based on sample size and year of study
A: Year of study and prevalence of hematuria in Idiopathic Hypercalciuria group.
B: Year of study and prevalence of hematuria in children.
C: Sample size and prevalence of hematuria in Idiopathic Hypercalciuria group.
D: Sample size and prevalence of hematuria in children.
Risk factors
Gender
In most of the studied groups, women had a higher chance of hematuria, except for idiopathic hypercalciuria and bladder cancer patients, which was more common in men, but this relationship was not statistically significant (P>0.05).
The odds ratio (OR) of women to men in the prevalence of hematuria in the general population (1.74, 95 % CI: 1.20–2.52, P=0.003), patients with beta-thalassemia major (2.02, 95 % CI: 1.11–3.65, P=0.020), children (2.61, 95 % CI: 1.19–5.71, P=0.016), the elderly (1.50, 95 % CI: 1.15–1.94, P=0.002), and taxi drivers (3.73, 95 %CI: 2.58–5.38, P<0.001) was obtained.
Publication Bias
Publication bias was not observed amongst the included studies according to the results of the Egger test (P=0.452).
Discussion
Microscopic hematuria is often asymptomatic and generally has a clinical prevalence of 4% to 5% (45). This percentage may be due to underlying kidney or urinary tract disease, so extensive urological and nephrological differential diagnosis should be considered for hematuria.
Microscopic hematuria can have many reasons. According to our results, the prevalence of hematuria in patients with beta-thalassemia major is 7.1%, patients with bladder cancer 69.8%, in children 1.6%, patients with idiopathic hypercalciuria equal to 16%, and finally in the general population was equal to 16.4%.
“Urinary bladder cancer is usually associated with asymptomatic hematuria; the percentage of hematuria in patients with bladder cancer is very high (up to 5% of patients with microscopic hematuria and approximately 10% of patients with gross hematuria have urinary tract cancer” (46). This condition is important because it can be a warning sign for the initial detection of bladder cancers. Other studies have also shown that the percentage of hematuria in patients with bladder cancer is high (3).
Older age, being a man, and greater hematuria increase the risk of having urinary tract cancer in individuals (47).
The prevalence of hematuria in children in this study was 1.6%, which is lower than the percentage of hematuria in a review study (10), which was equal to 4%. This could be due to the measurement of hematuria in RBCs above five in each microscopic field. Being a girl increases the probability of having microscopic hematuria by 2.61 times.
Moreover, the percentage of hematuria in the general population in this study was much higher than in other studies and was equal to 16.4%. This is due to the high percentage of lyrical (15), the percentage of hematuria was equal to 34.1%. One of the reasons for this high percentage is the high age of the people studying lyrics, colleagues, and factors such as kidney stones and so on. The percentage of hematuria in the general population in women was 1.74 times higher than men were. This study also showed that the percentage of hematuria in patients with idiopathic hypercalciuria was high and was equal to 16%. This issue has also been seen in another study (25).
On the other hand, the prevalence of hematuria in idiopathic hypercalciuria patients was much higher and was equal to 31%. This indicates an increase in hematuria in idiopathic hypercalciuria patients, so these numbers may vary based on differences in the design environment of the model and the data collection.
According to the data obtained in different groups, women generally have more hematuria than men (except in idiopathic hypercalciuria groups) do. According to this chart, being a woman in the two groups of bladder cancer and idiopathic hypercalciuria were 2.5% and 36% protective in causing hematuria, respectively, but from a statistical point of view, this ratio was not significant and it can be practically concluded that being a woman is the cause. There is a risk of developing hematuria in kidney disease. An increase in the number of deliveries and the use of vaginal estrogen was associated with an increase in hematuria in women (48).
Whereas hematuria is a usual disease, there is no evidence-based algorithm for diagnostic evaluation. Therefore, to detect or deny underlying disease that needs treatment all the causes of hematuria should be investigated and each individual risk factor must be considered (45).
Study Limitations
Our study was limited. One limitation of this study was the small number of studies performed on hematuria patients in Iran, which made our estimates difficult. Another limitation of this study was how hematuria-related factors were reported so that ultimately the only comparable factor for us was gender, and other factors such as hematuria-related risk factors could not be measured in Iran.
Conclusion
The prevalence of hematuria in the general population is relatively high. Hematuria is one of the most important risk factors of bladder cancer and CKD, so the presence of hematuria can be an appropriate predictor of bladder cancer. Therefore, the physician should emphasize the hematuria in all age groups.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
None.
Footnotes
Data availability
Resources of data and statistical analysis outputs of this study can be provided by the author of correspondence.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Plevová P, Gut J, Janda J. (2017). Familial hematuria: a review. Medicina (Kaunas), 53:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Davis R, Jones JS, Barocas DA, et al. (2012). Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol, 188(6 Suppl):2473–81. [DOI] [PubMed] [Google Scholar]
- 3.Ghandour R, Freifeld Y, Singla N, Lotan Y. (2019). Evaluation of Hematuria in a Large Public Health Care System. Bladder Cancer, 5:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno JA, Sevillano A, Gutierrez E, et al. (2019). Glomerular Hematuria: Cause or Consequence of Renal Inflammation? Int J Mol Sci, 20(9):2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossfeld GD, Litwin MS, Wolf JS, et al. (2001). Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology, 57:599–603. [DOI] [PubMed] [Google Scholar]
- 6.Sevillano AM, Gutierrez E, Yuste C, et al. (2017). Remission of Hematuria Improves Renal Survival in IgA Nephropathy. J Am Soc Nephrol, 28:3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivante A, Afek A, Frenkel-Nir Y, et al. (2011). Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA, 306:729–36. [DOI] [PubMed] [Google Scholar]
- 8.Deltas C, Pierides A, Voskarides K. (2013). Molecular genetics of familial hematuric diseases. Nephrol Dial Transplant, 28:2946–60. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi M, Ranjbaran H, Amiri MM, et al. (2012). Epidemiologic and socioeconomic status of bladder cancer in Mazandaran Province, northern Iran. Asian Pac J Cancer Prev, 13:5053–6. [DOI] [PubMed] [Google Scholar]
- 10.Bignall ONR, 2nd, Dixon BP. (2018). Management of Hematuria in Children. Curr Treat Options Pediatr, 4(3):333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qvigstad C, Tait RC, de Moerloose P, Holme PA. (2020). Hematuria in aging men with hemophilia: Association with factor prophylaxis. Res Pract Thromb Haemost, 4:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez AN, Lipsky MJ, Li G, et al. (2019). The Prevalence of Bladder Cancer During Cystoscopy for Asymptomatic Microscopic Hematuria. Urology, 126:34–38. [DOI] [PubMed] [Google Scholar]
- 13.Halpern JA, Chughtai B, Ghomrawi H. (2017). Cost-effectiveness of Common Diagnostic Approaches for Evaluation of Asymptomatic Microscopic Hematuria. JAMA Intern Med, 177:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barahimi H, Najafi I, Esmailian R, et al. (2011). Distribution of albuminuria and low glomerular filtration rate in a rural area, Shahreza, Iran. Iran J Kidney Dis, 5:374–9. [PubMed] [Google Scholar]
- 15.Mansour-Ghanaei F, Joukar F, Naghipour MR, et al. (2019). The PERSIAN Guilan Cohort Study (PGCS). Arch Iran Med, 22:39–45. [PubMed] [Google Scholar]
- 16.Mazhari R, Kimmel PL. (2002). Hematuria: an algorithmic approach to finding the cause. Cleve Clin J Med, 69(11):870, 872–4, 876 passim. [DOI] [PubMed] [Google Scholar]
- 17.Moudi E, Hosseini S-R, Bijani A. (2016). Higher rate of microscopic hematuria in elderly patients who take regular doses of aspirin: Result from AHAP Study. Caspian J Intern Med, 7(4):278–282. [PMC free article] [PubMed] [Google Scholar]
- 18.GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M-C, Wang J-H, Chen J-S, et al. (2020). Socio-Demographic Factors Affect the Prevalence of Hematuria and Proteinuria Among School Children in Hualien, Taiwan: A Longitudinal Localization-Based Cohort Study. Front Pediatr, 8:600907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlandi PF, Fujii N, Roy J, Chen HY, et al. (2018). Hematuria as a risk factor for progression of chronic kidney disease and death: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol, 19:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute JB. (2017). The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Analytical Cross Sectional Studies. North Adelaide, Australia The Joanna Briggs Institute. [Google Scholar]
- 23.Motamed S, Aminsharifi A, Vardanjani H. (2018). Bladder cancer in southwestern Iran: Histopathologic and clinical. Pakistan Journal of Medical and Health Sciences, 12(2):884–888. [Google Scholar]
- 24.Akhavan Sepahi M, Eftekhari SS, Rashidinia S, et al. (2017). Relationship between urinary reflux and nephrolithiasis in children-a cross-sectional study. Int J Pediatr, 5:4965–4973. [Google Scholar]
- 25.Esteghamati M, Ghasemi K, Nami M. (2017). Prevalence of idiopathic hypercalciuria in children with urinary system related symptoms attending a pediatric hospital in Bandar Abbas in 2014. Electron Physician, 9:5261–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghtaderi M, Noohi AH, Safaeyan B, et al. (2014). Screening for microscopic hematuria in school-age children of Gorgan City. Iran J Kidney Dis, 8:70–2. [PubMed] [Google Scholar]
- 27.Safaei Asl A, Heidarzadeh A, Maleknejad S, Moradi B. (2013). Hypercalciuria in school-aged children of Rasht: a single-center study. Iran J Kidney Dis, 7:265–7. [PubMed] [Google Scholar]
- 28.Shafi H, Ali Ramaji A, Akbarzadeh Pasha A, et al. (2013). A Survey on 175 Cases of Bladder Cancer in the Patients Who Referred to the Hospitals Affiliated to Babol University of Medical Sciences, Iran (2001–2011). Journal of Babol University of Medical Sciences, 15:116–122. [Google Scholar]
- 29.Madani A, Kermani N, Ataei N, et al. (2012). Urinary calcium and uric acid excretion in children with vesicoureteral reflux. Pediatr Nephrol, 27:95–9. [DOI] [PubMed] [Google Scholar]
- 30.Salehi A, Khezri AA, Malekmakan L, Aminsharifi A. (2011). Epidemiologic status of bladder cancer in Shiraz, southern Iran. Asian Pac J Cancer Prev, 12:1323–7. [PubMed] [Google Scholar]
- 31.Mahdavi-Mazdeh M, Saeed Hashemi Nazri S, Hajghasemi E, et al. (2010). Screening for decreased renal function in taxi drivers in Tehran, Iran. Ren Fail, 32:62–8. [DOI] [PubMed] [Google Scholar]
- 32.Emamghorashi F, Davami MH, Rohi R. (2010). Hypercalciuria in Jahrom's school-age children: what is normal calcium-creatinine ratio? Iran J Kidney Dis, 4:112–5. [PubMed] [Google Scholar]
- 33.Sepahi MA, Heidari A, Shajari A. (2010). Clinical manifestations and etiology of renal stones in children less than 14 years age. Saudi J Kidney Dis Transpl, 21:181–4. [PubMed] [Google Scholar]
- 34.Fallahzadeh MH, Fallahzadeh MK, Shahriari M, et al. (2010). Hematuria in patients with Beta-thalassemia major. Iran J Kidney Dis, 4:133–6. [PubMed] [Google Scholar]
- 35.Shajari A, Shajari H, Zade MH, Kamali K, Kadivar MR, Nourani F. (2009). Benefit of urinalysis. Indian J Pediatr, 76:639–41. [DOI] [PubMed] [Google Scholar]
- 36.Badeli H, Heydarzadeh A, Ahmadian M. (2009). Prevalence of hematuria and proteinuria in healthy 4 to 6 year old children in daycare centers of Rasht (Northern Iran). Iranian Journal of Pediatrics, 19:169–172. [Google Scholar]
- 37.Ali D, Mehran K, Moghaddam AG. (2008). Comparative evaluation of renal findings in Beta-thalassemia major and intermedia. Saudi J Kidney Dis Transpl, 19:206–9. [PubMed] [Google Scholar]
- 38.Ahmadzadeh A, Hakim Zm, Safa AA. (2008). Idiopathic hypercalciuria in Iranian children. (In Persian) [Google Scholar]
- 39.Sadeghi Majd S, Hadian MA, Rakhshani F. (2008). Prevalence of asymptomatic hematuria and proteinuria among school children in Zahedan. Zahedan Journal of Research in Medical Sciences, 10:59–64. [Google Scholar]
- 40.Esfahani ST, Madani A, Siadati AA, Nabavi M. (2007). Prevalence and symptoms of idiopathic hypercalciuria in primary school children of Tehran. Iranian Journal of Pediatrics, 17:353–358. [Google Scholar]
- 41.Safary M, Darogheh Z, Derakhshan M, Nabavizadeh S. (2005). Abnormal Urinary Findings In School Aged Children In Hamedan. Medical Journal of Mashhad University of Medical Sciences, 48:17–22. [Google Scholar]
- 42.Ghasemi K, Beigi S, Shojaee M. (2004). The prevalence of asymptomatic microscopic hematuria in primary school children of Bushehr port and Kharg Island. Iranian South Medical Journal, 7:54–60. [Google Scholar]
- 43.Vazirian S, Golpaygani M, Aminzadeh M, et al. (2004). Evaluation of Urinalysis Results in Asymptomatic Primary School Children in Kermanshah city (2003). J Kermanshah Univ Med Sci, 8:e81316. [Google Scholar]
- 44.Shahidi S, Salek M, Adilipour H. (2001). Hematuria in asymptomatic adults: its prevalence and its follow up. Journal of Research in Medical Sciences, 6. [Google Scholar]
- 45.Bolenz C, Schröppel B, Eisenhardt A, Schmitz-Dräger BJ, Grimm M-O. (2018). The Investigation of Hematuria. Dtsch Arztebl Int, 115(48):801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karnes RJ, Fernandez CA, Shuber AP. (2012). A noninvasive multianalyte urine-based diagnostic assay for urothelial cancer of the bladder in the evaluation of hematuria. Mayo Clin Proc, 87:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung H, Gleason JM, Loo RK, et al. (2011). Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. J Urol, 185:1698–703. [DOI] [PubMed] [Google Scholar]
- 48.Richter LA, Lippmann QK, Jallad K, et al. (2016). Risk factors for microscopic hematuria in women. Female Pelvic Med Reconstr Surg, 22(6):486–490. [DOI] [PubMed] [Google Scholar]